Abstract
Nuclear bud (NB) formation was investigated in blood erythrocytes of 1892 flounder Platichthys flesus, herring Clupea harengus, and eelpout Zoarces viviparus specimens that were collected at 82 sites representing different regions of the Baltic Sea in 2009–2011. This is the first attempt to evaluate the baseline levels of NB and rank the genotoxicity risk for native fish species. NB levels were compared to the previously published micronuclei (MN) data from the same individual fish specimens in order to compare the two methods of genotoxicity assessment and investigate the relationship between MN as the cytogenetic measure of genotoxicity and the DNA damage reflecting NB. In 2009–2011, elevated NB levels in 89.4 % of flounder sampling groups indicated high and extremely high genotoxicity risk levels. Herring and eelpout sampling groups showed elevated levels of NB, 74.6 and 45.7 %, respectively. In general, herring and eelpout NB measure was more sensitive as the genotoxicity biomarker than MN.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In various Baltic Sea sub-basins, large numbers of different toxic substances exceed the threshold levels indicating potential risk to the native species. In fish and mussel tissues, elevated levels of heavy metals, organometals, alkylphenols, phthalates, brominated compounds, polychlorinated biphenyls (PBC), dioxins, polycyclic aromatic hydrocarbons (PAHs), DDTs and chlorinated pesticides, and caesium-137 were found. The southern region of the Baltic Sea is polluted by all of the abovementioned substances, and many of them are recognized as genotoxic contaminants (HELCOM 2010; Schiedek et al. 2006; Baršienė et al. 2006a; Kopecka et al. 2006). Genotoxic compounds can bind to DNA causing the formation of DNA adducts, single- and double-strand breakages, modifications in DNA repair and crosslink consistent pattern, as well as alterations of cell functions, reproduction disturbances, growth inhibition, or even carcinogenesis (Ohe et al. 2004). Furthermore, contaminants are usually discharged in complex mixtures and as such can provoke interactions between substances and lead to unpredictability in genotoxic responses to pollution (Jha 2008).
Environmental genotoxicity in the Baltic Sea was earlier assessed in the Swedish part of the Gulf of Bothnia (Al-Sabti and Hardig 1990) and in Danish waters in Køge Bay, Little Belt, Store Belt, and Kattegat (Wrisberg et al. 1992). Later studies, carried out by the Institute of Ecology (Lithuania), covered the Lithuanian, Latvian, Estonian (Baršienė et al. 2004, 2006a, b, 2008, 2012a, b), Polish (Kopecka et al. 2006; Baršienė et al. 2006a, 2012a; Napierska et al. 2009), and German economic zones (Schiedek et al. 2006; Baršienė et al. 2006a, 2012a; Rybakovas et al. 2009). In most of these studies, formation of micronuclei (MN) and nuclear buds (NB) were used as genotoxicity assessment endpoints in fish and bivalve species. The data on the formation of abnormal nuclear structures, as readouts of the genomic instability in organisms inhabiting the Baltic Sea, indicated the impact of the environmental pollution (Baršienė et al. 2006a, b, 2012a, b; Rybakovas et al. 2009; Napierska et al. 2009; Kreitsberg et al. 2012). Furthermore, correlation between MN and NB induction in fish from different sites of the Baltic Sea suggested both of them being useful complementary assays for the environmental genotoxicity evaluation.
NB are morphologically similar to MN and differ only by comparatively thin nucleoplasmic connection with the main nuclei of cells. The NB is formed of amplified DNA sequences that may be extruded from the cell nucleus (Shimizu et al. 1998). The formation of NB may reflect unequal capacity of organisms to expel damaged, amplified, improperly replicated, or condensed DNA, as well as chromosome fragments without telomeres and centromeres (Lindberg et al. 2007). Pampalona et al.’s (2010) studies have confirmed NB origin from broken chromatin after the breakage of nucleoplasmic bridges and stressed that analysis of nuclear buds would be of great interest as a biomarker of genomic instability, especially for the assessment of DNA damage relevant to cancer development.
