Abstract
Desalination is a promising sustainable solution to meet growing water needs of cities across the United States. However, the environmental impacts of the resulting filtrate (brine) discharged to surface water need to be evaluated before large-scale desalination can be successful in the United States. Developing fish are especially sensitive to changes in salinity and varying ionic composition. Limited research is available on the impacts of hypersalinity on chronic vertebrate embryonic development, particularly on sublethal effects. To investigate this, Japanese medaka (Oryzias latipes) embryos were treated with: (1) graphite filtered freshwater; (2) artificial seawater [17, 35, 42, 56, and 70 parts per thousand (ppt)]; (3) effluent from a desalination facility at Monterey Bay Aquarium, CA, diluted to 75, 50, and 25% with 35 ppt artificial seawater to simulate mixing (39, 42, 46, and 50 ppt); (4) artificial San Joaquin River water (CA, USA) (9, 13, and 17 ppt); and (5) artificial San Joaquin River water diluted to 75, 50, and 25% with artificial seawater to simulate estuarine mixing in the San Francisco Bay (13, 19, 24, and 30 ppt). Percent hatch, survival post hatch, deformities, swim bladder inflation, and median day to hatch were recorded to calculate EC50 (50% effect concentration) and NOEC (no observable effect concentration) values. No significant difference was observed between artificial seawater and Monterey Bay aquarium effluent (EC50 = 45–55 ppt). However, San Joaquin River water decreased survival post hatch and increased deformities in comparison to artificial seawater and San Joaquin River water mixed with seawater, suggesting that unique ion compositions may play a role in embryo and larval toxicity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Population growth, drought, and climate change have placed strain on water resources around the world, leading to great interest in alternative methods of potable water production, such as desalination of seawater and brackish groundwater. Many countries currently utilize desalination, and several desalination facilities are already active along the California coast. However, 20 additional facilities are being considered for production within the next 15 years (Cooley et al. 2006). Although desalination has the potential to service millions of CA residences with potable water, possible environmental impacts need to be evaluated to ensure proper regulation. Concerns associated with desalination include high energy costs and toxicity of reverse osmosis (RO) reject or effluent (aka brine) to receive surface waters (Lattemann and Hopner 2008). The majority of proposed desalination facilities intend to discharge effluent directly into the open ocean. However, other facilities have indicated that brine also may be discharged into rivers and estuaries of the San Francisco Bay Delta (Cooley et al. 2006). Embayment locations have decreased dissolution and dispersal potential, which can possibly result in an increase in ambient salinity (Jenkins et al. 2013). Furthermore, desalination of brackish groundwater will produce reject with a different ionic content from seawater, which may have different toxicity to aquatic organisms.
Embryonic and larval developmental periods in teleosts often are the most sensitive to environmental stressors (von Westernhagen 1998). Although studies have been conducted on the toxicity of desalination brine to marine invertebrate development (Voorhees et al. 2013) and the acute toxicity of ion imbalance to freshwater organisms (Goodfellow et al. 2000), there is a lack on information of brine toxicity to euryhaline vertebrate development. Furthermore, few studies have addressed sublethal impacts of desalination effluent on developing organisms.
To better characterize the risks that may arise from various “brines” in CA waterways, we examined the impacts of artificial seawater, reverse osmosis (RO) brine from a facility at Monterey Bay Aquarium (CA, USA), and an artificial brackish water in the San Joaquin River, CA, on the embryonic development of the euryhaline fish model Japanese medaka (Oryzias latipes). The flexibility of the Japanese medaka to a wide-range of salinities allows for direct comparisons between freshwater, ambient seawater, and greater salinities. We assessed both lethal (percentage hatch and survival post hatch) and sublethal (percentage deformities, swim bladder inflation, median day to hatch) endpoints to provide CA regulators with information regarding potential ecological risks associated with brine discharge from desalination.
