Abstract
The phthalate esters are a group of industrial chemicals considered to have endocrine-disrupting properties. The most common tonnage product among these, di-2-ethylhexyl phthalate (DEHP), is widely spread in the environment. The objectives with the present work were to study uptake and metabolism of orally administered DEHP and its major metabolite mono-2-ethyl hexyl phthalate (MEHP) and to evaluate the impact of early life exposure on sex differentiation in Atlantic salmon. The feeding with contaminated diet started immediately after yolk sac resorption and continued for 4 weeks. Nominal concentrations of DEHP in the diet were 400 (measured 359), 800 (measured 827), and 1500 (measured 1648) mg DEHP/kg and a control group was fed food mixed with solvent. After the exposure period, fish were fed non-contaminated diet until final sampling 4 months post-exposure. There were no effects on growth or survival of the fish and no late effects on hepatosomatic index or sex ratio. However, the histological examination of gonads from fish exposed to 1500 mg DEHP/kg revealed a small but significant incidence (3%) of intersex fish (ovo-testis). Chemical residues of DEHP and MEHP were analyzed weekly during the first 3 months of the post-exposure period. Both DEHP and MEHP were rapidly eliminated to near background levels within one week post exposure. The study indicates that exposure of Atlantic salmon to relatively high concentrations of DEHP during a sensitive part of the life cycle may interfere with gonad differentiation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Environmental pollutants that can interfere with endocrine functions in animals have raised concern during the last decades. These so-called endocrine-disrupting chemicals include compounds with the ability to mimic natural hormones, block the action of natural hormones, or interfere with the synthesis and/or degradation of natural hormones (Soto et al. 1995). A group of widely used industrial chemicals considered to have endocrine-disrupting properties are the phthalate esters. These chemicals mainly serve as important additives in polyvinylchloride (PVC) resins and are used as plasticizers in various products, e.g., industrial hardware, food packaging, medical products, and toys (Staples et al. 1997; Balafas et al. 1999; Wahl et al. 1999; Earls et al. 2003). Since the phthalate esters are not chemically bound to the plastic material, they can leach into the environment (Page and Lacroix 1995). The most common tonnage product among the phthalate esters, di-2-ethylhexyl phthalate (DEHP), has been found in both fresh and marine water environments (Giam et al. 1975; Tan 1995) as well as in food (Page and Lacroix 1995) and considerable concern has been focused on its potential risk to human and wild life health.
DEHP, via its metabolite mono-2-ethyl hexyl phthalate (MEHP), has been shown to be a peroxisome proliferator (PP) by activating peroxisome proliferator-activated receptors in rodents (PPARs) (e.g., Moody and Reddy 1978; Lapinskas et al. 2005) and long-term exposure of rodents to DEHP has resulted in development of hepatocarcinomas (Reddy and Lalwani 1983). In fish, peroxisome proliferation has been reported after exposure to phthalates (i.e., dibutylphthalate) (Ortiz-Zarragoitia and Cajaraville 2000) and PPARs with similar sequence and distribution as in mammals have recently been found in a few fish species, including Atlantic salmon (Salmo salar) (Ruyter et al. 1997; Andersen et al. 2000). Effects of DEHP on reproduction and development have been shown in several vertebrates but the mechanism of action remains unclear. The estrogenic activity of DEHP has been tested in different in vitro assays showing weak or no affinity to the estrogen receptor (ER) at high concentrations (Harris et al. 1997; Blom et al. 1998; Zacharewski et al. 1998; Metcalfe et al. 2001). In rat, maternal exposure to DEHP resulted in lowered testosterone levels and reproductive tract malformations in androgen-dependent tissues in male offspring (Gray et al. 1999; Parks et al. 2000). These anti-androgenic effects appear to be mediated independent of the androgen receptor (AR) (Gray et al. 1999; Parks et al. 2000). In a study with female rats, DEHP exposure resulted in decreased serum levels of 17β-estradiol (E2), prolonged estrous cycle, and anovulation (Davis et al. 1994). In vitro studies on rat granulosa cells have shown that MEHP decreases aromatase RNA message and protein levels in a dose-dependent manner (Lovekamp and Davis 2001), which might explain the lowered serum E2 levels observed in vivo. Recent studies suggest that the inhibitory effect of peroxisome