Abstract
Ria de Aveiro (mainly Laranjo basin, Portugal) has been subjected to mercury contamination from a chlor-alkali plant, currently presenting a well-described mercury gradient. This study aimed to assess mercury genotoxicity in this area by measuring the frequency of erythrocytic nuclear abnormalities (ENA) in the European sea bass (Dicentrarchus labrax), addressing the relation with total mercury concentration in the blood and the modulatory role of seasonal variables. Fish were collected, in warm and cold periods, at three locations differing in their distances to the main mercury source: reference (R), moderately (M), and highly (H) contaminated sites. Genotoxicity was detected in both degrees of contamination (M and H) and in both periods of the year (warm and cold), which is in line with the greater levels of mercury measured in fish blood. No significant seasonal variations were observed for mercury bioaccumulation or ENA frequency. The apparent low imperviousness of ENA frequency to seasonal factors reinforced its consistency as a genotoxicity biomarker, thus enabling a clearer identification of cause-and-effect relationships. Overall, the results reflected a serious environmental risk to native ichthyofauna at Laranjo basin due to mercury contamination, showing a potential of mercury to induce genetic damage in fish blood cells through clastogenic and/or aneugenic actions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Mercury has been identified as one of the most pernicious environmental threats to aquatic biota. The major sources of mercury pollution are the chloridealkaline industry, mining, atmospheric residues coming from garbage burning, and fossil fuels (Wiener et al. 2003). In the aquatic compartment, mercury undergoes various chemical and biological transformations, with microbial methylation being the most relevant step. This process promotes the formation of organic mercury, the most toxic form, which is able to enter and biomagnify in food webs (Scudder et al. 2009). Nevertheless, bioaccumulation potential is not only influenced by mercury speciation but also by a range of environmental, ecological, and biological factors (Harmelin-Vivien et al. 2009; Piraino and Taylor 2009).
During the past few years, several studies have been published supporting the idea that relatively low concentrations of mercury may be at the origin of genotoxic processes (Amorim et al. 2000; Westphal et al. 2003; Cebulska-Wasilewska et al. 2005; Crespo-López et al. 2007). Moreover, a review performed by de Flora et al. (1994) indicated that mercury compounds often exert clastogenic effects in eukaryotes, mainly binding thiol groups and acting as spindle inhibitors, thereby causing aneuploidy and/or polyploidy. Despite the relative abundance of investigations performed on this subject in a large variety of test systems (from bacteria to humans), a scarcity of studies in fish still remains. Both organic and inorganic mercury compounds (Zoll et al. 1988; Al-Sabti 1994), as well as elemental mercury (Nepomuceno et al. 1997), have been shown to be chromosomal genotoxicants, eliciting in vivo formation of erythrocytic micronuclei (MN) in fish. In vitro experiments with gill cell suspensions exposed to mercuric chloride detected a high rate of DNA breaks (single- and double-stranded) as measured by comet assay (Arabi 2004; Arabi and Alaeddini 2005). Guilherme et al. (2008) also suggested that aneugenicity and clastogenicity are mechanisms that lead to chromosomal damage by mercury in Liza aurata. More recently, Pereira et al. (2010) showed that mercury uptake from the water fraction is sufficient to increase DNA damage in the same fish species.
Knowledge of the effects of mercury in a wide range of species, with different ecological and biological features, is ecotoxicologically relevant to provide a more comprehensive picture of the risk to native ichthyofauna associated with mercury contamination. In this direction, a differential sensitivity of fish species toward the induction of erythrocytic MN and other nuclear abnormalities has been reported by Sanchez-Galan et al. (1999) and Ayllón and Garcia-Vazquez (2000). However, reports on the evaluation of mercury genotoxicity in fish under realistic field conditions have been restricted to a small range of species.
The role of seasonality in determining the bioavailability of contaminants has been found insufficiently described and contradicting: Some studies described differences in metal body burdens in aquatic biota within the year or between seasons (Otchere 2003; Tekin-Ozan et al. 2008), whereas others reported no marked seasonal effects (Pereira et al. 2006). In contrast, bearing in mind the crucial role of the environmental factors affecting fish physiology, it is essential to evaluate if season-related factors, other than mercury accumulation, influence the genotoxic responses in fish.
