Abstract
Mercury (Hg) is one of the major aquatic contaminants even though emissions have been reduced over the years. Despite the relative abundance of investigations carried out on Hg toxicity, there is a scarcity of studies on its DNA damaging effects in fish under realistic exposure conditions. This study assessed the Hg genotoxicity in Golden grey mullets (Liza aurata) at Laranjo basin, a particularly contaminated area of Ria de Aveiro (Portugal) well known for its Hg contamination gradient. (1) Fish were seasonally caught at Laranjo basin and at a reference site (S. Jacinto), and (2) animals from the reference site were transplanted and caged (at bottom and surface), for 3 days, in two different locations within Laranjo basin. Using the comet assay, blood was analyzed for genetic damage and apoptotic cell frequency. The seasonal survey showed greater DNA damage in the Hg-contaminated area for all sampling seasons excluding winter. The temporal variation pattern of DNA lesions was: summer ≈ autumn > winter > spring. Fish caged at Laranjo also exhibited greater DNA damage than those caged at the reference site, highlighting the importance of gill uptake on the toxicity of this metal. No increased susceptibility to apoptosis was detected in either wild or caged fish, indicating that mercury damages DNA of blood cells by a nonapoptotic mechanism. Both L. aurata and the comet assay proved to be sensitive and suitable for genotoxicity biomonitoring in mercury-contaminated coastal systems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The aquatic environment, estuaries in particular, constantly receives genotoxic chemicals from either industrial or municipal waste waters (Rank and Jensen 2003) which may cause damage to biota. The assessment of DNA in individual cells, following exposure to pollutants, has been used as a valuable ecotoxicological tool concerning molecular genotoxicity biomarkers (Mitchelmore et al. 1998; Coughlan et al. 2002). In this context, the single-cell gel electrophoresis (SCGE) or comet assay appears to be a quick, simple, reliable, and sensitive technique to detect and measure genetic damage in almost any type of eukaryotic cell, using a small number of cells (Collins et al. 1997). This technique has proved its applicability in monitoring the effects of DNA-damaging agents in several marine and freshwater fish (Nacci et al. 1996; Belpaeme et al. 1998; Sumathi et al. 2001; Lee and Steinert 2003; Kim and Hyun 2006). The alkaline version developed by Singh et al. (Singh et al. 1988) allows for the detection of DNA single-strand breaks (SSBs), alkali-labile sites, DNA-DNA/DNA-protein cross-linking, and SSBs associated with incomplete excision repair sites (Tice et al. 2000). These events can lead to DNA fragmentation, which also occurs when cells die via apoptosis (Tice et al. 2000; Meintieres et al. 2003), also detectable by the comet assay (Meintieres et al. 2003).
Heavy metals are an important group of environmental contaminants that should be considered when assessing genotoxicity in aquatic organisms, including fish (Ayllon and Garcia-Vazquez 2000). Mercury (Hg) is one of the most toxic metals described (Ayllon and Garcia-Vazquez 2000); it accumulates in fish tissues mainly in the form of methylmercury (MeHg) (Wiener et al. 2002). The widespread geographic extension and adverse consequences of pollution by this metal are still an issue of concern and continue to prompt considerable scientific investigation. Hg’s toxicity and clastogenicity have been described in various studies but little has been done regarding in vivo genotoxicity assessment in fish. For example, mercury nitrate and metallic mercury (Hg0) were demonstrated to be micronucleus (MN) inducers in mollie (Poecilia latipinna) (Ayllon and Garcia-Vazquez 2000) and carp (Cyprinus carpio) (Nepomuceno et al. 1997), respectively. In addition, MeHg proved to be teratogenic as well as a MN and chromosomal aberration inducer in killifish (Fundulus heteroclitus) embryos (Weis and Weis 1977; Perry et al. 1988).
Testing different substances for their genotoxicity in fish is often performed under laboratory conditions. Such laboratory experimentation compromises the relevance of data since a reliable prevision of the substances’ toxicant effects is complicated by a deviation from real exposure situations. On the other hand, field data interpretation by itself is often a very difficult task. Thus, the combination of field-survey studies with in situ caging experiments has been proposed as the ideal biomonitoring strategy from an ecotoxicological point of view (Pacheco et al. 2005). In the present study, the DNA damage induced by environmental Hg was assessed in wild and transplanted Golden grey mullets (Liza aurata) along a contaminated area of Ria de Aveiro, Laranjo basin (LAR; Portugal), using the comet assay. This species is abundant in the Atlantic and Mediterranean European coastal waters, easy to catch, and well represented in both clean and contaminated areas. Additionally, heavy metal accumulation was previously proved to occur in members of the Mugilidae family, namely, L. aurata (Blasco et al. 1998).
In the present work we aimed (i) to detect DNA damage induction, as well as susceptibility to apoptosis, in L. aurata blood cells due to the presence of mercury at LAR, in either wild or in situ caged fish; (ii) to evaluate the environmental health status of the study area and the risk of Hg contamination for fish populations; and (iii) to propose the comet assay applied to L. aurata as a biomonitoring methodology to evaluate metal genotoxicity.