Experimental Design and Methods
Embryo Collection and Exposure
Japanese medaka (Oryzias latipes) were cultured at the University of California-Riverside at 27 °C. Tanks were aerated under 14-h light and 10-h dark cycles. Adults were fed twice a day a diet of live brine shrimp. We specifically used Japanese medaka, because they are a euryhaline species that are widely accepted as a model for testing toxicity.
Embryos were pooled from all adults and separated at random for treatment at the 2–4-cell stage in 60- × 15-mm petri dishes with 15–20 eggs per dish containing 10 mL of test solution. The viable eggs were separated according to Kirchen and West (1976). Four treatment waters were used in this study: freshwater, artificial seawater, artificial San Joaquin River, CA, water, and RO effluent brine discharged from a facility at Monterey Bay Aquarium, CA. Medium–hard, graphite filtered freshwater treatments were used as a quality control with each replicate. If embryo survival in freshwater was below 80%, the replicate was discarded. Instant Ocean Aquarium Salts were used to prepare the artificial seawater (Table 1). All water dilutions used in the study were prepared daily from stock waters before treatment and aerated to 70% saturation before treatment. Water and dishes were replaced daily until the end of the experiment. Full strength seawater (100%) was assumed to be at 35 ppt. Accordingly, artificial seawater treatments were prepared at: 17 ppt (50%), 35 ppt (100%), 42 ppt (130%), 56 ppt (160%), and 70 ppt (200%) (n = 3–5).
Artificial San Joaquin River (SJR) saltwater was prepared based on water from the Westlands Water District (Table 1). This district resides around 10-km south of Mendota within San Joaquin River Drainage Basin, CA. Use of this water is relevant, because desalination projects are proposed for this area. Full-strength SJR water is 13 ppt (Table 1) as measured with a refractometer. Accordingly, the artificial SJR water was concentrated from 13 to 17 ppt via evaporation by heating or diluted to 9 ppt to ascertain the toxicity of SJR brine alone (n = 5). The low solubility of CaSO4 present in the water prevented concentration above 17 ppt. Subsequently, to simulate discharge of SJR brine into marine environments, full-strength artificial SJR water (13 ppt) was diluted 25% (19 ppt), 50% (24 ppt), and 75% (30 ppt) with full-strength artificial seawater (35 ppt) (n = 4).
The Monterey Bay Aquarium, CA, maintains a small seawater desalination facility to produce industrial grade water, which is not treated with any chemicals prior to RO concentration. The aquarium provided RO desalination brine at 50 ppt to compare with artificial seawater, which was stored at 4 °C throughout the experiment. Full-strength Monterey Bay Aquarium (MB) brine was sequentially diluted with full-strength seawater to simulate mixing within an ocean environment. Effluent was diluted 25% (46 ppt), 50% (42 ppt), and 75% (39 ppt) in 35 ppt artificial seawater (n = 5).
Embryos were observed daily under a compound microscope for hatch, mortality, and deformities. Nonanesthetized larvae were examined for spinal (curvatures, such as lordosis, kyphosis, and scoliosis), craniofacial (ocular abnormalities and abnormalities in head shape), cardiac (edema), fin (fin shape), and yolk sac (edema) malformations. The presence of an inflated swim bladder was noted, because the swim bladder is important for buoyancy and balance control; however, it was scored separately from total deformities, because this occurs naturally in control fish and often is reversible. In addition, the survival of the hatched larvae was monitored for 3 days post hatch then sacrificed. Larvae were not fed during this period.
Ion and Water Quality Measurements
All tested waters were analyzed for major ionic constituents. Cations (Ca2+, Na+, Mg2+, Sr2+, and K+) were analyzed with inductively coupled plasma optical emission spectroscopy (ICP-OES) (Perkin Elmer Optima 3000 DV, Waltham, MA), while anions (Cl− and SO4 2−) were analyzed with an IonPac ASIV high capacity anion-exchange column on a Dionex 500-ion chromatograph (Sunnyvale, CA). Nitrogen species were measured with aquarium test strips (Tetra) for prepared waters and according to Kingsley et al. (2014) for brine from Monterey Bay Aquarium. Results compared with nominal concentrations are summarized in Table 1.