Therefore, the present study was conducted, including contrasting periods (warm and cold), at Laranjo basin, which is an area of a coastal lagoon (Ria de Aveiro, Portugal) historically impacted by a chlor-alkali plant effluent. Although effluent releases ceased in 1994, high mercury concentrations are still found in the fine surface sediments of this area, creating a contamination gradient. As a progression of a previous survey (Guilherme et al. 2008) in which mercury genotoxicity was shown in L. aurata, a species with an omnivorous diet, feeding mainly on planktonic communities and detritus (Martinho et al. 2008; França et al. 2009), the present work focused on a fish with a different feeding behavior, i.e., Dicentrarchus labrax (European sea bass), which has an opportunistic diet, including benthic invertebrates and small fish (Martinho et al. 2008). Considering mercury’s affinity to sediment, the study of a species having less contact with this environmental matrix (a source of metal that is exported to the overlying water) is crucial to evaluate the risk to native ichthyofauna associated with metal remobilization after 13 years without new discharges. Hence, the frequency of erythrocytic nuclear abnormalities (ENA) was determined in wild fish as a marker of chromosomal damage to determine the influence of seasonal variables on both mercury accumulation and subsequent genotoxic action. In addition, the concomitant evaluation of external levels of contamination, blood mercury levels, and ENA frequency will provide a causal interpretation of this genotoxic response.
Materials and Methods
Study Area and Sampling Procedures
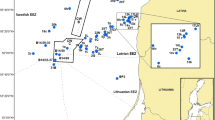
The study was performed in 2007 at Ria de Aveiro on the Portuguese northwestern coast (Fig. 1). This coastal lagoon received mercury-contaminated discharges resulting in the accumulation of approximately 25 tons of mercury in the Laranjo basin sediments and its upstream channel (Coelho et al. 2005). Sampling sites were selected in accordance with the existing contamination gradient: two sites were located at Laranjo basin: site M (the site with moderate contamination) and site H (the site with high contamination). Site H was located closer to the mercury source and at 2-km distance from M. An area close to the lagoon entrance (S. Jacinto) and far from the main polluting sources was chosen as the reference site (R). To assess the influence of contrasting environmental conditions, sampling was performed during both warm (July 2007) and cold (December 2007) periods. Water physicochemical parameters, such as temperature, dissolved (DO) oxygen, pH, salinity, and suspended particulate matter (SPM), were measured during high and low tide at a subsurface level; measurements of water-column depth and turbidity were taken as well. For mercury measurements, subsurface water samples (high and low tide) were collected in acid-washed plastic bottles, and five replicates from the surface sediment layer (approximately 2-cm depth) were collected at each site. All glassware and plastic implements were cleaned in accordance with Monterroso et al. (2003), and ultraclean laboratory procedures were followed during sample manipulations.
Fifteen juvenile specimens of D. labrax were caught at each sampling site during each season using a fishing rod. Fish had an average total length of 15.4 ± 3.5 cm during the warm period and 14.6 ± 1.4 cm during the cold period. After being caught, fish were dissected and blood collected from the posterior cardinal vein using a heparinized Pasteur pipette. Blood smears (n = 15) were immediately prepared and the remainder volume stored in microtubes and kept on ice. After blood sampling, fish were killed by severing the spinal cord. In the laboratory, blood samples were stored at −80 °C until further processing for total mercury analysis.
During the cold period, it was not possible to catch fish at site H; thus, the corresponding abiotic parameters were not determined.
Total Mercury Determination
Mercury in Water
Subsurface water samples were filtered through preweighed 0.45-μm Millipore cellulose acetate membrane filters, acidified with mercury-free HNO3 (Merck) to pH <2, and stored at 4 °C until analysis. Filters were reweighed after heating overnight at 60 °C and stored at 4 °C for SPM determinations. Water samples corresponding to high tide in site H were lost during the field campaign and could not be retrieved. The assessment of mercury in the water column was performed in the dissolved fraction as reactive mercury (R-Hg) and as total dissolved mercury (Dis-Hg). R-Hg represents the pool of mercury in the dissolved fraction that is bioavailable for the food web (Mason et al. 1996) and is characterized by easily reducible mercury species, such as inorganic dissolved species, elemental dissolved mercury, and labile mercury complexes (Fitzgerald et al. 2007). In addition, mercury in the particulate fraction (SPM-Hg) was also evaluated.
Mercury in the water column was analyzed by cold-vapor–atomic fluorescence spectrometry (CV-AFS) with a PSA 10.023 Merlin (P S Analytical, UK) equipped with a detector PSA model 10.003 (P S Analytical) using SnCl2 reduction (see Mucci et al. 1995). The detection limit (±SD) based on procedural blanks was 1.2 ± 0.3 ng L−1. The procedure and reagent contamination was followed by analysis of filtrate blanks and ultrapure water. For determination of SPM-Hg, filters were digested with HNO3 4 mol L−1, and the previously mentioned equipment was used (Pereira et al. 1998). The percentage of mercury associated with the particulate matter (% Hg particulate) was also calculated. The accuracy of the methods for mercury quantification was tested by fortification of samples (at two concentration levels within the range found in the samples); recovery efficiencies were always between 90 % and 100 % (n = 12 for Dis-Hg; n = 8 for SPM-Hg). Blank filters were used to investigate any possible contamination showing Hg levels between 3.5 and 9.4 % of the typical content in the sample filters.
