Abstract
By mimicking the info-chemicals emitted by grazers, the common anionic surfactant sodium dodecyl sulfate (SDS) can induce colony formation in the green algal genus Scenedesmus at environmentally relevant concentrations. The morphometric effects can hinder the feeding efficiency of grazers, reducing energy flow along the pelagic food chain from Scenedesmus to consumers. Despite this potential ecological risk, few studies exist on whether the SDS-triggered induction of colonies is common in other species of the family Scenedesmaceae. Here, we investigated the effects of SDS on the growth and morphology of three species of Scenedesmaceae (Desmodesmus subspicatus, Scenedesmus acutus, and Tetradesmus dimorphus) and on the clearance rates of Daphnia galeata grazing on the SDS-induced colonies. SDS triggered colony formation in all algal species at concentrations nonlethal to them (0.1–10 mg L−1) in 72 h; however, the induction levels of colony formation were generally lower than for those in the Daphnia culture medium. We also found that the SDS-induced colonial algae reduced D. galeata clearance rates. Our results highlight the potential effect of SDS on the Daphnia–Scenedesmaceae system by triggering the morphological response of Scenedesmaceae at concentrations below those that exert toxicity. Such disruptive effects of pollutants on predator–prey interactions should be considered within the framework of ecological risk assessments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Sodium dodecyl sulfate (SDS) is a common anionic surfactant for various applications: industry (e.g., cleaning agents and auxiliary agents of pesticides), medical care (e.g., pharmaceuticals), and household goods (e.g., personal care products and cosmetics). Owing to its extensive consumption, SDS can contaminate aquatic systems through the direct discharge of sewage effluents or by soil leaching (Rebello 2014). The toxicity of SDS to aquatic organisms has been investigated since the 1990s. For instance, the 50% effective or lethal concentration (EC50 or LC50) of SDS to fishes, invertebrates (mainly cladoceran crustaceans), and algae was 7.3–48 mg L−1 (e.g., Arezon et al. 2003; Hemmer et al. 2010; Reátegui-Zirena et al. 2013), 7.4–48 mg L−1 (e.g., Martinez-Jeronimo and Garcia-Gonzalez 1994; Bulus et al. 1996; Shedd et al. 1999), and 4.8–36.6 mg L−1 (Liwarska-Bizukojc et al. 2005; Mariani et al. 2006), respectively. Although SDS appears to be severely toxic to aquatic life at concentrations above 1 mg L−1, surfactants are generally present at concentrations below 0.5 mg L−1 in natural water because of their high biodegradability (Bondini et al. 2015; Jackson et al. 2016).
Despite SDS being almost harmless to aquatic organisms, several studies have mentioned its potential effects on the aquatic community through the impairment of predator–prey interactions (Lürling and Beekman 2002; Yasumoto et al. 2005; Zhu et al. 2020). In pelagic freshwater systems, the algal prey Scenedesmaceae and its grazer Daphnia are often used to describe the significance of a chemically induced defense (Van Donk 2007). For example, genus Desmodesmus and Scenedesmus exhibit polymorphology (change from a unicellular organism to multicellular colony) in response to info-chemicals (kairomones) emitted by Daphnia spp., in which the colonized algae can reduce the risk of being grazed (Lürling 2003). In a series of studies by Yasumoto et al. (2005, 2006, 2008a, 2008b), the Daphnia kairomones were identified as a group of aliphatic sulfates, which are compounds that are structurally analogous to synthesized anionic surfactants (Lürling 2012). Importantly, some anionic surfactants (e.g., SDS and mono- and didodecyl disulfanated diphenyloxide (FFD-6)) induce colony formation in Scenedesmaceae, even at very low concentrations (0.01–1 mg L−1, Yasumoto et al. 2005; Lürling et al. 2011). The colony formation induced by anionic surfactants in the absence of a grazer can impose unnecessary costs on Scenedesmaceae (e.g., enhanced sinking velocity to a lower unlit water layer, Lürling and Van Donk 2000). Even in a case where grazer’s info-chemicals were present, SDS enhanced and prolonged the expression level of colony formation (Zhu et al. 2020). Surfactant-induced colonial Scenedesmaceae can inhibit the feeding efficiency of planktonic grazers, leading to reduced energy flow from primary producers to consumers in the lake food chain (Lürling et al. 2011). Such interference effects by anionic surfactants on the colony formation of Scenedesmaceae should be considered when performing ecological risk assessments.