Materials and Methods
Study Area
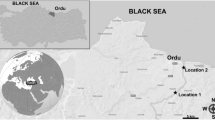
Laranjo basin (LAR), near Estarreja, is one of the most contaminated areas in Ria de Aveiro coastal lagoon, northwestern Portugal (Fig. 1). From the 1950s until 1994 this area continuously received discharges from a chlor-alkali plant contributing to the generation of a mercury-contamination gradient. The relatively recent improvements in the production process of this industry resulted in a significant decrease in mercury input into the lagoon. Nevertheless, high mercury levels are still present, mainly in sediments, due to its progressive deposition and resuspension, which is responsible for its exportation and increased bioavailability, mainly during periods of strong tidal currents (Pereira et al. 1997; Pereira et al. 1998). Since no other important sources of contaminants have been reported in this area, LAR is regarded as a “field laboratory,” offering to researchers a unique opportunity for the assessment of mercury toxicity (Guilherme et al. 2008a). An accompanying study detected total Hg levels in the sediment from 3.0 (LAR1) up to 7.7 (LAR2) ng/mg (dry weight) (Guilherme et al. 2008a), as well as elevated total Hg tissue loads in L. aurata inhabiting this area (e.g., 0.57 and 2.4 μg/g wet weight, respectively, in blood and liver) (Guilherme et al. 2008a, b).
Map of Ria de Aveiro (Portugal) with locations of fish capture and fish-caging sites (dark circles). Respective coordinates are as follows: reference site (REF)—40º40′26″N, 8º43′17″W; LAR1—40º43′24″N, 8º37′55″W; LAR2—40º43′49″N, 8º36′53″W. The LAR2 site adopted in the caging experiment coincides (≡) with the LAR site selected for sampling in the wild fish survey. The chlor-alkali plant location is not exactly to map scale, serving mainly to illustrate the relative position of caging sites
S. Jacinto was selected as the reference (REF) site due to its proximity to the lagoon entrance, the distance to the main polluting sources (Fig. 1), and its low contamination load (Pacheco et al. 2005).
Study Organism: Characterization and Sampling
The Golden grey mullet (Liza aurata), one of the most common fish in Ria de Aveiro, is a pelagic species that frequently contacts with sediments, feeding on small benthic organisms, detritus, and, occasionally, insects and plankton. Juvenile specimens were used to minimize the interference of variables such as gender and levels of accumulated contaminants.
Fish with an average weight of 13.5 ± 0.9 g and length of 12.1 ± 0.6 cm were caught at the REF site and contaminated areas during low tide, using a traditional beach-seine net called a chincha. After catching, fish were dissected and blood was collected from the posterior cardinal vein using heparinized Pasteur pipettes, stored in microtubes, and kept on ice. In the laboratory, samples were kept in the dark, diluted in saline solution (B. Braun Medical, Lda.), and then used for comet assay.
Experimental Design
Two experimental components were considered in this study: the first corresponding to a seasonal analysis of wild fish and the second corresponding to a field-caging experiment (Fig. 2). In both components, comet assay was performed in fish blood cells to assess DNA damage and the incidence of apoptotic cells (ACs). Blood of control animals (REF site) was exposed to hydrogen peroxide (75 μM H2O2, 1 h) (Sigma) and used as a positive control.
Wild Fish Survey Ten fish of similar dimensions (size and weight) per sampling moment were caught, as previously described, during the winter of 2004 (December) and the spring (March), summer (July), and autumn (September) of 2005 at LAR and S. Jacinto (REF site) (Fig. 1).
Field-Caging Experiment Fish were caught at the REF site (S. Jacinto; Fig. 1), transported to the laboratory in oxygenated saltwater (from the fishing site), and allowed to stabilize for 2 weeks prior to experimentation in order to reduce interindividual differences, to allow adaptation to confinement, and to reduce the levels of chemicals taken up previously. During stabilization, fish were kept in 80-L aquaria at natural temperature and photoperiod, in aerated (dissolved oxygen level = 8.4 ± 0.2 mg ∙ L−1) and filtered artificial seawater (Salsera, France; 23 ± 0.1 g ∙ L−1 salinity). Fish were fed daily with polychaete worms (Nereis sp.) collected in a clean area of Ria de Aveiro.
Subsequently, mullets were transferred to the field in oxygenated artificial seawater and caged (10 fish per 80-L cage), during 3 days, in two different locations at LAR, positioned at different relative distances from the metal contamination source (LAR1 and LAR2) (Figs. 1 and 2).
To study the effect of mercury uptake and genotoxicity, two cages were placed at two positions along the water column as follows: one at the surface and the other close to the sediment. Surface cages were maintained in a submerged position (30-cm depth) with a buoy-anchor system. Bottom cages were set at 15 cm from the sediment to avoid direct contact with it. Reference groups were caged at S. Jacinto. When transferred to cages, the animals were visually checked to be in perfect condition. During field exposure, fish were kept without any food supply. On the day of transfer to cages, 10 fish were sacrificed for analysis, constituting the t0 group. This part of the study was performed in December 2004.
Comet Assay
The alkaline version of SCGE—the comet assay—was performed with slight modifications of the Koppen and Verschaeve (Koppen and Verschaeve 1996) methodology. All procedures were performed under dim yellow light to prevent extra UV light-induced DNA damage. Agarose solutions were prepared in phosphate-buffered saline (without Ca2+ and Mg2+, pH 7.4; Bio Wittaker Europe, Cambrex Co.).