Mercury in Sediment and Fish Blood
At the laboratory, sediment samples were freeze-dried, well mixed, sieved through a 1 mm sieve and stored for total mercury determination (Sed-Hg). For total mercury analysis in D. labrax blood, samples were freeze-dried, homogenized, and weighed for fresh-weight determination. Sediment (n = 5 at each site) and blood samples (n = 15 at each site) were analyzed for total mercury determination by atomic absorption spectrometry with thermal decomposition with gold amalgamation using a LECO Advanced Mercury Analyzer AMA254 (LECO) (Costley et al. 2000). The accuracy and precision of the analytical methodology for total mercury determinations were assessed by replicate analysis of certified reference materials (CRMs; National Research Council Canada). The CRMs used were as similar as possible to the samples; hence, MESS-3 and PACS-2 (marine sediments) was used for sediments. The CRM used for blood samples, TORT-2 (lobster hepatopancreas), was selected in agreement with previous studies (Storelli et al. 2005; Guilherme et al. 2008; Ley-Quiñónez et al. 2011). Precision of the method was always >9 % (n = >5) with recovery efficiency between 83 % and 102 % (n = 50). Blanks between each sample were measured as part of the quality control and were always <1 % of the T-Hg.
ENA Frequency
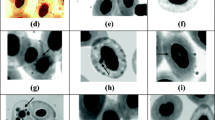
Genotoxicity was assessed by ENA assay, performed in mature peripheral erythrocytes, in accordance to the procedure adapted by Pacheco and Santos (1996). One blood smear per animal was fixed with methanol for 10 minutes and stained with Giemsa (5 %) for 30 minutes. NEA were scored in 1000 mature erythrocytes samples/fish to determine the frequency of the following categories: MN, lobed nuclei (L), binucleates or segmented nuclei (S), and kidney-shaped nuclei (K). In addition, notched nuclei (N) were also scored as suggested by Ayllón and Garcia-Vazquez (2001). The final result was expressed as the mean value (‰) of the sum for all of the individual lesions observed (MN + L + S + K + N).
Statistical Analysis
SigmaStat software (Systat Software) was used for statistical analyses. All of the data were first tested for normality and homogeneity of variance to meet statistical demands. One-way analysis of variance, followed by Tukey test, was used to compare sites (within the same season), and independent Student t test was performed to assess differences between periods (warm vs. cold) for the same site. Differences between means were considered significant at p < 0.05. Pearson’s correlation factor (r), as well as Spearman’s rank correlation coefficient, was determined for total mercury in blood versus ENA frequency.
Results
Environmental Physicochemical Characterization
Table 1 lists the general physicochemical characteristics of the water column in the three sampling sites (R, M, and H) at Ria de Aveiro. During the warm period, temperature, water depth, turbidity, and DO oxygen showed no clear trend of variation, whereas salinity decreased toward the contamination source. An increase in SPM was observed at the contaminated sites during low tide. During the cold period, a decrease in all of the studied parameters, except depth at both tides and turbidity at low tide, was observed at site M compared with reference site.
Mercury Levels in Water and Sediment
Table 2 lists the concentration of mercury in water and sediment (Sed-Hg) matrices measured during both warm and cold periods. During the warm period, an increase in R-Hg levels toward the contamination source was only discernible during low tide. Greater Dis-Hg concentrations in contaminated sites were only detected at M. The percentage of mercury associated with particulate matter in the water column (% Hg particulate) was always >90 %, without clear differences between site R and the contaminated sites. Concentrations of SPM-Hg increased toward the mercury source (site R < site M < site H) during both tides. Similarly, Sed-Hg clearly reflected the same contamination gradient.
During the cold period, greater R-Hg levels at site M were only observed during low tide, whereas at high tide a decrease was perceptible. The levels of Dis-Hg, % Hg particulate, and SPM-Hg at site M were greater at site R during both tide conditions. Sed-Hg showed a pronounced increase from site R to site M.
Total Mercury Levels in Blood
During the warm period, the analysis of total mercury (T-Hg) in whole blood (Table 2) showed significant increases in fish captured at site M (3.8 times) and site H (6.6 times) compared with those captured at R. Moreover, fish from site H also showed a significant increase compared with those collected at M. During the cold period, significant increment (2.6 times) was observed in fish from site M compared with those from site R. No difference in mercury accumulation was recorded when the two seasons were compared.
ENA Frequency
During the warm period, ENA frequency (Table 3) showed a significant increase at sites M and H compared with site R, representing increments of 1.9 and 2.4 times, respectively. Moreover, an increase in ENA frequency was also significant at site H compared with site M. During the cold period, a significant increase in ENA, corresponding to a 3.7 times increment, was observed at site M compared with site R.
The analysis of each nuclear lesion category individually (Table 3) showed that during the warm season, K, L, and M nuclei frequencies were significantly greater at site M compared with site R, whereas at site H, L, S, and N frequencies were significantly greater compared with site R. During the cold period, the nuclear lesion categories showing significant increase compared with site R were K, L, and N. No interseason differences in ENA values were recorded. Regarding the relationships between parameters (i.e., T-Hg in blood vs. ENA frequency), no significant correlations were found when tested using Pearson’s and Spearman’s rank tests.
