Abstract
Coastal and estuarine sediments play an important role in the biogeochemical cycle of mercury (Hg) in the aquatic environment. When contaminated, sediments can act as a potential source of Hg and may pose a long-term risk to aquatic biota. The aim of this research was to assess spatial and historical distribution of Hg in the sediments of the Krka River estuary, an environment that so far has been regarded as relatively unpolluted. To achieve this goal, 40 surface sediment samples and 7 sediment cores were collected along the entire estuary. Hg concentrations in the surface and deep sediments of the Krka River estuary were found in a broad range 0.042–57.8 mg kg−1, demonstrating significant spatial and temporal differences in Hg input to the estuarine sediments. Two distinct areas were distinguished; upper estuary where the Hg content was comparable to other unpolluted Adriatic sediments, and the lower estuary where sediment profiles reflected the history of anthropogenic Hg input associated with the city of Šibenik. The vertical Hg profile from the most affected area of the estuary, combined with 210Pb and 137Cs dating, demonstrated that a significant increase of Hg input started in late 1940s/early 1950s, mainly related to shipyard activities. This study provided more insight on the Hg concentration in the Krka River estuary, demonstrating that the high values obtained, although localized, were comparable to the ones found in some of the most contaminated sites in the Mediterranean.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Estuarine and coastal environments are often subjected to contamination by a variety of pollutants resulting from intense anthropogenic pressure (Kennish 1994; Chapman and Wang 2001; Emili et al. 2016). Among the contaminants present in aquatic environments, mercury (Hg) is of particular concern due to its high toxicity, high mobility and accumulative behaviour in the environment and biota (Covelli et al. 2012; Song et al. 2018; UNEP 2013). When introduced into the aquatic environment, Hg has a tendency to be sorbed to organic and inorganic particles thus settling to the bottom (Di Leonardo et al. 2006; Looi et al. 2015). Therefore, sediment is a major repository of anthropogenic Hg and a reliable archive of past Hg contamination (Stupar et al. 2014; Covelli et al. 2012; Ramalhosa et al. 2006). Sediments are also regions where net methylation of inorganic Hg to methylmercury (MeHg) occurs, making them an important reservoir for MeHg in aquatic systems (Shi et al. 2018). However, sediments cannot be observed not only as a sink but also as a possible long-term source (Cukrov et al. 2011; Tessier et al. 2011; Duan et al. 2015) from which Hg, as well as other contaminants, can be released to overlaying water through various biological and physico-chemical processes (Saulnier and Mucci 2000; Tankere-Muller et al. 2007; Duan et al. 2015).
The Krka River estuary is a highly stratified estuary located on the Eastern Adriatic Coast (Croatia). The largest part of its freshwater watercourse, the Krka River, is protected as a national park. Hence, there is no significant contamination entering the Krka River estuary from that direction, and the estuary is considered comparatively unpolluted. However, several studies evidenced anthropogenic impact in terms of elevated trace metal concentrations in the lower part of the estuary, around the largest settlement in the area—city of Šibenik (Bogner et al. 2004; Cukrov et al. 2008; Kwokal et al. 2002; Martinčić et al. 1989, 1990; Mikac et al. 1989). Electrode and ferroalloy production (Cindrić et al. 2015), untreated wastewater discharge (Mikac et al. 1989, 1996), naval repair shipyard and a phosphate transhipment port (Mikac et al. 1989, 2006) were major sources of contamination for the estuary. Currently, some of the sources are eliminated; the electrode and ferroalloy factory has been closed more than 20 years, and since 2007, wastewaters have been treated and discharged outside of the estuary. However, removing industry from the estuary, led to development of the nautical tourism—a serious periodic (seasonal) anthropogenic threat to the estuary ecosystem (Cindrić et al. 2015).
Over the past 30 years, several studies have been conducted on Hg in the sediments of the Krka River estuary. Kwokal et al. (2002) reported slightly elevated concentrations of Hg (1.42 mg kg−1) for the area around the city of Šibenik, while in other parts of the estuary, Hg values were in the range 0.101–0.418 mg kg−1. Similar values were reported by Mikac et al. (1989) and Martinčić et al. (1989), although the authors used only clay/silt (< 75 μm) fraction to reduce grain size influence. The highest concentrations (3 mg kg−1) were found in the Šibenik port area (Mikac et al. 2006).
Regarding the changes in contamination sources in the Krka River estuary and elevated Hg concentrations reported previously for some parts of estuary, a detailed study on Hg content in estuarine sediments was done with the following objectives: (1) make a high resolution spatial distribution map of Hg in the surface sediments of the Krka River estuary, allowing us to establish the main sources of Hg contamination within the estuary, (2) to assess historical Hg distribution in different parts of the estuary giving us insight on changes in Hg input to this environment over prolonged periods of time and (3) to assess the degree of contamination and potential ecotoxicity by calculating enrichment factor and comparing obtained concentrations with sediment quality criteria.