Although colony formation induced by grazer-released info-chemicals occurs in many species of the family Scenedesmaceae (five species of Desmodesmus and Scenedesmus, Lürling 2003; Tetradesmus dimorphus, Ha et al. 2004), the induction of colony formation mediated by anionic surfactants has been observed in only two species (S. obliquus and D. subspicatus, Lürling and Beekman 2002; Yasumoto et al. 2005). Therefore, there are limited studies whether anionic surfactants can trigger colony formation in various Scenedesmaceae species in the same manner with the grazer’s info-chemicals. Furthermore, a few studies have evaluated the grazers’ inhibitory effect on feeding rate via colony formation induced by anionic surfactants. For example, an FFD-6 induced colony of S. obliquus decreased the filtering rate of D. magna (Lürling et al. 2011). Compared with FFD-6 and Daphnia kairomones, SDS appears to exert only moderate activity on colony induction (Lürling and Beekman 2002; Yasumoto et al. 2005). Lower induction of colony formation by SDS may not be effecting the grazer’s feeding; however, such weak disruption effect of prey defense had not been studied much because previous experiments were performed under the conditions where the colony formation was clearly expressed.
SDS’s disruptive effects on predator–prey interactions through the induction of colony formation in Scenedesmaceae are problematic for the risk assessment of surfactants (e.g., Lürling et al. 2011). Hence, it is important to evaluate interspecific differences in SDS-mediated colony induction in Scenedesmaceae and any potential grazing interference effects. To elucidate the effects of SDS on the predator–prey interaction of Daphnia and Scenedesmaceae, we aimed to: (1) compare the effective concentration of SDS on growth inhibition and morphological change in three species of the family Scenedesmaceae (D. subspicatus, S. acutus, and T. dimorphus), and (2) evaluate the effects of SDS-induced colonies on D. galeata feeding rate.
Materials and Methods
Test Organisms
Single clones of D. subspicatus (NIES-802), S. acutus (NIES-95), and T. dimorphus (NIES-119) were obtained from the National Institute for Environmental Studies, Japan. The algal stocks were cultivated with autoclaved COMBO medium (Kilham et al. 1998) in a 1-L Erlenmeyer flask under constant laboratory conditions (22 ± 1 °C; light intensity of 60 μmol photons s−1 m−2; 16-h light to 8-h dark cycle). To maintain suspension of the algal cells and the exponential growth stage, stock cultures were manually shaken twice daily and culture water was replaced with fresh medium weekly.
Daphnia galeata was collected from Lake Kizaki (36°33′N, 137°50′E, Nagano Prefecture, Japan) with vertical tows of a Kitahara plankton net (22.5-cm mouth diameter, 0.1-mm mesh size). The stock culture of a single clonal line was established by isolating individuals from the original population. Daphnids were maintained under laboratory conditions (22 ± 1 °C; 16-h light to 8-h dark cycle) in 1-L glass beakers filled with autoclaved COMBO medium. The green alga Chlorella vulgaris (Chlorella Industry Co. Ltd, Fukuoka, Japan) was fed at a concentration of 5.0 × 105 cells mL−1 to the stock culture every two or three days. The culture medium was replaced with fresh medium once a week.
Experiment 1: Effect of SDS on Growth and Morphology of D. Subspicatus, S. Acutus, and T. Dimorphus
A 105 mg L−1 stock solution of SDS (≥ 99%; Merck KGaA, Darmstadt, Germany) was prepared using distilled water. Diluted stock solutions of five different SDS concentrations (10, 102, 103, 104, and 105 mg L−1) were also prepared by gradually diluting the original stock solution (105 mg L−1) with distilled water.
We performed culture experiments to investigate the effects of SDS on growth and morphology in D. subspicatus, S. acutus, and T. dimorphus, according to the Organization for Economic Cooperation and Development (OECD) test guideline no. 202 (OECD 2011). Each alga was obtained from those stock cultures and concentrated to approximately 106 cells mL−1 by centrifugation. The culture system was 100 mL of culture water—composed of 98 mL COMBO media, 1 mL concentrated alga, and 1 mL distilled water (for the control) or 1 mL SDS stock solution—in a 200-mL Erlenmeyer flask. The initial algal concentrations were 4.36 × 104 cells mL−1 for D. subspicatus, 3.25 × 104 cells mL−1 for S. acutus, and 1.25 × 104 cells mL−1 for T. dimorphus. The nominal concentrations of SDS in the experiments were 10–2–103 mg L−1 (common rate = 10; six treatments). The experiments were run for 72 h in triplicate under the same conditions as those used for the stock cultures. Water temperature, pH, and dissolved oxygen (DO) were measured for the initial condition of COMBO media and 72 h later in the controls, the lowest dose treatments, and the highest dose treatments.
Samples (0.3 mL) were collected daily for determination of cell density (cells mL−1) and morphological state. The number of cells and different morphologies (unicellular algae and two- to eight-celled colonies) were observed from at least 100 algal particles with a plankton counter (Matsunami Glass Ind. Ltd., Osaka, Japan) under a microscope at 200 × magnification. Chlorophyll a (Chl. a) concentrations (μg L−1) were also quantified at the beginning (in extra control samples, n = 3) and end of the experiment by ethanol extraction and fluorescence measurement in accordance with Marker et al. (1980).