Slide Preparation
Briefly, microscope slides were precoated with 1% normal-melting-point (NMP) agarose (Bio-Rad; 5 min at room temperature), and then a layer of cell suspension in 0.5% low-melting-point (LMP) agarose (Sigma) was added between a bottom layer of 0.8% NMP agarose and a top layer of 0.8% LMP agarose. After solidification on ice, microscope slides were immersed in cold lysing solution (2.5 M NaCl [Merck], 0.1 M Na2EDTA [Merck], 0.01 M Tris base [Qbiogene], pH 10, set with NaOH [Merck], 1% Na-lauroylsarcosinate [Sigma], 10% DMSO [Sigma], and 1% Triton X-100 [Sigma] in MQ water [Millipore]), in the dark, for a period of 1–24 h and then placed for 15 min in freshly prepared alkaline denaturing electrophoresis buffer (0.3 M NaOH, 0.001 M Na2EDTA, 0.1% 8-hydroxyquinoline [Sigma], and 2% DMSO in MQ water at 17°C). Electrophoresis ran at 0.7–1 V/cm (300 mA) for 10 min, after which slides were washed in cold Tris-HCl (0.4 M, pH 7.5), stained with ethidium bromide (Qbiogene; 20 μg ∙ mL−1), and stored in moistened boxes, with light protection, at 4°C, until observation.
Image Analysis
The alkaline comet assay allows for detection of DNA damage occurring as SBs by measuring the migration of DNA fragments from the nucleoid, visually resembling a comet. A LEICA DMLS fluorescence microscope (400 × magnification) was used for slide analysis. For each fish, two slides were prepared. Fifty cells (25 per individual) were randomly scored using a public domain NIH-Image program (Helma and Uhl 2000).
Image analysis was mainly made on the increased fluorescence in the tail region, referring to the percentage of DNA in the tail (TD%), the tail length (TL), or the product of both, called the tail moment (TM). TM was the chosen parameter for the comparative analysis between conditions due to its responsiveness. Data on TD and TL were measured (but not shown) in a preanalysis phase because, when using a derived parameter such as TM, the original parameters should be considered as suggested by Tice et al. (Tice et al. 2000). Control comet cells are represented by the nucleoid core only, normally with minimal DNA migration (Fig. 3, class 1). Any healthy cell typically contains a certain proportion of SBs in its DNA, the result of either spontaneous damage or DNA breakage necessary to DNA functions such as its synthesis (Koppen 1999).
Comet scale. A five-class classification based on tail DNA percentage (TD) adopted from Mitchelmore and Chipman (Mitchelmore and Chipman 1998). Comet classes: 1, no or minimal damage (<5%); 2, low damage (5–20%); 3, mid damage (20–40%); 4, high damage (40–75%); 5, extreme damage (>75%)
Besides TM, the frequency of ACs was also accessed through the comet assay data. The mean head intensity and area were considered together to better define an AC in this work. An interval of acceptance was defined by the mean ± standard deviation for both comet parameters, and values below this limit were highlighted and analyzed in detail. A positive result was considered if both parameters (mean head intensity and area) were demarked and confirmation was made by evaluation of the respective comet image in comparison to the fan-like pattern previously described by Tice et al. (Tice et al. 2000) or Meintieres et al. (Meintieres et al. 2003) (Fig. 3, class 5). These cells were included in the counting of 50 comets per individual but excluded from any TM image analysis or statistic calculations, as they represent dead/dying cells (Speit and Hartmann 2004).
Statistical Analysis
The significance of the differences, either between spatial distributions or between seasons, was evaluated using the nonparametric Kruskal-Wallis ANOVA and a posteriori the Mann-Whitney U-test, referring to STATISTICA 6 software (StatSoft, Inc., Tulsa, OK, USA).
Results
The hydrological parameters (temperature, dissolved oxygen, salinity, pH, turbidity, and depth) measured in the study areas at Ria de Aveiro concerning the wild fish survey and the caging experiment are reported in Tables 1 and 2, respectively. These parameters showed, in general, no important intersite differences within the same sampling season for temperature, dissolved oxygen, or pH. In winter and spring, LAR showed a lower salinity and depth, and a higher turbidity, compared to REF. A similar pattern was observed in the caging experiment comparing REF and LAR sites. Table 1 also reports common seasonal variations for most of the parameters.
DNA Damage in Wild Fish
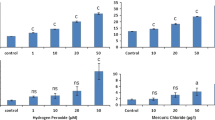
From the analysis of TM data (Fig. 4A), it is evident that animals from the contaminated site (LAR) showed significantly (p < 0.05) higher levels of DNA damage compared to those at REF in all sampling seasons excluding winter. The TM levels at LAR were as high as 2.0, 2.8, and 2.7 times the reference levels during spring, summer, and autumn, respectively.
DNA integrity in blood cells of wild Liza aurata collected seasonally at a mercury-contaminated site (LAR) and a reference site (REF) in the Ria de Aveiro. Results reflect (A) the tail moment (TM) and (B) the incidence of apoptotic cells (ACs) after the alkaline SCGE assay. The box-whisker plots for TM represent the median (□), 25th–75th percentiles (box), and average ± SD (bars). Data on ACs represent the average ± SD. Statistically significant differences are *versus REF site within each sampling season and aversus winter, bversus spring, and cversus summer
Analyzing the temporal variation patterns of DNA lesions in L. aurata, for both REF and LAR fish, the following order is seen: summer ≈ autumn > winter > spring. At both the REF and the LAR sites, DNA damage decreased significantly (p < 0.05) from winter to spring, increased in summer, and were maintained at a high level in autumn.
