Abstract
Wading birds are key components of wetlands, transporting matter, energy, contaminants and associated organisms among limnetic, estuarine, terrestrial and coastal ecosystems. The role of cocoi herons (Ardea cocoi) in connecting these ecosystems was studied in the only known estuarine and limnetic colonies in coastal plains of southern Brazil. Food obtained by parents around colonies when foraging for chicks and themselves was used to assess diet, by the analysis of 50 pellets, gastrointestinal contents and spontaneous regurgitated samples, as well as the analysis of stable isotopes (δ 13C and δ 15N) in the whole blood of 25 chicks. Cocoi herons were predominantly piscivorous in both environments, relying mainly on whitemouth croaker (Micropogonias furnieri) in the estuarine colony (47 % of samples and 36–56 % in the isotope mixing model), followed by Callinectes blue crabs and on trahira (Hoplias aff. malabaricus), in the limnetic colony (60 % of the dietary samples). However, for the limnetic colony, terrestrial and aquatic potential food sources contributed evenly to the isotopic mixing model. Furthermore, stable isotopes suggested that marine food sources, available on the coast 15 km away, were part of the diet of chicks in the limnetic colony. Complementary approaches used in the current study demonstrate that widely distributed, large-sized Ardea herons and other waterbirds are important for the connectivity of terrestrial and a range of aquatic environments. These birds contribute to the flow of matter and energy between wetlands and adjacent habitats and are key species for linking different food webs through complex and still poorly understood lateral subsides.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Most wading birds breed in large, multi-specific colonies. Chicks of colonial waterbirds are fed by prey obtained by parents around colonies, and thus, the ecological conditions of the surrounding environments are reflected in their tissues (Burger and Gochfeld 1993; Fasola et al. 1998; Green and Elmberg 2014). Even during the breeding period when adults are central place foragers (i.e. their foraging ranges are limited to areas around the nest), they are able to obtain and transport materials from a range of heterogeneous environments nearby (Green and Elmberg 2014; Britto and Bugoni 2015; Kloskowski and Trembaczowski 2015). For instance, chicks of Ardea herons are fed by prey captured in areas as far as 35 km from the colonies (Custer and Osborn 1978, Frederick 2002). The cocoi heron (Ardea cocoi Linnaeus, 1766) is the largest South American heron (Sick 1997). It inhabits wetlands, such as lakes, marshes, rivers and beaches, from the marine coast to inland waterbodies. Cocoi herons have a wide distribution across South America and are regarded as generalists and top predators in shallow water ecosystems. Data on its feeding and foraging ecology are essential for a comprehensive understanding of matter, energy and contaminant transport within and between these ecosystems. However, despite that this species is large, conspicuous and widespread, surprisingly little information is available of its basic biology (Kushlan and Hancock 2005).
Studies on cocoi herons are usually restricted to breeding and chick feeding observations (e.g. Borrero and Cruz-Millán 1982; Kushlan and Hancock 2005; González-Acuña et al. 2008; Ducommun and Beltzer 2010; Pretelli et al. 2012). Dietary studies suggest that the species is predominantly piscivorous, but their diet can also include insects, crustaceans, reptiles, amphibians and rodents as secondary food items (Borrero and Cruz-Millán 1982; Ducommun and Beltzer 2010; Pretelli et al. 2012). Herons, similar to storks, cormorants and several other waterbirds, regurgitate the food to feed chicks (Weller 1999) and can also regurgitate spontaneously when disturbed (Gianuca et al. 2012). Regurgitates represent partially digested prey, allowing species identification and quantification. On the contrary, pellets are hard, undigested remains combined with mucous that are regularly egested to avoid passing them through the digestive tract. Regurgitates and pellets have both been widely used for the study of the diet of Pelecaniformes (e.g. Hewson and Hancox 1979; Jakubas and Mioduszewska 2005; Rodríguez et al. 2007; Gianuca et al. 2012; Pretelli et al. 2012). For pellet analysis, hard remains, such as fish otoliths and bones, are used for species identifications and to assess individual fish size ingested (Barrett et al. 2007). However, these conventional approaches only reflect recently ingested food and could be biased towards prey with hard parts to the detriment of soft body food items (Barrett et al. 2007; Britto and Bugoni 2015).
Stable isotope analysis (SIA) is a complementary technique to traditional methods for the study of a consumers’ diet (Karnovsky et al. 2012) and is increasingly being used in studies of the feeding ecology of waterbirds (Hobson 2011). While traditional methods provide detailed information of recently ingested prey species and sizes, SIA provides clues on the proportions of assimilated food items at different timescales, as different tissues have variable turnover rates. In addition, SIA allows inferring trophic levels and the origins of carbon and nitrogen in the ecosystem (Peterson and Fry 1987).
Nitrogen isotope ratios (15N/14N or δ 15N) are frequently used to represent the vertical trophic structure in aquatic ecosystems (Vander-Zanden et al. 1997; Hussey et al. 2014), whereas carbon isotope ratios (13C/12C or δ 13C) are useful for the identification of food sources, such as coastal vs. oceanic, marine vs. limnetic or environments dominated by C3 or C4 photosynthetic plants (Peterson and Fry 1987; Wassenaar and Hobson 2000; Fry 2006; Barrett et al. 2007). The rationale of the technique is based on the assumption that stable isotope (SI) values of elements in animal tissues reflect the proportion of such elements in their food during the synthesis of tissues (Peterson and Fry 1987; Hobson 2009). As a consequence, inert tissues, such as feathers, fur or other keratin rich tissues, are used to infer the diet during the period of their formation, whereas tissue synthetized intermittently, such as blood and muscle, reflects the diet from sampling to a variable previous time window (Peterson and Fry 1987).
Despite several SIA-based studies that have investigated the diet of waterbirds, the technique has not been used to elucidate the feeding ecology of cocoi herons and their role in connecting environments. Furthermore, the diet of the species based on conventional methods is time- and place-restricted, usually limited to a list of ingested food items (e.g. Borrero and Cruz-Millán 1982), with limited sample sizes when quantification was attempted (Pretelli et al. 2012), or based on lethal methods applied to adults (Ducommun and Beltzer 2010). Furthermore, studies on the feeding ecology of the species in Brazil have not been conducted; thus, its role in aquatic ecosystems remains elusive.