Growth rates (μ, day−1) were calculated from changes in natural log-transformed algal biovolumes (cell density or Chl. a concentration) against time using the following equation:
where Vt is the final algal biovolume, V0 is the initial algal biovolume, and Δt is cultivation time (day).
Colony induction rate was determined as mean cells per particle (MCPs) using the following equation:
where NC is the total number of cells, and NP is the total number of colonies.
The initial and final SDS concentrations in the controls and each of the treatments were measured by methylene blue absorptiometry (Aomura et al. 1981), a spectrophotometric analysis of the ionic pair anionic surfactant–methylene blue extracted in chloroform. Water samples at the beginning of the experiments were 50 mL COMBO (control) and appropriate volumes of each SDS stock solution in the analytical quantitative range (2–50 μg L−1 as SDS). To remove algae, water samples collected at end of the experiments were filtered through a Whatman GF/C filter and then subjected to SDS analysis.
Cell-based and Chl. a-based growth rates (μ) for each algal species were statistically compared among treatments. Bartlett’s test was applied to the data set to evaluate whether equal variances could be assumed. A one-way ANOVA followed by the post hoc Tukey’s HSD test or Kruskal–Wallis rank sum test and the pairwise Welch’s t-test (P values were adjusted using Holm’s method) was conducted in accordance with the results of Bartlett’s test. Additionally, we estimated 72-h EC50 values of SDS for each algal species and their 95% confidence intervals (CIs) by fitting the cell-based growth rate to a three-parameter log-logistic model using the drc package (Ritz et al. 2016). In the estimation, SDS concentrations were applied as geometric mean of the beginning and end of the experiments (Table 1). The 72-h EC50 values estimated for different species were statistically compared via the ratio test (Ritz et al. 2006; Wheeler et al. 2006) using the EDcomp function in the drc package. The effects of time and SDS concentrations on the MCPs of each species were analyzed with a generalized linear model (GLM). We applied the identity-link and gamma distribution function to the GLM models. All statistical analyses above were performed using R software version 4.0.2 [R development Core Team, Vienna, Austria (http://www.R-project.org/)].
Experiment 2: Effect of SDS-Induced Colonies on D. Galeata Clearance Rates
A grazing experiment was conducted to evaluate the effect of SDS-induced colonies in the three tested algae on D. galeata clearance rates. To prepare unicellular and colonial prey, each algal species was previously cultured in the absence (controls) or presence of SDS (1 mg L−1) for 72 h under laboratory conditions. The MCPs and proportion of unicells and two- to eight-celled colonies in the controls and treatments for all species grown for 72 h were determined. Particle sizes of the unicells and two-, three-, four-, six-, and eight-celled colonies for each species were measured as surface-area dimensions (μm−2) using the Image J program ver. 1.51 K (National Institutes of Health, USA).
The grazing experiment was set up as a nested design with three factors: prey species (D. subspicatus, S. acutus, and T. dimorphus), algal condition (unicellular or colonial morph), and daphnid age (juvenile or adult). Daphnids aged 3 days (juveniles; mean body length = 1.0 mm, n = 10) and 7 days (adults; mean body length = 1.4 mm, n = 10) were collected from the stock cultures. The animals were rinsed with COMBO medium and then individually moved to a 15-mL plastic centrifuge tube containing 5 mL of the unicellular or colonial algal prey. Animal-free controls were served for each treatment. Treatments and controls were run for 2 h in triplicate at 22 °C in the dark, and the culture tubes were manually shaken every 30 min. Initially and after 2 h, the cell densities (cells mL−1) of all samples were measured. The initial SDS concentrations of the controls and treatments for each species were quantified via methylene blue absorptiometry; the values in the controls were below the lowest limit of quantification, and the values in the treatments were 1.0 × 10–2–2.0 × 10–2 mg L−1.
Clearance rates (CR, in mL h−1) were calculated using the following equations in reference to Lürling et al (2011):
where A0 is the initial algal concentration, AT is the final algal concentration in the treatments, AC,t is the final algal concentration in animal-free controls, Δt is the time (2 h), and V is the culture volume (5 mL).
In accordance with the results of Bartlett’s test, the differences in CRs and MCPs of the controls and treatments were compared using a one-way ANOVA or Kruskal–Wallis rank sum test. Statistical analyses were performed with R software.
Results
Effects of SDS on Growth and Morphology of D. Subspicatus, S. Acutus, and T. Dimorphus
Measured physicochemical characteristics (water temperature, pH, and DO) in Experiment 1 are shown in Table 1. The water temperature varied at less 1.0 °C during the experiments. The pH values at end of the experiments were 8.4–8.9 for the controls and the treatments 10–2 mg L−1, and 7.0–7.8 for the treatments 103 mg L−1. The DO values in the controls and the treatments 10–2 mg L−1 increased to 7.7–8.5 mg L−1. In the treatments 103 mg L−1, the DO values decreased to 6.0–6.6 mg L−1. These variations in controls and treatments did not seriously affect the algal growth conditions.