proliferators on aromatase activity in mammalian granulosa cells is mediated via the PPARs (Mu et al. 2000). Anti-estrogenic effects have been reported in a study on Japanese medaka (Oryzias latipes) water exposed to DEHP for three months post-hatching (Kim et al. 2002). Female fish treated with 1–50 μg DEHP/L showed reduced vitellogenin levels and retardation of oocyte development whereas no adverse effects were observed in males. Metcalfe et al. (2001) observed no skewed sex ratio or intersex gonads in Japanese medaka water exposed to 5000 μg DEHP/l. In a dietary study on Atlantic salmon, exposure to a nominal concentration of 1500 mg DEHP/kg food during the first four weeks after yolk-sac resorption resulted in a significantly higher proportion of phenotypic females (64%) and in an elevated hepatosomatic index (Norrgren et al. 1999). However, actual exposure levels were not measured either in food or fish, which makes it difficult to establish a lowest effect concentration. The major aim of the present study was to study uptake and elimination of DEHP and MEHP in Atlantic salmon after exposure to contaminated diets during the first four weeks after yolk-sac resorption. In order to study possible late effects, fish were examined after a four-month post-exposure period with focus on growth, relative liver weights, and gonad morphology.
Materials and Methods
Fish and Exposure
The fish used in the present study originated from feral Atlantic salmon (Salmo salar) from River Dalälven, Sweden. Hatching and subsequent rearing were conducted at the National Board of Fisheries, Älvkarleby, Sweden. Stock solutions of DEHP (Neste-Oxo AB, Sweden) were prepared in 99.5% ethanol. The ethanol solutions (0.5l) were sprayed 5–6 times onto 1 kg of granulated start feed (Aller Aqua, SGP 514, Denmark, 14% lipid content), to obtain nominal concentrations of 0, 400, 800, and 1500 mg DEHP/kg. The food was allowed to dry in fume hoods for one hour and manually mixed thoroughly between each spraying. The control diet was prepared in the same way with pure ethanol. The treated food was kept in fume hoods until the ethanol had evaporated completely (one week). At the start of the study, each experimental group consisted of approximately 1000 individuals originating from a mix of ten family groups. Each group was kept in a trough (1 m3) individually supplied with water from River Dalälven at a flow rate of 10 l/min. Light conditions in the rearing hall followed natural fluctuations. The water temperature ranged between 6.5 and 23.6°C during the experimental period from June to November, following the river water temperature. Dissolved oxygen and pH was measured regularly. Feeding with experimental diets started immediately after yolk-sac resorption, i.e., four weeks post hatch, and continued for four weeks. At the start of exposure, the mean weight of the fry was approximately 0.2 g. Fish were fed approximately 2% of their body weight daily using automatic feeders delivering food four times per day. After the exposure period, feeding continued with non-contaminated food for four months.
Chemical Analysis
Aliquots of the four diets were taken for DEHP analysis at the start and the end of the exposure period. In addition, sub-samples of fish were taken for analysis of DEHP and the metabolite mono-2-ethyl hexyl phthalate (MEHP) at weekly intervals from the first day post-exposure and for the following three months. The chemical analyses were made at the Research Institute for Chromatography, Belgium.
DEHP in diets were analyzed in duplicate by first extracting approximately 100 mg of sample with 10 ml of cyclohexane in an ultrasonic bath for one hour. The extract was then centrifuged and analyzed by GC-MS in selected ion mode (m/z 149). Analysis of DEHP in fish tissue involved two sequential extractions of approximately 3–15 g of macerated fish taken from 2–8 pooled individuals (in order to obtain a similar fat amount in all samples) with 10-ml aliquots of cyclohexane for 30 min in an ultrasonic bath. After centrifugation, the combined cyclohexane fractions were evaporated to dryness. The resulting residue was weighed to provide a determination of the lipid content of the samples. The fat residue was then re-dissolved in a sufficient volume of dichloromethane to yield a fat concentration of approximately 100 mg/ml. Deuterium labeled d4-DEHP (m/z/153) internal standard was added to 1 ml of the fat extract, which was then subjected to clean-up using gel permeation chromatography. The fraction containing DEHP was collected, evaporated to dryness under a gentle stream of nitrogen, re-dissolved in 0.1 ml of cyclohexane, and then analyzed using GC-MS.