Discussion
The susceptibility of wild fish to DNA damage can vary with species and with periods of the year, which is probably associated with seasonal effects in fish metabolism and baseline levels of defenses (including DNA-damage repair) as well as fluctuations of contaminant bioavailability (Oliveira et al. 2010). Hence, in following a previous study (Guilherme et al. 2008) focused on a species (L. aurata) with different feeding and ecological traits, the current investigation concerning sea bass (D. labrax) constitutes a further step to achieve the understanding of mercury genotoxic potential and the eventual modulatory role of seasonal variables.
In terms of the water physicochemical parameters currently determined, typical seasonal variations were evidenced by the greater water temperature and lower DO during the warm period. SPM concentrations were generally greater during the warm period. These patterns were common to all study sites, thereby decreasing the possibility of a direct influence of these variables on mercury bioavailability differentiated for each site. In contrast, it is possible that SPM is playing a critical function in transferring mercury along the water column because particulate mercury tends to be greater in conjunction with increased SPM, especially in shallow regions of estuaries where resuspension is easily enhanced (Coquery et al. 1997; Domagalski 2001), such as the Laranjo area. This evidence is in agreement with the previous findings of Mieiro et al. (2011) for the same geographic area showing no substantial intersite differences albeit some exceptions for SPM and salinity. Salinity variation was interpreted taking into account that mercury levels in water were low and thus not relevantly affected by this parameter.
In contrast to water, the sedimentary compartment reflected the historic mercury contamination in this area, showing increasing levels toward the mercury-contamination source. It is known that resuspension of mercury-rich particles by tidal movements may contribute significantly to the increase of mercury levels in the water column (Pereira et al. 2009). The assessment of methylmercury production in Ria de Aveiro indicated that it is present at levels <1 % (mercury in sediments occurs mainly in the inorganic form) (Válega et al. 2008).
Mercury levels in the environmental matrices, namely in sediment, confirmed that the studied area is still in a state of recovery. Seasonal variations of mercury levels would be expected in the water column rather than in sediment. However, a clear pattern was not observed because Dis-Hg was greater during cold period, whereas the opposite was found for SPM-Hg. In line with this difficulty in detecting seasonal variations of mercury bioavailability, the mercury accumulation in sea bass blood reflected no significant differences between warm and cold periods at the same site.
In contrast, an intersite comparison of mercury levels in D. labrax’s blood showed increased levels of mercury (T-Hg) at both sites M and H; moreover, mercury accumulation was according to the environmental gradient, thus indicating its ability to reflect the external levels of exposure.
Concomitantly with the increase of T-Hg levels in blood, ENA frequency increased at site M, during both periods, and at site H during the warm period only (the only period assessed at this site) compared with site R. However, no significant correlation was found between these two biological parameters, probably due to the low number of points considered in the statistical analysis. The genotoxic potential of mercury, as reported for other fish species in the same study area, viz. L. aurata (Guilherme et al. 2008), was strengthened by the present data on D. Labrax. Moreover, the results also provide a clearer picture, taking into account pollutant specificity, because evident mercury accumulation was shown in the current study that had not been reported in previous field studies (Pacheco et al. 2005; Mohmood et al. 2008), in which the interpretation of results was based just on the probability of mercury’s presence in the environment as well as the target tissue.
Corroborating the present results, laboratory experiments have also shown the genotoxic potential of mercury compounds in fish species, such as Poecilia latipinna exposed to mercury nitrate (Ayllón and Garcia-Vazquez 2000), Carassius auratus exposed to mercury chloride (Çavaş 2008), and Colossoma macropomum exposed to methylmercury (da Rocha et al. 2011). In other fish species, such as killifish (Fundulus heterociclitus), MN induction was obtained by exposure to methylmercury derivatives and was more active during a short period than inorganic mercury salts (Perry et al. 1988).
It seems uncontroversial that problems in segregating twisted and attached chromosomes or gene amplification by way of the breakage–fusion–bridge cycle could cause nuclear buds (L and blebbed nuclei) during the removal of amplified DNA from the nucleus (Tolbert et al. 1992; Shimizu et al. 1998, 2000). According to Serrano-García and Montero-Montoya (2001), the phenomena of budding nuclei and S cells have a similar origin as MN, i.e., they are regarded as genotoxic occurrences. Moreover, a positive and significant relationship between MN and nuclear bud induction was found by different investigators (Bolognesi et al. 2006; Ergene et al. 2007) suggesting that nuclear bud formation in erythrocytes may be a useful complementary assay for genotoxicity assessment in fish. Accordingly, analysis of the current results based on the frequency of each nuclear lesion category clearly showed that the L (budding nuclei) in particular displayed a pattern of response similar to that one displayed by the ENA frequency (a joint nuclear anomalies score), thus reinforcing the significance of total scoring as a genotoxic indicator.