Statistics
Data were tested for normality and homogeneity of variances, which was not met. The Kruskal–Wallis test was performed with a Dunn’s test post hoc and a Holm–Sidak correction. Statistical tests were performed in the statistical program Stata 13.1 (StataCorp LP) with the dunntest package. Statistical significance was determined at p ≤ 0.05. EC50 (50% effect concentration) values were calculated with the probit method, and 95% confidence intervals were calculated with the Litchfield–Wilcoxon method (Litchfield and Wilcoxon 1949). Calculated EC50 values that were greater than the highest test concentration are listed as greater than the highest concentration.
Results
In the present study, vertebrate developmental and larval toxicity of desalination brine was compared to artificial seawater and saltwater of differing ionic contents. Desalination reject brine and San Joaquin River water were diluted with full strength seawater to simulate mixing in marine waters. Artificial SJR water and artificial SJR mixed with seawater had no significant effect on percent hatch at any salinity (Fig. S1). In contrast, percent hatch was significantly decreased to 37% in 56 ppt seawater (160% full strength) and 22% in 70 ppt (200%; p = 0.03; Fig. S1). Similarly, significant decreases in percent hatch were observed with 50 ppt RO brine (100% effluent; p = 0.001; Fig. S1). EC50 values for the RO brine and seawater were 55.9 ppt (95% CI 48.1–65 ppt) and >50 ppt, respectively (Table 2).
Total deformities were measured following hatch in live larvae. All embryos treated with 70 ppt seawater were dead immediately post hatch and were not assessed for deformities. The percent of seawater deformities increased to 18% following exposure to 42 ppt seawater (p = 0.01) and to 50% in 56 ppt seawater (p = 0.001; Fig. S2). The percent of deformities increased to 22% when medaka were treated with 50 ppt of RO brine (p = 0.001; Fig. S2). Artificial SJR water mixed with seawater resulted in no increase in deformities; however, 17 ppt artificial SJR water alone caused 53% deformities (p = 0.0004; Fig. 1). This is in contrast to 17 ppt seawater exposures (50% concentrated), which failed to cause deformities. The EC50 values reflect this difference: >56 ppt for seawater; >50 ppt for RO brine; and >17 ppt for SJR (Table 2).
Percent total deformities ± standard error (SE) of Japanese medaka embryos following treatment with a artificial San Joaquin River saltwater at full strength (13ppt), concentrated (17 ppt), and diluted (9 ppt) (n = 5) and b with San Joaquin River water full strength (100%, 13 ppt), mixed with seawater, to 75% (19 ppt), 50% (24 ppt), and 25% (30 ppt) (n = 4). Differing letters indicate significant differences between treatments (p ≤ 0.05) following Dunn’s test
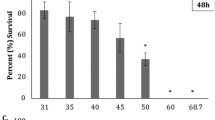
Swim bladder inflation is important for maintenance of buoyancy and balance in the water column for pelagic fish species. A concentration response of swim bladder inflation to salinity was observed in all saltwater/brine treatments except SRJ water mixed with seawater. Swim bladder inflation decreased significantly to 46% in embryos treated with 46 ppt RO brine (25% dilution) and 52% in 35 ppt artificial seawater (p = 0.0008 and 0.002 respectively; Fig. 2). Furthermore, artificial SJR water significantly decreased swim bladder inflation at 13 and 17 ppt to 73 and 53%, respectively (p = 0.01 and 0.0004; Fig. 2). Calculated EC50 values were >56 ppt for seawater, 45.1 ppt (95% CI 38–53.6) for desalination brine, and >17 ppt for artificial SJR water. This further suggests that artificial SJR water is more toxic to developing fish than seawater, based solely on salinity.