For MEHP analysis, 1 ml of the dichloromethane extract described above was spiked with 13C4-MEHP as an internal standard. The extract was evaporated to dryness with nitrogen. One milliliter of buffer (methanol/ammonium acetate, 25/75% by volume) was added to the remaining residue and vortexed for 10 sec. The extract was held in an ultrasonic bath for 15 min and then centrifuged during 10 min at 800 rpm. The supernatant was passed through a C18 silica gel solid phase cartridge, which was then eluted with a 75/25% methanol/ammonium acetate buffer. An aliquot of this elute was then analyzed by HPLC-MS using negative ion monitoring chemical ionization in the selection ion monitoring mode (m/z 277 for MEHP and m/z 281 for 13C4-MEHP). Further analytical details and method validation results are provided by Tienpont et al. (in prep.).
Biological Analysis
Sampling of fish for biological measurements was conducted four months post-exposure, i.e., at the end of October/beginning of November. Between 199 and 207 individuals per group were examined. Fish were anesthetized with MS222 and externally inspected. Individual body length and weight were recorded. After decapitation, the liver was dissected and weighed to calculate the hepatosomatic index (HSI = liver weight/total body weight × 100). The gonads were dissected and fixed in toto in phosphate-buffered formalin. After dehydration in a graded series of ethanol, the gonads were embedded in paraffin blocks. From each gonad, between 5 and 10 longitudinally 3–5-μm-thick step-sections were cut and placed on glass slides. The sections were stained with eosin-hematoxylin and examined under a light microscope. The histological evaluation included gonadal differentiation and presence of ovo-testis.
Statistics
A one-way analysis of variance (ANOVA) test was used to examine whether HSI, weight, and length of fish differed significantly (p < 0.05) between groups. The mortality was analyzed using a chi square test for significant (p < 0.05) differences between groups. For statistical analysis of the sex ratios, fish were classified as females, males, or intersex individuals. The number of fish with intersex gonads was compared with the total number of fish in each group. The number of males with precocious gonads, i.e., individuals with running milt, was compared with the total number of males in each group. Sex ratios, intersex ratios, and precocious ratios were analyzed using Fisher’s exact test for significant (p < 0.05) differences between groups.
Results
Chemical Analysis
The procedure used for analyzing DEHP in diets was found to give mean recoveries ranging from 87–111%. All groups were exposed to DEHP-concentrations that were close to nominal values. The measured mean concentrations of DEHP in the diet at start/finish of the exposure were 2/2, 346/371, 825/829, and 1661/1634 mg/kg.
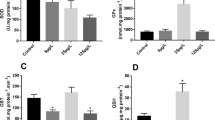
In fish tissue, the recoveries of DEHP ranged from 89–94% with a limit of quantification that was determined by the procedural blank of 0.001 mg/kgwet for a fish with a lipid content of 5%. The overall recoveries for the MEHP analysis in fish tissue ranged from 94–97% with a limit of quantification of 0.005 mg/kgwet for a fish with a lipid content of 5%. Tissue concentrations of both parent and metabolite compounds were shown to exhibit a dose-dependent relationship at the end of the exposure period. Fish fed with diets that were not artificially contaminated with DEHP still showed some background levels of both DEHP (0.016 mg/kg fish) and MEHP (0.020 mg/kg fish) (Table 1). At the end of the four-week exposure period, tissue concentrations of DEHP were approximately three times higher than MEHP (Table 1). During a subsequent depuration phase, DEHP and MEHP were rapidly eliminated from fish tissue by the first sampling time (one week) to near background levels observed in controls (Fig. 1).