According to Grisolia and ClMT (2000), one should be aware of the differential sensitivity and responses of fish species to genotoxicants, as well as their relationships within the aquatic ecosystem, to avoid permanent alterations of the health of icthyopopulations. Compared with a previous survey using L. aurata (Guilherme et al. 2008), in which genotoxicity was not detected during the winter (in a site corresponding to our site H), the current study reflected ENA induction in D. labrax during the cold period. This comparison reflects species specificities, pointing out D. labrax as a more sensitive and more vulnerable species compared with L. aurata.
The feeding ecology of juvenile D. labrax and L. aurata was recently compared (Mieiro et al. 2012). The diet of D. labrax was shown to be more diversified and essentially constituted crustaceans and polychaetes, and, to a lesser extent, fish, algae, mollusks, and echinoderms, whereas L. aurata’s diet was mainly composed of sediments and polychaetes. These previous results (Mieiro et al. 2012) showed that D. labrax accumulated greater levels of organic mercury in liver (the detoxifying organ) and in muscle (the reservoir organ) than L. aurata, suggesting that fish feeding strategy is the major factor determining mercury accumulation (Mieiro et al. 2012). Therefore, the current observations suggested that fish species having lesser direct contact with the sediment (viz. D. labrax) are not less susceptible to the genotoxic pressure associated with mercury, thus highlighting a risk to native ichthyofauna extensible to wide range of species.
Previous studies showed that genotoxicity in fish was related with sediment contamination (Costa et al. 2011) and, more specifically, with mercury levels in that compartment (Porto et al. 2005; Guilherme et al. 2008). This is not surprising taking into account that mercury methylation occurs (most often) at the sediment surface. In an attempt to evaluate the risk to ichthyofauna at Aveiro lagoon after 13 years without new mercury discharges, it was shown that this prolonged period of ecosystem recovery was not enough to eliminate the genotoxic risk associated with metal remobilization from the sediment.
Regarding the use of D. labrax as a target species for genotoxicity assessment, it is a eurythermic (5–28 °C) and euryhaline (3 to full-strength seawater) fish, and thus has the capability of living in a variety of environmental conditions. In addition, sea bass has shown its utility to detect the effects of a wide range of pollutants (Mohmood et al. 2008). Taking into account the previous description and goals of the present study, D. labrax justifies its selection as a sentinel for the evaluation of mercury genotoxicity regardless of the mobility often invoked as a limitation associated to fish species in general and to this species in particular.
Among the different abiotic factors, water temperature has been shown to greatly influence cell replication rates and DNA repair of poikilothermic organisms (Venier et al. 1997). In addition, Buschini et al. (2003) found a positive correlation between water temperature and DNA integrity loss in Dreissena polymorpha (zebra mussel). Recently, Pereira et al. (2010) suggested that water temperature should be regarded as an additive factor in the mercury-induced DNA-integrity loss in fish. Surprisingly, no seasonal variation in ENA frequency was observed in D. labrax, which, considering the absence of temporal alterations of mercury levels in fish blood, is indicative of a lack of impact of non–contaminant related seasonal factors, including temperature. The apparent low dependency of ENA frequency from seasonal abiotic variables allows the establishment of more straightforward cause-and-effect relationships with internal levels of exposure. This can be regarded as a positive feature, thus increasing the capacity of ENA assay to evaluate the extent of contamination in mercury-impacted systems, namely, when applied to D. labrax.
Conclusion
The results of the present work led to the following main findings:
-
1.
Uptake of mercury from the contaminated environment increased T-Hg levels in D. labrax’s blood and consequent genetic damage through clastogenic and/or aneugenic actions in the erythrocytes (measured as ENA frequency). Genotoxicity was detected at sites with both degrees of contamination (sites M and H) and during both periods (warm and cold), which is in line with the greater levels of mercury measured in fish blood. Nevertheless, no seasonal variations were observed for mercury bioaccumulation or ENA frequency.
-
2.
The usefulness of ENA assay on the assessment of aquatic genotoxicants was confirmed. The apparent low imperviousness of ENA frequency to seasonal factors reinforced its consistency as biomarker by enabling a clearer identification of cause-and-effect relationships.
-
3.
The need for this kind of data integration (i.e., assessment of mercury accumulation and genetic end points) to achieve more reliable and functionally relevant results was confirmed.
-
4.
D. labrax showed potential as a metal biosentinel because it is commonly found in both unpolluted and metal contaminated environments, is easy to catch, and is responsive in terms of the assessed parameters.
-
5.
Finally, the results reflected a serious environmental risk to native ichthyofauna at Laranjo basin due to mercury contamination.