Methods and materials
Study area
The Krka River estuary, situated in the karstic region of Croatia, is a typical stratified estuary with a fresh-brackish surface layer moving seawards and a bottom seawater layer moving upwards. It has characteristics of typical Mediterranean estuaries; weak tidal amplitude (0.2–0.5 m) and almost negligible tidal currents. Input of terrigenous matter into the Krka River estuary is very low (suspended particulate matter (SPM) generally lower than 5 mg L−1) (Cindrić et al. 2015), probably due to the fact that the Krka River drains mostly carbonate terrain and has tufa barriers along the stream which significantly reduce suspended material transport. The main source of terrigenous material is a small tributary, the Guduča River, which inflows in the upper estuary (Cukrov and Barišić 2006; Juračić and Pravdić 1991; Prohić and Juračić 1989). According to previous studies (Cukrov and Barišić 2006; Cukrov et al. 2009) there are differences in type and rate of sedimentation between the upper and the lower part of the estuary. In the upper part, especially in the Prokljan Lake, the sediment is a mixture of marine carbonates and terrigenous material. The sedimentation rate in this area varies from 2 to 4–5 mm/year, depending on the distance from the mouth of Guduča River. The lower estuary, Šibenik Bay, is characterized by marine biogenocarbonate sedimentation with sedimentation rates of less than 1 mm/year (Cukrov et al. 2007). Furthermore, there are differences in granulometric composition of sediments in the estuary with mean grain size increasing from Prokljan Lake (7 μm) toward the sea (410 μm) (Prohić and Juračić 1989). The coarsening of sediments seaward indicates either a different source of sediment particles or a non-depositional environment.
Sampling
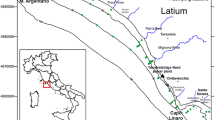
Surface sediment samples were collected at 40 locations along the entire Krka River estuary, using Uwitec gravity corers (PVC tube, φ = 9 cm, length = 60 cm). GPS instrument Garmin GPSMap 76 CSx (Kansas City, MO, USA) (accuracy ± 5 m) was used to precisely determine each location (Fig. 1). The uppermost 5 cm of the sediment cores were subsampled directly onboard, immediately transported to the laboratory and stored at − 20 °C until further treatment. Samples were freeze-dried, sieved under 2 mm and a subsample of the < 2 mm fraction was ground in a Planetary Ball Mill PM 100 (Retch) for subsequent analysis of aluminium (Al) and lithium (Li) content, organic carbon and total Hg.
Map of the sampling locations in the Krka River estuary; dark grey squares mark surface sediment sampling locations, sampling locations of the cores are represented with labels next to the symbol. All cores, except K32a (represented with a black square), were taken at same location as the surface samples
Seven sediment cores (K1, K7, K8, K20, K22, K32a and K36) were taken to trace historical Hg contamination in the study area. The sampling sites were selected in such a way that different areas of sedimentation were present, as well as areas exposed to various anthropogenic threats. Cores were sampled by a scuba diver using hand-driven Plexiglas corers, except for core K8 (Fig. 1) which was sampled using a gravity corer (Plexiglas tube, φ = 10 cm, length = 60 cm). Immediately after the sampling, sediment cores were sliced (each 1 or 2 cm, depending on the core), and same treatment protocol as described above was applied on obtained slices.
Granulometric and geochemical analyses
The particle size distribution was determined using a laser diffraction particle-size analyser LS 13320 (Beckman Coulter Inc.). Samples were prepared for analysis as follows: freeze-dried sediment samples were dispersed in deionized water and briefly treated in an ultrasonic bath (3 min) prior to measurement. The range of analysis was from 0.4–2000 μm.
The organic carbon (Corg) was determined using NCS Flash 2000 analyzer (Thermo Scientific) at a combustion temperature of 950 °C, after the acidification of samples with 6 M HCl (trace metal grade).
Semi-total elements (Al and Li) concentrations were measured after aqua regia and microwave digestion, by High Resolution Inductively Coupled Plasma Mass Spectrometer (HR ICP-MS, Element 2, Thermo). For the digestion of the samples, ~ 100 mg of sediment was added to 15 mL Teflon liners with 10 mL of aqua regia (HNO3:HCl 1:3, Fisher Scientific® Trace Analysis grade). Samples were mineralised using a microwave oven (UltraWAVE, Milestone), controlled by a two-step temperature programme. The temperature was first linearly increased from room temperature to 240 °C in 20 min and subsequently maintained at 240 °C for the next 20 min. After thermal treatment, the homogeneous solution was diluted to 25 mL and diluted 10 more times prior to analysis by the HR ICP-MS. For validation of the extraction method, certified material was used (PACS-2, National Research Council of Canada) (Online Resource 1).
Total Hg concentrations were determined in untreated samples (~ 10–100 mg) using an Advanced Mercury Analyser AMA 254 (ALTEC, Czech Republic). The AMA 254 is based on thermal decomposition of the sample, followed by collection of the evolved Hg vapor on a gold amalgamator. Total Hg is then determined by a standard atomic absorption spectrophotometer at 253.7 nm. To ensure accuracy and precision of the analysis, replicated measurements of the international reference materials (PACS-3, MESS-3 and MESS-4, National Research Council of Canada) (Online Resource 1), blanks and duplicated samples were conducted.
Radiometric analysis
The K32a core, located in a small bay with a repair shipyard and marina, was chosen for the radiometric analysis due to the lack of the knowledge on sedimentation rate in this particular area. For the other parts of the estuary, there is an available data from previous research (Cukrov 2006; Cukrov et al. 2007).