Biological Analysis
The mortality in all groups was low, i.e., 3 to 4%. No dose-dependent difference in weight was observed four months post-exposure. The overall means were 11.7 ± 1.6 g (weight), 10.1 ± 0.5 cm (length), and the HSI ranged from 1.1 to 1.2. The histological evaluation of the gonads revealed no skewed sex ratios (Fig. 2). The proportion of females ranged from 49 to 51%. The majority of the females had lamellae-structured ovaries dominated by oocytes in the perinucleolar stage characterized by a relatively large centrally located nucleus with numerous peripherally nucleoli (Fig. 3). A small number of the fish in each group also defined as females had less developed ovaries not differentiated to lamellar structures with a relatively low number of perionucleolar oocytes. Testes were, in general, anatomically thread-like structures in an early stage of differentiation into a tubular system (Fig. 4). However, in the groups exposed to nominal concentrations of 800 and 1500 mg DEHP/kg food, 1% of the fish (2 out of 199 and 3 out of 202, respectively) had relatively large testes with running milt (not statistically significant). Histologically, these were characterized by well-differentiated testicular tissue containing germ cells at different stages of maturation from spermatogonia to spermatozoa (Fig. 5). In the groups exposed to 800 and 1500 mg DEHP/kg feed, individuals with the simultaneous presence of both spermatozoa and oocytes (ovo-testis) were observed (Fig. 2). Oocytes were found both in fish with poorly differentiated testes with only a few spermatozoa (1 in the 800-group, and 3 in the 1500-group) and in males with well-developed testis and running milt (1 in the 800-group, and 3 in the 1500-group) (Fig. 5). The only significant difference was recorded in the group exposed to 1500 mg DEHP/kg, where 6 out of the 202 exposed fish had ovo-testis (p = 0.014).
Discussion
According to model calculations, exposure via diet serves as the major route of uptake for lipophilic compounds with log K ow > 5.0 (Thomann 1989). The water solubility of DEHP is approximately 3 μg/L and the log K ow is around 7 (Staples et al. 1997), which supports the use of dietary administration. Although DEHP is highly lipophilic, the bioaccumulation in fish has been reported to be low, which can be explained by an effective biotransformation (Barron et al. 1989). The metabolism of DEHP in fish is similar to mammals but appears to be dependent on species and exposure route (Barron et al. 1995). The first metabolic step in the biotransformation of DEHP is formation of mono-ethylhexyl phthalate (MEHP), followed by oxidation of MEHP by cytochrome P450, and excretion (Barron et al. 1995). In the present dietary study on Atlantic salmon, there was a rapid elimination of both DEHP and MEHP to near background levels within one week post-exposure, which suggests a low risk of bioaccumulation.
The sex differentiation in many fish species is labile and susceptible to several environmental factors, such as temperature, pH, density, and social hierarchy (Devlin and Nagahama 2002). Also, administration of hormones during sensitive parts of the life cycle is known to affect the sex differentiation in fish. The sensitive period for hormonal sex reversal in salmonids seems to vary between species (Pandian and Sheela 1995). In Atlantic salmon, exposure to 17β-estradiol (E2) via immersion of eyed eggs and alevins in combination with exposure via food (20 mg E2/kg) during the 21 first days of feeding resulted in all-female stocks (Johnstone et al. 1978). Exposure to E2, via food only, from day 0 to 80 or from day 15 to 45 of the first feeding period also resulted in 100% females. Sower et al. (1984) exposed Atlantic salmon via the food to 20 mg E2/kg for 60 days following yolk-sac resorption, which resulted in a skewed sex ratio (85% females) and in intersex fish (17%) nine months post-exposure. In a study by Norrgren et al. (1999), dietary exposure of Atlantic salmon to 30 mg E2/kg for 30 days following yolk-sac resorption resulted in 100% females. In the same study, exposure of Atlantic salmon to 1500 mg DEHP/kg food (nominal concentration) resulted, contradictory to the present study, in significantly female-biased sex ratio (64% females) (Norrgren et al. 1999). The exposure period was the same as in the present study. In both studies, food was prepared in the same way with