References
Al-Sabti K (1994) Micronuclei induced by selenium, mercury, methylmercury and their mixtures in binucleated blocked fish erythrocyte cells. Mutat Res 320(1–2):157–163
Amorim MIM, Mergler D, Bahia MO, Dubeau H, Miranda D, Lebel J et al (2000) Cytogenetic damage related to low levels of methyl mercury contamination in the Brazilian Amazon. An Acad Bras Cienc 72:497–507
Arabi M (2004) Analyses of impact of metal ion contamination on carp (Cyprinus carpio L.) gill cell suspensions. Biol Trace Elem Res 100(3):229–245
Arabi M, Alaeddini M (2005) Metal-ion-mediated oxidative stress in the gill homogenate of rainbow trout (Oncorhynchus mykiss): Antioxidant potential of manganese, selenium, and albumin. Biol Trace Elem Res 108(1):155–168
Ayllón F, Garcia-Vazquez E (2000) Induction of micronuclei and other nuclear abnormalities in European minnow Phoxinus phoxinus and mollie Poecilia latipinna: An assessment of the fish micronucleus test. Mutat Res 467(2):177–186
Ayllón F, Garcia-Vazquez E (2001) Micronuclei and other nuclear lesions as genotoxicity indicators in rainbow trout Oncorhynchus mykiss. Ecotoxicol Environ Safe 49(3):221–225
Bolognesi C, Perrone E, Roggieri P, Pampanin DM, Sciutto A (2006) Assessment of micronuclei induction in peripheral erythrocytes of fish exposed to xenobiotics under controlled conditions. Aquat Toxicol 78(Suppl):S93–S98
Buschini A, Carboni P, Martino A, Poli P, Rossi C (2003) Effects of temperature on baseline and genotoxicant-induced DNA damage in haemocytes of Dreissena polymorpha. Mutat Res 537(1):81–92
Çavaş T (2008) In vivo genotoxicity of mercury chloride and lead acetate: micronucleus test on acridine orange stained fish cells. Food Chem Toxicol 46(1):352–358
Cebulska-Wasilewska A, Panek A, Żabiński Z, Moszczyński P, Au WW (2005) Occupational exposure to mercury vapour on genotoxicity and DNA repair. Mutat Res 586(2):102–114
Coelho JP, Pereira ME, Duarte A, Pardal MA (2005) Macroalgae response to a mercury contamination gradient in a temperate coastal lagoon (Ria de Aveiro, Portugal). Estuar Coast Shelf Sci 65(3):492–500
Coquery M, Cossa D, Sanjuan J (1997) Speciation and sorption of mercury in two macro-tidal estuaries. Mar Chem 58(1–2):213–227
Costa PM, Neuparth TS, Caeiro S, Lobo J, Martins M, Ferreira AM et al (2011) Assessment of the genotoxic potential of contaminated estuarine sediments in fish peripheral blood: Laboratory versus in situ studies. Environ Res 111(1):25–36
Costley CT, Mossop KF, Dean JR, Garden LM, Marshall J, Carroll J (2000) Determination of mercury in environmental and biological samples using pyrolysis atomic absorption spectrometry with gold amalgamation. Anal Chim Acta 405(1–2):179–183
Crespo-López ME, Lima de Sá A, Herculano AM, Rodríguez Burbano R, Martins do Nascimento JL (2007) Methylmercury genotoxicity: A novel effect in human cell lines of the central nervous system. Environ Int 33(2):141–146
da Rocha CA, da Cunha LA, da Silva Pinheiro RH, de Oliveira Bahia M, Burbano RM (2011) Studies of micronuclei and other nuclear abnormalities in red blood cells of Colossoma macropomum exposed to methylmercury. Genet Mol Biol 34:694–697
De Flora S, Bennicelli C, Bagnasco M (1994) Genotoxicity of mercury compounds: a review. Mutat Res 317(1):57–79
Domagalski J (2001) Mercury and methylmercury in water and sediment of the Sacramento River Basin. California. Appl Geochem 16(15):1677–1691
Ergene S, Çavaş T, Çelik A, Köleli N, Kaya F, Karahan A (2007) Monitoring of nuclear abnormalities in peripheral erythrocytes of three fish species from the Goksu Delta (Turkey): Genotoxic damage in relation to water pollution. Ecotoxicology 16(4):385–391
Fitzgerald WF, Lamborg CH, Hammerschmidt CR (2007) Marine biogeochemical cycling of mercury. Chem Rev 107(2):641–662
França S, Costa MJ, Cabral HN (2009) Assessing habitat specific fish assemblages in estuaries along the Portuguese coast. Estuar Coast Shelf Sci 83(1):1–12
Grisolia CK, ClMT Cordeiro (2000) Variability in micronucleus induction with different mutagens applied to several species of fish. Genet Mol Biol 23:235–239
