Abstract
Mercury (Hg) contamination in coastal sediments has been widely studied in clay deposits; however, equivalent results on carbonated sediments are scarce. This article aims to study Hg distribution in Lebanese carbonate coastal marine sediments (Eastern Mediterranean) in order to characterize their contamination level and to explore the postdepositional mobility of methylmercury (MeHg) in the deposits. Vertical distribution profiles of total (HgT) and MeHg have been established for the solid phase of sediment cores collected in various near-shore environments chosen for their hypothetical various degrees of anthropization. In addition, dissolved MeHg was determined in sediment pore waters to test its mobility and potential availability for biota. Three sites on the Lebanese coasts—Akkar, Dora, and Selaata—were selected. Akkar is far from any direct contamination source, whereas Dora, located near the Beirut harbor, is a heavily urbanized and industrialized zone including a huge dump site, and Selaata is near a chemical plant that produces phosphate fertilizers. Particulate HgT concentrations in the sediments varied between <0.04 and 0.65 μg/g, with a proportion of MeHg lower than 1%. Based on a sediment quality guideline (MacDonald et al. 2000), we concluded that Dora bay sediments are heavily contaminated by Hg, with concentrations exceeding the “consensus-based threshold effect” level (0.17 μg/g) and almost reaching the “effects range-medium” level (0.71 μg/g). In spite of the low HgT concentration in Akkar and Selaata sediment (similar to natural carbonated sediment: 0.04 μg/g according to Turekian and Wedephol (1961), a closer analysis of the sediment core vertical profile allows one to observe an anthropogenic impact. This impact might be toxicologically insignificant; however, it allows tracing the time increase of Hg diffuse deposition. On the other hand, dissolved MeHg concentrations ranged from 0.04 to 0.09 and from 0.04 to 8.76 ng/l in the Selaata and the Dora sediments, respectively; MeHg vertical profiles in interstitial water enabled us to calculate diffusive fluxes of MeHg from the sediment varying from 0.3 to 1.0 ng/m2/day. Thus, the deposited sediments constitute a measurable source of bioavailable Hg for epibenthic organisms.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Mercury (Hg) is a highly toxic metal, which, because of its volatility, can be rapidly spread worldwide from its natural and anthropogenic sources. These properties, combined with its huge biomagnification in the food chain, make it the metal of the most environmental concern (e.g., Fitzgerald et al. 2007; Morel et al. 1998). The anthropogenic Hg sources are both diffuse and local. The diffuse sources are mainly the burning of fossil fuel, especially coal, the incineration of municipal solid waste, and the cement production; the local sources consist mainly of effluents from industrial activities, especially in developing countries (Pirrone et al. 2001; Sanchez Uria and Sanz-Mendel 1998).
Around the Mediterranean basin the anthropogenic Hg emissions have been estimated to be 106 × 103 kg/year—that is, one-third of the total European emissions (Pirrone et al. 2001). Other studies from the same research group concluded that the Mediterranean Sea region is not only affected by Hg released in its vicinity but also from air masses enriched in Hg from regions of northern and northeastern Europe (Pirrone et al. 2003). In addition, from a public health point of view, special interest concerning Hg has been focused on the Mediterranean area for decades when high concentrations of total mercury (HgT), mainly as methylmercury (MeHg), were found in Mediterranean fish (e.g., Baldi et al. 1983; Bernhard and Renzoni 1977). The question of the origin of the MeHg thus arose because the Hg deposition is thought to be mainly inorganic species. Albeit the recent evidence for Hg methylation in the Mediterranean water column (Cossa et al. 2009), the importance of off-shore sediments as a source of MeHg has even been estimated (Ogrinc et al. 2007) and could be significant at least for benthic communities. For coastal sediments, although the HgT distribution and MeHg mobility studies in the western part of the Mediterranean are now more than a few (e.g., Covelli et al. 1999; Muresan et al. 2007), equivalent results on the eastern coasts are still rare. Moreover, eastern Mediterranean sediments are particular because they are characterized by high carbonate content (e.g., Morse 2003), a chemical matrix poorly explored in term of Hg distribution and transformations. For the Lebanese coastal area, Nassif (2004) measured Hg concentrations up to 0.46 μg/g (dry weight) in marine sediments collected north of Beirut, a level that exceeds sediment quality guidelines, such as the one developed for the US Status and Trends Program (1999) and the French Geode Program (2000), which have been fixed at 0.17 and 0.4 μg/g (dry weight), respectively. In addition, for such contaminated sediments, the key process determining the risk is the net transformation of inorganic Hg to the highly toxic and bioavailable MeHg (Skyllberg et al. 2007).
We present the vertical distributions of HgT and MeHg in sediment cores collected at three sites on the Lebanese coast (eastern Mediterranean) in both solid phase and interstitial waters in order to explore the burial, transformations, and postdepositional mobility of this element. This should provide information about the anthropogenic impregnation of these coastal sediments and their role in providing MeHg species for the surrounding seawaters. This Hg speciation study is completed by ancillary data to characterize the sedimentary context (mineralogy and major elements composition).
Material and Methods
Study Area
The Lebanese coastal zone, which constitutes one part of the carbonate platform that covers most of the eastern Mediterranean basin, is the geological result of the carbonated marine sedimentation between the Jurassic and the Quaternary periods (Abdel-Rahman and Nader 2002). Geological surveys indicate the presence of thick layers of dolomite, marls, or chalk. These carbonate rocks are the only ones that reach today’s littoral. Volcanic rocks also exist in two different layers in the stratigraphic sequence. The volcanic complexes (basalts and ashes) of the Jurassic cover a low surface and are exposed to the level of the deep valleys of Mount Lebanon. The volcanic rocks (mainly basaltic) from the Quaternary Miocene age cover a large surface of the Akkar plate (Sanlaville 1977).