Concerning the frequency of ACs (Fig. 4B), no differences were found between fish groups from REF and those from LAR sites within the same sampling season. However, significant differences (p < 0.05) were found, for both REF and LAR fish, comparing spring to summer (increase) and summer to autumn (decrease). In addition, a significant difference was found between winter and summer (increase) for REF animals. The temporal pattern was similar to that previously obtained for DNA damage: summer > autumn > winter > spring.
The positive control with H2O2 revealed an average TM of 5119.01 (SD = 1385.31) and AC of 20% (SD = 13%). In terms of TM levels, no statistical differences were found between the positive control and the contaminated groups, but there was a statistically significant increase in damage in comparison to the negative control group (REF). The incidence of ACs was similar in all situations.
DNA Damage in Caged Fish
No significant differences were found between the 2-week-acclimated animals (t0) and the animals kept in the laboratory (LAB) or caged at the REF site for 3 more days, showing identical (low) damage levels. However, relatively high individual variability was still present in t0 animals.
Statistical analyses for comparison between locations were carried out separately for surface and bottom groups. From the analysis of TM results (Fig. 5A) it is evident that fish from LAR sites (LAR1 and LAR2) exhibited significantly higher genetic damage levels (p < 0.001) compared to fish from the REF site. The observed increase was 3.3, 1.7, and 2.5 times for LAR1 bottom, LAR2 surface, and LAR2 bottom, respectively. No significant differences were found between LAR1 and LAR2 bottom groups. In addition, a higher variability of results was observed at LAR sites.
DNA integrity in blood cells of Liza aurata caged (at surface and bottom), during 3 days, at different Ria de Aveiro locations, i.e., at a mercury-contaminated area (Laranjo basin; LAR1 and LAR2) and a reference site (REF). Results reflect (A) the tail moment (TM) and (B) the incidence of apoptotic cells (ACs) after the alkaline SCGE assay. The box-whisker plots for TM represent the median (□), 25th–75th percentiles (box), and average ± SD (bars). t0 represents the value at the beginning of the experiment; LAB, a 3-day laboratory control. Statistically significant differences are *versus REF site (surface or bottom, accordingly) and ♦surface versus bottom within the same site. n.m., not measured
Little can be said about the influence of the relative position in the water column on the incidence of genetic damage in these animals. The comparison is not feasible for LAR1, as the surface cage was lost; no significant differences were found between surface and bottom groups at LAR2. However, S. Jacinto (REF) bottom-caged fish showed significantly lower damage compared with surface-caged fish.
Data on the occurrence of ACs (Fig. 5B) did not reveal any significant or conclusive result in comparisons between REF and LAR groups, as well as surface and bottom groups at the same site.
Discussion
Although mercury’s spatial and biological distribution at Laranjo basin (LAR; Ria de Aveiro, Portugal) is well documented (Pereira et al. 1997; Coelho et al. 2005), there are some gaps in understanding its effects at the organism, population, and ecosystem levels. Various studies on mercury compounds and their genotoxic effects have been performed, evaluating a panoply of genetic endpoints. Clastogenic effects mostly associated with the spindle mechanism disturbance and the generation of reactive oxygen species, accompanied by glutathione depletion, which might also contribute to its genotoxicity, have been reported (De Flora et al. 1994). Organomercury compounds are the most predominant, as they are generally more toxic to aquatic organisms than the inorganic forms (Boening 2000). Nevertheless, the genetic effects of inorganic and organomercury are qualitatively comparable, suggesting the existence of a common genotoxic entity (De Flora et al. 1994).
The occurrence of mercury-induced DNA fragmentation has already been detected in vitro using the comet assay in a human cell line (Ben-Ozer et al. 2000) and carp gill cells (Arabi 2004). However, these studies have been conducted under artificial laboratorial conditions using cell lines instead of whole organisms and considering only the individual effect of each chemical compound tested. Only field studies can provide a final indication of the environmental health status and autochthonous population condition. Therefore, the proposed ideal strategy for environmental biomonitoring combines both survey campaigns and in situ caging experiments, after which animals’ responses are adequately interpreted (Pacheco et al. 2005). The comet assay has not been as extensively applied to field studies as it has to laboratory studies (Kim and Hyun 2006; Steinert et al. 1998; Klobucar et al. 2003; Rank et al. 2007). Hence, the novelty of our study is the adoption of a relatively recent technique—the comet assay—to assess mercury genotoxicity in wild and in situ-exposed L. aurata in an area that is well known for its mercury contamination gradient.
Though a previous study revealed that TL and TM can provide similar results (Duez et al. 2003), in the present study TM proved to be more responsive than TL and TD% (data not shown), and thus, only TM results are reported and discussed. Additionally, TM has the advantage of considering damage expressed as a short tail with a high fraction of DNA or a long tail with a low fraction of highly fragmented DNA (Salagovic et al. 1997). For statistical analysis, the median value was taken into consideration, as it proved to be a more accurate parameter than the mean value (data not shown), corroborating previous findings on this subject (Duez et al. 2003).
