Abstract
The Birama Swamp is the second largest wetland in the Caribbean region and it is inhabited by large populations of waterbirds. Here we report, for the first time, the foraging ecology and pollutant levels of three Ardeidae species: Cattle egret (Bubulcus ibis), Snowy egret (Egretta thula), and Tricolored heron (E. tricolor) breeding in this wetland using stable-isotope (δ 15N and δ 13C) and trace elements [mercury (Hg), lead (Pb), and selenium (Se)] analysis of chick feathers. Our results showed that individuals from all species occupied similar trophic levels. However, we found significant differences for δ 13C, with the highest values in cattle egret indicating its use of terrestrial habitats and a generalist and opportunist behavior. No significant differences were found for Pb among species. Yet, Hg levels were greater and similar in tricolored heron and snowy egret than in cattle egret, which was associated with their greater use of aquatic environments. Snowy egret had the lowest values of Se differing significantly with the other two species suggesting a different relative use of prey type. Modeling log-Hg concentration in relation to δ 15N and δ 13C showed an independent and significant relationship among species but without interaction with species level indicating that within a particular species, higher Hg levels were associated with higher δ 15N values. There was no interaction between δ 15N and δ 13C in the general linear models for Se and Pb in all species. We found an association between δ 15N and species in Pb for snowy egret. The foraging habitat use of these species and the low levels of pollutants, which are lower than in other similar habitats in other areas of the world, indicated that there is not risk of negative effects in juvenile birds of the Birama Swamp colony that may impair their survival. Our results can be used as a baseline to achieve management regulations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Tropical wetlands are among the world’s most productive and biologically diverse ecosystems, comparable with rainforests and coral reefs (Valiela et al. 2001). They provide water and resources that support countless species of microbes, plants and animals year round (Tavares et al. 2007; Yoon 2009). In addition, some anthropogenic systems (e.g., rice fields, fish ponds or salinas) can provide complementary habitats for many of these species due to the greater accumulation of resources during certain times of the year (Elphick and Oring 1998; Connor and Gabor 2006; Eadie et al. 2008; Fujioka et al. 2010; McKinney et al. 2011).
Natural ecosystems worldwide are suffering drastic alterations due to intense habitat transformation linked to human activities (e.g., agriculture, urbanization, coastal development, tourism pressure and fisheries) (Mitsch and Gosselink 2000; Valiela et al. 2001; Lee et al. 2006) and climate change (Lemoine et al. 2007; Gell et al. 2007; Montoya and Raffaelli 2010). As a result, species use of alternative and human-altered sites has increased as they become more abundant (Young and Chan 1997; Svazas and Stanevicius 2000; Takekawa et al. 2001; Amano 2009; Fasola et al. 2010). With new habitats, exposure to different pollutants from several sources (e.g., runoff, point source contaminant discharge, atmospheric deposition and soil erosion due to rainfall) has increased, thus influencing their bioavailability and entrance into the food webs (Birch et al. 1996; Berg and Steinnes 1997; Wang et al. 2004; Duman et al. 2007). As a result, some trace elements and organic pollutants are accumulated into the biota and transported to greater trophic levels through the food web (Haukås et al. 2007; Green et al. 2010). High concentration of pollutants is especially remarkable in long-lived organisms such as top predators that occupy high trophic levels and ultimately can affect their health and fitness (Burger 1993; Burger and Gochfeld 1995).
For these reasons the concern for the future of wetlands has grow during the last years, as well as the use of wildlife as bioindicators of ecosystem and human health (Peakall 1992; Burger and Gochfeld 2009; Perugini et al. 2011). One of the groups more widely used as biomonitors of environmental hazards are birds (e.g., Furness and Greenwood 1993; Burger 1993; Becker 2003; Piatt et al. 2007; Hofer et al. 2010). Most colonial waterbirds are top predators and thus tend to accumulate contaminants “moving” through the food webs. Thus, these species can be used as proxies of early environmental pollution. Pollutant monitoring programs of waterbirds are common and are advantageous as sampling can be relatively easy and can be performed with minimal invasiveness (using eggs, feathers, and blood samples).This group can also be very sensitive to pollutants displaying negative secondary effects at very low concentrations (Nisbet 1994). Because of their behavior, longevity and diet, members of the Ardeidae family are especially useful for contaminant studies (Burger et al. 1992a; Stewart et al. 1997). Nestlings are especially amenable subjects for monitoring studies, as bioaccumulation is minimal at this stage, and trace elements come mainly from food gathered by provisioning adults from local food webs.