Subsamples of dried sediment were placed in a cylindrical measuring vessel of 125 cm3 volume, hermetically closed and sealed. After the radiochemical equilibrium between 222Rn and its daughter 214Bi was established (approximately 4 weeks), the activities of radionuclides (226Ra, 214Bi, 210Pb and 137Cs) were determined by the gamma-spectrometric method. The measurements (counting) were performed using high-resolution HPGe (High Purity Germanium) detector (BE5030P, CANBERRA) (Broad Energy, resolution (FWHM) at 1.33 MeV (60Co) of 1.95 keV; relative efficiency of 48%). The spectra collection time was based upon the sample quantity, ranging from 80,000 to 200,000 s. For the processing of recorded spectra, the associated Genie 2000 computer software (Canberra) was used. The activity of 226Ra was determined by the activity of its daughter 214Bi, calculated from the energy photo peak of 609.4 keV. The activity of 210Pb was determined from the energy photo peak of 46.5 keV, while activity of the anthropogenic radionuclide 137Cs was calculated from the energy photo peak of 661.6 keV. The total budget of combined measurement uncertainty (k = 2) included the uncertainties of determining the net area of the photo peak, efficiency and the speed of counting, including the uncertainty of the background rate. Calibration of the efficiency of the measurement setup was made mathematically using the LabSOCS tool by modelling the measuring vessel and individual sample and using detector characterization provided by the manufacturer. The detector system performance and calibration were regularly monitored via intercomparison measurements, while the precision and accuracy were checked by the simultaneous measurement of IAEA reference materials as well as using gamma mixed standards supplied by Ecker & Ziegler (Analytics USA).
For the sediment age estimation, two short-lived radionuclides were used, 210Pb and 137Cs. 210Pb (T1/2 = 22.2 years) is a member of the natural decay chain of 238U, which has been widely used for very precise sediment dating on a 100-year time scale. In this study, for the age calculation the Constant Initial Concentration (CIC) model was applied (Robbins 1978). The value of supported 210Pb in each sample was assumed to be in equilibrium with the in situ 226Ra, and excess 210Pb was calculated by subtracting 226Ra activity from total 210Pb. The 210Pb dating results were validated by using chronostratigraphic dates based on records of the anthropogenic radionuclide 137Cs. 137Cs (T1/2 = 30.1 years) appears in environmental samples following the period of nuclear weapons testing beginning in the 1950s and peaking in 1963. After that time, activity of 137Cs decreased until a precipitous rise in 1986 as the consequence of the contamination caused by the nuclear incident in Chernobyl. Therefore, the maximum values of 137Cs activities were expected in the late 1980s and early 1990s, followed by a subsequent decline.
Results and discussion
Spatial distribution of Hg in surface sediments
Total Hg concentrations in the surface sediments of the Krka River estuary ranged from 0.058 mg kg−1 to 12.36 mg kg−1, with an average of 1.14 mg kg−1 (Table 1). From the Hg distribution map shown in Fig. 2, it is evident that the upper estuary is quite pristine (< 0.3 mg kg−1), comparable with other unpolluted sites in the Adriatic (Table 2). However, the Hg distribution in the lower part of the estuary demonstrates a strong influence from the city of Šibenik, resulting in elevated concentrations in the Šibenik Bay area. The localized “hot-spot” of Hg contamination is the nautical marina/repair shipyard (formerly a naval shipyard) area, where the highest Hg concentrations were reported. The connection between shipyard activities and elevated sediment Hg concentrations has been reported previously (Fairey et al. 1998; Canário et al. 2007; Cardellicchio et al. 2006). During shipbuilding and repairing processes, a huge volume of wastes and contaminants are released (Rahman and Karim 2015; Celebi and Vardar 2008), often leading to severe seabed contamination and negative impact on marine sediments in local waters. Among the contaminants commonly found near shipyards are toxic metals, TBT, PAH and other chlorinated organic components (Chiu et al. 2006; OECD 2010).
Although Hg concentrations in the surface sediments of the Krka River estuary obtained in this study partially correspond to the levels obtained in previous studies (Martinčić et al. 1989; Mikac et al. 1989, 2006; Kwokal et al. 2002), this research demonstrated that Hg contamination in the Šibenik area is more significant and widespread than previously considered. From the Hg distribution map, it can be concluded that there is no dispersal of Hg contamination outside of the estuary to the open sea. As it is often observed in systems contaminated by Hg (Canário et al. 2005, 2007), accumulation occurs mainly near the source, in this case the shipyard and transhipment port. The distribution pattern is most probably influenced by multiple factors: closed morphology of the estuary, bottom seawater layer moving landward preventing transport of Hg in a seaward direction (Mikac et al. 1989) and fast adsorption of Hg to the particles and deposition to the seabed (Bilinski et al. 1992). By studying adsorption on inorganic solid phases in Krka river water of various salinities (S = 3, 20 and 38), Bilinski et al. (1991) demonstrated the remarkable self-purification ability of the Krka River estuary with respect to Hg. The authors postulated that the surface sediments of the Krka River estuary containing calcite and aluminosilicates could be considered a sink for most trace metals, including Hg. Although sediments have been reported to be an important source of Hg to the water column (Cesário et al. 2016, 2017a, b; Shi et al. 2018), there are also contaminated coastal areas (Ramalhosa et al. 2006) where insignificant contribution of the Hg diffusion from the pore water to the overlying water column has been observed. In those areas, diffusion to the overlying waters appeared to be inhibited by presence of an oxic layer near the sediment-water interface (Gagnon et al. 1997; Mikac et al. 1999). The predominantly oxic character of the Krka River estuary surface sediments, supported by low Hg content in the water column (Mikac et al. 1989; Mikac and Kwokal 1997), suggests that presumably, significant remobilisation of the Hg from sediment to water column does not occur.