Chromatin extrusion parameters such as NB may correctly reflect the levels of environmental contamination in the field studies and indicate the overall fish health. In the Baltic Sea, increased frequency of NB was described in flounder (Platichthys flesus) from the Gulf of Gdansk, also in southern Baltic B01, BEEP5, B10 sites (Rybakovas et al. 2009; Napierska et al. 2009) in eelpout (Zoarces viviparus) from the Estonian zones of the Gulf of Finland and the Gulf of Riga (Kreitsberg et al. 2012). Particularly high levels of NB were found in dab (Limanda limanda) inhabiting oil and gas platform zones in the North Sea (Rybakovas et al. 2009), also in Atlantic cod (Gadus morhua) and mussels (Mytilus edulis) collected from aluminum smelter zone (Baršienė et al. 2010). Elevated levels of nuclear buds there were observed in the blue mussels (Mytilus trossulus) from the Gulf of Gdansk and Lithuanian coast in 2002–2003 (Baršienė et al. 2006a) also after the accidental oil spill in Lithuanian waters in 2008 (Baršienė et al. 2012b). Significantly increased frequencies of NB have been recorded in Nile tilapia Oreochromis niloticus (Çavaş and Ergene-Gözükara 2005a; Ergene et al. 2007a, b), in gray mullet Mugil cephalus (Çavaş and Ergene-Gözükara 2005b; Ergene et al. 2007a), in bleak Alburnus orontis, and in African catfish Clarias gariepinus (Ergene et al. 2007a), inhabiting contaminated sites in the Goksu and Berdan River estuaries and Mersin harbor (Turkey). NB has been the most frequently registered nuclear abnormalities in juvenile Senegalese soles Solea senegalensis exposed to sediments from the Sado River estuary, one of the largest estuarine areas in Europe (Costa et al. 2008).
The main objective of the present study was to evaluate environmental genotoxicity risk levels in different regions of the Baltic Sea using the nuclear bud assay in blood erythrocytes of the three most common native fish species. Moreover, the assessment of NB is a low-cost, non-invasive to the fish genotoxicity assay, which may be applied for an in situ evaluation of marine environment status. This is a first attempt to evaluate the background level NB frequencies and to rank the genotoxicity risk levels for fish species. Previously, our results on environmental genotoxicity risk assessment were published using the MN test (Baršienė et al. 2012a). In the present study, we investigate the NB, as validated and reproducible measure of genotoxicity, in the same fish erythrocyte samples collected in the various regions of the Baltic Sea in 2009–2011 and directly comparing the two approaches of genotoxicity assessment.
Materials and methods
Sampling
Total sampling at 82 Baltic Sea study locations was conducted for flounder (P. flesus), herring (Clupea harengus), and eelpout (Z. viviparus) in 2009–2011. The list of the fish sampling stations and their geographical coordinates are presented in Table 1. Samples were obtained from the research trawl catch surveys from June 2009 to March 2011 carried out by the RV Walther Herwig III and the RV Baltica as well as from local fishermen. Fish specimens of approximately the same size, both sexes, and in good health condition were used for the NB analysis. Fish otoliths were used for the age determination. The analysis of NB was carried out in P. flesus from 52 stations, in C. harengus from 59 stations, and in Z. viviparus from 29 stations. Since in some study stations the sampling of fish was done during two surveys in 2009, as well as in 2010, the NB analysis was carried out in a total of 168 sampling groups. The NB frequency was determined in 1892 fish specimens: 714 flounder, 759 herring, and 419 eelpout (Table 2).
Sample preparation and nuclear buds evaluation criteria
A drop of blood taken from the caudal vein of fish was directly smeared on glass slides and air-dried. Smears were fixed in methanol for 10 min and then stained with 5 % Giemsa solution in phosphate buffer pH = 6.8 for 8 min. The stained slides were analyzed under light microscopes (Olympus BX51 or Nikon Eclipse 50i) at final magnification of ×1000. Blind scoring of MN and other nuclear abnormalities was performed on coded slides. The evaluation of NB was performed following the criteria reported by Fenech et al. (2003). For each studied specimen of fish, 4000 intact erythrocytes were analyzed. Blood erythrocytes with large evagination of nuclear membrane forming narrow nucleoplasmic connection with the main nuclei as well as buds on thin nucleoplasmic filament were recorded as NB (Fig. 1).
Calculation of nuclear buds background level and mapping using GIS system
The environmental genotoxicity risk in each of the 82 studied stations was assessed on a basis of the established background response of NB incidences in flounder (<0.23 NB/1000 erythrocytes in offshore, <0.20 NB/1000 erythrocytes in coastal zones), in herring (<0.40 NB/1000 erythrocytes), and in eelpout (<0.40 NB/1000 erythrocytes). The background level of NB frequencies was calculated in the same way as for MN (Baršienė et al. 2012a). The empirical 90 % percentile (P90, value that separates the upper 10 % of all NB values in the group of data from the lower 90 %) of NB was determined in different fish species collected in the 2001–2010 period from the reference sites Kvädöfjärden, Palanga, Leba, Pärnu, 1a-1, 2a-1, and 2b-1 respectively, that are characterized by no known local sources of contamination and no impact from human and industrial activity. In general, an elevated NB frequency lies above the P90 percentile, whereas the majority of values below the P90 value belong to individuals that are unexposed, weakly exposed, and non-responding or adapted to stressful conditions.