The relationship between diet and contaminants has been analyzed through stable isotopes. Natural variation in stable isotope ratios of several elements [e.g., carbon (C), nitrogen (N)] has been increasingly used in trophic ecology studies during the last 20 years (Michener and Schell 1994; Jardine et al. 2006; Bucci et al. 2007; Inger and Bearhop 2008; Bond 2010). For example, nitrogen isotope (δ 15N) has been effective in quantifying the trophic level of organisms if adequate baselines are provided because enrichment of this isotope occurs across trophic levels at a constant rate (3.4 ‰) (Inger and Bearhop 2008). In contrast, the enrichment of carbon isotope (δ 13C) among different trophic levels is lower (i.e., approximately 1 ‰) (Inger and Bearhop 2008). This isotope is considered a valuable tracer for identifying different sources of primary production (Hobson et al. 2002; Hoekstra et al. 2003) with values being typically greater for aquatic than for terrestrial environments. Simultaneous use of stable isotope and trace-element analyses constitute a valuable tool in ecotoxicological studies to elucidate contaminant exposure through food webs (Borga et al. 2001; McIntyre and Beauchamp 2007; Hobson 2011).
In Cuba, coastal wetlands occupy 77.6 % of the 14,724 km2 of total wetlands in the country (CNAP 2002). It has been estimated that 41 % of species of Cuban birds depend of these ecosystems for their survival (Acosta and Mugica 2006), and most of them also breed in these areas. In the island, foraging habitats of waterbirds include both natural and artificial wetlands (e.g., rice fields). No previous dietary studies by stable isotopes analysis or pollution assessment have been undertaken for Cuban birds. Here we assess the foraging ecology and pollutant levels [trace elements mercury (Hg), lead (Pb), and selenium (Se)] of three Ardeidae species, Cattle egret (Bubulcus ibis), Snowy egret (Egretta thula), and Tricolored heron (E. tricolor), breeding at Birama Swamp (one of the largest Cuban wetlands), an area far from the urban and industrial centers but historically associated with large rice plantations.
Methods
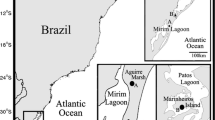
The study was performed in Birama Swamp, which is situated in the delta of the Cauto River (the longest river in Cuba), Granma province, (Fig. 1) in the eastern part of the country (20°32′N/77°01′W). This swamp covers an area of 67,500 ha, is surrounded by rice fields, and provides excellent conditions for several bird species, especially ardeids, to breed.
During the 2001 breeding season (May–July), we collected 5–10 fully grown scapular feathers from 67 2-week-old nestlings (1 Chick for each nest sampled; all from the same colony): 20 Tricolored heron, 26 Cattle egret, and 21 Snowy egret (Table 1). These feathers were kept in sealed plastic zip bags. Samples were processed and analyzed for trace elements and stable isotopes at the Serveis Científico Tècnics of the University of Barcelona, Spain.
Stable Isotope Analysis
Surface contamination was removed by washing feathers with 0.25 M sodium hydroxide solution. Feathers were then oven-dried at 60 °C before being grounded into a fine powder using an impact mill (6750 FREEZER/MILL, Spex CertiPrep, Metuchen, NJ, USA) that was operated at liquid nitrogen temperature (−195 °C). We weighed subsamples of powdered feathers (0.36 mg for δ 15N and δ 13C analysis), placed them into tin buckets, and crimped them for combustion for stable isotope analysis by elemental analysis–isotope ratio mass spectrometry using a Thermo Finnigan EA 1112 Series Flash Elemental Analyzer (Thermo Scientific, Lakewood, NJ, USA) coupled to a Delta isotope ratio mass spectrometer by way of a CONFLO III interface (Thermo Finnigan MAT, Bremen, Germany).
Stable isotope ratios were expressed in parts per thousand according to the following conventional equation (Eq. 1):
where X (‰) is δ 13C or δ 15N; Rsample is the corresponding ratio of 13C/12C or 15N/14N in the analyzed tissue; and Rstandard is the corresponding ratio of 13C/12C or 15N/14N related to the standard values. Rstandard values for δ 15N and δ 13C were those of Pee Dee Belemnite and atmospheric nitrogen. We used international standards in each batch of 12 samples to calibrate the system. The precision of measurements were 0.1 ‰ for δ 13C and 0.3 ‰ for δ 15N.
Trace-Element Analysis
We performed chemical determination of Se and heavy metals, such as Hg, and Pb, in feathers according to the following acid digestion protocol: 0.1 g of each sample was digested with 2 ml of HNO3 (70 %) and 1 ml of H2O2 (30 %) using a Teflon reactor for 12 h at 90 °C. The digested product was then diluted in 15 ml of distilled water, and the determination of Se and heavy metals was performed using a Optima 6000 ICP-MS (Perkin Elmer, Norwalk, CT, USA) induction coupled plasma-mass spectrometer. Accuracy of the analysis was checked by measuring certified reference tissue (human hair CRM 397 for feathers analysis). Mean recoveries were 101.9, 97.3, and 105.3 % for total Hg, Se, and Pb, respectively, and no corrections were applied to the original results. All concentrations are expressed in nanograms per gram on a dry-weight basis.