Since increased DNA migration could be alternatively associated with cytotoxicity, it is strongly recommended to address the possibility of cell death induction by means other than genotoxicity (Speit and Hartmann 2004). Several researchers adopted the comet assay to access eventually ongoing cytotoxicity events (for review see Lee and Steinert 2003). Hence, the analysis of TM should take into consideration the AC frequency in order to avoid misinterpretations. Moreover, the decision to ignore ACs in the present TM calculations provided lower and more consistent TM values.
Taking into account that hydrological abiotic conditions did not differ substantially between the study sites, the current discussion is based on the contamination scenario at each particular site. Moreover, mercury was assumed to be the main contaminant at LAR since no other contaminants were previously detected in that area, and thus mercury is taken to be responsible for the observed effects. This interpretation is corroborated by another study (Guilherme et al. 2008a) where increased blood mercury levels were detected in the same fish whose blood was assayed in the present study for comet assay.
DNA Damage in Wild Fish
Significantly higher levels of genetic damage were regularly found in wild fish from LAR, which can be attributed to the increased total mercury levels found in that location by Guilherme et al. (2008a), mainly in the sediment, pointing out Hg’s persistence and confirming previous findings of Pereira et al. (Pereira et al. 1997), Ramalhosa et al. (Ramalhosa et al. 2001), and Coelho et al. (Coelho et al. 2005).
A temporal analysis of the current data reveals that L. aurata from LAR showed the highest values of genetic injury in summer and autumn and the lowest in spring (i.e., summer ≈ autumn > winter > spring). These results seem to be in accordance to the findings of Guilherme et al. (2008a) showing a mercury-induced clastogenic/aneugenic action, measured as erythrocytic nuclear abnormality induction, only in summer and autumn. It must be taken into consideration that the interpretation of temporal response patterns, in the case of LAR fish, may be compromised by seasonal fluctuations in environmental mercury levels. However, the same temporal pattern (though less evident) is also perceptible in fish from the REF site, pointing out the modulatory effect of seasonal climatic variations on fish physiology. In this context, it must be emphasized that TM values measured along the year at the REF site perfectly matched the water temperature fluctuations recorded. This is in accordance with Venier et al. (Venier et al. 1997), who demonstrated that water temperature may influence cell replication rates and DNA repair of poikilothermal organisms, and with Buschini et al. (Buschini et al. 2003), who found a positive correlation between water temperature and DNA integrity loss in zebra mussels. Consequently, the interference of water temperature as an additive factor on the mercury-induced DNA integrity loss observed in LAR fish cannot be underestimated.
Data on AC incidence demonstrated that LAR fish have a slightly higher frequency compared to REF fish, though it was not possible to distinguish the groups based on statistically significant differences. On the other hand, significant temporal variations were detected for both control and mercury-exposed groups, which may indicate that the incidence of ACs depends on the seasonal fish health condition rather than on the environmental levels of Hg. The joint analysis of the current TM and AC data supports the idea that mercury damages blood cell DNA by a nonapoptotic mechanism, which is in accordance with the results obtained previously in human cell lines exposed to mercuric chloride (Ben-Ozer et al. 2000).
DNA Damage in Caged Fish
The significant DNA damage induction observed in fish caged at LAR1 and LAR2 should be regarded as an additional indication of a genotoxic action of mercury, reinforcing the current wild fish survey findings. The absence of erythrocytic nuclear abnormality induction observed in LAR1 and LAR2 fish by Guilherme et al. (2008a) suggests the higher suitability of the comet assay for detection of mercury genotoxicity.
The diet is expected to be a major source of Hg contamination in fish. However, considering that L. aurata feeding was almost completely limited due to caging, the current results emphasize the importance of uptake through the gills in mercury toxicity to fish. Furthermore, it was demonstrated that 3 days of exposure is enough for significant mercury uptake and expression of its genotoxic potential measured as DNA integrity loss in blood cells.
The Hg contamination gradient is more evident in the sediment, decreasing toward the sea, away from the contamination source. However, total Hg water content does not necessarily indicate total bioavailable Hg. The combination of different conditions favors both the Hg availability to biota and their vulnerability to Hg effects. Mercury bioaccumulation—and thus bioavailability—depend mainly on MeHg formation and uptake (Wiener et al. 2002). The uptake of MeHg by aquatic organisms is more rapid and extensive than that of inorganic mercury (Biesinger et al. 1982), and its formation, though also occurring in fish gills and gut (Boening 2000), depends mainly on soil-living, sulfur-reducing bacteria that are directly affected by environmental factors including redox potential, pH, salinity, and temperature (Wiener et al. 2002; Davis et al. 2003). In turn, other aquatic organisms such as fish are also affected (Boening 2000).
LAR2 had clearly higher levels of total Hg in the water and sediment in relation to LAR1 (Guilherme et al. 2008a). Nonetheless, exposure at LAR1 and LAR2 induced similar levels of genetic damage in L. aurata blood cells. Considering the previous statements, it can be hypothesized that the levels of biologically available Hg are similar at both sites, despite the reported differences in total Hg environmental levels, and thus induce comparable genetic damage.
The oxic-anoxic interface where MeHg formation occurs is very near the sediment surface (1 to 10 cm deep) (Davis et al. 2003). Changes in water and sediment movements (e.g., tides, freshwater influx, and erosion) may lead to the remobilization of contaminated sediment that has been deposited for decades, affecting adjacent areas. In addition, the sediment conditions are more suitable for Hg methylation (anoxia, low pH). Total mercury content at LAR2 was definitely higher in the bottom water (Guilherme et al. 2008a). Surprisingly, no significant differences related to water column position were found in the present study except for the REF site, where DNA integrity was higher at the bottom. This difference might just be a consequence of the high homogeneity of the REF samples, enhancing the power of the statistical tests, which was not observed at the contaminated sites. Even so, the levels of genetic damage in REF fish remain at the control level (LAB) in both the surface and the bottom groups.