DEHP delivered from the same producer but from different batches. Also the food was delivered from the same supplier but from different batches. However, in the study by Norrgren et al. (1999), no chemical analysis of the food was performed and chemicals known to have endocrine disrupting effects, i.e., nonylphenolethoxilate (NPEO) and E2 were tested parallel to the DEHP. In both studies, the river Dalälven Atlantic salmon stock was used and fish were kept under similar rearing conditions concerning photoperiod, stocking density, and water supply. Since river water was used, small variations in composition and temperature between the studies cannot be fully avoided. A slightly higher mean temperature (+1°C) was observed in the present study during the experimental feeding phase, which might affect the distribution and elimination of DEHP in the fish. In a study with rainbow trout, the capacity for elimination of DEHP increased linearly with increasing temperature (Barron et al. 1987). However, the capacity to store DEHP was even greater, which resulted in an increased potential for biological persistence and bioaccumulation with increased temperature. The exposure of the highly perfused tissues appeared to decrease with increasing temperature, while the exposure of poorly perfused tissues increased (Barron et al. 1987). This may be of toxicological importance since changes in temperature may alter the amount of DEHP entering target tissues, such as the liver and the gonads.
Although no skewed sex ratios were found in the present study, histological examination of the gonads four months post-exposure revealed a significant incidence of individuals with ovo-testis in the group exposed to1500 mg DEHP/kg. This dose level is approximately 1000 times higher than measured levels in aquatic prey (David and Sandra 2001; Vethaak et al. 2002). To our knowledge, juvenile hermaphroditism has not been observed in Atlantic salmon and no case of ovo-testis was found among the 207 individuals examined in the control group. Intersex may be induced in fish as a result of exposure to hormones during sensitive periods (Koger et al. 2000). In the UK rivers, high incidences of intersex (ovo-testis) in roach (Rutilus rutilus) have been associated with effluents from sewage treatment works (STW) known to contain estrogenic compounds (Jobling et al. 1998; Larsson et al. 1999). Furthermore, signs of feminization, i.e., ovo-testis and induction of vitellogenin in males, have been described in a number of fish species from industrialized estuaries containing endogenous hormones but also potential EDCs, such as nonylphenol and DEHP (Matthiessen et al. 2002). In Coho salmon (Oncorhynchus kisutch), lower concentrations of E2 increased the proportion of intersex fish compared to higher concentrations, which resulted in complete sex reversal (Goetz et al. 1979). Furthermore, lower concentrations of E2 have been reported to cause intersex in Atlantic salmon (Sower et al. 1984), whereas complete sex reversal has been observed after exposure to higher concentrations (Norrgren et al. 1999) or after a longer exposure period (Johnston et al. 1978). Since the actual DEHP dietary concentrations in the study by Norrgren et al. (1999) were not measured, an accurate comparison with the present study is difficult to perform. The lack of complete sex reversal and hence skewed sex ratios in the present study might be explained by the fact that the actual dose of DEHP was lower than in the study by Norrgren et al. (1999). The mechanism by which DEHP exerts its effects on development and reproduction in vertebrates is still unclear. However, most in vitro and in vivo studies suggest that the effects are not mediated via the androgen or estrogen receptors. Studies on rat indicate that maternal exposure to DEHP disrupts fetal male sexual differentiation by reducing testosterone to female levels (by an AR-independent mechanism) (Parks et al. 2000). If this is the case also for Atlantic salmon, lowered testosterone levels could explain the presence of intersex observed in the present study and the skewed sex ratio described in the study by Norrgren et al. (1999). In order to understand how DEHP and its metabolites act on the fish reproductive system, there is a need for more mechanistic studies and alternative test models based on species with short life cycles and well-characterized biology.