Statistical Procedures
Before data analysis, we used normal q–q plots to check the distributional characteristics of the analyzed variables. Those data that not fit to a normal model were normalized by logarithmic transformation, and standard parametric analyses were used. Homogeneity of variances was checked using Levene’s test. Standard one-way analysis of variance F test was used to compare results among species, and Student–Newman–Keuls procedure was used to make a posteriori comparisons between pairs. Welch’s approach and Tamhane test were used accordingly when variances were not homogeneous. We used Pearson’s correlation coefficient to evaluate the relationship between trace elements. To explore the relationship between isotopes and Se or Hg concentrations, we fitted a general linear model with species as a factor and isotopic values of C and N as covariates. PASW v18.0 (SPSS Inc., Chicago) statistical software package was used to carry out data analysis with α = 0.05.
Results
In the analysis of normality, isotope data showed a reasonable fit to the normal model. Conversely, Se and heavy metals concentrations showed skewed distributions. The descriptive statistics for δ 13C and δ 15N signatures—as well trace elements analyzed in feathers of nestlings of Tricolored heron, Cattle egret, and Snowy egret—are listed in Table 1. We found significant differences among δ 13C values (F 2,64 = 24.67; p < 0.001) with all pairwise differences being significant. The highest δ 13C values corresponded to Cattle egret followed by Snowy egret and the lowest values to Tricolored heron. No significant differences were found in mean value among the three species for δ 15N (F 2,64 = 1.10; p = 0.34). The relative variability of isotopes samples for each species, evaluated through coefficients of variation, was relatively low (suggesting a small degree of individual segregation) and ranged between 7 and 9 % for δ 13C and 5 and 11 % for δ 15N (Table 1).
The relationship between foraging habitat (through δ 13C) and trophic level (through δ 15N) occupied by the three species confirmed that most individuals sampled used similar trophic levels. No significant relationship was found between δ 13C and δ 15N, although greater variability was observed in Cattle egret (Fig. 2).
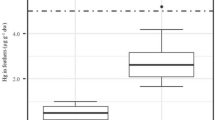
Hg concentrations in feathers of Tricolored heron and Snowy egret were similar, but levels were significantly lower in Cattle egret (F 2,64 = 79.88; p < 0.001) (Fig. 3). The geometric mean values of this heavy metal in the two egrets species were four-fold greater then those found in Cattle egret (95 % CI for the ratio 2.97–5.08). Pb concentration showed heterogeneity of variances, with greater variability among individuals of Snowy egret, but no significant differences were found among species (F 2,64 = 1.61; p = 0.21). Se concentration had the highest values in Tricolored heron and Cattle egret and the lowest values in Snowy egret with a significant differences between these two groups (F 2,64 = 13.9; p < 0.001).
Distribution of Hg, Pb, and Se concentrations in feathers of nestling Cattle egret (B. ibis, white), Snowy egret (E. thula, light gray), and Tricolored heron (E. tricolor, dark gray) from Birama Swamp, Cuba, 2001. *Outliers (Hg: F 2,64 = 79.88, p < 0.001; Pb: F 2,64 = 1.61, p = 0.21; Se: F 2,64 = 13.9, p < 0.001)
There was no significant relationship between Hg and Se log concentrations in any of the species [Cattle egret r = 0.18 (p = 0.4); Snowy egret r = 0.05 (p = 0.8); Tricolored heron r = 0.30 (p = 0.17)]. A significant and independent relationship was found when we modeled log-Hg concentration in relation to the isotopic signals of δ 15N and δ 13C without interaction with the species level (Fig. 4). Thus, within a particular species, greater Hg levels were associated with greater δ 15N values (slope = 0.066, p = 0.04). This relationship was especially strong in Cattle egret, the species that presented the wider range for δ 15N (Fig. 4).
In the case of general linear models of both Se and Pb, there was no interaction δ 15N * δ 13C and no effect over the δ 13C signature; thus, both elements were adjusted for δ 15N. The results for Se showed no significant interaction of δ 15N by species. Higher mean values of this metal were detected for Tricolored heron followed by Cattle egret with Snowy egret showing the lowest mean value. In the case of Pb, we found an association between δ 15N and species just for Snowy egret (F 2,67 = 4.97; p = 0.01) with a negative slope.