Percent swim bladder inflation ± SE in Japanese medaka embryos at hatch following treatment with a artificial seawater (n = 3–5), b RO reject mixed with artificial seawater (n = 5), c artificial San Joaquin River water (n = 4), and d San Joaquin River water mixed with seawater (n = 5). Differing letters indicate significant differences between treatments (p ≤ 0.05) following Dunn’s test
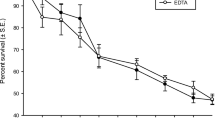
Embryos were monitored for 3 days post hatch (until feeding) for survival. Survival decreased to 78% in 42 ppt seawater (p = 0.04), 20% in 56 ppt (p = 0.0014) and 0% in 70 ppt (p = 0.00014; Fig. S3). RO brine treated larvae survival decreased to 90% in 50 ppt (full-strength effluent; p = 0.0014; Fig. S3). Although no decrease in survival was observed in artificial SJR water mixed with seawater embryos, artificial SJR water alone resulted in 17% survival at 9 ppt (p = 0.0065), 50% survival at 13 ppt (p = 0.04), and 1% survival at 17 ppt (p < 0.0001; Fig. 3). Due to the U-shaped nature of the dose response curve for artificial SJR water, which may be due to high variability, an EC50 for survival could only be calculated for seawater (49 ppt; 95% CI 46.5–51.6 ppt).
Percent survival 3 days post hatch ± SE of Japanese medaka embryos following treatment with a artificial San Joaquin River saltwater at full strength (13 ppt), concentrated (17 ppt), and diluted (9 ppt) (n = 5) and b with San Joaquin River water full strength (100%, 13 ppt), mixed with seawater to 75% (19 ppt), 50% (24 ppt), and 25% (30 ppt) (n = 4). Differing letters indicate significant differences between treatments (p ≤ 0.05) following Dunn’s test
Finally, the median day to hatch was calculated for each treatment group. No significant alteration in time to hatch was observed in artificial SJR water alone or when mixed with seawater (Fig. S4). However, RO brine significantly increased time to hatch from 9.1 ± 0.14 days post fertilization (dpf) in freshwater to 10.4 ± 0.25 to 10.8 ± 0.2 dpf at all concentrations tested (p < 0.005 for all; Fig. 4). Artificial seawater also significantly increased day to hatch beginning at 17 ppt to 10.25 ± 0.31 dpf, at 42 ppt to 10.4 ± 0.25 dpf, to 11 ± 0.37 dpf in 56 ppt exposures and to 14.5 ± 0.71 dpf in 70 ppt exposures (p = 0.047, 0.03, 0.007, and 0.007, respectively).
Median day to hatch ± SE of Japanese medaka embryos treated with a artificial seawater (17, 35, 42, 56, and 70 ppt corresponding to 50, 100, 130 160, and 200% concentrated) (n = 3–5); and b RO reject mixed with artificial seawater to 100% (50 ppt), 75% (46 ppt), 50% (42 ppt), and 25% (39 ppt) (n = 5). Differing letters indicate significant differences between treatments (p ≤ 0.05) following Dunn’s test
Discussion
Although data are available concerning acute saltwater impacts on invertebrate development, no true chronic vertebrate embryonic development tests have been conducted. Furthermore, most tests on euryhaline or marine organisms have been performed only with seawater effluent (Inoue and Takei 2009; Iso et al. 1994; Voorhees et al. 2013), whereas tests with effluent from brackish water have been conducted only with freshwater organisms (Mount et al. 1997; Soucek and Kennedy 2005; Wang et al. 2016). In the present research, we characterize EC50 and NOEC (no observable effect concentration) values (Table 2) for toxicity of three different saltwaters, including seawater desalination brine, vertebrate embryonic and larval development, and examine sublethal endpoints of toxicity. We accomplish this with a common, euryhaline model species—the Japanese medaka. Use of a euryhaline model is pertinent, because it is relevant for estuarine environments and allows for a wide range of salinities to be tested.