Guilherme S, Valega M, Pereira ME, Santos MA, Pacheco M (2008) Erythrocytic nuclear abnormalities in wild and caged fish (Liza aurata) along an environmental mercury contamination gradient. Ecotoxicol Environ Saf 70(3):411–421
Harmelin-Vivien M, Cossa D, Crochet S, Banaru D, Letourneur Y, Mellon-Duval C (2009) Difference of mercury bioaccumulation in red mullets from the north-western Mediterranean and Black seas. Mar Pollut Bull 58(5):679–685
Ley-Quiñónez C, Zavala-Norzagaray AA, Espinosa-Carreón TL, Peckham H, Marquez-Herrera C, Campos-Villegas L et al (2011) Baseline heavy metals and metalloid values in blood of loggerhead turtles (Caretta caretta) from Baja California Sur. Mexico. Mar Pollut Bull 62(9):1979–1983
Martinho F, Viegas I, Dolbeth M, Leitão R, Cabral HN, Pardal MA (2008) Assessing estuarine environmental quality using fish-based indices: performance evaluation under climatic instability. Mar Pollut Bull 56(11):1834–1843
Mason RP, Reinfelder JR, Morel FMM (1996) Uptake, toxicity, and trophic transfer of mercury in a coastal diatom. Environ Sci Technol 30:1835–1845
Mieiro CL, Pacheco M, Pereira M, Duarte A (2011) Mercury organotropism in feral European sea bass (Dicentrarchus labrax). Arch Environ Contam Toxicol 61(1):135–143
Mieiro CL, Coelho JP, Pacheco M, Duarte AC, Pereira ME (2012) Evaluation of species-specific dissimilarities in two marine fish species: mercury accumulation as function of metal levels in the consumed prey. Arch Environ Contam Toxicol 63(1):125–136
Mohmood I, Maria VL, Oliveira M, Ahmad I, Pacheco M, Santos MA (2008) Seasonal assessment of a contaminated coastal lagoon (Ria de Aveiro, Portugal) using Dicentrarchus labrax L. erythrocytic nuclear abnormalities. Fresenius Environ Bull 17(11B):1924–1931
Monterroso P, Abreu SN, Pereira E, Vale C, Duarte AC (2003) Estimation of Cu, Cd and Hg transported by plankton from a contaminated area (Ria de Aveiro). Acta Oecol 24:S351–S357
Mucci A, Lucotte M, Montgomery S, Plourde Y, Pichet P, VanTra H (1995) Mercury remobilization from flooded soils in a hydroelectric reservoir of northern Quebec, La Grande—2: Results of a soil resuspension experiment. Can J Fish Aquat Sci 52(11):2507–2517
Nepomuceno JC, Ferrari Í, Spanó MA, Centeno AJ (1997) Detection of micronuclei in peripheral erythrocytes of Cyprinus carpio exposed to metallic mercury. Environ Mol Mutagen 30(3):293–297
Oliveira M, Ahmad I, Maria VL, Ferreira CSS, Serafim A, Bebianno MJ et al (2010) Evaluation of oxidative DNA lesions in plasma and nuclear abnormalities in erythrocytes of wild fish (Liza aurata) as an integrated approach to genotoxicity assessment. Mutat Res 703(2):83–89
Otchere FA (2003) Heavy metals concentrations and burden in the bivalves (Anadara (Senilia) senilis, Crassostrea tulipa and Perna perna) from lagoons in Ghana: Model to describe mechanism of accumulation/excretion. Afr J Biotechnol 2(9):280–287
Pacheco M, Santos MA (1996) Induction of micronuclei and nuclear abnormalities in the erythrocytes of Anguilla anguilla L. exposed either to cyclophosphamide or to bleached kraft pulp mill effluent. Fresenius Environ Bull 5(11–12):746–751
Pacheco M, Santos MA, Teles M, Oliveira M, Rebelo JE, Pombo L (2005) Biotransformation and genotoxic biomarkers in mullet species (Liza sp.) from a contaminated coastal lagoon (Ria de Aveiro, Portugal). Environ Monit Assess 107(1–3):133–153
Pereira ME, Duarte AC, Millward GE, Vale C, Abreu SN (1998) Tidal export of particulate mercury from the most contaminated area of Aveiro’s Lagoon. Portugal. Sci Total Environ 213(1–3):157–163
Pereira E, Abreu SN, Coelho JP, Lopes CB, Pardal MA, Vale C et al (2006) Seasonal fluctuations of tissue mercury contents in the European shore crab Carcinus maenas from low and high contamination areas (Ria de Aveiro, Portugal). Mar Pollut Bull 52(11):1450–1457
Pereira C, Guilherme S, Barroso C, Verschaeve L, Pacheco M, Mendo S (2010) Evaluation of DNA damage induced by environmental exposure to mercury in Liza aurata using the comet assay. Arch Environ Contam Toxicol 58(1):112–122
Pereira ME, Lillebø AI, Pato P, Válega M, Coelho JP, Lopes C, Rodrigues S, Cachada A, Otero M, Pardal MA, Duarte AC (2009) Mercury pollution in Ria de Aveiro (Portugal): a review of the system assessment. Environ Monit Assess 155:39–49