Discussion
Wading-bird feeding strategies are broad and are related with a variety of factors that include prey availability, foraging behaviors, and habitat characteristics among others. The results obtained for δ 13C support reported differences in relation to foraging area and prey use among the three Ardeid species. Although Tricolored heron, Snowy egret, and Cattle egret are top predators (Palmer et al. 1997; Vander Zanden et al. 2006) and showed similar δ 15N values, each of them has a unique position in the community foraging structure. The lowest δ 13C values of Tricolored heron responds to their aquatic habits because they feed mainly on fishes (Denis and Jiménez 2009). Post (1990) and Frederick (1997) consider it a typical coastal piscivores species feeding mainly on poecilids (guppies, mollies, and platies) and ciprinodontiforms (rivulids, killifishes, and pupfishes) in all of its distribution range. For example, in the south center of Cuba, >90 % of the diet of this species consists of fishes (Acosta et al. 1990a; Denis and Jiménez 2009). The Snowy egret, with similar values of δ 13C signature, can also include crustaceans in its diet (Parnell et al. 2000). The work on conventional diet analysis performed by Denis and Jiménez (2009) for chicks in the same colony of Birama Swamp found that 99, 85, and 3.6 % of the number of prey consumed by Tricolored heron, Snowy egret, and Cattle egret, respectively, came from aquatic environments. Based on our results for δ 13C, i.e., the two species (Tricolored heron and Snowy egret) that fed mainly on aquatic prey showed strong trophic similarities, whereas Cattle egret showed a more terrestrial preference. Acosta et al. (1990a) found the same tendency based on stomach content analysis in adults of these Ardeid species that fed in Cuban rice paddies, an alternative foraging place.
Some birds shift their diets during breeding season either in response to physiological needs or to the growth requirements of their offspring (Newman and Unger 2009). For example, coastal nesting laughing gulls (Leucophaeus atricilla) and White ibis (Eudocimus albus) shift from a diet of primarily salt or brackish water preys to freshwater prey because their nestlings are salt intolerant (Johnston and Bildstein 1990; Dosch 1997). This can influence different δ 13C signatures between seasons even in the same species.
Although no differences in mean δ 15N values were found among species, it is important to highlight that these values respond to distinct strategies. δ 15N values for Cattle egret span >6 ‰, approximately two trophic levels. The wide dispersion of δ 15N and δ 13Cvalues in this species can be attributed to its generalist and opportunist behavior (Telfair II 2006). They feed on a wide variety of animal prey, preferably in dry areas and rice fields, where they consume mostly terrestrial prey, e.g., orthopterans, adult and larvae lepidoptera, arachnids, small rodents, and also some aquatic insects (Acosta et al. 1990b, 1994; Mugica et al. 2005). These results are consistent with those reported by Bryan et al. (2012) in individuals of Cattle egret of the southeastern United States.
In contrast, Snowy egret is mostly an aquatic feeder of a variety of prey (dragonflies, shrimp, and fish). Nevertheless, terrestrial preys (spiders and orthopterans) have also been found in its diet (Acosta et al. 1990a). We should also point out that there is a tendency in Tricolored heron and Snowy egret for certain feeding specialization (Frederick 1997; Parnell et al. 2000). However, frequent intraspecific variation may be related to physical condition, age, sex, or hierarchic position at the feeding site.
Because the bioaccumulation process is minimal in chick feathers, their Hg content is likely to reflect mostly local dietary inputs (Boncompagni et al. 2003; Sanpera et al. 2007; Abdennadher et al. 2010). The levels of this heavy metal differ according to the food source, being relatively greater for aquatic environments (Chen et al. 2009; Grigal 2002). It is known that inorganic Hg is most readily converted to methylmercury under anaerobic conditions in marine or freshwater systems, such as wetlands, lakes, and reservoirs; therefore, Hg can be easily incorporated in the diet aquatic organism and biomagnify at greater trophic levels (Rimmer et al. 2005; Driscoll et al. 2007). Still, the Hg values obtained in our study are lower compared with data from feathers of the same species in other parts of the world (see Table 2).
In contrast, δ 13C and δ 15N values showed an independent and significant relationship with Hg, although the effect did not vary among species. Individuals with more terrestrial feeding habits tend to show lower Hg levels than individuals with more aquatic foraging habitat preferences (see Fig. 4b). We already detected this trend at species level, where greater Hg values belongs to Tricolored heron and Snowy egret. Higher Hg levels were observed in the two species that feed on aquatic prey, which agrees with the results of Bloom (1992), who found that methylmercury is the dominant form found in fishes, accounting for ≥95 % of the total Hg burden. Because bioavailability is greater for birds feeding on aquatic prey, the Hg values were lower in Cattle egret.