In the current study, we determined the diet of cocoi heron chicks in limnetic and estuarine colonies in southern Brazil, using conventional and SIA methods, to infer the major food items of the species and their role in connecting coastal lowland environments. We hypothesize that chicks from the estuarine colony rely heavily on adjacent estuarine food sources, with a minor proportion of limnetic prey; on their turn, chicks from the limnetic colony only utilize limnetic prey. Furthermore, we expect that δ 13C values will be higher in chick blood from the estuarine colony, reflecting higher baseline values in comparison with limnetic or terrestrial food sources; δ 15N is expected to be similarly high, reflecting consumption of large prey at similar trophic levels in both environments.
Materials and Methods
Study Area
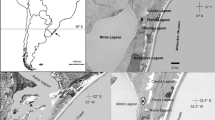
The study was conducted in two mixed colonies, one in a limnetic environment and one in an estuarine environment, both at Rio Grande Municipality, Rio Grande do Sul State, the southernmost in Brazil (Fig. 1). This region is regarded as an important area for heron conservation in the Americas (Kushlan and Hancock 2005). In both colonies, cocoi heron nests in mixed colonies with great egret Ardea alba Linnaeus, 1753, snowy egret Egretta thula (Molina, 1782), cattle egret Bubulcus ibis (Linnaeus, 1758), roseate spoonbill Platalea ajaja Linnaeus, 1758, and other large waterbird species. The limnetic colony was at Aguirre Marsh (32° 30′ S, 052° 32′ W), where the predominant vegetation was Schoenoplectus californicus C.A. Mey (Cyperaceae), with bushes of Cephalanthus glabratus Martius (Rubiaceae) over which the cocoi heron built nests. This colony was approximately 15 km from the Atlantic Ocean and 5 km from Mirim Lagoon, a large freshwater body between Brazil and Uruguay and a major waterbody in South America (Kotzian and Marques 2004). It was also nearby Taim Ecological Reserve, a fully protected 33,000-ha protected area, with vast wetlands.
The estuarine colony was located at Marinheiros Island (31° 58′ S, 052° 05′ W and 32° 02′ S, 052° 12′ W), the largest island of the Lagoa dos Patos Estuary. This island has an area of 40 km2, and the colony was located in its SW margin (32° 04′ S, 052° 09′ W) in a swamp composed mainly by Sebastiana brasiliensis L. and Sapium glandulosum L. (both Euphorbiaceae) trees, which covered approximately 70 % of the colony area (Gianuca 2010). The colonies were approximately 55 km apart from each other and likely spatially away from the foraging radius of herons during the breeding season (Custer and Osborn 1978; Frederick 2002) (Fig. 1).
Diet Sampling and Analysis
Spontaneous regurgitates of cocoi heron chicks were obtained during handling, as well as food remains and pellets found over or just below nests. Material was stored in plastic bags and frozen at −4 ° C until analysis. In the estuarine colony, a total of 30 samples (regurgitates, pellets and gastrointestinal contents of one dead chick) were obtained during the breeding seasons of 2008/2009, 2011/2012 and 2012/2013. In the limnetic heronry, 20 samples (regurgitates, pellets and stomach content of one chick found dead) were obtained during the 2011/2012 and 2012/2013 breeding seasons. Remains found in pellets, regurgitates and stomach contents were separated according to taxa, identified to the lowest taxonomic level possible and quantified (counted and weighted).
Fish were identified after consulting taxonomists of the Ichthyology Laboratory at Universidade Federal do Rio Grande (FURG), according to the identification guide by Corrêa et al. (2010). Estimation of the number of fish specimens ingested was based on the number of sagitta otoliths in severely digested samples or counting the individual partially digested fish. Insects were identified by experts from the Limnology Laboratory at FURG and quantified by counting the number of elytra or hemelytra. Crustaceans and reptiles were identified by experts at the Zoology Laboratory at FURG.
Blood Sampling for Stable Isotope Analysis
Chicks were captured in the nest by hand or with a dip net during the breeding seasons 2011/2012 and 2012/2013 in the estuary and in 2011/2012 in the limnetic colony. Isotopic values were pooled for analysis to increase sample sizes and because no marked inter-annual variation was detected for herons, as well as in other previous studies carried in our study area with similar or the same prey species (e.g. Silva-Costa and Bugoni 2013; Rodrigues et al. 2014). The nesting period and egg hatching were monitored aiming to sample chicks at least 3 weeks old to obtain blood that reflected the food ingested, instead of nutrients from egg yolk. The turnover rate of whole blood (half-life) in birds is ∼3–4 weeks (Bearhop et al. 2002). All birds were banded with metal rings to avoid resampling. Approximately 0.5 ml of blood was obtained with a syringe and needle from the brachial or tarsal vessel. A few whole blood drops were placed on glass slides and sun dried in the field. After drying, the blood was scraped with a spatula and placed inside plastic vials.
Muscle (fish, rodent) or whole body (insects, molluscs) of prey found in colonies or regurgitated by chicks was sampled for SIA. In addition, potential aquatic prey found in nearby colony areas was captured by dip nets, identified and stored in plastic bags. Muscle samples of recently dead road-killed rodents found near the limnetic colony were also collected. Most prey used in SI mixing models was obtained during the same breeding season, but some are from the bibliographic sources, previous to the current study (Table 1). In the laboratory, prey was measured and weighed. Lipids were extracted from these samples with petroleum ether for 4 h in a Soxhlet apparatus (Bugoni et al. 2010).