Enlarged liver has been observed in rodents after exposure to DEHP (Moody and Reddy 1978). Isenberg et al. (2001) demonstrated enhanced peroxisome proliferation and increased liver weight in rodents while exposed to DEHP but two weeks post-exposure the effects were reversed. In fish, increased relative liver weights have been reported after exposure to gemfibrozil, a known rodent peroxisome proliferator (Scarano et al. 1994) but also after exposure to estrogenic compounds (e.g., Haux and Nordberg 1985). A significant (p < 0.01) increase in relative liver weight (HSI) was seen in Atlantic salmon exposed to 1500 mg DEHP/kg (nominal concentration) four months post-exposure in the study by Norrgren et al. (1999) but not in the present study, which indicates different exposure conditions.
In summary, dietary exposure of Atlantic salmon to DEHP caused no late effects on growth, survival, or hepatosomatic index. Ovo-testis was observed in 3% of the fish exposed to a nominal concentration of 1500 mg DEHP/kg but no complete sex reversal resulting in skewed sex ratios was found. The environmental significance of the mild histological changes observed is lowered by the relatively high dose level used. The rapid elimination of both DEHP and MEHP to near background levels one week post-exposure suggests a short half life and a low risk of bioaccumulation in Atlantic salmon. However, it cannot be excluded that chronic exposure to DEHP, in contrast to the short-term exposure performed in the present study, may cause more pronounced effects.
References
Andersen Ø, Eijsink VGH, Thomassen M (2000) Multiple variants of the peroxisome proliferator-activated receptor (PPAR) γ are expressed in the liver of Atlantic salmon (Salmo salar). Gene 255:411–418
Balafas D, Shaw KJ, Whitfield FB (1999) Phthalate and adipate esters in Australian packaging materials. Food Chem 65:279–287
Barron MG, Tarr BD, Hayton WL (1987) Temperature dependence of di-2-ethylhexyl phthalate (DEHP) pharmacokinetics in rainbow trout. Toxicol Appl Pharmacol 88:305–312
Barron MG, Schultz IR, Hayton WL (1989) Presystemic branchial metabolism limits di-2-ethylhexyl phthalate accumulation in Fish. Toxicol Appl Pharmacol 98:49–57
Barron MG, Albro PW, Hayton WL (1995) Biotransformation of di(2-ethylhexyl) phthalate by rainbow trout. Environ Toxicol Chem 14(5):873–876
Blom A, Ekman E, Johannisson A, Norrgren L, Pesonen M (1998) Effects of xenoestrogenic environmental pollutants on the proliferation of human breast cancer cell line (MCF-7). Arch Environ Contam Toxicol 34:306–310
David F, Sandra P (2001) Phthalate esters in the environment. Monitoring program for the determination of phthalates in air, vegetation, cattle feed, milk and fish in the Netherlands (1999–2001). Report No. ECPI-2001-10. Research Institute of Chromatography, Kortrijk, Belgium
Davis BJ, Maronpot RR, Heindel JJ (1994) Di-(2-ethylhexyl) phthalate suppresses estradiol and ovulation in cycling rats. Toxicol Appl Pharmacol 128:216–223
Devlin RH, Nagahama Y (2002) Sex determination and sex differentiation in fish: an overview of genetic, physiological, and environmental influences. Aquaculture 208:191–364
Earls AO, Axford IP, Braybrook JH (2003) Gas chromatography-mass spectrometry determination of the migration of phthalate plasticisers from polyvinyl chloride toys and childcare articles. J Chromatogr 983A:237–246
Giam CS, Chan HS, Neff GS (1975) Sensitive methods for determination of phthalate ester plasticizers in open-ocean biota samples. Anal Chem 47(13):2225–2229
Goetz FW, Donaldson EM, Hunter GA, Dye HM (1979) Effects of estradiol-17β and 17α-methyl testosterone on gonadal differentiation in the Coho salmon, Oncorhynchus kisutch. Aquaculture 17:267–278