In each of the study stations, the percentage of the fish specimens with NB frequencies exceeding the background level of NB was assessed and mapped using GIS system. All the studied stations were grouped into a 5-grade scale, i.e., 0.0–19 % of specimens with NB frequencies higher than the background level was indicated as low, 20–39 % as moderate, 40–59 % as increased, 60–79 % as high, and 80–100 % as exceptionally high genotoxicity risk level.
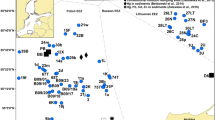
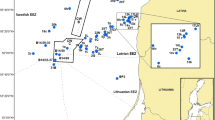
Considering that at some study stations, two or three fish species were collected, results from the flounder analysis are marked exactly under the geographical coordinates of the fish sampling. The other species are marked closely to the flounder. In the southern Baltic Sea study stations (B01, B12, B11, B10, SFI4), the sampling of fish was done two to four times; therefore, on the left side, data of earlier sampling (June and September 2009) are presented and, on the right side, the latter sampling (November and December of 2009). The map of 2009 reflects the results received in November and December, marked with black contour bookmarks. In the map of 2010–2011, such black contour bookmarks were used to express the responses in fish collected in May and August 2010. Genotoxicity risk zones were mapped utilizing the program ArcGIS Desktop by using ArcMap application.
Data treatment
Statistical analysis was carried out using the GraphPad PRISM 5.0 statistical package. The non-parametric Mann-Whitney U test was used to compare NB frequencies in the fish males and females. Pearson’s correlation analysis was performed to illustrate possible relationships between NB frequency and the fish biometrical measurements.
Results
The environmental genotoxicity levels were determined by counting nuclear buds in 62 sampling groups of flounder, herring, and eelpout collected in 2009. Six sampling groups were attributed to a low, 8 to a moderate, 18 to an increased, 19 to a high, and 11 to an extremely high genotoxicity risk level. Stations characterized as low genotoxicity risk were located singly in all studied zones of the Baltic Sea, such as the reference station Pärnu, the Swedish and Danish coastal waters, and the Wismar Bay. Low and moderate levels of environmental genotoxicity dominated in herring from the Gulf of Finland. An extremely high genotoxicity risk for flounder and herring was located predominantly in the southern Baltic Sea (Fig. 2).
In 2010–2011, the analysis of NB in erythrocytes and evaluation of environmental genotoxicity risk in 106 sampling groups of flounder, herring, and eelpout indicated that no station had low genotoxicity risk, and only 4 sampling groups attributed to a moderate, 7 to an increased, 16 to a high, and 79 to an extremely high genotoxicity risk level (Fig. 3). It should be pointed out that in 2010–2011, 93.5 % of the flounder (43 of 46 sampling groups) and 68.8 % (33 of 48 sampling groups) of the herring sampling groups were classified as living in areas with extremely high genotoxicity risk mainly located in the southern (especially in chemical munitions dumping sites) and eastern Baltic Sea offshore zones. No low genotoxicity risk stations were found in the analysis of eelpout NB, but 25 % (3 of 12 sampling groups) of eelpout groups were living in moderate and increased genotoxicity risk zones and 50 % of groups inhabited high and 25 % extremely high genotoxicity risk sites, located in the Gulf of Bothnia, the Roskilde area in Denmark, and Sorve station in Estonian coastal zone (Fig. 3, Table 3). When comparing responses of the fish species from the same study stations, interspecies differences were observed, and the genotoxicity risk level predominantly was highest in flounder (Figs. 2 and 3). The average means of NB frequencies were also higher in flounder compared to herring or eelpout.
Overall data for all three fish species collected in the period 2009–2011 indicated that 90 of 168 analyzed sampling groups (53.6 %) could be assigned to extremely high genotoxicity risk zones and only 3.6 % of them to low genotoxicity risk zones (Table 3). In 2009, 17.7 % were assigned to extremely high and 9.7 % to low genotoxicity risk zones, while in 2010–2011, the percentages were 74.5 and 0.0 %, respectively. Comparing environmental genotoxicity levels in fish from the same study stations between 2009 and 2010, an increase of genotoxicity risk was found in fish collected in 2010.
NB levels in studied fish species in 2009–2011 revealed that 89.4 % of flounder sampling groups could be attributed to high (13.6 %) and to extremely high (75.8 %) genotoxicity risk levels. In herring, 74.6 % of the sampling groups showed high (19.4 %) or extremely high (55.2 %) genotoxicity risk level; in eelpout, 45.7 % of the sampling groups showed such characteristics (Table 3).