Chick blood and prey samples were freeze-dried, ground and homogenized. Approximately 1 mg of the biological sample was placed in sterile tin capsules and sent to the Analytic Chemistry Laboratory at the University of Georgia, USA. An isotope ratio mass spectrometer coupled to an elemental analyzer was used for the SIA of carbon and nitrogen. Values are provided in delta notation (δ), by Eq. 1 from Bond and Hobson (2012), as follows:
where R = 13C/12C or 15N/14N. The international standard for carbon was Pee Dee Belemnite and for nitrogen was atmospheric air. Samples were shuffled to minimize drift. Internal laboratory standards were bovine (7.51 ‰ ± 0.10 for δ 15N and −21.25 ‰ ± 0.07 for δ 13C) and poplar (−2.4 ‰ ± 0.21 for δ 15N and −27.45 ‰ ± 0.04 for δ 13C). Standards were run every 12 unknown samples. The precision calculated from internal standards was ±0.1 ‰ for both δ 15N and δ 13C.
Data Analysis
The samples of regurgitates, pellets and gastrointestinal tracts from different breeding seasons were pooled for data analysis and compared between colonies. For each food item found, defined as a species or other taxonomic category, the following were calculated: the frequency of occurrence (FO), as the number of samples containing a given food item; the relative frequency of occurrence (FO%), as a percentage of the total number of samples examined; the number of individual prey (N) counted in samples, as well as its relative prey-specific numeric contribution (PN%); the mass of each food item (M) and its relative prey-specific contribution in all samples (PM%); and the prey-specific index of relative importance (PSIRI%), calculated as in Brown et al. (2012), an index that integrates all other parameters, as follows:
For reconstruction of the mass of each individual prey ingested by herons, the body mass of whitemouth croaker Micropogonias furnieri (Desmarest, 1823) was determined after the identification and measurement of sagitta otoliths of 34 individuals with a stereomicroscope with a millimetric scale and the application of otolith × body length/body mass allometric equations (Naves 1999), which resulted in body mass of 174 ± 216.7 g (mean ± 1 SD). Atherinidae fish found in the samples were weighed in scale, as there was minimum digestion sign, and resulted in a mass of 2.2 g. The body mass of trahiras Hoplias aff. malabaricus (Bloch, 1794) and pale catfish Rhamdia quelen (Quoy & Gaimard, 1824) under an advanced stage of digestion had their mass estimated as 169.6 ± 135.2 g and 208.2 g ± 235.2 g, respectively, corresponding to the body mass of 33 individuals (trahiras) and 3 individuals (pale catfish) of similar sizes found in the diet of herons or obtained in fish sampling, for which mass could be determined. Unidentified fish, as well as La Plata croaker Pachyurus bonariensis Steindachner, 1879, and marbled swamp eel Synbranchus marmoratus Bloch, 1795, had body masses estimated as 175.1 g based on the mean body mass of all the other fish identified in the sample.
For beetles (Coleoptera), the reconstructed body mass was obtained by measuring 30 individuals, with sizes similar to those found in heron diet, collected near the study sites and weighed in the laboratory, resulting in a mean of 0.3 ± 0.2 g. The mass of Callinectes blue crabs was inferred in the same way as described for beetles, based on ten individuals and resulting in a mass of 68.5 g. For rodents, we used the mass of 35.3 ± 31.7 g, based on 203 individuals of nine species of rodents associated with wetlands (Sponchiado et al. 2012) and with size similar to those found in heron diet samples. The nine species were as follows: Akodon azarae (Fischer, 1829), Akodon reigi González et al. 1998, Calomys laucha (Fischer, 1814), Deltamys kempi (Thomas, 1917), Oligoryzomys flavescens (Waterhouse, 1837), Oligoryzomys nigripes (Olfers, 1818), Oxymycterus nasutus (Waterhouse, 1837), Scapteromys tumidus (Waterhouse, 1837) and Wilfredomys oenax (Thomas, 1928). For the yellow-bellied liophis Erythrolamprus poecilogyrus (Wied-Neuwied, 1825), the mass was estimated as 23.7 ± 10.3 g, based on the mean body mass of 20 individuals of similar sizes sampled in the region and available at the Herpetological Collection at FURG.
Statistical Analysis
The δ 15N values of chicks were compared between the estuarine colony and the limnetic colony by Student’s t test. The residuals of δ 13C values were not normally distributed and homoscedastic; therefore, we used the non-parametric Mann–Whitney U test for comparisons. Both tests were conducted in BioEstat 5.0 software (Ayres et al. 2007).
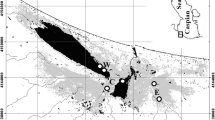
Based on δ 15N and δ 13C, the contribution of each prey group in the diet of cocoi heron chicks from each colony was estimated by Bayesian mixing models in R software, using the Stable Isotope Analysis in R (SIAR) package (Parnell et al. 2010). The isotopic values of 12 potential food items were used for modelling, of which four were collected in the estuarine colony, seven were collected around the limnetic colony, and one was collected in the marine beach adjacent to the limnetic colony (Table 1). Taxa were selected based on the data obtained from traditional methods used to assess the diet of cocoi heron at the study areas, as well as potential food items (e.g. lambari Bryconamericus iheringii [Boulenger, 1887]; one-sided livebearer Jenynsia multidentata [Jenyns, 1842] or habitat markers, such as the banded croaker Paralonchurus brasiliensis [Steindachner, 1875]) that were used as markers for the marine SI signature. Selection of potential food sources or habitat markers was based on availability of such information based on our sampling or the bibliography, as well as on cocoi heron habitats (e.g. predominant piscivorous). Selected sources and markers were also those abundant in the region, thus with high potential of being ingested by herons. Taxa with similar SI values or that were ecologically similar were pooled, as recommended by Phillips et al. (2005), and shown in Fig. 2. For limnetic heron chicks, the selected model had six potential food sources: insects (INS)—Belostomatidae water bugs; large limnetic fishes (LLG)—trahira and pale catfish; small limnetic fishes (SLF)—lambari; rodents (ROD)—Sigmodontinea and Cavia sp.; marine fishes (MAF)—banded croaker; and amphibians (ANP)—paradoxical frog Pseudis minuta Günther, 1858. The second model was based on SI values in the blood of chicks sampled at the estuarine colony, and the six sources used were as follows: INS, LLF and ROD as above, plus crustaceans (CRU)—blue crab Callinectes sp.; large estuarine fishes (LEF)—white croaker and the marine silverside Odontesthes argentinensis (Valenciennes, 1835); and small estuarine fishes (SEF)—one-sided livebearer (Fig. 3). Consumer–diet discrimination values used in the model were −0.3 ± 0.5 and 2.61 ± 0.5 ‰, for δ 15N and δ 13C, respectively, as mean ± 1 SD values of discrimination factors for penguins fed with whole fish in Cherel et al. (2005) (δ 13C = −0.81 ‰, δ 15N = 2.07 ‰ and δ 13C = 0.20 ‰, δ 15N = 2.72 ‰, for king penguin Aptenodytes patagonicus Miller, 1778, and rockhopper penguin Eudyptes chrysocome [Forster, 1781], respectively) and Williams et al. (2007) for tufted puffin Fratercula cirrhata (Pallas, 1769) (δ 13C = −0.30 and δ 15N = 3.05 ‰). These discrimination values were previously used for other waterbirds, predominantly piscivorous with generalist diet, the kelp gull Larus dominicanus Lichtenstein, 1823 (Silva-Costa and Bugoni 2013), great egrets and roseate spoonbills (Britto and Bugoni 2015).