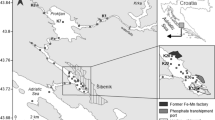
The prospected zone is the coastal area located between Tripoli and the Syrian boundary in the north and Beirut in the south (Fig. 1). This zone has been selected for its relatively high urban and industrial activities; it contains more than 30% of Lebanese industries and more than 40% of the population of Lebanon (Nakhlé 2003). For our study, three sites representative of the Lebanese coast were selected because they include urban, industrial, and apparently noncontaminated zones. Three bays were studied: from north to south: Akkar, Selaata, and Dora. Akkar was chosen because the site is located opposite the seemingly noncontaminated bay of Akkar, far from any great industrial activity or urbanization. The site of Selaata, north of the city of Batroun, is near a chemical plant (established in 1957), which produces phosphoric acid, triple superphosphate, aluminum sulfate, and sulfuric acid (Al-Hajj and Muscat 2000) and discharges phosphogypsum effluents into the sea through a pipe. Dora is a heavily urbanized and industrialized zone of the St Georges bay, adjacent to the Beirut harbor, characterized by a dump site peninsula that received 120 × 106 kg of industrial and municipal waste per year until 1998, the year of its closure (DR/ECODIT-IAURIF 1997). In addition, the St Georges bay receives the inputs from the drainage area of the Beirut River (0.1–10 m3/s), where various industries are located, including tanneries (Nakhlé 2003).
Sampling Methods
Sediment cores for the solid phase collection were taken from the three sites Akkar (A and A′), Dora (D and D′), and Selaata (S1, S3, S4, and S6) by divers during two periods. The number of cores collected was higher at Selaata than in the two other locations because we wanted to trace the possible contamination gradient from the mouth of the effluent pipe seaward. In February 2006, A′, D′, and S1 sediment cores were collected in order to analyze HgT and particulate MeHg in the solid fraction of the sediment. In October and November 2006, sediment cores were collected at A, D, S3, S4, an S6 for HgT analyses and to determine additional mineralogical, datation, major elements, and organic carbon (Table 1). For this purpose, acid-prewashed methacrylate tubes (40 cm long and with an inner diameter of 7 cm) were vertically introduced into the sediment, plugged on both sides with plastic caps, and brought back to the boat, where they were maintained in a vertical position in a refrigerated box until returning to the laboratory. In the second sediment sampling campaign, in addition to Akkar (A) and Dora (D) sediment cores, three cores were sampled at Selaata sediments (S3, S6, and S4), the first one facing the chemical power plant and in direct contact with its effluent, an the second located in a bay to the north of the Selaata plant, relatively protected from marine currents, where higher amounts of fine sediment were expected to be present. A supplementary S4 core, located between S3 and S6, was also collected for mineralogical analysis. In the laboratory, the sediment cores were sliced at 1-cm intervals for the first 5 cm and at 3-cm intervals to the bottom of the core. Subsamples were put into polyethylene bags and frozen at −18°C.
Sediment pore waters were collected in October 2007 at Dora and Selaata using multichambered in situ dialysis samplers (peepers) proposed by Hesslein (1976). This method is based on the equilibration of a contained quantity of deionized water with the surrounding interstitial water of the sediment through a dialysis membrane. The sampler (peeper) is composed of a methacrylate body with 40 wells drilled into it every 2 cm. These wells were covered by a polycarbonate membrane (0.45 μm) and filled with deionized water, Milli-Q® (Millipore). Two peepers were vertically introduced by divers into the sediment at the three sites, keeping six to eight wells above the sediment water interface to examine differences between overlying and pore-water MeHg concentrations. These peepers were maintained in the sediment for 2 weeks. However, due to sea storm, we were only able to recover two peepers at Dora (D and D′) and one from the sediment of Selaata (S6). Upon removal, interstitial waters in different compartments were extracted, using syringes, and stored in Teflon (FEP) tubes, as previously described. All of the plasticware used for collecting pore waters was acid-washed and rinsed with Milli-Q® before use.
Analyses
Prior to the analyses, the sliced frozen sediments were air-dried in an oven at 50°C until reaching constant weight, with frequent manual agitation in order to avoid their cementation. Then the sediment slices were dry-sieved (<63 μm) using an acid-precleaned nylon sieve, the fraction (<63 μm), stored in polyethylene vials, being used for further analysis. Major elements of sediment were measured to assess their relationship with HgT and MeHg distribution. The concentrations of Ca, Si, and Mg were determined using an inductively coupled plasma–mass spectrometer, and the concentrations of Fe and Al were determined by atomic absorption spectrophotometry. The detailed analytical procedures are given in the companion article onthe Pb contamination of the same sediments (Abi-Ghanem et al. 2009).
HgT and MeHg Analysis in the Solid Fraction of the Sediment
Total Hg analyses were carried out on a semiautomatic system—AMA-254® produced by ALTEC (Czech Republic). Aliquots of sediments were weighed and put into an Ni capsule and were automatically introduced into the system, where they were exposed to high temperature (550°C) so that the Hg is volatilized and carried by an oxygen current. Then elemental Hg is concentrated by amalgamation on a golden trap before being thermally dissociated and analyzed by atomic absorption spectrometry.
The method detection limit was 0.007 μg/g, calculated as 3.29 times the standard deviation of the blank. The reproducibility was 1.8% for material whose HgT concentration was 0.092 μg/g. Certified reference material MESS-2 was also used to assure the quality control of the method, and the values obtained (0.092 ± 0.002 μg/g) were always within the range of the certified values: 0.092 ± 0.009 μg/g. Statistical calculations were performed on the basis of six replicate analyses.