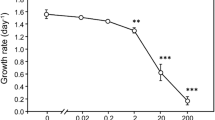
Both the cell-based and Chl. a-based growth rates of all tested species decreased with exposure to 102 mg L−1 SDS (Fig. 1a–c). Only the growth rate of D. subspicatus was nearly zero or below under 102 mg L−1 < of SDS concentration. The estimated 72-h EC50 values for each species were 23.2 mg L−1 (D. subspicatus), 157.5 mg L−1 (S. acutus), and 46.0 mg L−1 (T. dimorphus) (Table 2). The ratio test revealed that these EC50 values were significantly different (P < 0.05), indicating that sensitivity to SDS was as follows: D. subspicatus > T. dimorphus > S. acutus.
Growth rates (μ, day−1) based on the increase in cell density (filled circles) and on the increase in Chl.-a concentration (open circles) for a D. subspicatus, b S. acutus, and c T. Dimorphus incubated for 72 h in the controls and treatments (SDS concentrations of 10–2–103 mg L−1). Error bars show standard deviations (n = 3). Similar subscript letters (A–C and a–d) indicate homogenous groups that are not significantly different
The MCPs of each species changed over time and with SDS concentration. Increase in MCP appeared more rapidly in D. subspicatus and S. acutus (24 h later, Fig. 2a, b) compared with T. dimorphus (48 h later, Fig. 2c). In the controls, the MCPs of T. dimorphus varied over time (3.74–4.74, Fig. 2c, Table 3). Maximum MCP values were observed 48 h later in S. acutus (2.16, Fig. 2b) and 72 h later in both D. subspicatus (2.36, Fig. 2a) and T. dimorphus (6.22, Fig. 2c). GLM analysis revealed that the SDS concentrations to increase MCP were 0.1–10 mg L−1 for both D. subspicatus and T. dimorphus and 10–100 mg L−1 for S. acutus (Table 3). In higher dose levels than those SDS concentrations, MCP increases tended to be restricted in all species (Fig. 2a–c; Table 3).
Effect of SDS-Induced Colonies on D. Galeata Clearance Rates
Juvenile D. galeata feeding on SDS-induced colonial D. subspicatus and T. dimorphus in the treatments had significantly lower CRs than the daphnids in those controls (Fig. 3a, c). The reduced CRs in the treatments were also observed in adult daphnids feeding on D. subspicatus (Fig. 3a). Meanwhile, the CRs of the controls and treatments did not differ for S. acutus-fed daphnids, in spite of age (Fig. 3b). The MCPs of D. subspicatus and T. dimorphus in the treatments were significantly higher than those in the controls (Fig. 4a, c); however, the control and treatment MCPs of S. acutus were comparable (Fig. 4b). The higher MCP in the treatment population of D. subspicatus mostly resulted from a decreased proportion of unicells (12.6%) and increased proportion of four-celled colonies (7.7%) (Fig. 4d). In T. dimorphus, a reduction in unicells (9.3%) and an increase in four- and eight-celled colonies (17.8%) mainly contributed to the higher MCPs in the treatment (Fig. 4f). Differences in the surface-area dimensions of those unicells and colonies are presented in Table 4.
Mean clearance rates (CR, mL h−1) of juvenile and adult D. galeata grazing on food two types (unicells from the controls: white bars; colonies from the treatments: gray bars) of a D. subspicatus, b S. acutus, and c T. dimorphus. Error bars show standard deviations (n = 3). Asterisks denote a significant difference between the control and treatment. (** P < 0.01, *** P < 0.001)
Mean cells per particle (MCPs) and the proportions of unicells, two-, three-, four-, six-, and eight-celled colonies in a, d D. subspicatus populations, b, e S. acutus populations, and c, f T. dimorphus populations grown for 72 h in the absence (control; white bars) and presence of SDS (treatment; gray bars). Error bars show standard deviations (n = 3). Asterisks denote a significant difference between the control and treatment. (* P < 0.05, *** P < 0.001)
Discussion
The 72-h EC50 values of SDS for the three Scenedesmaceae species examined here ranged from 23.6 mg L−1 to 159.5 mg L−1 (Fig. 1a–c; Table 2), which were much greater than real-world surfactant concentrations (< 0.5 mg L−1, Bondini et al. 2015; Jackson et al. 2016). Still, SDS triggered colony formation in all tested species at concentrations 10–100 times lower than the 72-h EC50 (Fig. 2a–c, Table 3). Our results coincide with several other studies on S. obliquus; anionic surfactants (SDS or FFD-6) have consistently promoted colony formation in S. obliquus at concentrations below those at which growth was inhibited (Lürling and Beekman 2002; Lürling 2006; Lürling et al. 2011; Zhu et al. 2020). This indicates that the morphological response to anionic surfactants at harmless dose levels is common in the family Scenedesmaceae—at least in the genera Desmodesmus, Scenedesmus, and Tetradesmus. Given that colony formation of those genera following exposure to Daphnia culture medium has also been reported (Ha et al. 2004; Lürling 2003), the effect of the surfactant on Scenedesmaceae morphology may, not surprisingly, be attributed to its structural similarity to the compounds (aliphatic sulfates) emitted by Daphnia (Yasumoto et al. 2005, 2006, 2008a, 2008b). SDS also impeded morphological changes in all tested species at dose levels above the 72-h EC50 (Fig. 2a–c). Such limited induction of colony formation has also been found for other anionic surfactants (FFD-6, Lürling 2006; Lürling et al. 2011). Colonies of Scenedesmaceae are formed in an asexual reproduction process (production of daughter cells) of auto-sporulation in active growth periods (Lürling 2003). Therefore, such interference effects on colony formations may be the result of growth inhibition.
