Abstract
Aqueous exposures to selenomethionine (SeMet), the major form of selenium (Se) in the diet, represent a rapid and simplified method for determining the embryotoxic effects of SeMet. Using fathead minnows (Pimephales promelas) as a model test organism, the objective of this study was to evaluate the effects of waterborne exposure to elevated SeMet on embryos from fertilization to swim-up. Newly fertilized embryos were exposed for 6 days to 30, 90, 270, 810, 2430, 7290, 21,870, and 65,610 µg Se/L (as SeMet). Survival, hatchability, days to hatch, and the frequency and severity of deformities (total and type) were quantified. SeMet exposure reduced hatchability and days to hatch at concentrations ≥ 21870 µg/L. Significant decreases in survival and significant increases in the incidence and severity of deformities were observed at concentrations ≥ 810 µg/L. The results suggest that early life-stage fathead minnows are more tolerant to aqueous exposure to SeMet compared to medaka and zebrafish.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Selenium (Se) is an important micronutrient in all vertebrate organisms due to its involvement in the synthesis of select proteins and enzymes involved in redox metabolism (Young et al. 2010). However, its narrow range between essentiality and toxicity has generated concern as certain industrial activities have exacerbated the loading of Se into the aquatic environment (Lemly 2004). Oviparous vertebrates such as amphibians, reptiles, birds, and fishes are particularly susceptible to elevated dietary Se concentrations during early life stages, as Se is readily transferred from mother to embryo through egg yolk proteins (Janz et al. 2010). Exposure to selenomethionine (SeMet), the predominant form of Se in the diet, during sensitive developmental stages can result in teratogenic effects such as spinal deformities (lordosis, kyphosis, scoliosis), missing or misshapen fins, craniofacial malformations, as well as an increase in the incidence of edema (craniofacial, yolk sac, pericardial) and mortality in developing fish larvae (Lemly 1997; Janz 2012). Deformities are ecotoxicologically relevant as they can impair recruitment, diminishing population size and diversity over time (Lemly 2002).
Previous studies addressing the embryotoxic effects of Se have largely consisted of maternal dietary exposures (Janz et al. 2010), with more recent studies using yolk microinjections (Thomas and Janz 2016) and aqueous embryo exposures (Lavado et al. 2012; Arnold et al. 2016; Kupsco and Schlenk 2016a, b). Since previous research involving aqueous embryo exposures to Se utilized Japanese medaka (Oryzias latipes) and zebrafish (Danio rerio) as model test organisms, similar work on fish species native to North America has received little attention. Using fathead minnows (Pimephales promelas) as a model test organism, the purpose of this study was to evaluate the effects of elevated SeMet, in solution, on the hatchability, mortality, and incidence/severity of deformities in developing fathead minnow embryos exposed during early life stages.
Materials and Methods
Newly fertilized fathead minnow embryos were collected from an in house adult breeding colony that was maintained in an environmental chamber under controlled temperature (25 ± 1 °C) and photoperiod (16:8 light:dark) in the Aquatic Toxicology Research Facility (ATRF) at the Toxicology Centre, University of Saskatchewan (Saskatoon, Canada). Healthy embryos between stage 5–10 (within 4.5 h of fertilization) of embryonic development (USEPA 1996) were selected and randomly distributed into glass petri dishes containing 40 mL of test solution. Each individual petri dish contained 20–30 embryos, and there were 3–6 replicate petri dishes in each exposure group. Exposure proceeded for 6 days in E3 Embryo Medium (Cold Spring Harbor Protocols 2012) alone (control embryos) and E3 Embryo Medium spiked with nominal seleno-l-methionine (SeMet; ≥ 98% purity) (Sigma–Aldrich, Oakville, ON, Canada) concentrations of 30, 90, 270, 810, 2430, 7290, 21,870, and 65,610 µg Se/L. Dead embryos were removed, and 75% solution changes were performed daily. Following the 6-day exposure, remaining live embryos in each petri dish were euthanized with an overdose of buffered tricaine methanesulfonate (MS-222; 250 mg/L, pH 7.4) (Sigma–Aldrich, Oakville, ON, Canada), fixed in buffered 10% formalin for 18–24 h, and stored in 70% ethanol. The preserved larvae were then examined for malformations in a blind fashion using an Olympus model S261 dissecting microscope (Olympus, Melville, NY, USA).
In the present study, frequency analysis and the Graduated Severity Index (GSI) were used to quantify deformities in fry (Holm et al. 2003) (Fig. S1). During frequency analysis, larvae in each replicate were individually assessed and categorized as either deformed or not deformed. An overall incidence of deformities was thus calculated by dividing the number of individuals classified as deformed by the total number of individuals in that replicate. This process was then repeated for abnormalities in four distinct categories: skeletal curvatures (kyphosis, lordosis, and scoliosis), craniofacial, finfold, and edema.