Se is an essential trace element in animals because it forms part of some enzymatic systems (Spallholz and Hoffman 2002); however, it becomes toxic at concentrations only slightly greater than the normal level (Heinz 1996; Lam et al. 2005). In our study, the concentrations of this element were slightly greater in Tricolored heron than in Snowy egret. Although these two species have similar diets, they can have different proportional use of each type of prey. Therefore, feeding on different prey classes in the aquatic environment can result in different Se levels. Our results presented more variation in mean values than in other studies, at least in Cattle egret, with some values in the literature lower than in Cuba (Table 2).
Pb has been responsible for incidents of acute bird poisoning, including neurobehavioral effects (Dey et al. 2000; Burger & Gochfeld 2005). In our study, Pb concentrations in feathers were similar among species and had lower values than reported by other studies (Table 2) (Burger et al. 1992b). These levels fall within background levels of Pb in wild birds (Clark and Scheuhammer 2003); therefore, there is probably no risk of lethal and sublethal effects of this heavy metal in juvenile birds of the Birama Swamp colony that may impair their survival.
Our results may also be influenced by the fact that the three species share feeding sites most times, i.e., rice and other cultures surrounding nesting colonies, mainly those fields prepared for sowing. In these fields, there is a sudden increase in prey availability through exposure during mechanical tilling, and many subterranean prey are exposed and easily captured with low energy cost (Mugica et al. 2006). As a result, we can find some individuals with opportunistic behavior feeding on prey that are not common in the species diet. Our study period was also limited to 1 year and is indicative of the climatic conditions associated with this specific breeding season. It has been reported that annual differences in rainfall can greatly affect wetland availability and avian use of this site (Gaines et al. 2000; Gariboldi et al. 2001).
In general, the species analyzed showed low pollutant levels compared with natural standards and with data from other studies. All values obtained are lower than sublethal values reported for Hg, Se, and Pb (Burger and Gochfeld 1997, 2000; Boncompagni et al. 2003). This is not surprising because in Cuba, many agricultural activities, such as rice culture, are performed without excessive contaminant charge, such as chemical fertilizers, pesticides, and herbicides. Moreover, Birama Swamp is a natural and isolated area in remote eastern Cuba far away from sites with greater industrialization. Our results could be considered as a baseline for trace-element levels in conservation efforts in similar habitats, and managers can use these as a tool to achieve management regulations.
References
Abdennadher A, Ramírez F, Romdhane MS, Ruiz X, Jover L, Sanpera C (2010) Biomonitoring of coastal areas in Tunisia: stable isotope and trace element analysis in the Yellow-legged gull. Mar Pollut Bull 60:440–447
Acosta M, Mugica L (2006) Aves acuáticas en Cuba. http://www.birdlife.org/action/science/species/waterbirds/waterbirds_pdf/waterbirds_report_cuba_2006.pdf. Accessed 04 Oct 2012
Acosta M, Mugica L, Martínez P (1990a) Segregación del subnicho trófico en seis especies de ciconiformes cubanos. Ciencias Biológicas 23:68–81
Acosta M, Mugica L, Torres O, Abad Y (1990b) Alimentación de Bubulcus ibis ibis (Linneo) (Aves: Ardeidae) en la Provincia de Pinar del Río. Ciencias Biológicas 23:82–91
Acosta M, Mugica L, Valdés S (1994) Estructura trófica de una comunidad de aves acuáticas. Ciencias Biológicas 27:24–44
Bryan Jr. AL, Brant HA, Jagoe CH, Romanek CS, Brisbin Jr. IL (2012) Mercury concentrations in nestling wading birds relative to diet in the Southeastern United States: a stable isotope analysis. Arch Environ Contam Toxicol 63(1):144–152
Amano T (2009) Conserving bird species in Japanese farmland: past achievements and future challenges. Biol Conserv 142:1913–1921
Becker PH (2003) Biomonitoring with birds. In: Marker BA, Breure AM, Zechmeister HC (eds) Bioindicators and biomonitors: principles, concepts and applications. Elsevier Science, New York, pp 677–737