Gray LE Jr, Wolf C, Lambright C, Mann P, Price M, Cooper RL, Ostby J (1999) Administration of potentially antiandrogenic pesticides (procymidone, linuron, iprodione, chlozolinate, p,p’-DDE, and ketoconazole) and toxic substances (dibutyl- and diethylhexyl phthalate, PCB 169, and ethane dimethane sulphonate) during sexual differentiation produces diverse profiles of reproductive malformations in the male rat. Toxicol Ind Health 15:94–118
Harris CA, Henttu P, Parker MG, Sumpter JP (1997) The estrogenic activity of phthalate esters in vitro. Environ Health Perspect 105(8):802–811
Haux C, Norberg B (1985) The influence of estradiol-17β on the liver content of protein, lipids, glycogen and nucleic acids in juvenile rainbow trout, Salmo gairdnerii. Comp Biochem Physiol 81B(2):275–279
Isenberg JS, Kamendulis LM, Ackley DC, Smith JH, Pugh G Jr, Lington AW, McKee RH, Klaunig JE (2001) Reversibility and persistence of di-2-ethylhexyl phthalate (DEHP)- ands Phenobarbital-induced hepatocellular changes in rodents. Toxicol Sci 64:192–199
Jobling S, Nolan M, Tyler CR, Brighty G, Sumpter JP (1998) Widespread sexual disruption in wild fish. Environ Sci Technol 32:2498–2506
Johnstone R, Simpson TH, Youngson AF (1978) Sex reversal in salmonid culture. Aquaculture 13:115–134
Kim E-J, Kim J-W, Lee S-K (2002) Inhibition of oocyte development in Japanese medaka (Oryzias latipes) exposed to di-2-ethylhexyl phthalate. Environ Int 28:359–365
Koger CS, Teh SJ, Hinton DE (2000) Determining the sensitive developmental stages of intersex induction in medaka (Oryzias latipes) exposed to17β-estradiol or testosterone. Mar Environ Res 50:201–206
Larsson DGJ, Adolfsson-Erici M, Parkkonen J, Petterson M, Berg AH, Olsson P-E, Förlin L (1999) Ethinyloestradiol: an undesired fish contraceptive? Aquat Toxicol 45:91–97
Lapinskas PJ, Brown S, Leesnitzer LM, Blanchard s, Swanson C, Cattley RC, Corton JC (2005) Role of PPARα in mediating the effects of phthalates and metabolites in the liver. Toxicology 207:149–163
Lovekamp TN, Davis BJ (2001) Mono-(2-ethylhexyl) phthalate suppresses aromatase transcript levels and estradiol production in cultured rat granulosa cells. Toxicol Appl Pharmacol 172:217–224
Matthiessen P, Allen Y, Bamber S, Craft J, Hurst M, Hutchinson T, Feist S, Katsiadaki I, Kirby M, Robinson C, Scott S, Thain J, Thomas K (2002) The impact of oestrogenic and androgenic contamination on marine organisms in the United Kingdom: summary of the EDMAR programme. Mar Environ Res 54:645–649
Metcalfe CD, Metcalfe TL, Kiparissis Y, Koenig BG, Khan C, Hughes RJ, Croley TR, March RE, Potter T (2001) Estrogenic potency of chemicals detected in sewage treatment plant effluents as determined by in vivo assays with Japanese medaka (Oryzias latipes). Environ Toxicol Chem 20(2):297–308
Moody DE, Reddy JK (1978) Hepatic peroxisome (Microbody) proliferation in rats fed plasticizers and related compounds. Toxicol Appl Pharmacol 45:497–504
Mu Y-M, Yanase T, Nishi Y, Waseda N, Oda T, Tanaka A, Takayanagi R, Nawata H (2000) Insulin sensitizer, troglitazone, directly inhibits aromatase activity in human ovarian granulosa cells. Biochem Biophys Res Commun 271:710–713
Norrgren L, Blom A, Andersson PL, Börjeson H, Larsson DGJ, Olsson P-E (1999) Effects of potential xenoestrogens (DEHP, nonylphenol and PCB) on sexual differentiation in juvenile Atlantic salmon (Salmo salar). Aquat Ecosyst Health Manage 2:311–317
Ortiz-Zarragoitia M, Cajaraville MP (2000) Environmental estrogenic compounds alter peroxisomal functions in fish. Comp Biochem Physiol A 126:S1–S113