EC50 values measured in this study ranged between 45 and 60 ppt for artificial seawater and RO reject from Monterey Bay. Overlapping 95% confidence intervals indicated no difference between the RO reject and seawater toxicity to embryos and larvae. Although reverse osmosis may concentrate trace toxicants present at low levels, this appears to not have impacted toxicity and the results were expected considering no chemical treatment was added to the effluent. This study is one of the few to examine sublethal indicators of brine toxicity. No difference in EC50 or NOEC was observed between percent hatch, survival, and deformities. However, NOEC values were lower for failure of swim bladder inflation, suggesting effects following alterations in salinity above 35 ppt seawater. Uninflated swim bladders may negatively impact fish; for instance, Japanese medaka larvae with uninflated swim bladders consumed more oxygen than those with inflated swim bladders (Marty et al. 1995). This metric may be a good indicator of sublethal effects from brine discharge. Additionally, the median day to hatch was significantly increased by exposures of greater than 35 ppt. Increased time to hatch may decrease larval competitiveness and survival in the wild (Kestemont et al. 2003).
Current regulations on brine discharge in California are characterized as increases in salinity over ambient or by absolute salinity. The U.S. EPA has set a limit of an increase <4 ppt, whereas the San Diego Regional Water Quality Control Board and Santa Ana Regional Water Quality Control Board set an absolute salinity of <40 ppt in 2006 and 2012, respectively, for oceanic discharge (Jenkins et al. 2013). Other countries utilizing desalination as a water source, such as Australia, Oman, or Japan suggest increments of <1 or 2 ppt for oceanic discharge (Jenkins et al. 2013). Embryos used in the current study were spawned in freshwater and no significant toxicity was observed in 35 ppt seawater. Hence, according our results and assuming a seawater salinity of 35 ppt, the high flexibility of Japanese medaka embryos and larvae indicates that they would be protected to seawater effluent under these guidelines.
Previous studies have been performed investigating the lethal effects of seawater on marine/euryhaline embryos and larvae. Inoue and Takei (2009) reported that Japanese medaka embryos spawned in 50% seawater hatched at a rate of 90%, and a rate of 60% in full seawater. Conversely, in the current study, percentage hatch was unaffected by 35 and 17 ppt seawater. Time to hatch was affected in both our study and the Inoue and Takei (2009) study approximately 1–2 days. Studies on artificial seawater also have been conducted in flounder (Pleuronectes yokohumae) eggs and larvae (Iso et al. 1994). Saltwater acclimated flounder larvae experienced a mortality rate of 12% in 50 ppt seawater and 100% in 60 ppt (Iso et al. 1994). Furthermore, embryo percent hatch remained at 100% at 60 ppt but decreased to 0% at 70 ppt (Iso et al. 1994).
While informative, the above experiments were performed on marine organisms with only artificial seawater and give no indication of differences between RO brine and seawater toxicity. Short-term development tests on purple sea urchin, red abalone, sand dollar, and bay mussels have been conducted with desalination reject brine from Monterey Bay and indicated EC50 values between 36 and 43 ppt (Voorhees et al. 2013). The invertebrate embryos used in these tests were spawned in seawater, whereas the medaka embryos used in this study were spawned in freshwater. Thus, the medaka embryos were subjected to greater overall changes in salinity than the invertebrates at fertilization, yet greater EC50 values were measured. This suggests that marine invertebrates are more sensitive to alterations in salinity than euryhaline Japanese medaka. However, larval assays performed with saltwater acclimated topsmelt, suggested an EC50 of 62 ppt (Voorhees et al. 2013). This value is greater than the EC50 values calculated for larval survival here (49 ppt), suggesting that medaka larvae are more sensitive to high salinities. This difference could be due differences in methods, as topsmelt were transferred into the test solution as larvae, whereas the Japanese medaka were reared in the corresponding salt and spawned in freshwater.