Particulate MeHg analyses were conducted on the sediment fractions finer than 63 μm from Akkar, Dora, and Selaata after propylation and isotopic dilution and detection by gas chromatography–inductively coupled plasma–mass spectrometry (GC-ICPMS). This method was slightly modified from Monperrus et al. (2005). A known quantity of Me202Hg was added to the sediments, MeHg was extracted using HNO3 (6 M; Merck, Suprapur®), and propylated by the use of 100 μl of 4% sodium tetrapropylborate (Galab). The propylated MeHg extracted into iso-octane (0.3 ml iso-octane/300 mg sediments) and the detection was carried out by GC-ICPMS (GC-Focuswith X-series; Thermo Electron). The ratio of the surface areas of peaks corresponding to Me200Hg and Me202Hg makes it possible to calculate the original concentration of MeHg in the sediments. The detection limit for the MeHg method was 0.02 ng/g and the precision was 20% for concentrations higher than 1 ng/g and 30% for the lower values. The accuracy of the MeHg analysis was established using Certified Reference Material IAEA-405. The average concentration for IAEA-405 was 5.49 ± 0.4 ng/g compared to the certified value of 5.49 ± 0.3 ng/g.
Dissolved MeHg Analysis
Dissolved methylmercury (MeHgD) contained in interstitial water collected from Dora and Selaata sediments was analyzed with a technique combining hydruration and cryogenic chromatography. The protocol applied was suggested by Tseng et al. (1998) and modified by Cossa et al. (2003). It is based on the use of NaBH4 to transform MeHg into volatile MeHgH; however, Hg2+ is also transformed into volatile HgH2 and Hg0 within this reaction. These volatile compounds are then concentrated by a cryogenic procedure (−196°C) on a column filled with Chromosorb W/AW-DMCS (60/80 mesh impregnated with 15% OV-3) in a nitrogen bath before being progressively released by heating the column from −196°C to 90°C. These compounds are thermally decomposed into Hg0 within a furnace (800°C) and then transported in a vapor state by a current of helium (35 ml/min) to an atomic fluorescence spectrophotometer (AFS). The blank of the method was checked regularly and was often close to the picogram level for MeHg. The absence of certified reference material for marine interstitial water samples did not enable us to verify the accuracy of the method. The reproductibility of the method, as described earlier, varies from 15% for samples with a concentration near the limit of detection, to 6% for samples with a 0.1-ng/l MeHg concentration or more (Cossa et al. 2003). The field of linearity ranged from the limit of detection (0.02 ng/l for a 10 ml sample) to 20 ng/l.
Result and Discussion
Sediment Characteristics
Mineralogical analyses and sediment dating have already been published in a companion article (Abi-Ghanem et al. 2009). Briefly, the results showed a high contribution of dolomite, calcite, and quartz at the three sites. Ankerite and fluorite were more specific in Akkar and Selaata sediments, respectively. The 210Pbex and 137Cs measurements suggested that Akkar (A) and Dora (D) are mainly composed of detritic sediments from the coastal erosion (or anthropogenic quarry material) with very little influence of biogenic marine origin and recent continental alluvial deposits. At Selaata, 210Pbex measurements do not exhibit any linear decrease with depth. This suggests that the origin of the 210Pbex is to be found in the waste from the Selaata phosphoric acid plant (Abi-Ghanem et al. 2009).
The major element composition of the sediment is summarized by a ternary plot (Fig. 2) in which three different sample groups are to be distinguished, corresponding to the sediments of Akkar (A), Dora (D), and Selaata (S6 and S3). Akkar sediments are the most highly enriched in CaCO3 and MgCO3 (70–80%), whereas the Dora sediments show the highest content in SiO2 (45–60%). The Selaata sediments show levels between those of the Akkar and Dora sediments. It is important to note that the contribution of Fe and Al is similar for the three groups of sediment, with mass percentages, expressed as Fe2O3 + Al2O3, ranging from 5 to 15%. It could be concluded that the chemical composition of the sediments is consistent with the mineralogical results. Vertical profiles of Ca, Mg, Si, Fe, and Al in Akkar (A), Dora (D), and Selaata (S3 and S6) sediments are discussed in detail in another article (Abi-Ghanem et al. 2009).
HgT Distributions
Total mercury (HgT) concentration profiles are presented in Fig. 3 for Akkar (Fig. 3a), Dora (Fig. 3b), and Selaata (Fig. 3c). Because natural variations of Hg concentrations in sediments can result from differences in the grain size, the mineralogy, and the redox of the sediment, we tried to limit these sources of variability by applying the grain-size normalization approach, completed by a geochemical correction; this treatment should allow better insight into the anthropogenic influence on the Hg distribution in the sediment cores. Thus, HgT concentrations were determined in the sediment fraction (<63 μm) according to Ackermann et al. (1983) and were geochemically normalized by the use of a tracer element (Kersten and Smedes 2002) for HgT concentrations at A, D, S3, and S6 sediment cores. In addition, Al was chosen as a normalizer element to take into account the clay mineral indicator capacity of the sediment.