Berg T, Steinnes E (1997) Use of mosses (Hylocomium splendens and Pleurozium schreberi) as biomonitors of heavy metal deposition: from relative to absolute deposition values. Environ Pollut 98:61–71
Beyer WN, Spalding MG, Morrison D (1997) Mercury concentrations in feathers of wading birds from Florida. Ambio 26:97–100
Birch GF, Evenden D, Teutsch ME (1996) Dominance of point source in heavy metal distributions in sediments of a major Sydney estuary (Australia). Environ Geol 28:169–174
Bloom NS (1992) On the chemical form of mercury in edible fish and marine invertebrate tissue. Can J Fish Aquat Sci 49:1010–1017
Boncompagni E, Muhammad A, Jabeen R, Orvini E, Gandini C, Sanpera C et al (2003) Egrets as monitors of trace-metal contamination in wetlands of Pakistan. Arch Environ Contam Toxicol 45:399–406
Bond AL (2010) Relationships between stable isotopes and metal contaminants in feathers are spurious and biologically uninformative. Environ Pollut 158:1182–1184
Borga K, Gabrielsen GW, Skaare JU (2001) Biomagnification of organochlorines along a Barents Sea food chain. Environ Pollut 113:187–198
Bucci JP, Rebach S, DeMaster D, Showers WJ (2007) A comparison of blue crab and bivalve delta N-15 tissue enrichment in two North Carolina estuaries. Environ Pollut 145:299–308
Burger J (1993) Metals in avian feathers: bioindicators of environmental pollution. Rev Environ Toxicol 5:203–311
Burger J, Gochfeld M (1995) Heavy metals concentrations in eggs of Herring gulls (Larus argentatus): temporal differences from 1989 to 1994. Arch Environ Contam Toxicol 29:192–197
Burger J, Gochfeld M (1997) Risk, mercury levels, and birds: relating adverse laboratory effects to field biomonitoring. Environ Res 75:160–172
Burger J, Gochfeld M (2000) Metal levels in feathers of 12 species of seabirds from midway atoll in the northern Pacific Ocean. Sci Total Environ 257:37–52
Burger J, Gochfeld M (2005) Effects of lead on learning in herring gulls: an avian wildlife model for neurobehavioral deficits. Neurotoxicology 26:615–624
Burger J, Gochfeld M (2009) Comparison of arsenic, cadmium, chromium, lead, manganese, mercury and selenium in feathers in Bald eagle (Haliaeetus leucocephalus), and comparison with Common eider (Somateria mollissima), Glaucous-winged gull (Larus glaucescens), Pigeon guillemot (Cepphus columba), and Tufted puffin (Fratercula cirrhata) from the Aleutian Chain of Alaska. Environ Monit Assess 152:357–367
Burger J, Parsons K, Benson T, Shukla T, Rothstein D, Gochfeld M (1992a) Heavy metal and selenium in young cattle egrets from colonies in the northeast United States, Puerto Rico and Egypt. Arch Environ Contam Toxicol 23:435–439
Burger J, Schreiber EA, Gochfeld M (1992b) Lead, cadmium, selenium and mercury in seabird feathers from the tropical mid-Pacific. Environ Toxicol Chem 11:815–822
Chen CY, Dionne M, Mayes BM, Ward DM, Sturup S, Jackson BP (2009) Mercury bioavailability and bioaccumulation in estuarine food webs in the Gulf of Maine. Environ Sci Technol 43:1804–1810
Clark AJ, Scheuhammer AM (2003) Lead poisoning in upland-foraging birds of prey in Canada. Ecotoxicology 12:23–30
CNAP (2002) Sistema Nacional de Áreas Protegidas. Cuba. Plan 2003–2008. Escandón Impresores. Sevilla, España
Connor K, Gabor S (2006) Breeding waterbird wetland habitat availability and response to water-level management in Saint John River floodplain wetlands, New Brunswick. Hydrobiologia 567:169–181
Denis D, Jiménez A (2009) Nestling diet in five species of herons and egrets in Birama swamp, Cuba. J Carib Ornithol 22(1):26–31
Dey PM, Burger J, Gochfeld M, Reuhl KR (2000) Developmental lead exposure disturbs expression of synaptic neural cell adhesion molecules in herring gull brains. Toxicology 146:137–147
Dosch JJ (1997) Salt tolerance of nestling Laughing Gulls: an experimental field investigation. Colon Waterbirds 20:449–457
Driscoll C, Han YJ, Chen CY, Evers DC, Lambert KF, Holsen TM et al (2007) Mercury contamination in forest and freshwater ecosystems in the northeastern United States. Bioscience 57:17–28
Duman F, Aksoy A, Demirezen D (2007) Seasonal variability of heavy metals in surface sediment of lake Sapanca, Turkey. Environ Monit Assess 133:277–283
Eadie JM, Elphick CS, Reinecke KJ, Miller MR (2008) Wildlife values of North American rice lands. In: Manley SW (ed) Conservation in rice lands of North America. The Rice Foundation, Stuttgart, pp 7–90