δ 15N and (δ 13C) values of potential food items (filled symbols; mean ± 1 standard deviation) and values in the whole blood of cocoi heron chicks (empty symbols) in limnetic (a) and estuarine (b). Source values were corrected for a consumer–diet discrimination factor (−0.3 ± 0.5 ‰ for δ 15N and 2.61 ± 0.5 ‰ for δ 13C). Graphs are shown in different scales for clarity
Results
Diet
A total of 36 prey was found in 20 samples obtained in the limnetic colony, with fish dominating (29 individuals of two species), followed by rodents (five individuals). In the estuarine colony, the diet analysis of heron chicks resulted in 66 prey in 30 analyzed samples, similarly dominated by fish (53 individuals of 4 species), followed by crustaceans (ten individuals) (Table 2). Crustaceans and reptiles were found only in samples from the estuary and insects exclusively in samples from the limnetic colony. The most frequent prey in the limnetic environment was trahira (FO% = 60), followed by pale catfish (FO% = 40), which were also the most common in number, together accounting for 80.6 % of individual prey ingested. The whitemouth croaker and Callinectes blue crabs were the dominant food items in the estuarine samples, accounting for 66.7 % of all individual prey.
The prey-specific index of relative importance (PSIRI%) also indicated fish (80.3 %), whitemouth croaker (PSIRI = 40.2 %), crabs (PSIRI 13.1 %), rodents (PSIRI = 6.0 %) and the yellow-bellied liophis (PSIRI = 0.6 %) as the main food items in the estuarine colony. Chick herons in the limnetic colony also had fish (PSIRI = 71.6 %) and trahira (PSIRI = 40.6 %) as the major food items. Second in the ranking were rodents (PSIRI = 18.4 %), followed by insects (PSIRI = 10.0 %) (Table 2). Coleoptera beetles had the minimum size and body mass of 0.8 cm and 0.3 g, respectively, while the maximum was a trahira with 35-cm total length and 435 g. The most common estuarine fish, the whitemouth croaker, for which body sizes were possible to calculate through regression equation, was 201.4 ± 82.2 mm, n = 34. No taxon identified to species or genus level was found in both colonies, indicating no overlap in diet at this level.
Stable Isotope Analysis
The means ± 1 SD of SI values in the whole blood of cocoi heron chicks from the estuarine colony were δ 15N = 13.5 ± 0.6 ‰ and δ 13C = −15.1 ± 0.6 ‰ (n = 14), whereas in the limnetic environment, they were δ 15N = 11.6 ± 0.5 ‰ and δ 13C = −22.3 ± 1.4 ‰ (n = 11). Values differed between colonies for both δ 15N (t = 8.2, df = 23, P < 0.0001) and δ 13C (U = 0, P < 0.0001) (Fig. 2). The mixing model in SIAR indicated that chicks from the limnetic colony had small limnetic fishes as the main food source, with the 95 % credibility interval (CI) = 5–45 %, followed by amphibians (CI = 3–40 %), rodents (CI = 1–34 %), insects (CI = 1–31 %) and large limnetic fishes (CI = 1–29 %). Marine fishes, despite not being recorded in the diet through conventional methods, had a CI similar to other food sources (CI = 4–25 %). In the estuary, large estuarine fishes (LEF) were the main source of prey in mixing models with CI = 35–55 %, similar to crustaceans (CI = 35–52 %). Other sources included in the model had estimated contributions <10 % (Figs. 3 and 4).
Output of Bayesian stable isotope mixing models in SIAR package with intervals of credibility of 95, 75 and 25 % (grey shades in the centre, adjacent and borders, respectively). Insects (INS), large limnetic fish (LLF), small limnetic fish (SLF), rodents (ROD), marine fish (MAF), amphibians (ANP), crustaceans (CRU), large estuarine fish (LEF) and small estuarine fish (SEF). The graph shows the estimated contribution of different potential food sources for the isotopic values measured in the whole blood of cocoi heron (Ardea cocoi) chicks in limnetic (a) and estuarine (b) colonies, respectively
Discussion
Our results based on conventional dietary methods coupled with SIA confirmed that the cocoi heron is a generalist, but predominantly piscivorous forager in both estuarine and limnetic areas. The analysis of pellets and regurgitates pooled provided a moderately accurate estimate of diet. Because pellets may overemphasize prey with hard parts, soft prey was only detectable in spontaneous regurgitates. Notwithstanding, SIA provided information about the food sources effectively assimilated by consumers, complementing the dietary information provided by both conventional methods.