The severity of abnormalities was also assessed using severity scores adapted from a graduated severity index system described previously (Holm et al. 2003). Severity scores ranged from 0 to 2, with a score of 0 representing normal; 1—moderate; and 2—severe. Total GSI, which describes the overall severity of deformities in each treatment, was calculated by summing the severity score assigned to each category (kyphosis, lordosis, scoliosis, craniofacial, finfold, and edema) for each individual fry in the replicate. The sums calculated for each fry were then averaged to obtain an overall severity for each replicate. In addition to total GSI, the severity of each distinct type of deformity was calculated by averaging the severity scores assigned to each fry in the category of interest in each replicate.
Stock solution samples were filtered and acidified with 2% high purity nitric acid (Fisher Scientific, Hampton, NH, USA) for total Se analysis. Pooled larvae collected for total Se analysis were euthanized with MS-222, collected in microcentrifuge tubes, and freeze dried. Dried samples were then digested using high purity nitric acid and 30% hydrogen peroxide. Inductively coupled plasma-mass spectrometry (ICP-MS) was used to quantify total Se in both water and tissue samples as described previously (Thomas and Janz 2016). Instrument performance was verified using the standard reference material 1640a solution (National Institute of Standards and Technology), with recoveries of 102% (SD; ± 2.65%) and 104% (SD; ± 3.46%) for water samples and 92.5% (SD; ± 1.92%) for tissue samples. The tissue digestion process was verified using a certified reference material (TORT-2, lobster hepatopancreas, NRC, Ottawa, ON, Canada) for which a recovery of 97.8% (SD; ± 2.05%) was obtained. Instrument detection limit differed with each run, with a detection limit of 0.029–0.164 µg/L for water samples and 0.026 mg/kg for tissue.
All results were reported as mean ± standard error, and all analyses were performed using GraphPad Prism Version 7.03 (GraphPad Software: La Jolla, CA, USA). All data were tested for normality and homogeneity of variance using the Shapiro–Wilk normality test and either the Browne-Forsythe test or Bartlett’s test as appropriate. When data distribution was normal and exhibited homogeneity of variance, one-way analysis of variance (ANOVA) followed by Dunnett’s post hoc test was used to test for significant differences between treatments and the control group. When data distribution was non-normal, or data could not be satisfactorily transformed, a non-parametric Kruskal–Wallis one-way ANOVA by ranks was used followed by Dunn’s multiple comparisons post hoc test. Alpha values were two-tailed and set at 0.05. A nonlinear regression, dose–response analysis without constraints was performed on the percent cumulative mortality data to determine the LC50 for SeMet exposure.
Results and Discussion
In a selenium-contaminated aquatic system, a developing embryo would be exposed to predominantly inorganic species of selenium such as selenite and selenate (Young et al. 2010). However, these species lack biological relevance as the ecologically relevant Se exposure pathway for embryos is maternal transfer of SeMet accumulated through diet (Janz 2012). As a result, previous research addressing the embryotoxic effects of Se has largely consisted of yolk microinjections or adult feeding/breeding studies; however, these methods tend to be time consuming, labor intensive, and require specific skills and equipment to conduct. As such, aqueous SeMet exposures were selected as they offer a more rapid and simplified method for determining the effects of SeMet on developing embryos.
Although the chorion has the potential to act as a barrier to xenobiotics during waterborne exposures (Finn 2007), previous work has suggested that the chorion is water permeable and potentially exhibits some degree of permeability to smaller organic molecules (Alderdice 1988; Finn 2007) such as SeMet. To determine if Se uptake across the chorion was occurring, total Se was quantified in pooled embryos following 6 days of aqueous exposure at concentrations of 810, 2430, 7290, and 21,870 µg/L (Table 1). Total Se in control embryos was 2.26 µg Se/g dry mass (d.m.), while embryos treated aqueously with 810, 2430, 7290, and 21,870 µg Se/L exhibited tissue concentrations of 90.68, 53.40, 63.68, and 88.66 µg/g d.m., respectively, indicating bioaccumulation in larvae, although not in a clear exposure concentration-dependent manner. To verify exposure concentrations, test solutions were analyzed for total Se using ICP-MS. Measured concentrations were all in agreement with nominal concentrations (Table 1).