We detected a species-specific difference in the 72-h EC50 of SDS in the tested algae. Specifically, S. acutus was approximately 6.8 and 3.5 times less sensitive to SDS than were D. subspicatus and T. dimorphus, respectively (Table 2). In accordance with this trend, the morphological response of S. acutus to SDS was insensitive compared with those of the other species (Table 3). The sensitivity to SDS in another freshwater green alga Raphidocelis subcapitata (the 72-h LC50 = 36.6 mg L−1) was also comparable to those of D. subspicatus and T. dimorphus (Liwarska-Bizukojc 2005), but not S. acutus. We found no other study addressing differences in sensitivity to SDS within Scenedesmaceae, with the exception of Lürling (2006), who reported extremely less sensitivity in S. obliquus to the anionic surfactant FFD-6; growth inhibition was found at 103–104 mg L−1 of FFD-6. These results suggest that the genus Scenedesmus may have higher tolerance to anionic surfactants than other genera of the family Scenedesmaceae. While further investigation is needed to elucidate the differential sensitivities to SDS in Scenedesmaceae, our microscopic observations confirmed that there is no association between sensitivity to SDS and individual cell size; the particle size of unicells were ranked as follows: T. dimorphus > S. acutus > D. subspicatus (Table 4). Anionic surfactants can denature and bind protein in the cell wall (such as endogenous thylakoid proteins) and then affect chlorophyll synthesis (Chawla et al. 1987; Lewis 1990). Generally, algae with thicker cell walls are less likely to be affected by surfactants (Lweis 1990). Therefore, the species-specific sensitivity to SDS among the three Scenedesmaceae species may be resulted from the difference in the thickness of cell wall rather than individual cell size.
The induction level of colony formation in D. subspicatus by SDS nearly coincides with that of a previous study, where MCP was approximately 1.5–2.5 (Fig. 2a, Yasumoto et al. 2005). Those MCPs were smaller than those observed in Daphnia culture water (3.5 by Yasumoto et al. 2005). Scenedesmus acutus and T. dimorphus also showed smaller MCP with SDS exposure (1.4–2.1 for S. acutus and 5.8–6.2 for T. dimorphus, Fig. 2b, c) than with filtrates of the Daphnia culture medium (3.0 for S. acutus by Lürling and Van Donk 1996; 7.0 for T. dimorphus by Ha et al. 2004). Zhu et al. (2020) also reported that SDS exposure resulted in approximately 2.0–2.5 of MCPs in S. obliquus, whereas the Daphnia culture medium increased the MCPs to 4.0. These results indicate that SDS moderately induces the colonial morphology of Scenedesmaceae. In contrast, another anionic surfactant, octyl sodium sulfate, appeared to be highly active in producing colonial Scenedesmaceae, comparable with the Daphnia culture medium (Yasumoto et al. 2005; Yokota and Sterner 2011). The specific factors that determine colony formation remain unclear; however, differences in chemical structure of anionic surfactants, such as the number of double bonds, the presence/absence of a methyl group terminus and an alkyl chain length could be important factors (Yasumoto et al. 2005).
Despite the moderate activity of SDS on Scenedesmaceae morphology, interference effects on Daphnia feeding ability were found for the algal prey that had formed colonies following SDS pre-exposure (D. subspicatus and T. dimorphus); the CRs in juvenile or adult daphnids feeding on the colonial algae were reduced (Fig. 3a, c; Fig. 4a, c). Although SDS may have directly affected D. galeata, measured concentrations of SDS in the grazing experiments (1.0 × 10–2–2.0 × 10–2 mg L−1) were far below the levels toxic to Daphnia. For instance, the 48-h EC50 (or LC50) values of SDS to D. obtusa and D. magna were 9.8 mg L−1 and 24.8 mg L−1, respectively (Bulus et al. 1996; Martinez-Jeronimo and Garcia-Gonzalez 1994). Meanwhile, the anionic surfactant FFD-6 reduced the CRs of D. magna at a fivefold lower dose than the 48-h LC50 of D. magna (Lürling et al. 2011). Surfactants can indirectly affect the filtering efficiency of Daphnia by changing the surface charge of food particles (from neutral to net negative charges) and by increasing the wettability of both grazers and algal prey (Gerritsen and Porter 1982). A certain quantity of SDS may have absorbed onto the algae because the lower concentrations of SDS (10–2–101 mg L−1) dramatically decreased 72 h after exposure (Table 1). However, we also observed identical CRs between the controls and treatments for D. galeata feeding on S. acutus (Fig. 3b), indicating that the toxic effects on daphnids were small in the grazing experiments.