Although no differences in desalination brine and artificial seawater were noted, significant differences between artificial SJR alone, seawater alone, and seawater mixed with artificial SJR were observed. For instance, 17 ppt artificial SJR water resulted in 50% deformities and 1% survival post hatch, whereas treatment with 17 ppt seawater generated no deformities and 84% survival. Furthermore, a hormetic dose response curve was observed for survival of larvae treated with artificial SJR water. This may be due to a lack of necessary ions in the 9 ppt treatments, which were supplemented at 13 ppt but toxic at 17 ppt. Artificial SJR water mixed with seawater produced results similar to seawater alone. These differences suggest that the ion ratios and imbalance are larger drivers of embryo and larval toxicity, rather than the total dissolved solids. Furthermore, sublethal indicators of toxicity (deformities and swim bladder inflation) were a more sensitive metric than percent hatch in the current study, suggesting that they may be of use in assessing desalination impacts of brackish groundwater.
In a study on coho salmon (Oncorhynchus kisutch Walbaum), embryos were exposed to 2500 mg/L (2.5 ppt) TDS mimicking ionic content of mining effluent in Alaska (Stekoll et al. 2009). The solution was sulfate-dominated, primarily composed of CaSO4, Na2SO4, and MgSO4, similar to the water used in this study. Approximately 50% mortality was observed in embryos treated from fertilization to swim up (Stekoll et al. 2009). Researchers further calculated EC50 values for the impacts of individual ions on coho salmon fertilization and found the order of toxicity to be Ca2+ > K+ > Mg2+ > SO4 2− > Na+ with a range of 102 mg/L (0.102 ppt) to 4744 mg/L (4.7 ppt) (Stekoll et al. 2009). Similar to the results observed, embryos exposed through swim-up experienced high post-hatch mortality (Stekoll et al. 2009).
In stenohaline freshwater organisms, combinations of various salts were tested for toxicity to Daphnia magna, Ceriodaphnia dubia, and Pimephales promelas (Mount et al. 1997). LD50 values for the combination of NaCl and Na2SO4, the primary salts present in the artificial San Joaquin water, were approximately 6000 mg/L (6 ppt) for the 96-h fathead minnow test and 5700 mg/L (5.7 ppt) for the 48-h Daphnia magna test (Mount et al. 1997). Ion toxicity modeling suggested that in order of toxicity, K+ > HCO3− = Mg2+ > Cl− > SO42−, while Ca2+ and Na+ were not significant (Mount et al. 1997). With the exception of Ca2+, agreement exists between this model and the one determined for coho salmon (Stekoll et al. 2009).
However, other research suggests that ion ratios may be more important than concentration in determining toxicity. In manufacturing effluent, high ratios of Ca2+ to Na+ were found to be responsible for fathead minnow toxicity (Dorn and Rodgers Jr 1989). Ca2+ to Na+ ratios of 15:1 caused high mortality, while 1:20 did not (Dorn and Rodgers Jr 1989). EC50 values for Ca2+ were calculated for Daphnia pulex, Mysidopsis bahia, and Pimephales promelas and ranged between 266 (0.27 ppt) and 927 mg/L (0.926 ppt) (Goodfellow et al. 2000). However, these values were all based on adult 96-h exposures to larval and juvenile freshwater organisms and likely vary for euryhaline or saltwater organisms during embryonic development. Very low levels of K+ and Mg2+ are found in the artificial SJR water, indicating low potential for toxicity; however, 392 mg/L (0.392 ppt) Ca2+ present in the artificial SJR is greater than the calculated EC50 values for coho salmon and fathead minnows (Goodfellow et al. 2000; Stekoll et al. 2009).