Elphick C, Oring L (1998) Winter management of Californian rice fields for waterbirds. J Appl Ecol 35:95–108
Fasola M, Rubolini D, Merli E, Boncompagni E, Bressan U (2010) Long-term trends of heron and egret populations in Italy, and the effects of climate, human-induced mortality, and habitat on population dynamics. Popul Ecol 52:59–72
Frederick PC (1997) Tricolored Heron (Egretta tricolor). In: Pool A (ed) The birds of North America online. Cornell Lab of Ornithology, Ithaca, NY. http://bna.birds.cornell.edu/bna/species/306. Accessed 04 Oct 2012
Fujioka M, Lee SD, Kurechi M, Yoshida H (2010) Bird use of rice fields in Korea and Japan. Waterbirds 33(Special Publ 1):8–29
Furness RW, Greenwood JJD (1993) Birds as monitors of environmental change. Chapman & Hall, London
Gaines KF, Bryan AL Jr, Dixon PM (2000) The effects of drought on foraging habitat selection of breeding Wood Storks in coastal Georgia. Waterbirds 23:64–73
Gariboldi JC, Bryan AL Jr, Jagoe CH (2001) Annual and regional variation in mercury concentrations in Wood Stork nestlings. Environ Toxicol Chem 20:1551–1556
Gell P, Fritz S, Battarbee R, Tibby J (2007) LIMPACS—Human and climate interactions with lake ecosystems: setting research priorities in the study of the impact of salinization and climate change on lakes, 2005–2010. Hydrobiologia 591:99–101
Green ID, Diaz A, Tibbett M (2010) Factors affecting the concentration in seven-spotted ladybirds (Coccinella septempunctata L.) of Cd and Zn transferred through the food chain. Environ Pollut 158:135–141
Grigal DF (2002) Inputs and outputs of mercury from terrestrial watersheds: a review. Environ Rev 10:1–39
Haukås M, Berger U, Hop H, Gulliksen B, Gabrielsen GW (2007) Bioaccumulation of per- and polyfluorinated alkyl substances (PFAS) in selected species from the Barents Seafood web. Environ Pollut 148:360–371
Heinz GH (1996) Selenium in birds. In: Beyer WN, Heinz GH, Redmon-Norwood AW (eds) Environmental contaminants in wildlife: interpreting tissue concentrations. CRC, Boca Raton, pp 447–458
Hobson KA (2011) Isotopic ornithology: a perspective. J Ornithol 152(Suppl 1):S49–S66
Hobson KA, Fisk A, Karnovsky N, Holst M, Gagnon JM, Fortier M (2002) A stable isotope (delta C-13, delta N-15) model for the North Water food web: implications for evaluating trophodynamics and the flow of energy and contaminants. Deep Sea Res Part II Top Stud Oceanogr 49:5131–5150
Hoekstra PF, O’Hara TM, Fisk AT, Borga K, Solomon KR, Muir DCG (2003) Trophic transfer of persistent organochlorine contaminants (OCs) within an Arctic marine food web from the southern Beauforte Chukchi Seas. Environ Pollut 124:509–522
Hofer C, Gallagher FJ, Holzapfel C (2010) Metal accumulation and performance of nestlings of passerine bird species at an urban brownfield site. Environ Pollut 158:1207–1213
Inger R, Bearhop S (2008) Applications of stable isotope analyses to avian ecology. Ibis 150:447–461
Jardine T, Kidd K, Fisk A (2006) Applications, considerations, and sources of uncertainty when using stable isotope analysis in ecotoxicology. Environ Sci Technol 40:7501–7711
Johnston JW, Bildstein KL (1990) Dietary salt as a physiological constraint in white Ibis breeding in an estuary. Physiol Zool 63:190–207
Lam JCW, Tanabe S, Lam MHW, Lam PKS (2005) Risk to breeding success of waterbirds by contaminants in Hong Kong: evidence from trace elements in eggs. Environ Pollut 135(3):481–490
Lee SY, Dunn RJK, Young RA, Connolly RM, Dale PER, Dehayr R et al (2006) Impact of urbanization on coastal wetland structure and function. Austral Ecol 31:149–163
Lemoine N, Bauer HG, Peintinger M, Bohning-Gaese K (2007) Effects of climate and land-use change on species abundance in a central European bird community. Conserv Biol 21:495–503
McIntyre JK, Beauchamp DA (2007) Age and trophic position dominate bioaccumulation of mercury and organochlorines in the food web of Lake Washington. Sci Total Environ 372(2–3):571–584
McKinney RA, Raposa KB, Cournoyer RM (2011) Wetlands as habitat in urbanizing landscapes: patterns of bird abundance and occupancy. Landsc Urban Plan 100:144–152
Michener RH, Schell DM (1994) Stable isotope ratios as tracers in marine aquatic food webs. In: Lajtha K, Michener RH (eds) Stable isotopes in ecology and environmental science. Blackwell Scientific, London, pp 138–157
Mitsch WJ, Gosselink JG (2000) Wetlands, 3rd edn. Van Nostrand, New York