Akkar (see Fig. 3a)
The HgT concentrations varied from 0.012 to 0.035 μg/g in core A and from 0.009 to 0.027 μg/g in core A′. These concentrations are within the range of the Earth’s crust values and comparable to Hg concentrations in sandstone and carbonate rocks (0.03 and 0.04 μg/g, respectively, according to Turekian and Wedephol (1961) and in some uncontaminated sediment from the eastern Mediterranean coasts (Table 2). They are also comparable to values detected in deep-sea Mediterranean sediment (Cossa and Coquery 2005). Compared to the “background” level of coastal Sicilian sediments of 0.038 μg/g calculated by Di Leonardo et al. (2006) and that are chemically and mineralogically close to the Lebanese sediments, the deepest levels of Akkar sediments have a much lower Hg concentration and, thus, it would be reasonable to consider the very low Hg concentration observed at the deepest level (28 cm), as the Hg background of this coastal zone. However, whereas HgT concentration levels in Akkar sediments suggest that the Hg anthropogenic inputs are quite insignificant, the vertical profile of HgT concentrations clearly shows a regular concentration increase from the bottom of the core up to 10 cm below the surface (see Fig. 3a), the origin of which should be explained. To question the importance of anthropogenic influence. we adopted the approach described by Covelli et al. (2001), who used the comparison of Hg and other trace metals enrichment factors (EFs). The EF of an element is the ratio between the potential enriched concentration of the element and the baseline, which is the background value of the element, these later concentrations being normalized to a conservative element. The above metal concentrations are normalized to Al and are subdivided to the baseline, which is the concentration of the element that corresponds to the deepest layer and which is also normalized to Al. This method, applied by Covelli et al. (2001), helps to obtain a nondimensional EF:
where M is the concentration of the potentially enriched element and N is the concentration of the normalizing element. Figure 4 shows the Hg EF distributions, varying up to 4, obtained from the Akkar (A) sediment core. Even if this enrichment is small compared to a heavily impacted area such as the Trieste Gulf [24-fold according the Covelli et al. (2001) study], we address the question as to the anthropogenic influence on the 4-fold variation encountered. Hg EF presented vertical profiles similar to those obtained for Pb (see Fig. 4), which suggests similar sources for the two metals. Pb sources in these sediments were determined by stable isotopic analyses presented in another article (Abi-Ghanem et al. 2009). We showed that, based on the 206Pb/207Pb values, the Pb at the 28-cm level naturally originates from Miocene silicate rocks, whereas in the upper sediment levels, the contribution of industrial and gasoline Pb was detected. Thus, we can infer from these results that Hg and lead EF variations respond to similar histories of human impact. Indeed, Pirrone et al. (2001) estimated that 1.5 × 103 kg of anthropogenic Hg per year are emitted in the atmosphere of Lebanon, coming from fossil fuel combustion, the incineration of municipal solid waste, cement production, and other miscellaneous minor sources. Anthropogenic Hg emitted into the atmosphere can be transferred to terrestrial and water receptors by dry deposition, by wet scavenging followed by precipitation, and by gaseous Hg exchange at the air–water interface. It might be concluded that even if the HgT concentrations in the solid phase of the sediment from Akkar are quite low compared to many coastal environments, the current influence of diffuse anthropogenic Hg via the atmosphere can be clearly detected. The current anthropic contribution appears to be of at least a similar magnitude as that of the natural contribution. Unfortunately, the 210Pb and 137Cs data obtained from this core do not permit the determination of the period when the anthropization began.
Dora (see Fig. 3b)
High HgT concentrations typical of a contaminated environment (see Table 2) were detected at Dora D and D′ sediment cores where Hg concentrations fluctuate from 0.37 to 0.65 and from 0.1 to 0.5 μg/g, respectively (see Fig. 3b). These high Hg concentrations are, especially in Dora’s marine water column, contaminated with Hg (7–8 ng/l) (Nakhlé 2003). Hg can reach Dora Bay in several ways: (1) atmospheric inputs, (2) the huge dump site nearby, which is in direct contact with marine water, and (3) through Beirut and the Antélias rivers, where several sources of untreated urban waste waters are discharged. Data concerning dissolved and particulate Hg in the Antélias River have shown concentrations reaching 3.1 ng/l and 2.1 ng/g, respectively (Nakhlé 2003). These values are higher than values corresponding to other Lebanese rivers and are comparable to values detected in some other polluted rivers in the world. In spite of the differences in the concentrations, both Dora HgT profiles, D and D′, present three major peaks, at ~ 4, 10, and 23 cm depth (see Fig. 3b). In addition, the persistence of elevated Hg concentrations even at the deepest layers of the sediment cores indicates that we did not reach the background levels in Dora sediments. Normalizing Hg to Al in the Dora D sediment core allows the smoothing out of the Hg fluctuations, but at least two peaks persist, whereas the mineralogy does not change significantly with depth (Abi-Ghanem et al. 2009). These changes are most likely related to the varying sources of wastes discharged nearby during the activity of the Dora dumpsite (1980–1998). Clearly, the Dora bay is heavily contaminated by Hg. Sedimentary Hg mobility and potential bioavailability will be further clarified thanks to MeHg analysis in the solid phase and in the interstitial water of the sediments (discussed later).
Selaata (see Fig. 3c)
At Selaata, HgT concentrations varied from 0.02 to 0.06 μg/g. These concentrations are comparable to values obtained by Nassif (2004) in Selaata sediments and are, in most of the sediment layers, superior to values detected in other Lebanese sediments (Akkar-Batroun) (see Table 2). Thus, in spite of the fact that these concentration levels are of lower orders of magnitude than the Hg concentration in contaminated areas (see Table 2), we cannot dismiss the fact that Hg enrichment occurs in some sediment layers, all the more so because Hg-enriched phosphogypsum deposits are present (Jackson et al. 1986; METAP/Tebodin 1998). Figure 3c illustrates the HgT variation in the two Selaata cores (S3 and S6). At S3, facing the Selaata chemical plant effluent and in direct contact with the phosphogypsum discharged, the Hg profile is characterized by a two-layer distribution, the rupture zone being located between 6 and 8 cm and the superficial 6-cm layers of the S3 core being the most highly enriched in Hg (0.059 μg/g). Normalizing Hg to Al in the S3 core (Fig. 3c) does not eliminate the two-layer structure. This two-layer structure has also been observed by Fakhri (2005), who studied the organic matter in a station near the Selaata sediments. It was attributed to changes in the nature of the waste discharged by the phosphorgypsum plant probably linked to different phosphate ore sources. This hypothesis, which is also the reason for the Hg vertical profile obtained in S3, cannot be verified historically because of difficulties in obtaining information on any modifications in the process applied in the Selaata chemical power plant. However, based on our mineralogical analysis, the Selaata sediment core, even at the deepest layers, is rich in fluorite (25–55%), which is a compound naturally present in the phosphate rock, Ca3(PO4)2, processed in the Selaata plant (Al-Hajj and Muscat 2000) (see Fig. 3c). This finding testifies to the dominance of industrial deposits in the sediments studied. In addition, the vertical profile of excess 210Pb indicates that the sediments are recent. Thus, the vertical Hg profile, or any other major and trace element profile, will be largely influenced by the composition of the Ca3(PO4)2 used as raw material in the Selaata chemical plant. In the S6 sediment core, the two-layer profile was not observed. The HgT concentrations exhibit several smaller fluctuations within the first 8 cm and then they increase slowly toward the deepest (8–16 cm) layers (Fig. 3c). This Hg profile, which persists after normalizing Hg to Al, can be explained by the position of S6 in a bay north of the Selaata plant and thus relatively more protected than S3 from the direct discharges of phosphogypsum and associated impurities. This position of S6 allows HgT in the sediments to attain a more constant profile.