Cocoi heron seems to occupy a top predator position not only in aquatic ecosystems, by preying on large carnivorous fish such as trahira, pale catfish and whitemouth croaker, but also in terrestrial adjacent habitats, preying on snakes and rodents. In both estuarine and limnetic colonies, cocoi heron relied mainly on abundant food resources close to the breeding grounds. This is supported by the fact that the main prey in the estuary, the whitemouth croaker and blue crab, is abundant in estuarine shallow waters and largely exploited by artisanal fisheries in both the estuary and adjacent coast (Garcia et al. 2001; Vianna and D’Incao 2006; Costa et al. 2014; Rodrigues and D’Incao 2014). Similar situation was observed in the limnetic area, where cocoi heron relied on trahira and pale catfish, which are commonly found in wetlands around the colony (Garcia et al. 2006) and are also important resources for inland artisanal fisheries—trahira accounts for 67 % of the fish landings from Mirim Lagoon, followed by pale catfish with 14.3 % (Piedras et al. 2012). Taking into account that artisanal fisheries targeting pink shrimp Farfantepenaeus paulensis (Perez-Farfante, 1967) in the Patos Lagoon Estuary discards around 200 t of whitemouth croaker (<25-cm total length) per summer/autumn (Vieira et al. 1996) and that cocoi heron can feed on dead floating fish (Borrero and Cruz-Millán 1982), the possibility of herons feeding on discards cannot be ignored. Two other similar-sized and ecologically equivalent herons, the great blue heron Ardea herodias (Linnaeus, 1758) and the grey heron Ardea cinerea (Linnaeus, 1758), from North America and Old World, respectively (Kushlan and Hancock 2005), were reported following fishing vessels for feeding on discarded fish (Ewins and Hennessey 1992; Dies 1999a), as well as feeding on dead floating fish on shallow waters (Dies 1999b). Overall, results on the diet and feeding ecology based on SIA of cocoi herons studied in southern Brazil contribute to a better understanding of the role of ecologically similar species in other ecosystems and continents.
In addition to the large fish commonly available around colonies, herons also ingested terrestrial prey, such as rodents and reptiles, and relied on marine food items obtained far from breeding sites, evidencing their capacity exploring both alternative prey and relative distant foraging areas. The few available studies on the feeding ecology of the cocoi heron found similar results. Borrero and Cruz-Millán (1982), through direct visual observations, reported that chicks were fed fish and less frequently amphibians and crustaceans in a Colombian estuary. Ducommun and Beltzer (2010) analyzed the gastrointestinal tracts of adults shot during the non-breeding season and reported fish as the main food item, including trahira, while Pretelli et al. (2012) examined chick pellets, both in limnetic areas of Argentina, and reported fish as the main food item (including trahira), in addition to less frequently alternative prey such as rodents, frogs, freshwater crabs and insects. The two similar herons mentioned before, the great blue heron and the grey heron, also have a predominantly piscivorous diet, but prey items also include a wide range of mammals, birds, reptiles, amphibians, crustaceans and insects (Hewson and Hancox 1979; Collazo 1985; Jakubas and Mioduszewska 2005; Gwiazda and Amirowicz 2006). The flexibility in diet observed in cocoi heron and closely related species elsewhere allows herons to successfully respond to inter-annual and seasonal changes in prey availability. During the non-breeding season, when energy demands are lessened, herons can be more flexible in their diet and explore alternative prey more often (Kushlan and Hancock 2005). In our study system, at least in the estuarine colony, herons utilize the breeding area as roosting site during the non-breeding period (Gianuca 2010). Thus, they are expected to forage in the same areas, exploring different food items according to seasonal changes in prey availability (Frederick 2002; Kushlan and Hancock 2005). Britto and Bugoni (2015) studied the diet of the great egret in the same colonies of the present study and mainly found fish in the diet of estuarine great egrets, while insects dominated in the limnetic colony. This result differed from cocoi herons, for which the chick diet was mainly composed of fish in both locations. For both species, the importance of marine items in the limnetic colony was found, despite unexpectedly. This is in line with frequent records of cocoi herons foraging in marine beaches in the region (Vooren and Chiaradia 1990; Belton 1994) and with studies demonstrating that Ardea herons forage in areas as far as 38 km from the colonies (Custer and Osborn 1978; Frederick 2002), much farther than the 15 km between the studied site and the Atlantic Ocean beaches. In our study, the connectivity, and thus the transport of marine-derived nutrients and energy, was limited to a few individuals, but it is probably much stronger in colonies near the coast or located in estuaries. Notwithstanding, the lack of overlap in food items identified at the species level between colonies, added to the predominance of locally abundant prey in the diet analyses of both sites, indicates that cocoi herons primarily exploited food resources near to breeding grounds. This pattern was expected, because despite herons, in general, can perform long foraging flights, colony site choice is strongly linked to the proximity of high-quality foraging habitats; hence, they commonly forage near colonies (0–5 km) (Custer and Osborn 1978; Smith 1995; Frederick 2002; Kelly et al. 2008). Such pattern was also found in great egrets in both locations, but not in roseate spoonbills, whose diet was composed of limnetic food items even when nesting in the estuary (Britto and Bugoni 2015). This was likely due to osmoregulatory constraints in the early life of spoonbills (Johnston and Bildstein 1990), while heron and egret nestlings seem to better tolerate salt in their diets much earlier in the growth phase.
SIA suggested that cocoi herons in the limnetic colony used all of the sources included in the model evenly. This confirmed that adults foraged in all environments around the colony, including limnetic, terrestrial and marine beaches, which explained why the SI values were in between limnetic and marine values. The importance of limnetic food items, such as small fish, in the SI mixing model and its absence in regurgitates and pellets could be explained by an early chick diet based mainly on small fish, as described for grey herons (Moser 1985) and white-tailed tropicbird Phaethon lepturus (Daudin, 1802) (Ramos and Pacheco 2003). For both species, adults select small prey for younger chicks, increasing the size of the food items as nestlings mature and are able to ingest larger prey due to increased gape size (Moser 1985; Ramos and Pacheco 2003). Because SIA reflected the diet over a larger time window than regurgitates, the SIA results indicate the chick diet just after hatching. On the contrary, the limited importance of large limnetic fish in SIA, but its predominance in conventional methods, was also consistent with the diet switch of chicks and the different windows of time of both methods.