Taken as a whole, our results indicate that in the Baltic Sea, extremely high genotoxicity risk zones for flounder were found in 6 stations (37.5 %) out of 16 studied in 2009 and 38 stations (92.7 %) out of 41 studied in 2010–2011, and for herring, 4 (22.2 %) out of 18 stations in 2009 and 33 (76.7 %) out of 43 stations in 2010–2011. In contrast, for eelpout, only 3 stations were characterized by an extremely high genotoxicity risk in 2010, out of 13 stations studied (23.1 %), and none of those were found in 2009. In 2010–2011, eelpout inhabited 6 locations (46.2 %) attributed to high genotoxicity risk level (Figs. 2 and 3).
In herring and eelpout, erythrocyte NB induction was more prominent and that of MN. There were less herring and eelpout sampling groups with low and moderate damage representing NB levels and more groups attributed to high and exceptionally high genotoxicity levels. NB analysis showed that 74.6 % of the herring sampling groups belonged to high and exceptionally high genotoxicity risk, while MN test has indicated only 46.3 % of such groups. NB analysis revealed high and exceptionally high genotoxicity risk for 45.7 % eelpout groups, and MN test only for 8.6 % of the fish groups. Nevertheless, in flounder, the induction of NB and MN was approximately at the same levels (Table 3).
The application of Pearson correlation analysis of NB frequency and fish age, total length, total weight, liver weight, and condition factor (CF) in herring showed strong positive correlations (r values over 0.6) in 16 out of 67 studied sampling groups. In eelpout, the positive correlations between NB and biological variables were found in specimens collected from four study stations, located in Swedish, Estonian, and Latvian zones of the Baltic Sea The positive correlation with fish age was found in herring and flounder sampled from the southern Baltic Sea (stations 17a, 19a, 23a). Statistically significant differences in NB frequency between fish males and females were found only in herring collected from GOR1 (p = 0.0397; Mann-Whitney U test) and in flounder from 27LT (p = 0.002) stations.
Discussion
Data of the present study pointed out to the utility of the NB assay in genotoxicity assessment. The assay was applied for three native fish species for the evaluation of the environmental genotoxicity risk in different regions of the Baltic Sea. The NB assay showed very high genotoxicity levels in fish analyzed from some of the studied stations. Up to 53.6 % fish sampling groups were collected from locations attributed to extremely high genotoxicity risk zones, and only 3.6 % of them were found in low genotoxicity risk zones. In 2009, 17.7 % were assigned to extremely high and 9.7 % to low genotoxicity risk zones; however in 2010–2011, the percentages were 74.5 and 0.0 %, respectively. In 2009–2011, NB levels in 89.4 % of flounder sampling groups were attributed to high and to extremely high genotoxicity risk levels, 74.6 and 45.7 % in herring and eelpout sampling groups, respectively. It is particular of interest that elevated concentration of BDE-47, Cu, and Pb was detected in the muscle tissues of flounder, caught in 2010 and 2011 from the southern Baltic Sea area (Waszak et al. 2012; Polak-Juszcak 2013).
MN test is one of the most frequently used and validated tool to evaluate cytogenetic damage in marine fish (Al-Sabti and Hardig 1990; Çavaş and Ergene-Gözükara 2005b; Baršienė et al. 2004, 2006b, c, 2012a; Kopecka et al. 2006; Schiedek et al. 2006; Rybakovas et al. 2009; Bolognesi and Hayashi 2011; Costa et al. 2011). Using MN approach, we have previously described the genotoxicity risk for native fish species inhabiting various regions of the Baltic Sea (Baršienė et al. 2012a). In the present study, the measurements of NB were performed in the same fish samples as the MN. In order to quantify the environmental genotoxicity risk, the same ranking system was used for both NB and MN parameters.
Studies indicated that both assays are simple, rapid, non-invasive, reproducible, and low-cost techniques for the assessment of cytogenetic and DNA and can be used in indigenous fish species for the evaluation of environmental genotoxicity. Taking into consideration that MN response is a transient marker dependent of the mitosis, NB assay can be used as an express tool for the genotoxicity evaluation in non-dividing cells. Since the NB formation usually is apparent earlier than MN induction, NB assay can reflect early effects of genotoxic agents and can be used for the assessment of accidental spills of DNA damaging contaminants (Baršienė et al. 2012b). In addition, NB assay as an index of accumulated DNA damage could be good approach to identify the integrated response to existing mixtures of contaminants in marine environment.