In accordance with the classical hypothesis that the colony-formed Scenedesmus have enhanced grazing resistance to herbivorous zooplanktons (e.g., Hessen and Van Donk 1993), the reduction in D. galeata CRs may have resulted from the increased particle size of algal food in the treatments (Fig. 3a, c). The D. subspicatus population in the treatment had a higher proportion of four-celled colonies (Fig. 4d) of which the apparent algal particle size (as the surface area, 223.1 ± 27.3 μm2) was approximately five times larger than that of the unicells (42.6 ± 8.0 μm2) (Table 4). Similarly, the proportion of larger-sized colonies in the T. dimorphus population increased in the treatment (Fig. 4f). The four-celled (1,673.2 ± 361.9 μm2) and eight-celled (3,825.8 ± 718.5 μm2) colonies differed markedly from the unicells (322.5 ± 76.5 μm2) (Table 4). Such particle size increases of entire algal populations can inhibit the feeding efficiency of small grazers. For example, Daphnia smaller than 1.2 mm exhibited lower CRs when they were feeding on colonial S. obliquus that have a mean particle volume of 150–300 μm3 (Lürling 2003). Because the MCPs highly correlate with the mean particle volume (e.g., Lürling 2006), colonial Scenedesmaceae with MCPs of even 1.5–3.0 can affect small grazers. In the present study, juvenile D. galeata (body length = 1.0 mm) was in small size class to being affected by colonial algal prey. In contrast, larger-sized cladocerans (approximately 1.6–2.5 mm) readily consumed colonial Scenedesmus (Lürling 2003). Although we detected a reduction in CRs in adult D. galeata feeding on colonial D. subspicatus (Fig. 3a), the size class of those daphnids (1.4-mm body length) were also below the large-sized zooplankton.
The potential ecological risks of several contaminants involve not only direct toxicity to aquatic organisms (i.e., detrimental effects on survival, growth, and reproduction), but also the inhibition of adaptive phenotypic responses by interfering with the natural chemical information transfer between species (Lürling 2012). Some studies have pointed out that such effects tend to occur even at nonlethal concentrations (e.g., Hanazato 2001; Boyd 2010; Lürling 2012; Van Donk et al. 2016). In accordance with these studies, we demonstrated the general morphometric effects of SDS on three Scenedesmaceae species at nonlethal concentrations. Although SDS moderately changed the morphology of tested algae, the colonized D. subspicatus and T. dimorphus suppressed the CRs of the consumer D. galeata. Inhibited feeding by colonial algal prey can decrease somatic growth and reproduction in Daphnia (Lürling and Van Donk 1996), resulting in reduced population growth. While our findings suggest that SDS indirectly affects Daphnia–Scenedesmaceae systems by promoting colony formation, recent studies have also demonstrated that some pollutants can impair the inducible defense in Scenedesmus against Daphnia grazing cues at harmless concentrations (Huang et al. 2016; Zhu et al. 2019, 2020). Interfered induction of colonies can expose Scenedesmaceae to higher grazing risk, leading to population collapse of the algae. Colony formation should only act as an adaptive response to a reliable cue (i.e., info-chemicals emitted by grazers) because of the associated unnecessary costs (e.g., enhanced sinking velocity and reduction in surface-to-volume ratios, Lürling and Van Donk 2000). This means that both pollutant-induced and -impaired colonization would have a maladaptive consequence for Scenedesmaceae. At the population level, the grazer-induced formation of protective colonies can work as a stabilizing factor to population size fluctuations of both predator and prey (Verschoor et al. 2004). The interference effects of pollutants on colony induction can disrupt population stability by causing mismatches in response timing to appropriate predator density in prey alga (Miner et al. 2005). Such potential risks of pollutants are not covered by the traditional endpoints (mortality and reproductive output) in standard toxicity tests. In future research, population- or community-level assessments are needed to detect the disruptive effects of pollutants on predator–prey interactions.