AC frequencies remained very low in all groups, reinforcing the previous result from a wild L. aurata survey that, although mercury may damage DNA, apoptosis does not seem to be markedly involved. Moreover, it seems that the stress induced by handling and caging did not affect AC incidence, which is a positive aspect of this experimental approach.
General Discussion
L. aurata proved its sensitivity as a model species for monitoring metal genotoxicity at different degrees of contamination. A relatively high baseline of DNA lesions was found in wild L. aurata. On the other hand, transplanted fish had a 2-week recovery period prior to caging and exposure, allowing for the reduction of existing damage induced by chemicals taken up previously.
Blood is an easily accessible tissue with central physiologic functions, providing an important source of cells for genotoxicity studies (Singh 2000). In the present study, blood proved to be suitable for the purpose of monitoring Hg genotoxicity, as it significantly responded to Hg contamination.
Despite the restriction of exposure routes in caged fish (only via water) in comparison to wild fish (via water and food), a similar increase (two to three times) in DNA damage from REF to LAR sites was found in both studies in the present work. It seems that, independently of the time and type of exposure, Hg damage induction in L. aurata reached a plateau.
Metal detoxification processes such as cellular sequestration, influx-efflux balance, and a combination of the two (Kraemer et al. 2005), together with DNA repair systems, are responsible for the prevention of DNA damage, thereby determining fish adaptation to contaminated environments. Hence, although L. aurata is commonly found at LAR, the occurrence of increased DNA damage levels suggests that the combined action of the previous defense mechanisms was not entirely effective.
Both experiments performed in the present work revealed Hg persistence and contamination at LAR, clearly affecting the studied population in terms of genetic integrity. The relatively rapid response (in 3 days) emphasizes the acute toxicity of Hg, revealing that the levels found at LAR should undoubtedly be of great concern when considering the local species’ conservation and biodiversity.
The genotoxic potential of Hg already demonstrated by in vitro experiments with gill cell suspensions measured by the comet assay (Ben-Ozer et al. 2000; Arabi and Alaeddini 2005) was confirmed in both parts of the present study.
Mercury contamination issues in Aveiro lagoon were previously reported to be confined to LAR (Coelho et al. 2005). The REF site chosen within the lagoon (S. Jacinto) fits the clean area prerequisites, as fish caged at that site did not show significant differences from what was observed in the laboratory controls (LAB and t0). Furthermore, it was demonstrated that cage confinement did not constitute an extra stress factor for the considered biomarker, allowing the cause-effect linkage of mercury contamination and DNA damage.
However, it is recommended that appropriate cytotoxicity tests should be adopted as a complement of genotoxicity assays. The alkaline comet assay is not as sensitive or reliable in detecting cytotoxicity (Meintieres et al. 2003) and the neutral version has been indicated for that purpose (e.g., Tice et al. 2000). However, the results presented here should not be underestimated. Moreover, current overall results support the idea of Ben-Ozer et al. (Ben-Ozer et al. 2000) that DNA damage in surviving cells is a more sensitive indicator of environmental insult than apoptosis.
Conclusion
This study provides new information on mercury genotoxicity under realistic exposure conditions, evaluated by the comet assay, as higher DNA damage was found in both wild and caged fish. No increased susceptibility to apoptosis was detected, indicating that mercury damages DNA of blood cells by nonapoptotic mechanisms. Mercury uptake from the water per se (dissolved and associated with suspended particulate matter) was shown to be sufficient to increase genetic damage significantly. The comet assay applied to L. aurata blood cells was shown to be sensitive and suitable for genotoxicity biomonitoring in mercury-contaminated coastal systems. Finally, the results point out the environmental risk to native ichthyofauna at LAR due to mercury contamination.