Perry DM, Weis JS, Weis P (1988) Cytogenetic effects of methylmercury in embryos of the killifish Fundulus heteroclitus. Arch Environ Contam Toxicol 17(5):569–574
Piraino MN, Taylor DL (2009) Bioaccumulation and trophic transfer of mercury in striped bass (Morone saxatilis) and tautog (Tautoga onitis) from the Narragansett Bay (Rhode Island, USA). Mar Environ Res 67(3):117–128
Porto JIR, Araujo CSO, Feldberg E (2005) Mutagenic effects of mercury pollution as revealed by micronucleus test on three Amazonian fish species. Environ Res 97(3):287–292
Sanchez-Galan S, Linde AR, Garcia-Vazquez E (1999) Brown trout and European minnow as target species for genotoxicity tests: differential sensitivity to heavy metals. Ecotoxicol Environ Saf 43(3):301–304
Scudder BC, Chasar LC, Wentz DA, Bauch NJ, Brigham ME, Moran PW, et al. (2009) Mercury in fish, bed sediment, and water from streams across the United States, 1998–2005. United States Geological Survey Scientific Investigations Report
Serrano-García L, Montero-Montoya R (2001) Micronuclei and chromatid buds are the result of related genotoxic events. Environ Mol Mutagen 38(1):38–45
Shimizu N, Itoh N, Utiyama H, Wahl GM (1998) Selective entrapment of extrachromosomally amplified DNA by nuclear budding and micronucleation during S phase. J Cell Biol 140(6):1307–1320
Shimizu N, Shimura T, Tanaka T (2000) Selective elimination of acentric double minutes from cancer cells through the extrusion of micronuclei. Mutat Res 448(1):81–90
Storelli MM, Storelli A, Giacominelli-Stuffler R, Marcotrigiano GO (2005) Mercury speciation in the muscle of two commercially important fish, hake (Merluccius merluccius) and striped mullet (Mullus barbatus) from the Mediterranean Sea: estimated weekly intake. Food Chem 89(2):295–300
Tekin-Ozan S, Kir I, Ayvaz Y, Barlas M (2008) Influence of seasons on heavy metal levels in carp (Cyprinus carpio L.) tissues from Kovada Lake (Turkey). Adv Food Sci 30(3):140–144
Tolbert PE, Shy CM, Allen JW (1992) Micronuclei and other nuclear anomalies in buccal smears: methods development. Mutat Res 271(1):69–77
Válega M, Lillebø AI, Pereira ME, Corns WT, Stockwell PB, Duarte AC et al (2008) Assessment of methylmercury production in a temperate salt marsh (Ria de Aveiro Lagoon, Portugal). Mar Pollut Bull 56(1):153–158
Venier P, Maron S, Canova S (1997) Detection of micronuclei in gill cells and haemocytes of mussels exposed to benzo[a]pyrene. Mutat Res 390(1–2):33–44
Westphal G, Asgari S, Schulz T, Bünger J, Müller M, Hallier E (2003) Thimerosal induces micronuclei in the cytochalasin B block micronucleus test with human lymphocytes. Arch Toxicol 77(1):50–55
Wiener J, Krabbenhoft D, Heinz GA, Scheuhammer A (2003) Ecotoxicology of mercury. In: Hoffman DJ, Rattner BA, Burton GA, Cairns J (eds) Handbook of ecotoxicology. CRC Press, Boca Raton, FL, pp 407–461
Zoll C, Saouter E, Boudou A, Ribeyre F, Jaylet A (1988) Genotoxicity and bioaccumulation of methyl mercury and mercuric chloride in vivo in the newt Pleurodeles waltl. Mutagenesis 3(4):337–343
Acknowledgments
This work was financed by the Portuguese Foundation for Science and Technology through a doctoral Grant to I. Mohmood (Grant No. SFRH/BD/74410/2010) and postdoctoral grants to C. L. Mieiro (Grant No. SFRH/BPD/79445/2011), J. P. Coelho (Grant No. SFRH/BPD/48449/2008), and N. Anjum (Grant no. SFRH/BPD/64690/2009) along with CESAM–University of Aveiro. This study was conducted in accordance with European Union Directive 2010/63/EU, on the protection of animals used for scientific purposes, under the supervision of a team member (Mário Pacheco) authorized by the competent authorities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mohmood, I., Mieiro, C.L., Coelho, J.P. et al. Mercury-Induced Chromosomal Damage in Wild Fish (Dicentrarchus labrax L.) Reflecting Aquatic Contamination in Contrasting Seasons. Arch Environ Contam Toxicol 63, 554–562 (2012). https://doi.org/10.1007/s00244-012-9799-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-012-9799-7