Vertical distribution of Hg in sediment cores
Vertical Hg distribution profiles in the 7 sediment cores taken in the Krka River estuary are presented in Fig. 3. Values were found in a broad range from 0.042–57.4 mg kg−1, with distinct differences between cores from the upper and lower part of the estuary. Low Hg values, ranging from 0.042–0.192 mg kg−1 were observed in the sediment cores from the upper part of the estuary: K1—downstream from the last waterfall, K7—Prokljan Lake and K8—tributary Guduča River. These values are in good agreement with Hg concentrations reported for the unpolluted Adriatic sediments (Table 2). In the deeper levels of K7 core, values (0.042–0.068 mg kg−1) corresponded to the background values of the area. At − 24 cm, there was an increase to 0.124 mg kg−1, and from then to present, values remain fairly invariable (0.176–0.192 mg kg−1). Cukrov et al. (2007) reported that sedimentation rates in the Prokljan Lake were 3–4 mm/year, indicating that a sudden increase in Hg concentrations happened 60–80 years ago. Considering that there are no significant sources of Hg in the area of Prokljan Lake, the somewhat increased concentrations could be attributed to the bottom seawater layer moving up the estuary bringing Hg from the downstream polluted area.
Concentrations of Hg in the K20 sediment core were found to range 0.719–2.08 mg kg−1. The sedimentation rate in this area, < 1 mm/year was determined by Cukrov et al. (2007). Because of the large amounts of dross that can be found in the sediment in front of the old factory of electrodes and ferroalloys, it is not possible to assume continuous sedimentation and discuss the historical input of Hg. Nevertheless, an Hg decrease was observed in the upper layers of the sediment core, indicating a possibility that sediment registered a decrease of Hg input at this particular location, as a consequence of the removal of the source of pollution.
Concentrations of Hg in the Šibenik port area were found to range from 2.61–3.53 mg kg−1. These results were in good agreement with previous research (Mikac et al. 2006). Considering a sedimentation rate of 4–5 mm/year in the port area (Cukrov 2006), it can be concluded that this area was an active source of Hg contamination for at least the last 35 years.
By far, the highest Hg concentrations, reaching 57.4 mg kg−1, were obtained in the samples from the K32a core. According to the 210Pb and 137Cs sediment age estimation (Fig. 4), a significant increase in Hg values occurred around 1950, coinciding with the beginning of the operation of the naval repair shipyard. A continuous increase in concentrations can be observed until the mid-70s, followed by invariable values for the next 25 years. Subsequently, the decrease in Hg concentrations occurred, most probably due to the decline of the shipyard business during the late 90s and early 2000s. Reasons for the increase of Hg content in the uppermost 2 cm of the core section remain unclear. However, some authors (Rasmussen 1994; Canário et al. 2005; Chatterjee et al. 2009) suggest that increased concentrations in the surface layer can result from post-depositional diagenetic processes that remobilize Hg from the deeper sediments, causing upward migration in the sediment column.
The extremely high Hg concentrations were limited to the narrow area surrounding the repair shipyard. However, the observed values were in range with some of the most contaminated sites regarding Hg in the Adriatic, such as Kaštela Bay where sediments were contaminated due to the uncontrolled effluent of a PVC chlor-alkali plant and the Gulf of Trieste where high Hg concentrations in the sediment were a consequence of long-term mining activity (Idrija mine, Slovenia) (Table 2).
Relationship between Hg-Al, Hg-fine fraction and Hg-Corg
Although many studies have reported the dependence of Hg concentrations in the sediment on organic carbon content (Mikac et al. 1999; Ramalhosa et al. 2006; Wu et al. 2013; García-Ordiales et al. 2016; Duan et al. 2015; Gao et al. 2016), the Al content (Mikac et al. 1999; Canário et al. 2007) or the fine sediment fraction (Chatterjee et al. 2009; Jin et al. 2012; Duan et al. 2015), there was no significant correlation (p > 0.05) of these parameters with Hg concentration in this research (Online Resource 2). The lack of correlations suggests that variation of Hg content along the profiles reflects changes in the anthropogenic Hg input.
Enrichment factor
Calculating normalized enrichment factor is a common approach for the assessment of the anthropogenic impact on the sediments (Zhang et al. 2007; Varol 2011; Cukrov et al. 2014). Normalization is based on a conservative element such as Al, Fe or Li. Enrichment factor is determined according to the equation:
Where X is a conservative element for normalization; (Me/X) sample is the metal/X ratio in the sample of interest; and (Me/X) background is the natural background value of the metal/X ratio. Based on the enrichment factor, pollution can be classified in one of the five categories (Sutherland 2000): enrichment factor (EF) < 2, deficiency to low enrichment; EF 2–5, moderate enrichment; EF 5–20, significant enrichment; EF 20–40, very high enrichment; EF > 40, extremely high enrichment.
In this study, a layer 34–36 cm of sediment core taken at the location K1 was chosen as the natural background value. According to previous research (Cukrov et al. 2007), sedimentation in this area is 2 mm y−1. Thus, the chosen layer has been deposited before industrial development in the study area. The conservative element Li was chosen over Al or Fe, because of possible anthropogenic input of those elements to the Krka River estuary.