In summary, the HgT concentrations found in the sediment cores of the three bays on the Lebanese coast studied, which ranged from 0.01 to 0.65 μg/g, testified to various degrees of anthropogenic contamination. At Akkar, the sediment recently deposited gave evidence of a limited diffuse anthropogenic contribution; at Dora Bay, the sediments are heavily polluted; and at Selaata, the Hg contamination is directly related to the industrial waste flowing out into the bay. For comparison with our results, the few Hg studies of eastern Mediterranean coastal sediments showed some degree of Hg contamination (0.05–0.70 μg/g) in the inner part of Haifa Bay, whereas concentrations in the outer coastal sediments range between values lower than 0.001 μg/g and up to 0.054 μg/g (Herut et al. 1993; Krumgalz and Fainshtein 1991). North of this area, along the Lebanese coast, Nassif (2004) measured the Hg concentration in surface sediments ranging from 0.01 to 0.46 μg/g, with the highest concentrations obtained at Antélias, the highly urbanized and industrialized zone located near Beirut, and in the Selaata Port sediments (see Table 2).
Methylmercury
Distributions
Particulate MeHg concentrations were measured in the solid phase of Akkar (A′), Dora (D′), and Selaata (S1) sediments. Concentrations obtained for Akkar and Dora sediments are presented in Figure 5; MeHg concentrations in Selaata sediments were below the detection limit (0.02 ng/g). These aforementioned low particulate MeHg concentrations in Selaata sediments can be due to the fact that the studied deposits result mostly from the wastes discharged by the nearby phosphogypsum industry. MeHg concentrations in the solid phase of the Akkar A′ sediment core varied from 0.03 to 0.22 ng/g, with high MeHg concentrations obtained at the 2.5–10.5-cm sediment layers. However, higher MeHg concentrations were obtained in Dora sediment (0.07–0.5 ng/g), for which the MeHg depth profile is characterized by a downcore decrease (Fig. 5).
The MeHg concentrations are very low in comparison with some coastal polluted sediment in the Mediterranean Sea, such as the Gulf of Trieste, where particulate MeHg concentrations vary from 0.2 to 60.1 ng/g (Covelli et al. 2001). However, they are in the range of concentrations in the coastal Mediterranean sediments of the same region (0.09–0.61 ng/g; Shoham-Frider et al. 2007), or in western Mediterranean sediments of the Thau lagoon (0.08–2.75 ng/g; Muresan et al. 2007), or in other unimpacted coastal areas in the world: 0.05–1.5 ng/g (Bay of Fundy; Sunderland et al. 2006). Significant (p < 0.01) positive correlation was found between MeHg and HgT in the solid phase of Akkar sediment ([MeHg] (ng/g) = 0.005 [HgT] (ng/g)–0.008; R 2 = 0.5; n = 24). This latter significant positive correlation are often detected at low levels of HgT (Marvin-Dipasquale et al. 2009), which is not the case of Dora sediment, for which no significant positive correlation was found between particulate MeHg and HgT. This suggests that in polluted environment, such as Dora, inorganic Hg is not a limiting factor for Hg methylation.
The MeHg concentrations in the solid phase of Akkar and Dora sediments correspond to 0.2–1% of HgT in the Akkar sediments and to 0.03–0.35% of HgT in the Dora sediments. These percentages, which can be used as a proxy for the rate of mercury methylation (Drott et al. 2008; Hammerschmidt and Fitzgerald 2004, 2006), suggest that MeHg production in the Dora sediments could be relatively low, although higher variable methylation rates were obtained in the Akkar sediment core, especially in the upper 18-cm levels of the sediment core. The relatively low contribution of MeHg to HgT in the solid phase of the Dora sediments could be due to Dora’s higher HgT concentrations and might also indicate a limited methylation rate in the presence of high sulfide concentrations (Benoit et al. 1999; Castelle et al. 2007).
The MeHgD concentrations were determined in the interstitial waters of the Dora and Selaata sediments and in the overlying waters, in order to explore the possible evasion of this Hg species from these contaminated sediments. In the interstitial waters, the MeHgD concentrations ranged from 0.04 to 0.09 and from 0.04 to 8.76 ng/l in the Selaata and the Dora sediments, respectively; in the overlying water, they were below the limit of detection (0.020 ng/l). The levels are similar to those expected in coastal sediments (Muresan et al. 2007). In the interstitial waters the variation of MeHgD exhibited subintersurface maxima peaking at 8.76 ng/l in core D at the Dora site (Fig. 6), which suggest that Hg in situ methylation at those depths is due to the activity of sulfate reducing bacteria in the suboxic zone of the sediment (e.g., Compeau and Bartha 1985; Langer et al. 2001; Muresan et al. 2007).