Comparison of the genotoxicity levels determined by NB and MN in the same fish specimens revealed that NB parameter indicated less sampling groups with low and moderate genotoxicity level while high and extremely high genotoxicity level was observed more frequently. It should be stressed that comparison of MN and NB formation in blood erythrocytes of eelpout, collected from the Danish coast, showed that the correlation between different contaminant concentrations and NB levels was observed more frequently and at higher significance levels than MN induction. Besides, experimental data revealed strong correlation between NB levels and phenanthrene (three-ring PAH) and pyrene (four-ring PAH) metabolite concentrations in gibel carp (Carassius auratus gibelio) bile (Kreitsberg et al. 2013).
In general, exceptionally high genotoxicity risk as measured by NB in flounder and herring was found predominantly in the southern Baltic Sea, especially in stations closely located to the chemical munition dumping sites. Low genotoxicity risk zones were located singly in all studied areas of the Baltic Sea, and most of them correspond to the eelpout data from the reference station Pärnu, the Swedish and Danish coastal waters, and the Wismar Bay. In 2009, low and moderate levels of environmental genotoxicity dominated in herring from the Gulf of Finland.
NB induction by pollutants has been extensively studied, and the validation of the NB assay was performed repeatedly in a variety of laboratory exposure studies using contaminants from different chemical groups (Cavaş and Ergene-Gözükara 2003, 2005b; Baršienė et al. 2006c; Cavaş 2008; de Campos Ventura et al. 2008). Statistically significant increase of NB has been shown in turbot exposed to 0.5 ppm crude oil spiked with PAHs and alkylphenols (Baršienė et al. 2006c), in O. niloticus treated with different concentrations of atrazine herbicide (de Campos Ventura et al. 2008), after 3-, 6-, and 9-day exposure to petroleum refinery and textile mill effluents (Cavaş and Ergene-Gözükara 2003, 2005b), also in goldfish Carassius auratus auratus after treatment with 1, 5, and 10 μg/l mercury chloride and with 100 μg/l lead acetate concentrations (Cavaş 2008). Significant increase of NB was observed in mussels treated with 5 ppb of tetrabromodiphenyl ether 47 (Baršienė et al. 2006d), also after 48-h exposure to 12 μg/l of fluoranthene, to mixture containing 12 μg/l of fluoranthene and 4 μg/l benzo(a)pyrene, or mixture of 12 μg/l of pyrene and 4 μg/l benzo(a)pyrene, as well as to mixture containing 12 μg/l of fluoranthene, 12 μg/l of pyrene, and 4 μg/l benzo(a)pyrene (Baršienė et al. unpublished data).
After 3-week exposure of turbot (Scophthalmus maximus) to 0.5 ppm crude Norwegian oil, we found significant increase of NB and strong correlation between NB induction in blood erythrocytes and PAH bile metabolites: fluorescent aromatic compounds (FACs) (290/335) (r = 0.979, p = 0.021 Pearson correlation; R 2 = 0.9579 linear regression), FACs (341/381) (r = 0.993, p = 0.007; R 2 = 0.9863), and FACs (380/430) (r = 0.988, p = 0.012; R 2 = 0.9759) (Baršienė et al. 2006c). Furthermore, a strong positive linear correlation (R 2 = 0.929) between NB and 16 PAH body burden levels has been described in mussels caged in the Ekofisk oil platform (North Sea) pollution gradient, as well as between MN and 16 PAH body burden (Sundt et al. 2011). Therefore, including NB assay into a system assessing the biological impact of contaminants on marine organisms would highlight the ecological importance of the DNA damage in heavily polluted marine areas. Considering the danger from dumped chemicals in marine environment, the implementation of laboratory-controlled studies using environmentally realistic doses of these genotoxic compounds will help describe cause-effect relationships between concentrations of hazardous compounds in fish tissues and genotoxicity risk levels.
Conclusions
The study is the first attempt to evaluate the background levels of NB in blood erythrocytes of flounder, herring, and eelpout inhabiting 82 study stations located in different sub-regions of the Baltic Sea. The background NB response was used for the establishment of low, moderate, increased, high, and exceptionally high genotoxicity risk levels for the fish species. In the study period of 2009–2011, 53.6 % of analyzed sampling groups could be assigned to extremely high genotoxicity risk zones and only 3.6 % of them to low genotoxicity zones. Thus, the data presented in this study indicate the existence of high genotoxicity levels in the different regions of the Baltic Sea. The analysis of NB employed as biomarker for wide-scale genotoxicity risk evaluation serves as good measure of genotoxicity and is very useful in environmental monitoring using the native fish species. The present findings suggest that the NB assay is informative and should be integrated with other genotoxicity parameters such as micronuclei and nucleoplasmic bridges, thus acting as a useful guide to detect the effects of long-term genotoxicity consistent with fish tissue contamination.