The δ 15N and δ 13C values in the whole blood of chicks sampled in the estuarine colony closely agreed with the results from conventional dietary methods. Both techniques indicated the predominance of whitemouth croakers and blue crabs as major prey/food sources. Despite the limited importance of small estuarine fish in the SI mixing model, consumption of these food sources by chicks during early development stages is plausible, as its isotopic signature is probably similar to that of blue crabs, although it was not measured in the current study. Rapid digestion of small fish and their identifiable remains also contributed to its absence in regurgitates and pellets.
The SIA indicated that chicks from the limnetic colony rely on more variable food items, with relatively important contributions of limnetic and marine fish, amphibians, rodents and insects. However, in the estuary, large estuarine fish and crustaceans were by far the most important food sources. This pattern is likely reflecting a primary difference between these two environments. Estuaries offer predictable foraging areas for herons through the season, like extensive shallow waters and tidal creeks, with high biomass of relatively few dominant euryhaline species (Day-Jr. et al. 2013). By contrast, inland wetlands offer a wider range of prey items (higher biodiversity) and foraging conditions and prey abundance are largely influenced by rainfall, thus less predictable than in estuaries (Powell 1987; Frederick et al. 1992, Frederick 2002; Maccarone and Brzorad 2005; Day-Jr. et al. 2013).
The current study clearly demonstrated the efficacy and robustness of the simultaneous use of SIA and traditional methods for the study of the foraging ecology of cocoi herons in a suite of environments, presumably with distinct SI signatures. Both techniques agreed that cocoi heron had a predominantly piscivorous diet, based on large and carnivorous fish, but also rodents and snakes, confirming our hypothesis of cocoi heron occupying higher food web positions. Furthermore, the main fish species in the diet were commercially important, widely fished and marketable species in the region. The use of food resources around colonies has been demonstrated by both methodological approaches, supporting our prediction of higher δ 13C values in blood of chicks from the estuarine colony. However, herons in the limnetic colony use a much wider range of foraging habitats than only limnetic ones, as had been hypothesized. Overall, cocoi herons play an important role in connecting marine, terrestrial, estuarine and limnetic environments during their breeding period. This further clarifies the role played by widely distributed, large-sized, Ardea herons and other waterbirds, on the connectivity between terrestrial and a range of aquatic environments, thus contributing to the flow of matter and energy between ecosystems around the world. The current study demonstrates that wading birds are key species in connecting different food webs (e.g. terrestrial, aquatic–limnetic, estuarine and coastal) through complex and still poorly understood lateral subsides. This theme deserves further studies using complementary approaches as those used here, in addition to remote tracking, genetic and trace element analysis, among others.
References
Ayres, M., M. Ayres-Jr, D.L. Ayres, and A.A. Santos. 2007. BIOESTAT—Aplicações estatísticas nas áreas das ciências bio-médicas. Belém: Ong Mamirauá.
Barrett, R.T., K. Camphuysen, T. Anker-Nilssen, J.W. Chardine, R.W. Furness, S. Garthe, O. Hüppop, O. Leopold, W.A. Montevecchi, and R.R. Veit. 2007. Diet studies of seabirds: a review and recommendations. ICES Journal of Marine Science 64: 1675–1691.
Bearhop, S., S. Waldron, S.C. Votier, and R.W. Furness. 2002. Factors that influence assimilation rates and fractionation of nitrogen and carbon stable isotopes in avian blood and feathers. Physiological and Biochemical Zoology 75: 451–458.
Belton, W. 1994. Aves do Rio Grande do Sul: distribuição e biologia. São Leopoldo: Unisinos.
Bond, A.L., and K.A. Hobson. 2012. Reporting stable-isotope ratios in ecology: recommended terminology, guidelines and best practices. Waterbirds 35: 324–331.
Borrero, J.I., and C.A. Cruz-Millán. 1982. Notas sobre la historia de la garza morena Ardea cocoi (Aves) en Colombia. Acta Biologica Colombiana 1: 51–75.
Britto, V.O., and L. Bugoni. 2015. The contrasting feeding ecology of great egrets and roseate spoonbills in limnetic and estuarine colonies. Hydrobiologia 744: 187–210.
Brown, S., J. Bizarro, G. Cailliet, and D. Ebert. 2012. Breaking with tradition: redefining measures for diet description with a case study of the Aleutian skate Bathyraja aleutica (Gilbert 1896). Environmental Biology of Fishes 95: 3–20.
Bugoni, L., R.A.R. McGill, and R.W. Furness. 2010. The importance of pelagic longline fishery discards for a seabird community determined through stable isotope analysis. Journal of Experimental Marine Biology and Ecology 391: 190–200.
Burger, J., and M. Gochfeld. 1993. Heavy metal and selenium levels in feathers of young egrets and herons from Hong Kong and Szechuan, China. Archives of Environmental Contamination and Toxicology 25: 322–327.
Cherel, Y., K.A. Hobson, and S. Hassani. 2005. Isotopic discrimination between food and blood and feathers of captive penguins: implications for dietary studies in the wild. Physiological and Biochemical Zoology 78: 106–115.
Collazo, J.A. 1985. Food habits of nesting great blue herons at Heyburn State Park, Idaho. Northwest Science 59: 144–146.
Corrêa, F., M.C. Claudino, and A.M. Garcia. 2010. Guia fotográfico e aspectos da biologia dos principais peixes de água doce do Parque Nacional da Lagoa do Peixe, RS. Cadernos de Ecologia Aquática 5: 28–43.
Costa, M.D.P., J.H. Muelbert, L.E. Moraes, J.P. Vieira, and J.P. Castello. 2014. Estuarine early life stage habitat occupancy patterns of whitemouth croaker Micropogonias furnieri (Desmarest, 1830) from the Patos Lagoon, Brazil. Fisheries Research 160: 77–84.