In addition to Ca2+, a major component of the SJR water is SO4 2−. A comprehensive study on sulfate toxicity to freshwater species (mussels, midges, fathead minnows, and cladoceran) has been performed (Wang et al. 2016). Chronic development tests with fathead minnows suggested growth/weight EC20s of 305–477 mg/L (0.305–0.477 ppt) for survival and 106–185 mg/L (0.106–0.185 ppt) (Wang et al. 2016). Short-term (7–14 day) embryonic LC50 values ranged from 478 to 644 mg/L (0.478–0.644 ppt). These values are substantially lower than those used in this study, most likely because the species tested were stenohaline freshwater organisms compared with the euryhaline organism tested. However, Wang et al. (2016) reported that increasing the concentration of K+ from 1 to 3 mg/L (0.003 ppt) decreased sulfate toxicity, while increasing Cl− had no effect. The artificial SJR water used here contained very high ratios of SO4 2− to K+, which may have contributed to toxicity.
In contrast, other studies on sulfate toxicity to a freshwater invertebrate, Hyalella azteca, found that SO4 2− toxicity decreased with increasing levels of Cl− by 5.5-fold (Soucek and Kennedy 2005). Furthermore, sulfate toxicity was also reduced with increasing water hardness as CaCO3, which was not present in the prepared SJR water. Additional studies on the impacts of these factors in H. azteca and D. magna, indicated that chloride concentrations from 5 to 25 mg/L (0.005–0.025 ppt) decreased SO4 2− toxicity, whereas greater concentrations caused it to increase, suggesting an additive effect (Soucek 2007). Although SO4 2− levels predominate in SJR water, based on these studies, its possible that the Cl− levels also may have an effect. We can conclude that further chronic testing of ion ratios on vertebrate development of a variety of endemic species is necessary to understand fully the impacts of brackish water desalination.
Overall our results indicate that developing euryhaline organisms (specifically Japanese medaka) will be protected from seawater brine toxicity under the current regulations. However, a general TDS measurement for National Pollutant Discharge Elimination System (NPDES) permits is insufficient for brine of different ionic contents, and alternative regulations need to be in place for brackish groundwater desalination. Recently, researchers have advocated for the use of site-specific and ion-specific standards for adequate ecosystem protection (Cañedo-Argüelles et al. 2016), with which we agree in the case of desalination. Further research on ion imbalance and endemic species is necessary to determine site-specific impacts of brackish water desalination brine. Additionally, the implications of ion imbalance need to be considered for estuarine disposal of groundwater desalination reject.
Supporting Information
Supplemental data concerning embryo hatch, survival and deformities of artificial seawater and desalination brine treated embryos, and day to hatch of artificial San Joaquin River water treated embryos is available.
References
Cañedo-Argüelles M, Hawkins CP, Kefford BJ, Schäfer RB, Dyack BJ, Brucet S, Buchwalter D, Dunlop J, Frör O, Lazorchak J, Coring E, Fernandez HR, Goodfellow W, González Achem AL, Hatfield-Dodds S, Karimov BK, Mensah P, Olson JR, Piscart C, Prat N, Ponsá S, Schulz C-J, Timpano AJ (2016) Ion-specific standards are needed to protect biodiversity. Science 351:914–916
Cooley H, Gleick PH, Wolff G (2006) Desalination, with a grain of salt: a California perspective. Pacific Institute for Studies in Development, Environment, and Security, Oakland
Dorn PB, Rodgers JH Jr (1989) Variability associated with identification of toxics in NPDES effluent toxicity tests. Environ Toxicol Chem 8:893–902
Goodfellow WL, Ausley LW, Burton DT, Denton DL, Dorn PB, Grothe DR, Heber MA, Norberg-King TJ, Rodgers JH Jr (2000) Major ion toxicity in effluents: a review with permitting recommendations. Environ Toxicol Chem 19:175–182