References
Al-Sabti, K., & Hardig, J. (1990). Micronucleus test in fish for monitoring the genotoxic effects of industrial waste products in the Baltic Sea, Sweden. Comparative Biochemistry and Physiology, Part C, 97, 179–182.
Baršienė, J., Lazutka, J., Šyvokienė, J., Dedonytė, V., Rybakovas, A., Bjornstad, A., & Andersen, O. K. (2004). Analysis of micronuclei in blue mussels and fish from the Baltic and the North Seas. Environmental Toxicology, 19, 365–371. doi:10.1002/tox.20031.
Baršienė, J., Schiedek, D., Rybakovas, A., Šyvokienė, J., Kopecka, J., & Förlin, L. (2006a). Cytogenetic and cytotoxic effects in gill cells of the blue mussel Mytilus spp. from different zones of the Baltic Sea. Marine Pollution Bulletin, 53, 469–478. doi:10.1016/j.marpolbul.2005.11.015.
Baršienė, J., Lehtonen, K., Koehler, A., Broeg, K., Vourinen, P. J., Lang, T., Pempkowiak, J., Šyvokienė, J., Dedonytė, V., Rybakovas, A., Repečka, R., Vountisjarvi, H., & Kopecka, J. (2006b). Biomarker responses in flounder (Platichthys flesus) and mussel (Mytilus edulis) in the Klaipėda-Būtingė area (Baltic Sea). Marine Pollution Bulletin, 53, 422–436. doi:10.1016/j.marpolbul.2006.03.009.
Baršienė, J., Dedonytė, V., Rybakovas, A., Andreikėnaitė, L., & Andersen, O. K. (2006c). Investigation of micronuclei and other nuclear abnormalities in peripheral blood and kidney of marine fish treated with crude oil. Aquatic Toxicology, 78, 99–104. doi:10.1016/j.aquatox.2006.02.022.
Baršienė, J., Šyvokienė, J., & Bjornstad, A. (2006d). Induction of micronuclei and other nuclear abnormalities in mussels exposed to bisphenol A, diallyl phthalate and tetrabromodiphenyl ether-47. Aquatic Toxicology, 78, 105–108. doi:10.1016/j.aquatox.2006.02.023.
Baršienė, J., Andreikėnaitė, L., Garnaga, G., & Rybakovas, A. (2008). Genotoxic and cytotoxic effects in bivalve mollusks Macoma balthica and Mytilus edulis from the Baltic Sea. Ekologija, 54, 44–50. doi:10.2478/V10055-008-0009-x.
Baršienė, J., Bjornstad, A., Rybakovas, A., Šyvokienė, J., & Andreikėnaitė, L. (2010). Environmental genotoxicity and cytotoxicity studies in mussels and fish inhabiting northern Atlantic zones impacted by aluminum industry. Ekologija, 56, 116–123. doi:10.6001/ekologija.v56i3-4.1299.
Baršienė, J., Rybakovas, A., Lang, T., Grygiel, W., Andreikėnaitė, L., & Michailovas, A. (2012a). Risk of environmental genotoxicity in the Baltic Sea over the period of 2009–2011 assessed by micronuclei frequencies in blood erythrocytes of flounder (Platichthys flesus), herring (Clupea harengus) and eelpout (Zoarces viviparus). Marine Environmental Research, 77, 35–42. doi:10.1016/j.marenvres.2012.01.004.
Baršienė, J., Rybakovas, A., Garnaga, G., & Andreikėnaitė, L. (2012b). Environmental genotoxicity and cytotoxicity studies in mussels before and after the oil spill in marine oil terminal (Baltic Sea). Environmental Monitoring and Assessment, 184, 2067–2078. doi:10.1007/s10661-011-2100-0.
Bolognesi, C., & Hayashi, M. (2011). Micronucleus assay in aquatic animals. Mutagenesis, 26, 205–213. doi:10.1093/mutage/geq073.
Cavaş, T. (2008). In vivo genotoxicity of mercury chloride and lead acetate: micronucleus test on acridine orange stained fish cells. Food and Chemical Toxicology, 46, 352–358. doi:10.1016/j.fct.2007.08.015.
Cavaş, T., & Ergene-Gözükara, S. (2003). Micronuclei, nuclear lesions and interphase silver-stained nucleolar organizer regions (AgNORs) as cyto-genotoxicity indicators in Oreochromis niloticus exposed to textile mill effluent. Mutation Research, 538, 81–91. doi:10.1016/S1383-5718(03)00091-3.