Custer, T.W., and R.G. Osborn. 1978. Feeding habitat use by colonially-breeding herons, egrets and ibises in North Carolina. Auk 95: 733–743.
Day-Jr., J.W., A. Yáñez-Arancibia, W.M. Kemp, and B.C. Crump. 2013. Introduction of estuarine ecology. In Estuarine ecology, ed. J.W. Day-Jr, A. Yáñez-Arancibia, W.M. Kemp, and B.C. Crump, 1–18. Hoboken: Wiley-Blackwell.
Dies, J.I. 1999a. Grey herons following fishing boats and diving for discarded fish. British Birds 92: 679.
Dies, J.I. 1999b. Large concentration of grey herons feeding on dead fish. British Birds 92: 679.
Ducommun, M.P., and A.H. Beltzer. 2010. Feeding ecology of cocoi heron (Ardea cocoi) in the flood valley of the Paraná River. Avian Biology Research 3: 115–121.
Ewins, P.J., and B. Hennessey. 1992. Great blue herons Ardea herodias, feeding at a fishing vessel offshore in Lake Erie. Canadian Field-Naturalist 106: 521–522.
Fasola, M., P.A. Movalli, and C. Gandini. 1998. Heavy metal, organochlorine pesticide, and PCB residues in eggs and feathers of herons breeding in northern Italy. Archives of Environmental Contamination and Toxicology 34: 87–93.
Frederick, P.C. 2002. Wading birds in the marine environment. In Biology of marine birds, ed. E.A. Schreiber and J. Burger, 618–655. Boca Raton: CRC Press.
Frederick, P.C., R. Bjork, G.T. Bancroft, and G.V.N. Powell. 1992. Reproductive success of three species of herons relative to habitat in southern Florida. Colonial Waterbirds 15: 192–201.
Fry, B. 2006. Stable isotope ecology. New York: Springer.
Garcia, A.M., J.P. Vieira, and K.O. Winemiller. 2001. Dynamics of the shallow-water fish assemblage of the Patos Lagoon estuary (Brazil) during cold and warm ENSO episodes. Journal of Fish Biology 59: 1218–1238.
Garcia, A.M., D.J. Hoeinghaus, J.P. Vieira, K.O. Winemiller, D.M.L. Motta-Marques, and M.A. Bemvenuti. 2006. Preliminary examination of food web structure of Nicola Lake (Taim Hydrological System, south Brazil) using dual C and N stable isotope analyses. Neotropical Ichthyology 4: 279–284.
Gianuca, D. 2010. Abundância e ecologia reprodutiva de Pelecaniformes em uma colônia na Ilha dos Marinheiros, estuário da Lagoa dos Patos, RS. MSc. Dissertation, Universidade Federal do Rio Grande – FURG.
Gianuca, D., A.T. Gianuca, and C.M. Vooren. 2012. Abundance, breeding and food of the little blue heron Egretta caerulea (Aves, Ardeidae) in the Patos Lagoon estuary, a recently colonized area in southern Brazil. Iheringia, Série Zoologia 102: 19–25.
González-Acuña, D., R.A. Figueroa, A. González, C. Barrientos, K. Ardilles, and L. Moreno. 2008. Biología reproductiva de la garza cuca (Ardea cocoi) en el centro-sur de Chile. Ornitología Neotropical 19: 485–493.
Green, A.J., and J. Elmberg. 2014. Ecosystem services provided by waterbirds. Biological Reviews 89: 105–122.
Gwiazda, R., and A. Amirowicz. 2006. Selective foraging of grey heron (Ardea cinerea) in relation to density and composition of the littoral fish community in a submontane dam reservoir. Waterbirds 29: 226–232.
Hewson, R., and M. Hancox. 1979. Prey remains in grey heron pellets from north-east Scotland. Bird Study 26: 29–32.
Hobson, K.A. 2009. Trophic interactions between cormorants and fisheries: towards a more quantitative approach using stable isotopes. Waterbirds 32: 481–490.
Hobson, K.A. 2011. Isotopic ornithology: a perspective. Journal of Ornithology 152: 49–66.
Hussey, N.E., M.A. MacNeil, B.C. McMeans, J.A. Olin, S.F.J. Dudley, G. Cliff, S.P. Wintner, S.T. Fennessy, and A.T. Fisk. 2014. Rescaling the trophic structure of marine food webs. Ecology Letters 17: 239–250.
Jakubas, D., and A. Mioduszewska. 2005. Diet composition and food consumption of the grey heron (Ardea cinerea) from breeding colonies in northern Poland. European Journal of Wildlife Research 51: 191–198.
Johnston, J.W., and K.L. Bildstein. 1990. Dietary salt as a physiological constraint in white ibis breeding in an estuary. Physiological Zoology 63: 190–207.
Karnovsky, N.J., K.A. Hobson, and S.J. Iverson. 2012. From lavage to lipids: estimating diets of seabirds. Marine Ecology Progress Series 451: 263–284.
Kelly, J.P.D., K. Etienne Stralberg, and M. McCaustland. 2008. Landscape influence on the quality of heron and egret colony sites. Wetlands 28: 257–275.
Kloskowski, J., and A. Trembaczowski. 2015. Fish reduce habitat coupling by a waterbird: evidence from combined stable isotope and conventional dietary approaches. Aquatic Ecology 49: 21–31.
Kotzian, H.B., and D.M. Marques. 2004. Lagoa Mirim e a convenção Ramsar: um modelo para ação transfronteiriça na conservação de recursos hídricos. REGA 1: 101–111.
Kushlan, J.A., and J.A. Hancock. 2005. The herons. Oxford: Oxford University Press.
Maccarone, A.D., and J.N. Brzorad. 2005. Foraging microhabitat selection by wading birds in a tidal estuary, with implications for conservation. Waterbirds 28: 383–391.
Moser, M.E. 1985. Prey profitability for adult grey herons Ardea cinerea and the constraints on prey size when feeding young nestlings. Ibis 128: 392–405.