Page BD, Lacroix GM (1995) The occurrence of phthalate ester and di-2-ethylhehyl adipate plasticizers in Canadian packaging and food sampled in 1985-1989: a survey. Food Addit Contam 12(1):129–151
Pandian TJ, Sheela SG (1995) Hormonal induction of sex reversal in fish. Aquaculture 138:1–22
Parks LG, Ostby JS, Lambright CR, Abbott B, Klinefelter GR, Barlow NJ, Gray LE Jr (2000) The plasticizer diethylhexyl phthalate induces malformation by decreasing fetal testosterone synthesis during sexual differentiation in the male rat. Toxicol Sci 58:339–349
Reddy JK, Lalwani ND (1983) Carcinogenesis by hepatic peroxisome proliferators: evaluation of the risk of hypolipidemic drugs and industrial plasticizers to humans. CRC Critic Rev Toxicol 12:1–58
Ruyter B, Andersen Ø, Dehli A, Östlund Farrants A-K, Gjøen T, Thomassen MS (1997) Peroxisome proliferator activated receptors in Atlantic salmon (Salmo salar): effects on PPAR transcription and acyl-CoA oxidase activity in hepatocytes by peroxisome proliferators and fatty acids. Biochim Biophys Acta 1348:331–338
Scarano LJ, Calabrese EJ, Kostecki PT, Baldwin LA, Leonard DA (1994) Evaluation of a rodent peroxisome proliferator in two species of freshwater fish: Rainbow trout (Onchorynchus mykiss) and Japanese Medaka (Oryzias latipes). Ecotox Environ Saf 29:13–19
Soto AM, Sonnenschein C, Chung KL, Fernandez MF, Olea N, Olea Serrano F (1995) The E-screen assay as a tool to identify estrogens; an update on estrogenic environmental pollutants. Environ Health Perspect 103(7):113–122
Sower SA, Dickhoff WW, Flagg TA, Michall JL, Mahnken CVW (1984) Effects of estradiol and diethylstilbestrol on sex reversal and mortality in Atlantic salmon (Salmon salar). Aquaculture 43:75–81
Staples CA, Peterson DR, Parkerton TF, Adams WJ (1997) The environmental fate of phthalate esters: A literature review. Chemosphere 35(4):667–749
Tan GH (1995) Residue levels of phthalate esters in water and sediment samples from the Klang river basin. Bull Environ Contam Toxicol 54:171–176
Thomann RV (1989) Bioaccumulation model of organic chemical distribution in aquatic food chains. Environ Sci Technol 23:699–707
Tienpont B, David F, Sandra P (in prep.). In preparation for J Chromatogr
Vethaak AD, Rijs GBJ, Schrap SM, Ruiter H, Gerristen A, Lahr J (2002) Estrogens and xeno-estrogens in the aquatic environment of the Netherlands, occurrence, potency and biological effects, RIZA/RIKZ Report no 2002.001, Dictorate-General for Public Works and Waste Management
Wahl HG, Hoffman A, Häring H-U, Leibich HM (1999) Identification of plasticizers in medical products by a combined direct thermodesorption-cooled injection system and gas chromatography-mass spectrometry. J Chromatogr 847A:1–7
Zacharewski TR, Meek MD, Clemons JH, Wu ZF, Fielden MR, Matthews JB (1998) Examination of the in vitro and in vivo estrogenic activities of eight commercial phthalate esters. Toxicol Sci 46:282–293
Acknowledgements
This work was financially supported by the European Council for Plasticizers and Intermediates (ECPI) and by grants from the ReproSafe-program at the Swedish environmental Protection Agency. The Swedish Board of Fisheries, Älvkarleby is acknowledged for keeping the fish and for support during sampling.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Norman, A., Börjeson, H., David, F. et al. Studies of Uptake, Elimination, and Late Effects in Atlantic Salmon (Salmo salar) Dietary Exposed to Di-2-Ethylhexyl Phthalate (DEHP) During Early Life. Arch Environ Contam Toxicol 52, 235–242 (2007). https://doi.org/10.1007/s00244-005-5089-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-005-5089-y