References
Arabi M (2004) Analyses of impact of metal ion contamination on carp (Cyprinus carpio L.) gill cell suspensions. Biol Trace Elem Res 100(3):229–246. doi:10.1385/BTER:100:3:229
Arabi M, Alaeddini MA (2005) Metal-ion-mediated oxidative stress in the gill homogenate of rainbow trout (Oncorhynchus mykiss)—antioxidant potential of manganese, selenium, and albumin. Biol Trace Elem Res 108(1–3):155–168. doi:10.1385/BTER:108:1-3:155
Ayllon F, Garcia-Vazquez E (2000) Induction of micronuclei and other nuclear abnormalities in European minnow Phoxinus phoxinus and mollie Poecilia latipinna: an assessment of the fish micronucleus test. Mutat Res 467:177–186
Belpaeme K, Cooreman K, Kirsch-Volders M (1998) Development and validation of the in vivo alkaline comet assay for detecting genomic damage in marine flatfish. Mutat Res 415:167–184
Ben-Ozer EY, Rosenspire AJ, McCabe MJ Jr, Worth RG, Kindzelskii AL, Warra NS, Petty HR (2000) Mercuric chloride damages cellular DNA by a non-apoptotic mechanism. Mutat Res 470:19–27
Biesinger KE, Anderson LE, Eaton JG (1982) Chronic effects of inorganic and organic mercury on Daphnia magna: toxicity, accumulation, and loss. Arch Environ Contam Toxicol 11:769–774. doi:10.1007/BF01059166
Blasco J, Rubio JA, Forja J, Gomez-Parra A, Establier R (1998) Heavy metals in some fishes of the Mugilidae family from salt-ponds of Cadiz bay, SW Spain. Ecotoxicol Environ Restoration 1(2):71–77
Boening DW (2000) Ecological effects, transport, and fate of mercury: a general review. Chemosphere 40:1335–1351. doi:10.1016/S0045-6535(99)00283-0
Buschini A, Carboni P, Martino A, Poli P, Rossi C (2003) Effects of temperature on baseline and genotoxicant-induced DNA damage in haemocytes of Dreissena polymorpha. Mutat Res 537:81–92
Coelho JP, Pereira ME, Duarte A, Pardal MA (2005) Macroalgae response to a mercury contamination gradient in a temperate coastal lagoon (Ria de Aveiro, Portugal). Estuar Coast Shelf Sci 65:492–500. doi:10.1016/j.ecss.2005.06.020
Collins AR, Dobson VL, Duinska M, Kennedy G, Stetina R (1997) The comet assay: what can it really tell us? Mutat Res 375:183–193. doi:10.1016/S0027-5107(97)00013-4
Coughlan BM, Hartl MGJ, O’Reilly SJ, Sheehan D, Morthersill C, van Pelt F, O’Halloran J, O’Brien NM (2002) Detecting genotoxicity using the Comet assay following chronic exposure of Manila clam Tapes semidecussatus to polluted estuarine sediments. Mar Pollut Bull 44:1359–1365. doi:10.1016/S0025-326X(02)00254-0
Davis JA, Yee D, Collins JN, Schwarzbach SE, Luoma SN (2003) Potential for increased mercury accumulation in the estuary food web. In: Brown LR (ed) Issues in San Francisco estuary tidal wetlands restoration. San Francisco Estuary and Watershed Science 1(1):article 4
De Flora S, Bennicelli C, Bagnasco M (1994) Genotoxicity of mercury compounds. A review. Mutat Res 317:57–79
Duez P, Dehon G, Kumps A, Dubois J (2003) Statistics of the comet assay: a key to discriminate between genotoxic effects. Mutagenesis 2(18):159–166. doi:10.1093/mutage/18.2.159
Guilherme S, Valega M, Pereira ME, Santos MA, Pacheco M (2008a) Erythrocytic nuclear abnormalities in wild and caged fish (Liza aurata) along an environmental mercury contamination gradient. Ecotoxicol Environ Saf 70(3):411–421. doi:10.1016/j.ecoenv.2007.08.016
Guilherme S, Válega M, Pereira ME, Santos MA, Pacheco M (2008b) Antioxidant and biotransformation responses in Liza aurata under environmental mercury exposure—relationship with mercury accumulation and implications for public health. Mar Pollut Bull 56(5):845–859. doi:10.1016/j.marpolbul.2008.02.003
Helma C, Uhl M (2000) A public domain image-analysis program for the single-cell gel-electrophoresis (comet) assay. Mutat Res 466:9–15
Kim I-Y, Hyun C-K (2006) Comparative evaluation of the alkaline comet assay with the micronucleus test for genotoxicity monitoring using aquatic organisms. Ecotoxicol Environ Saf 64(3):288–297. doi:10.1016/j.ecoenv.2005.05.019
Klobucar GIV, Pavlica M, Erben R, Papes D (2003) Application of the micronucleus and comet assays to mussel Dreissena polymorpha haemocytes for genotoxicity monitoring of freshwater environments. Aquat Toxicol 64:15–23. doi:10.1016/S0166-445X(03)00009-2
Koppen G (1999) Single cell gel electrophoresis/comet assay for plants—a tool to assess DNA integrity. Mol. Ph.D. thesis. Vlaamse Instelling voor Technologisch Onderzoek (VITO), Belgium
Koppen G, Verschaeve L (1996) The alkaline comet test on plant cells: a new genotoxicity test for DNA strand breaks in Vicia faba root cells. Mutat Res 360:193–200
Kraemer LD, Campbell PGC, Hare L (2005) Dynamics of Cd, Cu and Zn accumulation in organs and sub-cellular fractions in field transplanted juvenile yellow perch (Perca flavescens). Environ Pollut 138:324–337. doi:10.1016/j.envpol.2005.03.006
Lee RF, Steinert S (2003) Use of the single cell gel electrophoresis/comet assay for detecting DNA damage in aquatic (marine and freshwater) animals (Review). Mutat Res 544:43–64. doi:10.1016/S1383-5742(03)00017-6