Çavaş, T., & Ergene-Gözükara, S. (2005a). Induction of micronuclei and nuclear abnormalities in Oreochromis niloticus following exposure to petroleum refinery and chromium processing plant effluents. Aquatic Toxicology, 74, 264–271. doi:10.1016/j.aquatox.2005.06.001.
Çavaş, T., & Ergene-Gözükara, S. (2005b). Micronucleus test in fish cells: a bioassay for in situ monitoring of genotoxic pollution in the marine environment. Environmental and Molecular Mutagenesis, 46, 64–70. doi:10.1002/em.20130.
Costa, P. M., Lobo, J., Caeiro, S., Martins, M., Ferreira, A. M., Caetano, M., Vale, C., Del Valls, T. A., & Costa, M. H. (2008). Genotoxic damage in Solea senegalensis exposed to sediments from the Sado Estuary (Portugal): effects of metallic and organic contaminants. Mutation Research, 654, 29–37. doi:10.1016/j.mrgentox.2008.04.007.
Costa, P. M., Neuparth, T. S., Caeiro, S., Lobo, J., Martins, M., Ferreira, A. M., Caetano, M., Vale, C., Del Valls, T. A., & Costa, M. H. (2011). Assessment of the genotoxic potential of contaminated estuarine sediments in fish peripheral blood: laboratory versus in situ studies. Environmental Research, 111, 25–36.
de Campos Ventura, B., de Fransceschi de Angelis, D., & Marin-Morales, M. A. (2008). Mutagenic and genotoxic effects of the Atrazine herbicide in Oreochromis niloticus (Perciformes, Cichlidae) detected by the micronuclei test and the comet assay. Pesticide Biochemistry and Physiology, 90, 42–51. doi.org/10.1016/j.pestbp.2007.07.009.
Ergene, S., Çavaş, T., Celik, A., Koleli, N., Kaya, F., & Karahan, A. (2007a). Monitoring 410 of nuclear abnormalities in peripheral erythrocytes of three fish species from the Goksu Delta (Turkey): genotoxic damage in relation to water pollution. Ecotoxicology, 16, 385–391. doi:10.1007/s10646-007-0142-4.
Ergene, S., Çavaş, T., Celik, A., Koleli, N., & Aymak, C. (2007b). Evaluation of river water genotoxicity using the piscine micronucleus test. Environmental and Molecular Mutagenesis, 48, 421–429. doi:10.1002/em.20291.
Fenech, M., Chang, W. P., Kirsch-Volders, M., Holland, N., Bonassi, S., & Zeiger, E. (2003). HUMN project: detailed description of the scoring criteria for the cytokinesis-block micronucleus assay using isolated human lymphocyte cultures. Mutation Research, 534, 65–75. doi:10.1016/S1383-5718(02)00249-8.
HELCOM, (2010). Hazardous substances in the Baltic Sea—an integrated thematic assessment of hazardous substances in the Baltic Sea. Baltic Sea Environmental Proceedings No. 120B.
Jha, A. N. (2008). Ecotoxicological applications and significance of the comet assay. Mutagenesis, 23, 207–221. doi:10.1093/mutage/gen014.
Kopecka, J., Lehtonen, K., Baršienė, J., Broeg, K., Vuorinen, P. J., Gercken, J., & Pempkowiak, J. (2006). Measurements of biomarker levels in flounder (Platichthys flesus) and mussel (Mytilus trossulus) from the Gulf of Gdańsk (southern Baltic). Marine Pollution Bulletin, 53, 406–421. doi:10.1016/j.marpolbul.2006.03.008.
Kreitsberg, R., Tuvikene, A., Baršienė, J., Fricke, N. F., Rybakovas, A., Andreikėnaitė, L., Rumvolt, K., & Vilbaste, S. (2012). Biomarkers of environmental contaminants in the coastal waters of Estonia (Baltic Sea): effects on eelpout (Zoarces viviparus). Journal of Environmental Monitoring, 14, 2298–2308. doi:10.1039/C2EM30285C.
Kreitsberg, R., Baršienė, J., Freiberg, R., Andreikėnaitė, L., Tammaru, T., Rumvolt, K., & Tuvikene, A. (2013). Effects of hypoxia and contaminated sediments on the gibel carp (Carassius auratus gibelio) in laboratory exposure based on multiple biomarkers. Ecotoxicology and Environmental Safety, 98, 227–235. doi:10.1016/j.ecoenv.2013.08.016.