References
Aomura K, Ashidate N, Aisawa T, Fujita M, Goto K, Hasebe K, Hikime S, Ikehata A, Kawamura S, Kimura M et al (1981) Mizu no Bunseki. The Japan Society for Analytical Chemistry, Hokkaido, Kagaku-Dojin, Kyoto, pp 374–378
Arenzon A, Pinto RF, Colombo P, Raya-Rodriguez MT (2003) Assessment of the freshwater annual fish Cynopoecilus melanotaenia as a toxicity test organism using three reference substances. Environ Toxicol Chem 22(9):2188–2190
Bondi CA, Marks JL, Wroblewski LB, Raatikainen HS, Lenox SR, Gebhardt KE (2015) Human and environmental toxicity of sodium lauryl sulfate (SLS): evidence for safe use in household cleaning products. Environ Health Insights 9:EHI-31765
Boyd RS (2010) Heavy metal pollutants and chemical ecology: exploring new frontiers. J Chem Ecol 36(1):46–58
Bulus Rossini GD, Ronco AE (1996) Acute toxicity bioassay using Daphnia obtusa as a test organism. Environ Toxicol Water Qual 11(3):255–258
Chawla G, Viswanathan PN, Devi S (1987) Biochemical studies on the toxicity of linear alkylbenzene sulphonate to Scenedesmus quadricauda in culture. Environ Exp Bot 27(3):311–323
Gerritsen J, Porter KG (1982) The role of surface chemistry in filter feeding by zooplankton. Science 216:1225–1227
Ha K, Jang MH, Takamura N (2004) Colony formation in planktonic algae induced by zooplankton culture media filtrate. J Freshw Ecol 19(1):9–16
Hanazato T (2001) Pesticide effects on freshwater zooplankton: an ecological perspective. Environ Pollut 112:1–10
Hemmer MJ, Barron MG, Greene RM (2010). Comparative toxicity of Louisiana sweet crude oil (LSC) and chemically dispersed LSC to two Gulf of Mexico aquatic test species. USEPA Dispersed Oil Toxicity Testing
Hessen DO, Van Donk E (1993) Morphological changes in Scenedesmus induced by substances released from Daphnia. Arch Hydrobiol 127:129–140
Huang Y, Nan H, Zhu X, Li B, Zhang Z, Yang Z (2016) Waterborne copper impairs grazer-induced colony formation and photosynthetic efficiency in Scenedesmus obliquus. Limnol Oceanogr 61(2):625–634
Jackson M, Eadsforth C, Schowanek D, Delfosse T, Riddle A, Budgen N (2016) Comprehensive review of several surfactants in marine environments: fate and ecotoxicity. Environ Toxicol Chem 35(5):1077–1086
Kilham SS, Kreeger DA, Lynn SG, Goulden CE, Herrera L (1998) COMBO: a defined freshwater culture medium for algae and zooplankton. Hydrobiologia 377(1):147–159
Lewis MA (1990) Chronic toxicities of surfactants and detergent builders to algae: a review and risk assessment. Ecotoxicol Environ Saf 20(2):123–140
Liwarska-Bizukojc E, Miksch K, Malachowska-Jutsz A, Kalka J (2005) Acute toxicity and genotoxicity of five selected anionic and nonionic surfactants. Chemosphere 58(9):1249–1253
Lürling M (2003) Phenotypic plasticity in the green algae Desmodesmus and Scenedesmus with special reference to the induction of defensive morphology. Annales De Limnologie-Int J Limnol 39:85–101
Lürling M (2006) Effects of a surfactant (FFD-6) on Scenedesmus morphology and growth under different nutrient conditions. Chemosphere 62:1351–1358
Lürling M (2012). Infodisruption: pollutants interfering with the natural chemical information conveyance in aquatic systems. Brönmark C, Hansson LA (Eds.), Chemical Ecology in Aquatic Systems, Oxford University Press, New York, 250–271.
Lürling M, Beekman W (2002) Extractable substances (anionic surfactants) from membrane filters induce morphological changes in the green alga Scenedesmus obliquus (Chlorophyceae). Environ Toxicol Chem 21(6):1213–1218
Lürling M, Van Donk E (1996) Zooplankton-induced unicell-colony transformation in Scenedesmus acutus and its effect on growth of herbivore Daphnia. Oecologia 108(3):432–437
Lürling M, Van Donk E (2000) Grazer-induced colony formation in Scenedesmus: are there costs to being colonial? Oikos 88(1):111–118
Lürling M, De Lange HJ, Peeters E (2011) Effects of an anionic surfactant (FFD-6) on the energy and information flow between a primary producer (Scenedesmus obliquus) and a consumer (Daphnia magna). Ecotoxicology 20(8):1881–1889
Mariani L, De Pascale D, Faraponova O, Tornambè A, Sarni A, Giuliani S, Ruggiero G, Onorati F, Magaletti E (2006) The use of a test battery in marine ecotoxicology: the acute toxicity of sodium dodecyl sulfate. Environ Toxicol 21(4):373–379
Marker AFH, Nusch EA, Rai H, Riemann B (1980) The measurement of photosynthetic pigments in freshwaters and standardization of methods: conclusions and recommendations. Archiv Für Hydrobiologie Beihefte Ergebnisse Der Limnologie 14:91–106