The obtained enrichment factor for the surface sediments clearly demonstrated differences between the upper part of the estuary—from the last waterfall to the end of Prokljan Lake and the lower part of the estuary—downstream of Prokljan Lake (Table 1). In the upper part of the estuary, there was no enrichment (EF < 2) at most of the sampling stations. Down from Prokljan Lake, there was enrichment at all sampling locations, ranging from moderate enrichment to extremely high enrichment (up to 150.7) in the area around Šibenik. The extremely high enrichment was connected to the former naval repair shipyard—currently a nautical marina/shipyard area and transhipment port. The rest of the area surrounding the city of Šibenik showed very high enrichment. A similar trend was observed in the enrichment factor of the sediment cores; cores from the upper part of the estuary (K1, K7 and K8) showed low to moderate enrichment, while cores from the lower part of the estuary (K20, K22, K32 and K36), all showed significant to extremely high enrichment. By far, the highest enrichment (up to 1158) was observed in the core taken from the marina (former navy yard), where Hg concentrations were elevated almost 1000-fold in comparison with background values.
Assessment of ecotoxicological risk
On their own, the data on total metal concentrations in the sediment are not sufficient to assess potential toxicity for organisms living in or near this ecosystem. For this purpose, interpretive tools such as various sediment quality guidelines (SQGs) are required to predict the biological effect of present metals on benthic organisms (Burton 2002). Commonly in use are those proposed by Long et al. (1995). Based on ecotoxicological data from North American marine ecosystems, these authors defined two threshold values: the effects-range-low (ERL) and the effects-range-median (ERM), calculated as the 10th and the 50th percentiles of the effect dataset. The ERL value indicates the concentration limit for each pollutant above which negative impacts on organisms are possible, while the ERM value is the concentration limit above which adverse effects on organisms are expected. The ERL and ERM values for Hg are 0.15 mg kg−1 and 0.71 mg kg−1, respectively. The percentages of sampling stations in the Krka River estuary corresponding to SQG ranges are given in Table 1.
All sediment samples from cores collected in the Šibenik Bay (K20, K22, K32 and K36) exceeded the ERM value, indicating that in terms of Hg, sediments pose a high risk of adverse effects on biota in the lower part of the Krka River estuary. This conclusion is also evident from the comparison of Hg concentrations in the surface sediment with SQGs, where 37.5% of samples have values higher than ERM, and the same percentage of samples have values between ERL and ERM, most of which were taken downstream from Prokljan Lake. Of all of the samples subsampled from cores K1 and K8, only one exceeded the ERL values, indicating no expected negative impact on the biota, supporting the pristine condition of the upper estuary. Almost 40% of the subsamples of the core from the Prokljan Lake (K7) exceeded the ERL values, indicating the possibility of harmful effects of the sediment to benthic organisms.
Conclusion
In order to assess the history of contamination and the current status of the semi-enclosed area of Krka River estuary, a detailed study on Hg concentrations in the sediments was performed. This study of both surface and deep sediments has shown that the Krka River estuary can be characterized by the Hg content into two discreet regions: (1) the upper part of the estuary, from the last Krka River waterfall to Prokljan Lake, where Hg concentrations were in range of those reported for unpolluted Adriatic sediments and (2) the lower part of the estuary, downstream from Prokljan Lake, where increased Hg concentrations were found due to input from various anthropogenic sources connected to the city of Šibenik. The most significant source of Hg to the estuarine sediment was the area of the former naval repair shipyard, active repair shipyard and marina, followed by transhipment port.
The spatial Hg distribution of surface sediments demonstrated that there was no spreading of Hg outside of the estuary. Most probably, this is not only a result of the enclosed morphology of the estuary but also due to water movement in the area, i.e. due to bottom seawater layer moving up into the estuary. The somewhat elevated concentrations in Prokljan Lake may most likely be attributed to water movement transporting Hg from the downstream polluted area.
The pollution assessment by EF and ecotoxicological assessment by SQG have shown serious Hg pollution in the entire Šibenik area, as well as a high risk of negative effects on the biota. The Hg pollution status in the lower part of the Krka River estuary was much more severe than expected, and it must be considered a serious environmental problem. Although some research suggests that there is no significant transfer of Hg from sediment to the water column, the high concentrations obtained in some parts of the estuary have to be considered a threat to this ecosystem. Despite of the fact that some sources of pollution in the area have been eliminated, a decrease in surface layer concentrations was not observed, indicating there are still active sources contributing to the Hg content in the Krka River estuary.