Havonec TA (2015) Synthetic sea salts: are they all equal? A primer on Instant Ocean®: the premier sea salt for your marine aquarium. Marineland, Spectrum Brands, Blacksburg
Inoue K, Takei Y (2009) Diverse adaptability in oryzias species to high environmental salinity. Zool Sci 19:727–734
Iso S, Suizu S, Maejima A (1994) The lethal effect of hypertonic solution and avoidance of marine organisms in relation to the discharged brine from desalination plant. Desalination 97:389–399
Jenkins S, Paduan J, Roberts P, Schlenk D, Weiss J (2013) Management of brine discharges to coastal waters: recommendations of a science advisory panel. Southern California Coastal Water Research Project, Costa Mesa
Kestemont P, Jourdan S, Houbart M, Melard C, Paspatis M, Fontaine P, Cuvier A, Kentouri M, Baras E (2003) Size heterogeneity, cannibalism and competition in cultured predatory fish larvae: biotic and abiotic influences. Aquaculture 227:333–356
Kingsley E, Modisette N, Phillips R (2014) Development and use of nitrate and total ammonia testing procedures at the Monterey Bay Aquarium. 2nd Aquality Symposium: Water Quality Treatment in Zoos and Aquariums
Kirchen RV, West WR (1976) The Japanese medaka: its care and development. Carolina Biological Supply Company, Burlington
Lattemann S, Hopner T (2008) Environmental impact and impact assessment of seawater desalination. Desalination 220:1–15
Litchfield JT Jr, Wilcoxon F (1949) A simplified method for evaluating dose-effect experiments. J Pharmacol Exp Ther 96:99–113
Marty GD, Hinton DE, Cech JJ (1995) Oxygen consumption by larval Japanese Medaka with inflated or uninflated swim bladders. Trans Am Fish Soc 124:623–627
Mount DR, Gulley DD, Hockett JR, Garrison TD, Evans JM (1997) Statistical models to predict the toxicity of major ions to Ceriodaphnia dubia, Daphnia magna and Pimephales promelas (fathead minnows). Environ Toxicol Chem 16:2009–2019
Schlenk D, Zubcov N, Zubcov E (2003) Effects of salinity on the uptake, biotransformation, and toxicity of dietary seleno-l-methionine to rainbow trout. Toxicol Sci 75:309–313
Soucek DJ (2007) Comparison of hardness- and chloride-regulated acute effects of sodium sulfate on two freshwater crustaceans. Environ Toxicol Chem 26:773–779
Soucek DJ, Kennedy AJ (2005) Effects of hardness, chloride, and acclimation on the acute toxicity of sulfate to freshwater invertebrates. Environ Toxicol Chem 24:1204–1210
Stekoll MS, Smoker WW, Failor-Rounds BJ, Wang IA, Joyce VJ (2009) Response of the early developmental stages of hatchery reared salmonids to major ions in a simulated mine effluent. Aquaculture 298:172–181
von Westernhagen H (1998) Sublethal effects of pollutants on fish eggs and larvae. In: Hoar DS, Randall DJ (eds) Fish physiology, vol XIa. Academic Press, San Diego, p 253
Voorhees JP, Phillips BM, Anderson BS, Siegler K, Katz S, Jennings L, Tjeerdema RS, Jensen J, Carpio-Obeso MD (2013) Hypersalinity toxicity thresholds for nine California ocean plan toxicity test protocols. Arch Environ Contam Toxicol 65:665–670
Wang N, Dorman RA, Ingersoll CG, Hardesty DK, Brumbaugh WG, Hammer EJ, Bauer CR, Mount DR (2016) Acute and chronic toxicity of sodium sulfate to four freshwater organisms in water-only exposures. Environ Toxicol Chem 35:115–127
Acknowledgements
This research was supported by the National Water Research Institute and Southern California Salinity Coalition Fellowship, a National Research Service Award Institutional Training Grant (2T32ES018827-06), and the University of California-Riverside/Agricultural Experiment Station Resource Allocation Program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kupsco, A., Sikder, R. & Schlenk, D. Comparative Developmental Toxicity of Desalination Brine and Sulfate-Dominated Saltwater in a Euryhaline Fish. Arch Environ Contam Toxicol 72, 294–302 (2017). https://doi.org/10.1007/s00244-016-0354-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-016-0354-9