Montoya JM, Raffaelli D (2010) Climate change, biotic interactions and ecosystem services. Philos Trans R Soc Lond B Biol Sci 365:2013–2018
Mugica L, Acosta M, Denis D, Jiménez A (2005) Variación estacional en la dieta de 6 especies del gremio Zancudas, Aves: Ciconiiformes. Biología 19:40–49
Mugica L, Acosta M, Denis D (2006) Disponibilidad de presas para las aves acuáticas en los campos inundados de la arrocera Sur del Jíbaro durante el ciclo de cultivo del arroz. J Carib Ornithol 19:102–108
Newman MC, Unger MA (2009) Fundamentals of ecotoxicology, 3rd edn. Lewis, Washington, DC
Nisbet ICT (1994) Effects of pollution on marine birds. In: Nettleship D, Burger J, Gochfeld M (eds) Seabirds on islands. Birdlife conservation series 1. Birdlife International, Cambridge, pp 8–25
Palmer MA, Ambrose RF, Poff NL (1997) Ecological theory and community restoration ecology. Restor Ecol 5:291–300
Parnell JF, Ai DG, Parsons KC, Master TL (2000) Snowy egret (Egretta thula). In: Poole A (ed) The birds of North America online. Cornell Lab of Ornithology, Ithaca, NY. http://bna.birds.cornell.edu/bna/species/489. Accessed 04 Oct 2012
Peakall DB (ed) (1992) Animal biomarkers as pollution indicators. Ecotoxicology series 1. Chapman & Hall, London
Perugini M, Manera M, Grotta L, Abete MC, Tarasco R, Amorena M (2011) Heavy metal (Hg, Cr, Cd, and Pb) contamination in urban areas and wildlife reserves: honeybees as bioindicators. Biol Trace Elem Res 140:170–176
Piatt JF, Harding AMA, Shultz M, Speckman SG, van Pelt TI, Drew GS et al (2007) Seabirds as indicators of marine food supplies: cairns revisited. Mar Ecol Prog Ser 352:221–234
Post W (1990) Nest survival in a large ibis–heron colony during a three-year decline to extinction. Colon Waterbirds 13:50–61
Rimmer CC, McFarland KP, Evers DC, Miller EK, Aubry Y, Busby D et al (2005) Mercury levels in Bicknell’s thrush and other insectivorous passerine birds in montane forests of the northeastern United States and Canada. Ecotoxicology 14:223–240
Sanpera C, Ruiz X, Moreno R, Jover L, Waldron S (2007) Mercury and stable isotopes in feathers of Audouin’s Gulls as indicators of feeding habits and migratory connectivity. Condor 109(2):268–275
Spallholz JE, Hoffman DJ (2002) Selenium toxicity: cause and effects in aquatic birds. Aquat Toxicol 57:27–37
Stewart FM, Phillips RA, Catry P, Furness RW (1997) Influence of species, age and diet on mercury concentrations in Shetland seabirds. Mar Ecol Prog Ser 151:237–244
Svazas S, Stanevicius V (2000) The waterbirds of the large fish pond complexes in Lithuania. Acta Ornithol 35(1):45–54
Takekawa JY, Corinna TL, Pratt RT (2001) Avian communities in baylands and artificial salt evaporation ponds of the San Francisco Bay estuary. Hydrobiologia 466:317–328
Tavares PC, Kelly A, Lopes RJ, Pereira ME, Duarte AC, Furness RW (2007) The influence of dietary specialization and trophic status on mercury levels in two species using common coastal wetland, Himantopus himantopus and Sterna albifrons. Ardeola 54:275–288
Telfair II RC (2006) Cattle Egret (Bubulcus ibis). In: Poole A (ed) The birds of North America online. Cornell Lab of Ornithology, Ithaca, NY. http://bna.birds.cornell.edu/bna/species/113. Accessed 04 Oct 2012
Valiela I, Bowen J, York J (2001) Mangrove forests: one of the worlds threatened major tropical environments. Bioscience 51:807–815
Vander Zanden MJ, Olden JD, Gratton C (2006) Foodweb approaches in restoration ecology. In: Falk DA, Palmer MA, Zedler JB (eds) Foundations of restoration ecology. Island Press, Washington, DC, pp 165–189
Wang Q, Kim D, Dionysiou DD, Sorial GA, Timberlake D (2004) Sources and remediation for mercury contamination in aquatic systems—a literature review. Environ Pollut 131(2):323–336
Yoon CG (2009) Wise use of paddy rice fields to partially compensate for the loss of natural wetlands. Paddy Water Environ 7:357–366
Young L, Chan G (1997) The significance of drained fish ponds for wintering waterbirds at the Mai Po Marshes, Hong Kong. Ibis 139(4):694–698
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rodríguez, A., Acosta, M., Mugica, L. et al. Assessment of Trace Elements and Stable Isotopes of Three Ardeid Species at Birama Swamp, Cuba. Arch Environ Contam Toxicol 65, 24–32 (2013). https://doi.org/10.1007/s00244-013-9887-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-013-9887-3