References
Bilinski H, Kozar S, Plavšić M, Kwokal Z, Branica M (1991) Trace-metal adsorption on inorganic solid-phases under estuarine conditions. Mar Chem 32:225–233. https://doi.org/10.1016/0304-4203(91)90040-4
Bilinski H, Kwokal Z, Branica M (1992) Processes affecting the fate of mercury in the Krka River estuary. Water Res 26:1243–1253. https://doi.org/10.1016/0043-1354(92)90185-7
Bogner D, Ujević I, Zvonarić T, Barić A (2004) Distribution of selected trace metals in coastal surface sediments from the middle and south Adriatic Sea Fresen. Environ Bull 13:1281–1287
Burton GA Jr (2002) Sediment quality criteria in use around the world. Limnology 3:65–76. https://doi.org/10.1007/s102010200008
Canário J, Vale C, Caetano M (2005) Distribution of monomethylmercury and mercury in surface sediments of the Tagus Estuary (Portugal). Mar Pollut Bull 50:1142–1145. https://doi.org/10.1016/j.marpolbul.2005.06.052
Canário J, Prego R, Vale C, Branco V (2007) Distribution of mercury and monomethylmercury in sediments of Vigo Ria , NW Iberian Peninsula Water. Air Soil Poll 182:21–29. https://doi.org/10.1007/s11270-006-9317-5
Cardellicchio N, Buccolieri A, Di Leo A, Spada L (2006) Heavy metals in marine sediments from the mar piccolo of Taranto (Ionian Sea, Southern Italy). Ann Chim-Rome 96:727–741. https://doi.org/10.1002/adic.200690075
Celebi UB, Vardar N (2008) Investigation of VOC emissions from indoor and outdoor painting processes in shipyards. Atmos Environ 42:5685–5695. https://doi.org/10.1016/j.atmosenv.2008.03.003
Cesário R, Monteiro CE, Nogueira M, O’Driscoll NJ, Caetano M, Hintelmann H, Mota AM, Canário J (2016) Mercury and methylmercury dynamics in sediments on a protected area of Tagus Estuary (Portugal). Water Air Soil Pollut 227:475. https://doi.org/10.1007/s11270-016-3179-2
Cesário R, Hintelmann H, O’Driscoll NJ, Monteiro CE, Cetano M, Nogueira M, Mota AM, Canário J (2017a) Biogeochemical cycle of mercury and methylmercury in two highly contaminated areas of Tagus Estuary (Portugal). Water Air Soil Pollut 228:257. https://doi.org/10.1007/s11270-017-3442-1
Cesário R, Poissant L, Pilote M, O’Driscoll NJ, Moya AM, Canário J (2017b) Dissolved gaseous mercury formation and mercury volatilization in intertidal sediments. Sci Total Environ 603-604:279–289. https://doi.org/10.1016/j.scitotenv.2017.06.093
Chapman PM, Wang FY (2001) Assessing sediment contamination in estuaries. Environ Toxicol Chem 20:3–22. https://doi.org/10.1002/etc.5620200102
Chatterjee M, Canário J, Sarkar SK, Branco V, Bhattacharya AK, Satpathy KK (2009) Mercury enrichments in core sediments in Hugli-Matla-Bidyadhari estuarine complex, north-eastern part of the Bay of Bengal and their ecotoxicological significance. Environ Geol 57:1125–1134. https://doi.org/10.1007/s00254-008-1404-z
Chiu SW, Ho KM, Chan SS, So OM, Lai KH (2006) Characterization of contamination in and toxicities of a shipyard area in Hong Kong. Environ Pollut 142:512–520. https://doi.org/10.1016/j.envpol.2005.10.038
Cindrić AM, Garnier C, Oursel B, Pižeta I, Omanović D (2015) Evidencing the natural and anthropogenic processes controlling trace metals dynamic in a highly stratified estuary: the Krka River estuary (Adriatic, Croatia). Mar Pollut Bull 94:199–216. https://doi.org/10.1016/j.marpolbul.2015.02.029
Covelli S, Faganeli J, Horvat M, Brambati A (2001) Mercury contamination of coastal sediments as the result of long-term cinnabar mining activity (Gulf of Trieste, northern Adriatic Sea). Appl Geochem 16:541–558. https://doi.org/10.1016/S0883-2927(00)00042-1
Covelli S, Fontolan G, Faganeli J, Ogrinc N (2006) Anthropogenic markers in the Holocene stratigraphic sequence of the Gulf of Trieste (northern Adriatic Sea). Mar Geol 230:29–51. https://doi.org/10.1016/j.margeo.2006.03.013
Covelli S, Langone L, Acquavita A, Piani R, Emili A (2012) Historical flux of mercury associated with mining and industrial sources in the Marano and Grado Lagoon (northern Adriatic Sea). Estuar Coast Shelf S 113:7–19. https://doi.org/10.1016/j.ecss.2011.12.038
Cuculić V, Cukrov N, Kwokal Z, Mlakar M (2009) Natural and anthropogenic sources of Hg, Cd, Pb, Cu and Zn in seawater and sediment of Mljet National Park, Croatia. Estuar Coast Shelf S 81:311–320. https://doi.org/10.1016/j.ecss.2008.11.006
Cukrov N (2006) Krka River estuary, trap for radionuclides. Dissertation, University of Zagreb (in Croatian)
Cukrov N, Barišić D (2006) Spatial distribution of K-40 and Th-232 in recent sediments of the Krka River estuary. Croat Chem Acta 79:115–118
Cukrov N, Barišić D, Juračić M (2007) Calculated sedimentation rate in the Krka River Estruary using vertical Diostribution of 137Cs. In: CIESM congress Rapp. Comm Int Mer Médit 38:81
Cukrov N, Frančišković-Bilinski S, Mikac N, Roje V (2008) Natural and anthropogenic influences recorded in sediments from the Krka river estuary (eastern Adriatic coast), evaluated by statistical methods. Fresen Environ Bull 17:855–863