Fluxes
The possible diffusion flux of MeHgD from the Dora and Selaata sediments to the overlying water column is estimated by applying Fick’s First Law. The concentration gradient between MeHgD concentrations in the upper 2-cm sediment layer of Dora (D) and Selaata (S6) and the overlying water is used to estimate MeHgD diffusive flux at the sediment–water interface. In fact, if we consider that the transfer of MeHgD from sediments to the water column is proportional to the concentration gradient, we can apply Fick’s First Law (Eq. 1) described by Berner (1980) to calculate MeHgD flux (Choe et al. 2004; Hammerschmidt and Fitzgerald 2006; Muresan et al. 2007; Ogrinc et al. 2007):
where Ø is the porosity of the sediment (volume of pore water/volume of bulk sediment), C is the concentration of MeHgD in pore-water sediment, C W is the concentration of the overlying water (ng/l), h is the depth of the sediment (cm), and D S is the whole sediment molecular diffusion coefficient (cm2s).
The benthic flux estimation will allow us to obtain a first-order approximation of the transport of MeHg from Dora and Selaata sediments to the overlying water column, especially because studies conducted by Choe et al. (2004) have shown that sediments with a high estimated diffusive flux also have a high directly measured diffusive flux and vice versa. Because there are no data available concerning the D S in our sediments, we used values calculated for comparable Mediterranean sediments at the Thau Lagoon (France) (Muresan et al. 2007) with the assumption that MMHgD was as methylmercury chloride (D S = 1.84 × 10−5 cm2 s). Similarly, the sediment porosity was estimated to be 0.853. The concentration of MeHgD obtained from the 2-cm sediment layers is used for the pore-water value and is applied to the 2-cm depth for the flux calculation. The concentrations of MeHg in the overlying water C W is fixed at 0.02 ng/l because measured values were below the detection limit of <0.04 ng/l.
Results of calculated diffusive fluxes for Dora and Selaata sediments are presented in Table 3. Although the Dora diffusive flux is almost three times higher than the Selaata diffusive flux, Dora and Selaata diffusive fluxes (Table 3) are comparable to MeHg diffusive flux in other coastal Mediterranean sediments, such as the Thau Lagoon (0.8 ± 0.4 ng/m2/day; Muresan et al. 2007), and are comparable to the lowest values obtained at deep Mediterranean sediment layers (0.6–6.4 ng/m2/day; Ogrinc et al. 2007) and at the Lavaca Bay (0.4–1400 ng/m2/day; Gill et al. 1999), and they are 10–102 less than those measured or calculated in other near-shore polluted sediments such as the Gulf of Trieste in the northern Adriatic Sea, where MeHg diffusive flux reaches values of 40 ng/m2/day (Covelli et al. 1999). In spite of the low estimated molecular diffusive flux of MeHg measured here, the deposited sediments constitute a measurable source of bioavailable Hg for epibenthic organisms. Indeed, according to Benoit et al. (2009), the estimated diffusive flux constitutes only a small fraction (<10%) of the real diffusive flux, and benthic organisms can assimilate MeHg directly from deep anoxic sediment layers and thus transfer it to fish through the aquatic food web and to human consumers.
Summary and Conclusions
The sediment cores from the three bays along the studied Lebanese coast (Akkar, Dora, and Selaata) are mostly composed of carbonate materials and quartz, with the specific presence of fluorite at the Selaata Point. Chemical composition, dating, and mineralogy demonstrate that the sediment in the Akkar bay, located away from any direct source of contamination, comes mostly from the erosion of local rocks, whereas in the Dora bay, located near an enormous dumping site, is subject to industrial and urban contamination, and at the Selaata Point, located near a chemical plant, they are typically characteristic of phosphogypsum industrial wastes. Analyses of HgT in the solid phase of the sediment allow us to point out an important Hg contamination at Dora, near the huge dump site, which functioned from 1980 to 1998. The highest measured level (0.65 μg/g) encompasses the sediment quality guidelines developed for the National Status and Trends Program in the United States by the National Oceanographic and Atmospheric Administration (NOAA 1999) based on works by MacDonald et al. (2000). Concentrations most often exceed the “consensus-based threshold effect” level (0.17 μg/g) and the highest almost reach the “effects range-medium” level (0.71 μg/g). In spite of the low HgT concentrations in Akkar and Selaata sediment [similar to natural carbonated sediment: 0.04 μg/g according to Turekian and Wedephol (1961)], a closer analysis of sediment core vertical profiles allow one to observe an anthropogenic impact. This impact might be toxicologically insignificant but allows one to trace the time increase of Hg diffuse deposition. At Selaata, the Hg contamination is directly related to the phosphogypsum waste flowing out into the bay. However, the HgT encountered was low compared to what should be expected from the exploitation of phosphate rocks in other parts of the world. At Akkar, even if the HgT in the sediments ranged within the Earth’s crust values, a close examination of the results, by using a comparison of the Hg profile with the Pb stable isotopic profiles obtained for the same core (Abi-Ghanem et al. 2009), allows one to suggest an anthropogenic contribution.
The MeHg measurements in those sediments indicate relatively low levels (0.03–0.5 μg/g) and show that this species represents less than 1% of the HgT present in the solid phase. Dissolved MeHg concentrations, estimated as a “risk assessment” complementary method (Syllberg et al. 2007), were measured in the pore waters of the Dora and the Selaata sediments (0.04–8.76 ng/l); the concentrations present vertical profiles allowing the calculation of low diffusive fluxes out of the sediment toward the water column (0.7 and 0.3 ng/m2/day) at the Dora and Selaata sediments, respectively. These fluxes constitute sources for the exposition of coastal ecosystems to this toxic chemical, which is further added to the MeHg incorporated via the benthic food webs.