Meintieres S, Nesslany F, Pallardy M, Marzin D (2003) Detection of ghost cells in the standard alkaline comet assay is not a good measure of apoptosiss. Environ Mol Mutagen 41:260–269. doi:10.1002/em.10156
Mitchelmore CL, Chipman JK (1998) Detection of DNA strand breaks in brown trout (Salmo trutta) hepatocytes and blood cells using the single cell gel electrophoresis (comet) assay. Aquat Toxicol 41:161–182. doi:10.1016/S0166-445X(97)00064-7
Mitchelmore CL, Birmelim C, Livingstone DR, Chipman JK (1998) Detection of DNA strand breaks in isolated mussel (Mytilus edulis L.) digestive gland cells using the comet assay. Ecotoxicol Environ Saf 41:51–58. doi:10.1006/eesa.1998.1666
Nacci DE, Cayula S, Jackim E (1996) Detection of DNA damage in individual cells from marine organisms using the single cell gel assay. Aquat Toxicol 35(3–4):197–210. doi:10.1016/0166-445X(96)00016-1
Nepomuceno JC, Ferrari I, Spano MA, Centeno AJ (1997) Detection of micronuclei in peripheral erythrocytes of Cyprinus carpio exposed to metallic mercury. Environ Mol Mutagen 30:293–297. doi:10.1002/(SICI)1098-2280(1997)30:3<293::AID-EM7>3.0.CO;2-M
Pacheco M, Santos MA, Teles M, Oliveira M, Rebelo JE, Pombo L (2005) Biotransformation and genotoxic biomarkers in mullet species (Liza sp.) from a contaminated coastal lagoon (Ria de Aveiro, Portugal). Environ Monit Assess 107:133–153. doi:10.1007/s10661-005-5308-z
Pereira ME, Duarte AC, Millward GE, Abreu SN, Reis MC (1997) Distribution of mercury and other heavy metals in the Ria de Aveiro. Quimica Analitica 16(S1):S31–S35
Pereira ME, Duarte AC, Millward G, Vale C, Abreu SN (1998) Tidal export of particulate mercury from the most contaminated area of Aveiro’s Lagoon, Portugal. Sci Total Environ 213:157–163. doi:10.1016/S0048-9697(98)00087-4
Perry DM, Weis JS, Weis P (1988) Cytogenetic effects of methylmercury in embryos of the killifish, Fundulus heteroclitus. Arch Environ Contam Toxicol 17(5):569–574. doi:10.1007/BF01055824
Ramalhosa E, Monterroso P, Abreu S, Pereira E, Vale C, Duarte A (2001) Storage and export of mercury from a contaminated bay (Ria de Aveiro, Portugal). Wetlands Ecol Manage 9:311–316. doi:10.1023/A:1011864702531
Rank J, Jensen K (2003) Comet assay on gill cells and hemocytes from the blue mussel Mytilus edulis. Ecotoxicol Environ Saf 54:323–329. doi:10.1016/S0147-6513(02)00006-4
Rank J, Lehtonen KK, Strand J, Laursen M (2007) DNA damage, acetylcholinesterase activity and lysosomal stability in native and transplanted mussels (Mytilus edulis) in areas close to coastal chemical dumping sites in Denmark. Aquat Toxicol 84(1):50–61. doi:10.1016/j.aquatox.2007.05.013
Salagovic J, Maes A, Van Gorp U, Verschaeve L, Kalina I (1997) The cell cycle positions influence DNA migration as measured with the alkaline comet assay in stimulated human lymphocytes. Folia Biol 43(2):79–82
Singh NP (2000) Microgels for estimation of DNA strand breaks, DNA protein crosslinks and apoptosis. Mutat Res 455:111–127. doi:10.1016/S0027-5107(00)00075-0
Singh NP, McCoy T, Tice RR, Schneider EL (1988) A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res 175:184–192. doi:10.1016/0014-4827(88)90265-0
Speit G, Hartmann A (2004) The comet assay—a sensitive test for the detection of DNA damage repair. Methods Mol Biol 291:85–96
Steinert SA, Streib-Montee R, Leather JM, Chadwick DB (1998) DNA damage in mussels at sites in San Diego Bay. Mutat Res 399(1):65–85. doi:10.1016/S0027-5107(97)00267-4
Sumathi M, Kalaiselvi K, Palanivel M, Rajaguru P (2001) Genotoxicity of textile dye effluent on fish (Cyprinus carpio) measured using the comet assay. Bull Environ Contam Toxicol 66(3):407–414. doi:10.1007/s00128-001-0020-3
Tice RR, Agurel E, Anderson D, Burlinson B, Hartman A, Kobayashi H, Rojas E, Ryu J-C, Sasaki YF (2000) Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ Mol Mutagen 35:206–221. doi:10.1002/(SICI)1098-2280(2000)35:3<206::AID-EM8>3.0.CO;2-J
Venier P, Maron S, Canova S (1997) Detection of micronuclei in gill cells and haemocytes of mussels exposed to benzo[a]pyrene. Mutat Res 390:33–44
Weis P, Weis JS (1977) Methylmercury teratogenesis in the killifish, Fundulus heteroclitus. Teratology 16(3):317–325. doi:10.1002/tera.1420160311
Wiener JG, Krabbenhoft DP, Heinz GH, Scheuhammer AM (2002) Ecotoxicology of mercury. In: Hoffman DJ, Rattner BA, Burton GA Jr, Cairns J Jr (eds) Handbook of ecotoxicology, 2nd edn. CRC Press, Boca Raton, pp 409–463
Acknowledgment
The authors are very thankful for the technical assistance of Mr. Aldiro Pereira.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pereira, C.S.A., Guilherme, S.I.A.G., Barroso, C.M.M. et al. Evaluation of DNA Damage Induced by Environmental Exposure to Mercury in Liza aurata Using the Comet Assay. Arch Environ Contam Toxicol 58, 112–122 (2010). https://doi.org/10.1007/s00244-009-9330-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-009-9330-y