Cukrov N, Mlakar M, Cuculić V, Barišić D (2009) Origin and transport of U-238 and Ra-226 in riverine, estuarine and marine sediments of the Krka River. Croatia J Environ Radioactiv 100:497–504. https://doi.org/10.1016/j.jenvrad.2009.03.012
Cukrov N, Frančišković-Bilinski S, Hlača B, Barišić D (2011) A recent history of metal accumulation in the sediments of Rijeka harbor, Adriatic Sea, Croatia. Mar Pollut Bull 62:154–167. https://doi.org/10.1016/j.marpolbul.2010.08.020
Cukrov N, Frančišković-Bilinski S, Bogner D (2014) Metal contamination recorded in the sediment of the semi-closed Bakar Bay (Croatia). Environ Geochem Hlth 36:195–208. https://doi.org/10.1007/s10653-013-9558-3
Di Leonardo R, Tranchida G, Bellanca A, Neri R, Angelone M, Mazzola S (2006) Mercury levels in sediments of central Mediterranean Sea: a 150+ year record from box-cores recovered in the Strait of Sicily chemosphere 65:2366-2376. https://doi.org/10.1016/j.chemosphere.2006.04.076
Duan LQ, Song JM, Yu Y, Yuan HM, Li XG, Li N (2015) Spatial variation, fractionation and sedimentary records of mercury in the East China Sea. Mar Pollut Bull 101:434–441. https://doi.org/10.1016/j.marpolbul.2015.09.050
Emili A, Acquavita A, Covelli S, Spada L, Di Leo A, Giandomenico S, Cardellicchio N (2016) Mobility of heavy metals from polluted sediments of a semi-enclosed basin: in situ benthic chamber experiments in Taranto’s Mar Piccolo (Ionian Sea, Southern Italy) environ Sci Pollut R 23:12582-12595. https://doi.org/10.1007/s11356-015-5281-1
Fabbri D, Felisatti O, Lombardo M, Trombini C, Vassura I (1998) The lagoon of Ravenna (Italy): characterisation of mercury-contaminated sediments. Sci Total Environ 213:121–128. https://doi.org/10.1016/S0048-9697(98)00083-7
Fairey R, Roberts C, Jacobi M, Lamerdin S, Clark R, Downing J, Long E, Hunt J, Anderson B, Newman J, Tjeerdema R, Stephenson M, Wilson C (1998) Assessment of sediment toxicity and chemical concentrations in the San Diego Bay region, California, USA. Environ Toxicol Chem 17:1570–1581. https://doi.org/10.1002/etc.5620170819
Gagnon C, Pelletier E, Mucci A (1997) Behaviour of anthropogenic mercury in coastal marine sediments. Mar Chem 59:159–176. https://doi.org/10.1016/S0304-4203(97)00071-6
Gao B, Han LF, Hao H, Zhou HD (2016) Pollution characteristics of mercury (Hg) in surface sediments of major basins. China Ecol Indic 67:577–585. https://doi.org/10.1016/j.ecolind.2016.03.031
García-Ordiales E, Esbrí JM, Covelli S, López-Berdonces MA, Higueras PL, Loredo J (2016) Heavy metal contamination in sediments of an artificial reservoir impacted by long-term mining activity in the Almadén mercury district (Spain). Environ Sci Pollut R 23:6024–6038. https://doi.org/10.1007/s11356-015-4770-6
Jin HF, Liebezeit G, Ziehe D (2012) Distribution of total mercury in surface sediments of the Western Jade Bay, Lower Saxonian Wadden Sea, Southern North Sea. B Environ Contam Tox 88:597–604. https://doi.org/10.1007/s00128-012-0530-1
Juračić M, Pravdić V (1991) The role of suspended matter in assessing the assimilative capacity case study of two estuaries in the Adriatic Sea. Chemistry and Ecology 5:241–248. https://doi.org/10.1080/02757549108035253
Kennish MJ (1994) Pollution in estuaries and coastal marine waters. J Coastal Res Special Issue 12:27–49
Kwokal Z, Frančišković-Bilinski S, Bilinski H, Branica M (2002) A comparison of anthropogenic mercury pollution in Kaštela Bay (Croatia) with pristine estuaries in Öre (Sweden) and Krka (Croatia). Mar Pollut Bull 44:1152–1157. https://doi.org/10.1016/S0025-326X(02)00134-0
Long ER, Macdonald DD, Smith SL, Calder FD (1995) Incidence of adverse biological effects within ranges of chemical concentrations in marine and estuarine sediments. Environ Manage 19:81–97. https://doi.org/10.1007/Bf02472006
Looi LJ, Aris AZ, Yusoff FM, Hashim Z (2015) Mercury contamination in the estuaries and coastal sediments of the Strait of Malacca Environmental monitoring and assessment 187:4099. https://doi.org/10.1007/s10661-014-4099-5
Martinčić D, Kwokal Z, Stoeppler M, Branica M (1989) Trace-metals in sediments from the Adriatic Sea Sci Total Environ 84:135–147. https://doi.org/10.1016/0048-9697(89)90378-1
Martinčić D, Kwokal Z, Branica M (1990) Distribution of zinc, lead, cadmium and copper between different size fractions of sediments II. The Krka River estuary and the Kornati-Islands (central Adriatic Sea). Sci Total Environ 95:217–225. https://doi.org/10.1016/0048-9697(90)90066-4
Mikac N, Kwokal Z (1997) Distribution of mercury species in the water column of the stratified Krka River Estuary. Croat Chem Acta 70:271–288
Mikac N, Kwokal Z, May K, Branica M (1989) Mercury distribution in the Krka River estuary (Eastern Adriatic Coast). Mar Chem 28:109–126. https://doi.org/10.1016/0304-4203(89)90190-4
Mikac N, Kwokal Z, Martinčić D, Branica M (1996) Uptake of mercury species by transplanted mussels under estuarine conditions (Krka river estuary). Sci Total Environ 184:173–182. https://doi.org/10.1016/0048-9697(96)05078-4