Naves, L. 1999. Ecologia alimentar do Talha-mar, Rhynchops nigra (Aves: Rhynchopidae), na desembocadura da Lagoa dos Patos. MSc. Dissertation, Universidade Federal do Rio Grande – FURG.
Parnell, A.C., R. Inger, S. Bearhop, and A.L. Jackson. 2010. Source partitioning using stable isotopes: coping with too much variation. PloS One 5, e9672.
Peterson, B.J., and B. Fry. 1987. Stable isotopes in ecosystem studies. Annual Review of Ecology and Systematics 18: 293–320.
Phillips, D.L., S.D. Newsome, and J.W. Gregg. 2005. Combining sources in stable isotope mixing models: alternative methods. Oecologia 144: 520–527.
Piedras, S.R.N., J.D. Santos, J.M. Fernandes, R.A. Tavares, D.M. Souza, and J.L.O.F. Pouey. 2012. Caracterização da atividade pesqueira na Lagoa Mirim, Rio Grande do Sul. Revista Brasileira de Agrociência 18: 107–116.
Powell, G.V.N. 1987. Habitat use by wading birds in a subtropical estuary: implications of hydrography. Auk 104: 740–749.
Pretelli, M.G., M.L. Josens, and A.H. Escalante. 2012. Breeding biology at a mixed-species colony of great egret and cocoi heron in a pampas wetland of Argentina. Waterbirds 35: 35–43.
Ramos, J.A., and C. Pacheco. 2003. Chick growth and provisioning of surviving and nonsurviving white-tailed tropicbirds (Phaethon lepturus). Wilson Bulletin 115: 414–422.
Rodrigues, L.R., N.F. Fontoura, and D.M.L.M. Marques. 2014. Food-web structure in a subtropical coastal lake: how phylogenetic constraints may affect species linkages. Marine and Freshwater Research 65: 453–465.
Rodrigues, M.A., and F. D’Incao. 2014. Biologia reprodutiva do siri-azul Callinectes sapidus no estuário da Lagoa dos Patos, RS, Brasil. Boletim do Instituto de Pesca, São Paulo 40: 223–236.
Rodríguez, A., B. Rodríguez, B. Rumeu, and M. Nogales. 2007. Seasonal diet of the grey heron Ardea cinerea on an oceanic island (Tenerife, Canary Islands): indirect interaction with wild seed plants. Acta Ornithologica 42: 77–87.
Sick, H. 1997. Ornitologia brasileira. Rio de Janeiro: Nova Fronteira.
Silva-Costa, A., and L. Bugoni. 2013. Feeding ecology of kelp gulls (Larus dominicanus) in marine and limnetic environments. Aquatic Ecology 47: 211–224.
Smith, J.P. 1995. Foraging flights and habitat use of nesting wading birds (Ciconiiformes) at Lake Okeechobee, Florida. Colonial Waterbirds 18: 139–158.
Sponchiado, J., G.L. Melo, and N.C. Cáceres. 2012. Habitat selection by small mammals in Brazilian Pampas biome. Journal of Natural History 46: 1321–1335.
Vander-Zanden, M.J.V., G. Cabana, and J.B. Rasmussen. 1997. Comparing trophic position of freshwater fish calculated using stable nitrogen isotope ratios (δ 15N) and literature dietary data. Canadian Journal of Fisheries and Aquatic Sciences 54: 1142–1158.
Vianna, M., and F. D’Incao. 2006. Evaluation of by-catch reduction devices for use in the artisanal pink shrimp (Farfantepenaeus paulensis) fishery in Patos Lagoon, Brazil. Fisheries Research 81: 331–336.
Vieira, J.P., M.C. Vasconcellos, R.E.E. Silva, and L.G. Fisher. 1996. A rejeição da pesca do camarão-rosa (Penaeus paulensis) no estuário da Lagoa dos Patos, RS, Brasil. Atlantica 18: 123–142.
Vooren, C.M., and A. Chiaradia. 1990. Seasonal abundance and behavior of coastal birds on Cassino Beach, Brazil. Ornitología Neotropical 1: 9–24.
Wassenaar, L.I., and K.A. Hobson. 2000. Stable-carbon and hydrogen isotope ratios reveal breeding origins of red-winged blackbirds. Ecological Applications 10: 911–916.
Weller, M.W. 1999. Wetland birds: habitat resources and conservation implications. Cambridge: Cambridge University Press.
Williams, C.T., C.L. Buck, J. Sears, and A.S. Kitaysky. 2007. Effects of nutritional restriction on nitrogen and carbon stable isotopes in growing seabirds. Oecologia 153: 11–18.
Acknowledgments
The authors are grateful to the colleagues that supported our field and lab work, especially Cindy Barreto, Cícero Faria, Suzana Martins, Milena Caregnato, Helen Assis, Paulo Azambuja, Guilherme Nunes and Leonardo Furlanetto. We are also grateful to Daniel Loebmann (ICB-FURG), Edélti Albertoni (ICB-FURG), Fabiano Corrêa (IO-FURG), Rodrigo Bastos (PUC-RS) and Vinicius Condini (IO-FURG) for help with the identification of food items. Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio) allowed the study to be carried out through License SISBIO No. 32424-2). CEMAVE/ICMBio provided metal bands, and ESEC Taim/ICMBio provided logistic support during fieldwork. Finally, we are grateful to Dr. Rafael A. Dias and MSc. Rodrigo F. Bastos for their critical review of a previous version of this paper and three anonymous reviewers for insightful suggestions. L. Bugoni is a research fellow from the Brazilian CNPq (Proc. No. 308697/2012-0).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Marianne Holmer
Rights and permissions
About this article
Cite this article
Faria, F.A., Silva-Costa, A., Gianuca, D. et al. Cocoi Heron (Ardea cocoi) Connects Estuarine, Coastal, Limnetic and Terrestrial Environments: an Assessment Based on Conventional Dietary and Stable Isotope Analysis. Estuaries and Coasts 39, 1271–1281 (2016). https://doi.org/10.1007/s12237-016-0073-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-016-0073-5