References
Abdel-Rahman A, Nader F (2002) Characterization of the Lebanese Jurassic-Cretaceous carbonate stratigraphic sequence: a geochemical approach. Geol J 37:69–91
Abi-Ghanem C, Chiffoleau JF, Bermond A, Nakhlé K, Borschneck D, Khalaf G, Cossa D (2009) Lead and its isotopes in the sediment of three sites of the Lebanese coasts: estimation of contamination sources. Appl Geochem 24:1990–1999
Ackermann F, Bergmann M, Schleichert GU (1983) Monitoring of heavy metals in coastal and estuarine sediments—a question of grain size: <20 μm versus <60 μm. Environ Technol Lett 4:317–328
Al-Hajj Z, Muscat C (2000) From Europe to the Mediterranean. A Greenpeace report focusing on pollution by the fertilizer industry and the shift of such dirty technologies from Europe to developing countries in the Mediterranean
Baldi F, Bargagli R, Focardi S, Fossi C (1983) Mercury and chlorinated hydrocarbons in sediments from the bay of Naples and adjacent marine areas. Marine Pollut Bull 14:108–111
Benoit JM, Gilmour CC, Mason RP, Heyes A (1999) Sulfide controls on mercury speciation and bioavailability to methylating bacteria in sediment pore waters. Environ Sci Technol 33:951–957
Benoit JM, Shull DH, Harvey RM, Beal SA (2009) Effect of bioirrigation on sediment-water exchange of methylmercury in Boston Harbor, Massachusetts. Environ Sci Thechnol 43:3669–3674
Berner RA (1980) Early diagenesis: a theoretical approach. Princeton University Press, Princeton, NJ
Bernhard M, Renzoni A (1977) Mercury concentration in Mediterranean marine organisms and their environment: natural and anthropogenic origin. Thalass Jugosl 13:265–300
Castelle S, Schafer J, Blanc G, Audry S, Etcheber H, Lissalde J-P (2007) 50-Year record and solid state speciation of mercury in natural and contaminated reservoir sediment. Appl Geochem 22:1359–1370
Choe K-Y, Gill GA, Lehman RD, Han S, Heim WA, Coale KH (2004) Sediment–water exchange of total mercury and monomethyl mercury in the San Francisco Bay Delta. Limnol Ocean 49:1512–1527
Compeau GC, Bartha R (1985) Sulfate-reducing bacteria: principal methylators of mercury in anoxic estuarine sediment. Appl Environ Microbiol 50:498–502
Cossa D, Coquery M (2005) The Mediterranean mercury anomaly, a geochemical or a biological issue. In: Saliot A (ed) The Mediterranean Sea. Handbook of environmental chemistry, vol Vol V. Springer, New York, pp 177–208
Cossa D, Averty B, Bretaudeau J, Sénard AS (2003) Spéciation du mercure dissous dans les eaux marines. Dosage du mercure total, gazeux, réactif, mono et diméthylmercure. Méthodes d’analyse en milieu marin. Co-édition Ifremer et ministère de l’Ecologie et du développement durable
Cossa D, Averty B, Pirrone N (2009) The origin of methylmercury in open Mediterranean waters. Limnol Ocean 54:837–844
Covelli S, Faganelli J, Horvat M, Brambati A (1999) Porewater distribution and benthic flux measurements of mercury and methylmercury in the Gulf of Trieste (North Adriatic Sea). Estuar Coast Shelf Sci 48:415–428
Covelli S, Faganelli J, Horvat M, Brambati A (2001) Mercury contamination of coastal sediments of long-term cinnabar mining activity (Gulf of Trieste, northern Adriatic Sea). Appl Geochem 16:541–558
Di Leonardo R, Tranchida G, Bellanca A, Neri R, Angelone M, Mazzola S (2006) Mercury levels in sediments of central Mediterranean sea: a 150+ year record from box-cores recovered in the Strait of Sicily. Chemosphere 65:2366–2376
DR/ ECODIT-IAURIF (1997) Council for development and reconstruction. Regional environmental assessment report on the coastal zone of Lebanon. Final report, Prepared by ECODIT-IAURIF, Beirut
Drott A, Lambertsson L, Bjorn E, Skyllberg U (2008) Do potential methylation rates reflect accumulated methyl mercury in contaminated sediments? Environ Sci Technol 42:153–158
Fakhri M (2005) Interactions de deux sources continentales, naturelle et anthropogénique, sur les caractéristiques physico-chimiques et biologiques du milieu marin de Batroun au Liban Nord (Méditerranée Orientale). PhD thesis, University of the Mediterranean, Aix Marseille II, France
Fitzgerald WF, Lamborg CH, Hammerschmidt CR (2007) Marine biogeochemical cycling of mercury. Chem Rev 107:641–662
French Geode Program (2000) French law on water n° 92.3 (3rd January 1992), Decree of June the 14th 2000). J Officiel, République Française. http://www.ifremer.fr/delcc/cycleau/reglementation/dragages.htm
Gill GA, Bloom NS, Cappellino S, Driscoll CT, Mason R, Rudd JWM (1999) Sediment–water fluxes of mercury in Lavaca Bay, Texas. Environ Sci Technol 33:663–669
Hammerschmidt CR, Fitzgerald WF (2004) Geochemical controls on the production and distribution of methylmercury in near-shore marine sediments. Environ Sci Technol 38:1487–1495
Hammerschmidt CR, Fitzgerald WF (2006) Methylmercury cycling in sediments on the continental shelf of southern New England. Geochim Cosmochim Acta 70:918–930
Herut B, Hornung H, Krom MD, Kress N, Cohen Y (1993) Trace metals in shallow sediments from the Mediterranean coastal region of Israel. Marine Pollut Bull 26:675–682
Hesslein RH (1976) An in-situ sampler for close interval pore water studies. Limnol Oceanogr 21:912–914
Jackson M, Hancock D, Schulz R, Talbot V, William D (1986) Rock phosphate: the source of mercury pollution in a marine ecosystem at Albany, Western Australia. Marine Environ Res 18:185–202