Mikac N, Niessen S, Ouddane B, Wartel M (1999) Speciation of mercury in sediments of the Seine estuary (France). Appl Organomet Chem 13:715–725. https://doi.org/10.1002/(SICI)1099-0739(199910)13:10<715::AID-AOC918>3.0.CO;2-4
Mikac N, Roje V, Cukrov N, Foucher D (2006) Mercury in aquatic sediments and soils from Croatia Arhiv za higijenu rada i toksikologiju 57:325
OECD (2010) Environmental and climate change issues in the shipbuilding industry. Organisation for Economic Co-operation and Development
Prohić E, Juračić M (1989) Heavy-metals in sediments - problems concerning determination of the anthropogenic influence - study in the Krka River estuary. Eastern Adriatic Coast, Yugoslavia Environ Geol Water S 13:145–151. https://doi.org/10.1007/BF01664699
Rahman A, Karim MM (2015) Green shipbuilding and recycling: issues and challenges International. Journal of Environmental Science and Development 6:838. https://doi.org/10.7763/IJESD.2015.V6.709
Ramalhosa E, Segade SR, Pereira E, Vale C, Duarte A (2006) Mercury cycling between the water column and surface sediments in a contaminated area. Water Res 40:2893–2900. https://doi.org/10.1016/j.watres.2006.05.023
Rasmussen PE (1994) Current methods of estimating atmospheric mercury fluxes in remote areas. Environmental Science & Technology 28:2233–2241. https://doi.org/10.1021/es00062a006
Robbins JA (1978) Geochemical and geophysical applications of radioactive lead. In: Nriagu JO (ed) Biogeochemistry of lead in the environment. Elsevier Scientific, Amsterdam, pp 285–393
Saulnier I, Mucci A (2000) Trace metal remobilization following the resuspension of estuarine sediments: Saguenay Fjord. Canada Appl Geochem 15:191–210. https://doi.org/10.1016/S0883-2927(99)00034-7
Shi XM, Mason RP, Charette MA, Mazrui NM, Cai PH (2018) Mercury flux from salt marsh sediments: insights from a comparison between 224Ra/228Th disequilibrium and core incubation methods. Geochim Cosmochim Ac 222:569–583. https://doi.org/10.1016/j.gca.2017.10.033
Song ZC, Li P, Ding L, Li ZG, Zhu W, He TR, Feng XB (2018) Environmental mercury pollution by an abandoned chlor-alkali plant in Southwest China. J Geochem Explor 194:81–87. https://doi.org/10.1016/j.gexplo.2018.07.017
Sprovieri M, Feo ML, Prevedello L, Manta DS, Sammartino S, Tamburrino S, Marsella E (2007) Heavy metals, polycyclic aromatic hydrocarbons and polychlorinated biphenyls in surface sediments of the Naples harbour (Southern Italy). Chemosphere 67:998–1009. https://doi.org/10.1016/j.chemosphere.2006.10.055
Stupar YV, Schafer J, Garcia MG, Schmidt S, Piovano E, Blanc G, Huneau F, Le Coustumer P (2014) Historical mercury trends recorded in sediments from the Laguna del Plata Cordoba, Argentina. Chem Erde-Geochem 74:353–363. https://doi.org/10.1016/j.chemer.2013.11.002
Sutherland RA (2000) Bed sediment-associated trace metals in an urban stream Oahu, Hawaii. Environ Geol 39:611–627. https://doi.org/10.1007/s002540050473
Tankere-Muller S, Zhang H, Davison W, Finke N, Larsen O, Stahl H, Glud RN (2007) Fine scale remobilisation of Fe, Mn, Co, Ni, Cu and Cd in contaminated marine sediment. Mar Chem 106:192–207. https://doi.org/10.1016/j.marchem.2006.04.005
Tessier E, Garnier C, Mullot JU, Lenoble V, Arnaud M, Raynaud M, Mounier S (2011) Study of the spatial and historical distribution of sediment inorganic contamination in the Toulon bay (France). Mar Pollut Bull 62:2075–2086. https://doi.org/10.1016/j.marpolbul.2011.07.022
UNEP (2013) Global mercury assessment 2013: sources, emissions, Releases and Environmental Transport. UNEP Chemicals Branch, Geneva, Switzerland
Varol M (2011) Assessment of heavy metal contamination in sediments of the Tigris River (Turkey) using pollution indices and multivariate statistical techniques. J Hazard Mater 195:355–364. https://doi.org/10.1016/j.jhazmat.2011.08.051
Wu FC, Xu LB, Liao HQ, Guo F, Zhao XL, Giesy JP (2013) Relationship between mercury and organic carbon in sediment cores from lakes Qinghai and Chenghai, China. J Soil Sediment 13:1084–1092. https://doi.org/10.1007/s11368-013-0694-2
Zhang LP, Ye X, Feng H, Jing YH, Ouyang T, Yu XT, Liang RY, Gao CT, Chen WQ (2007) Heavy metal contamination in western Xiamen Bay sediments and its vicinity, China. Mar Pollut Bull 54:974–982. https://doi.org/10.1016/j.marpolbul.2007.02.010
Acknowledgements
Authors are thankful to the Public Institute Nature of Šibenik-Knin County for the use of the boat for the sampling and to Xiaogang Chen, Jasmin Pađan and Domagoj Živković for assistance with sampling.
Funding
This work has been supported by Croatian Science Foundation under the project IP-2014-09-7530 (MEBTRACE) and by the French Government through short term internship grants.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Severine Le Faucheur
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cukrov, N., Doumandji, N., Garnier, C. et al. Anthropogenic mercury contamination in sediments of Krka River estuary (Croatia). Environ Sci Pollut Res 27, 7628–7638 (2020). https://doi.org/10.1007/s11356-019-07475-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-07475-y