Martínez-Jerónimo F, García-González R (1994) Effect of food concentration on the chronic toxicity of sodium dodecyl sulphate to Daphnia magna. J Aquat Ecosyst Health 3(4):247–253
Miner BG, Sultan SE, Morgan SG, Padilla DK, Relyea RA (2005) Ecological consequences of phenotypic plasticity. Trends Ecol Evol 20:685–692
Organisation for Economic Co-operation and Development (OECD) (2011). OECD guidelines for testing of chemicals, no. 201: Freshwater Alga and Cyanobacteria, Growth Inhibition Test. OECD, Paris
Reátegui-Zirena EG, Whatley A, Chu-Koo F, Stewart PM (2013) Acute toxicity testing of crude oil using Piaractus brachypomus, and Pimephales promelas. Int J Environ Protection 3(12):1
Rebello S, Asok AK, Mundayoor S, Jisha MS (2014) Surfactants: toxicity, remediation and green surfactants. Environ Chem Lett 12(2):275–287
Ritz C, Cedergreen N, Jensen JE, Streibig JC (2006) Relative potency in nonsimilar dose–response curves. Weed Sci 54:407–412
Ritz C, Strebig JC, Ritz MC (2016) Package ‘drc.’ Mountain View, CA, USA, Creative Commons
Shedd TR, Widder MW, Toussaint MW, Sunkel MC, Hull E (1999) Evaluation of the annual killifish Nothobranchius guentheri as a tool for rapid acute toxicity screening. Environ Toxicol Chem 18(10):2258–2261
Van Donk E (2007) Chemical information transfer in freshwater plankton. Ecol Inform 2(2):112–120
Van Donk E, Peacor S, Grosser K, Domis LNDS, Lürling M (2016). Pharmaceuticals may disrupt natural chemical information flows and species interactions in aquatic systems: ideas and perspectives on a hidden global change. Rev Environ Contam Toxicol 91–105
Verschoor AM, Vos M, Van Der Stap I (2004) Inducible defences prevent strong population fluctuations in bi- and tritrophic food chains. Ecol Lett 7(12):1143–1148
Wheeler MW, Park RM, Bailer AJ (2006) Comparing median lethal concentration values using confidence interval overlap or ratio tests. Environ Toxicol Chem 25:1441–1444
Yasumoto K, Nishigami A, Yasumoto M, Kasai F, Okada Y, Kusumi T, Ooi T (2005) Aliphatic sulfates released from Daphnia induce morphological defense of phytoplankton: isolation and synthesis of kairomones. Tetrahedron Lett 46(28):4765–4767
Yasumoto K, Nishigami A, Kasai F, Kusumi T, Ooi T (2006) Isolation and absolute configuration determination of aliphatic sulfates as the Daphnia kairomones inducing morphological defense of a phytoplankton. Chem Pharm Bull 54:271–274
Yasumoto K, Nishigami A, Aoi H, Tsuchihashi C, Kasai F, Kusumi T, Ooi T (2008a) Isolation and absolute configuration determination of aliphatic sulfates as the Daphnia kairomones inducing morphological defense of a phytoplankton—Part 2. Chem Pharm Bull 56:129–132
Yasumoto K, Nishigami A, Aoi H, Tsuchihashi C, Kasai F, Kusumi T, Ooi T (2008b) Isolation of new aliphatic sulfates and sulfamate as the Daphnia kairomones inducing morphological change of a phytoplankton Scenedesmus gutwinskii. Chem Pharm Bull 56:133–136
Yokota K, Sterner RW (2011) Trade-offs limiting the evolution of coloniality: ecological displacement rates used to measure small costs. Proc Roy Soc B Biol Sci 278(1704):458–463
Zhu X, Wang Y, Hou X, Kong Q, Sun Y, Wang J, Huang Y, Yang Z (2019) High temperature promotes the inhibition effect of Zn2+ on inducible defense of Scenedesmus obliquus. Chemosphere 216:203–212
Zhu X, Wang Z, Sun Y, Gu L, Zhang L, Wang J, Huang Y, Yang Z (2020) Surfactants at environmentally relevant concentrations interfere the inducible defense of Scenedesmus obliquus and the implications for ecological risk assessment. Environ Pollut 261:114131
Acknowledgements
This study was supported by a Grant-in-Aid for Japan Society for the Promotion of Sciences (JSPS) fellows (Grant No. JP20J11681). We thank Natalie Kim, PhD, from Edanz Group (https://en-author-services.edanz.com/ac) for editing a draft of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no conflict of interests in relation to this work.
Rights and permissions
About this article
Cite this article
Oda, Y., Sakamoto, M. & Miyabara, Y. Colony Formation in Three Species of the Family Scenedesmaceae (Desmodesmus subspicatus, Scenedesmus Acutus, Tetradesmus Dimorphus) Exposed to Sodium Dodecyl Sulfate and its Interference with Grazing of DAPHNIA Galeata. Arch Environ Contam Toxicol 82, 37–47 (2022). https://doi.org/10.1007/s00244-021-00890-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-021-00890-8