Lindberg, H. K., Wang, X., Järventaus, H., Falck, G. C. M., Norppa, H., & Fenech, M. (2007). Origin of nuclear buds and micronuclei in normal and folate-deprived human lymphocytes. Mutation Research, 617, 33–45. doi:10.1016/j.mrfmmm.2006.12.002.
Napierska, D., Baršienė, J., Mulkiewicz, E., Podolska, M., & Rybakovas, A. (2009). Biomarker responses in flounder Platichthys flesus from the Polish coastal area of the Baltic Sea and application in biomonitoring. Ecotoxicology, 18, 846–859. doi:10.1007/s10646-009-0328-z.
Ohe, T., Watanabe, T., & Wakabayashi, K. (2004). Mutagens in surface waters: a review. Mutation Research, 567, 109–149. doi:10.1016/j.mrrev.2004.08.003.
Pampalona, J., Soler, D., Genesca, A., & Tusell, L. (2010). Telomere dysfunction and chromosome structure modulate the contribution of individual chromosomes in abnormal nuclear morphologies. Mutation Research, 683, 16–22. doi:10.1016/j.mrfmmm.2009.10.001.
Polak-Juszcak, L. (2013). Trace metals in flounder, Platichthys flesus (Linnaeus, 1758), and sediments from the Baltic Sea and the Portuguese Atlantic coast. Environmental Science and Pollution Research, 20, 7424–7432. doi:10.1007/s11356-013-1762-2.
Rybakovas, A., Baršienė, J., & Lang, T. (2009). Environmental genotoxicity and cytotoxicity in the offshore zones of the Baltic and North Seas. Marine Environmental Research, 68, 246–256. doi:10.1016/j.marenvres.2009.06.014.
Schiedek, D., Broeg, K., Baršienė, J., Lehtonen, K. K., Gercken, J., Pfeifer, S., Vuontisjärvi, H., Vuorinen, P. J., Dedonytė, V., Koehler, A., Balk, L., & Schneider, R. (2006). Biomarker responses as indication of contaminant effects in blue mussel (Mytilus edulis) and female eelpout (Zoarces viviparus) from the southwestern Baltic Sea. Marine Pollution Bulletin, 53, 387–405. doi:10.1016/j.marpolbul.2005.11.013.
Shimizu, N., Itoh, N., & Vahl, G. M. (1998). Selective entrapment of extrachromosomally DNA by nuclear budding and micronucleation during the S-phase. Journal of Cell Biology, 140, 1307–1320. doi:10.1083/jcb.140.6.1307.
Sundt, R. C., Pampanin, D. M., Grung, M., Baršienė, J., & Ruus, A. (2011). PAH body burden and biomarker responses in mussels (Mytilus edulis) exposed to produced water from a North Sea oil field: laboratory and field assessments. Marine Pollution Bulletin, 62, 1498–1505. doi:10.1016/j.marpolbul.2011.04.009.
Waszak, I., Dabrowska, H., & Góra, A. (2012). Bioaccumulation of polybrominated diphenyl ethers (PBDEs) in flounder (Platichthys flesus) in the southern Baltic Sea. Marine Environmental Research, 79, 132–141. doi:10.1016/j.marenvres.2012.06.006.
Wrisberg, M. N., Bilbo, C. M., & Spliid, H. (1992). Induction of micronuclei in hemocytes of Mytilus edulis and statistical analysis. Ecotoxicology and Environmental Safety, 23, 191–205. doi:10.1016/0147-6513(92)90058-B.
Acknowledgments
This study was funded mainly by Lithuanian Science Council (MIP-62/2010 GENCITOX and MIP-033/2012 GENOTOX-CG projects) and by BONUS BEAST project (FP/2007-2013 under grant agreement no. 217246). We are thankful to Thomas Lang (Thünen Institute of Fisheries Ecology, Germany) and Aleksandras Rybakovas for collecting samples during Walther Herwig III cruises and helping in the analysis of samples, Lars Förlin (Goteborg University, Sweden) for providing material from four Swedish stations, Jens Gercken (Institute for Applied Ecology, Germany) for providing material from three stations in the Wismar Bay, and Arvo Tuvikene (Estonian University of Life Sciences, Estonia) for providing material from five stations at the Estonian coast. The authors would like to thank Dalia Baršytė Lovejoy (Toronto University) for the language check.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Baršienė, J., Butrimavičienė, L., Michailovas, A. et al. Assessing the environmental genotoxicity risk in the Baltic Sea: frequencies of nuclear buds in blood erythrocytes of three native fish species. Environ Monit Assess 187, 4078 (2015). https://doi.org/10.1007/s10661-014-4078-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-014-4078-x