Kersten M, Smedes F (2002) Normalization procedures for sediment contaminants in spatial and temporal trend monitoring. J Environ Monit 4:109–115
Krumgalz BS, Fainshtein G (1991) Trace metals and organic matter in nearshore sediment cores from the Eastern Mediterranean (Haifa Bay of Israel). Marine Environ Res 31:1–15
Langer CS, Fitzgerald WF, Visscher PT, Vandal GM (2001) Biogeochemical cycling of methylmercury at Barn Island Salt Marsh, Stonington, CT, USA. Wet Ecol Manag 9:295–310
MacDonald DD, Ingersoll CG, Berger TA (2000) Development and evaluation of consensus-based sediment quality guidelines for freshwater ecosystems. Arch Environ Contam Toxicol 39:20–31
Marvin-Dipasquale M, Lutz AM, Brigham EM, Krabbenhoft PD, Aiken RG, Orem HW, Hall DB (2009) Mercury cycling in stream ecosystems. 2. Benthic methylmercury production and bed sediment—pore water partitioning. Environ Sci Technol 43:2726–2732
METAP/Tebodin (1998) Ministry of Environment. Industrial pollution control Lebanon. Prepared by Tebodin and financed by the World Bank and UNIDO
Monperrus M, Tessier E, Veschambre S, Amouroux D, Donnard O (2005) Simoultaneous speciation of mercury and butyltin compounds in natural waters and snow by propylation and species-specific isotope dilution mass spectrometry analysis. Anal Bioanal Chem 381:854–862
Morel MMF, Kraepiel MLA, Amyot M (1998) The chemical cycle and bioaccumulation of mercury. Annu Rev Ecol Syst 29:543–566
Morse JW (2003) Formation and diagenesis of carbonate sediments. In: Lollar SB, Holland HD, Turequian KK (eds) Treatise on geochemistry, vol VII. Elsevier, Amsterdam, pp 67–85
Muresan B, Cossa D, Jézéquel D, Prévot F, Kerbellec S (2007) The biogeochemistry of mercury at the sediment–water interface in the Thau lagoon. 1. Partition and speciation. Estuar Coast Shelf Sci 72:472–484
Nakhlé K (2003) Le mercure, le cadmium et le plomb dans les eaux littorales libanaises: Apports et suivi au moyen de bioindicateurs quantitatifs (Eponges, bivalves et gasteropodes). PhD thesis, University of Paris 7 Denis Diderot, France
Nassif N (2004) Pollutions chimiques en milieu marin: essai de modélisation et approche réglementaire. PhD thesis, AgroParisTech, France
NOAA (1999) National Oceanographic and Atmospheric Administration. ccma.nos.noaa.gov/publications/sqg.pdf
Ogrinc N, Monperrus M, Kotnik J, Fajon V, Vidimova K, Amouroux D, Kocman D, Tessier E, Zizek S, Horvat M (2007) Distribution of mercury and methylmercury in deep-sea surficial sediments of the Mediterranean Sea. Marine Chem 107:31–48
Pirrone N, Costa P, Pacyna JM, Ferrara R (2001) Mercury emissions to the atmosphere from natural and anthropogenic sources in the Mediterranean region. Atmos Environ 35:2997–3006
Pirrone N, Ferrara R, Hedgecock IM, Kallos G, Mamane Y, Munthe J, Pacyna JM, Pytharoulis I, Sprovieri F, Voudouri A, Wangberg I (2003) Dynamic processes of mercury over the Mediterranean region: results from the Mediterranean Atmospheric Mercury Cycle System (MAMCS) project. Atmos Environ 37(Suppl 1):S21–S39
Sanchez Uria JE, Sanz-Mendel A (1998) Inorganic and methylmercury speciation in environmental samples. Talanta 47:509–524
Sanchiz C, Garcia-Carrascosa AM, Pastor A (2000) Heavy metal content in soft-bottom marine macrophytes and sediments along the Mediterranean coast of Spain. Marine Ecol 21:1–16
Sanlaville P (1977) Etude géomorphologique de la région du littorale du Liban, vol I. The Lebanese University, Beyrouth
Shoham-Frider E, Shelef G, Kress N (2007) Mercury speciation in sediments at a municipal sewage sludge marine disposal site. Marine Environ Res 64:601–615
Sunderland EM, Gobas APCF, Branfireun AB, Heyes A (2006) Environmental controls on the speciation and distribution of mercury in coastal sediments. Marine Chem 102:111–123
Syllberg U, Drott A, Lambertsson L, Björn E, Karlsson T, Johnson T, Heinemo S-Å, Holmström H (2007) Net methylmercury production as a basis for improved risk assessment of mercury-contaminated sediments. Ambio 36:437–442
Tomiyasu T, Matsuyama A, Eguchi T, Fuchigami Y, Okiki K, Horvat M, Rudi R, Akagi H (2006) Spatial variations of mercury in sediment of Minamata Bay, Japan. Sci Total Environ 368:283–290
Tseng CM, de Diego A, Pinaly H, Amouroux D, Donard OFX (1998) Cryofocusing coupled to atomic adsorption spectrometry for rapid and simple mercury speciation in environmental matrices. J Anal Atom Spectrom 13:755–764
Turekian KK, Wedephol KH (1961) Distribution of the elements in some major units of the earth’s crust. Geol Soc Am Bull 72:175–192
Acknowledgments
We gratefully acknowledge the skillful help of E. Teirik, D. Auger, B. Averty, and E. Rozuel in the sampling operation and for their analytical assistance. This research was funded by the CEDRE project (Convention No. 05 E F42/L6), the cooperative research program between the Lebanese CNRS and Ifremer (Convention Cadre No. 07/1216532), and the AUF, which provides funding for student residence time in France.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abi-Ghanem, C., Nakhlé, K., Khalaf, G. et al. Mercury Distribution and Methylmercury Mobility in the Sediments of Three Sites on the Lebanese Coast, Eastern Mediterranean. Arch Environ Contam Toxicol 60, 394–405 (2011). https://doi.org/10.1007/s00244-010-9555-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-010-9555-9