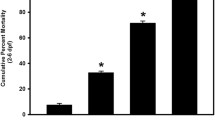
SeMet exposures ≥ 810 µg/L significantly decreased embryo survival, with percentages of 22.3 ± 7.6%, 28.7 ± 9.9%, 47.3 ± 9.0%, and 95.8 ± 2.2% (p = 0.0141, 0.0178, < 0.0001, and < 0.0001, respectively) compared to 2.50 ± 1.5% in the controls (Fig. S2). An LC50 value of 17,763 µg/L (95% confidence interval of 9761–42,503 µg/L) was calculated for SeMet-induced mortality (Fig. 1). While Arnold et al. (2016) reported a significant elevation in mortality at a concentration of 100 µg SeMet/L in zebrafish, previous research involving aqueous SeMet embryo exposures with Japanese medaka reported significant elevations in mortality at concentrations ≥ 490 µg/L (Kupsco and Schlenk 2016a, b). Thus, it appears that fathead minnow embryos are equally and less sensitive to aqueous SeMet exposures than medaka and zebrafish, respectively.
Dose–response curve illustrating mean (± SE) percent cumulative mortality (1–6 dpf) of fathead minnows exposed to increasing concentrations of l-selenomethionine (SeMet) via embryo aqueous exposure. Dotted lines represent the 95% confidence interval (CI), and error bars are standard error. Calculated LC50 = 17,763 µg/L (95% CI = 9761–42,503 µg/L)
The percentage of total deformities increased significantly at concentrations ≥ 810 µg SeMet/L, with percentages of 67.7 ± 12% and 79.9 ± 3.4% (p = 0.0277 and 0.0102, respectively) compared to 34.1 ± 4.3% in the controls (Fig. S3). In comparison, aqueous SeMet embryo exposures with Japanese medaka were reported to cause a significant increase in the percentage of total deformities at SeMet concentrations ≥ 490 µg/L (Kupsco and Schlenk 2016a, b). Despite the elevated proportion of deformed individuals in the controls (34.1 ± 4.3%), previous Se research conducted with the same fathead minnow cultures used in the present study reported background rates of 16.6 ± 5.8%, with the rate of deformities ranging from 0 to 29% in the controls (Lane et al. 2017). The elevated and variable rate of deformities observed in control fish is likely related to variations in egg quality (McDonald and Chapman 2009), which differs across individual eggs as well as eggs collected from different females. Furthermore, previous Se research conducted in field-based settings have found similar deformity rates at reference sites. For example, Holm et al. (2005) found that background rates of deformities (edema, skeletal, finfold, and craniofacial) in rainbow trout (Oncorhynchus mykiss) offspring from one of two reference creeks ranged from 7.9 to 42.2%. Similarly, Rudolph et al. (2008) reported background rates of 37.4 ± 3.6% for skeletal deformities in cutthroat trout (Oncorhynchus clarkii) fry from a reference lake.
In addition to total deformities, the percentage of edema, craniofacial and fin malformations, as well as spinal curvatures in the form of kyphosis, lordosis, and scoliosis were also calculated (Fig. 2). The incidence of edema increased significantly, and in a concentration-dependent manner, at concentrations ≥ 810 µg/L, with percentage malformations ranging between 50.9 ± 14% and 73.0 ± 2.9% (p < 0.0209, 0.0152, 0.0294, and 0.0020, respectively) compared to 14.4 ± 4.6% in the controls. Fin malformations increased significantly at 810 and 21,870 µg/L, with percentages of 27.8 ± 6.2% and 38.7 ± 0.89% (p = 0.0214 and 0.0073, respectively) compared to 6.32 ± 3.7% in the controls. For spinal deformities, the percentage of scoliosis increased significantly at 810 and 2430 µg/L, with percentages of 18.8 ± 3.6% and 20.5 ± 7.1% (p = 0.0224 and 0.0137, respectively) compared to 2.82 ± 1.3% in the controls. Previous studies have reported that spinal deformities tend to be the most common type of deformity observed in Se-exposed embryos, with lordosis representing the most prevalent form of spinal deformity (Muscatello et al. 2006; Kupsco and Schlenk 2016b). However, edema and fin/craniofacial malformations appeared to be more prevalent than spinal deformities in the present study, and lordosis did not appear to be more common than scoliosis or kyphosis (Fig. 2). However, it is important to note that factors such as the developmental stage at which exposure was conducted has the potential to influence the specific types of deformities observed in developing fish larvae. For example, aqueous SeMet exposures conducted with Japanese medaka during stages 9, 17, 25, 29, 34, and 38 of development, which range from the late morula stage (256–512 cell stage) to the spleen development stage (Iwamatsu 2004) (i.e. between 5 and 192 h post fertilization), resulted in differing expression of certain types of deformities depending on the stage at which treatment was initiated (Kupsco and Schlenk 2016b). It is unsurprising then that different types of deformities were observed in the Japanese medaka, as exposures in the present study were conducted on embryos between stages 5–10 (4 cell-high blastula cell stages; i.e. within 4.5 h post fertilization) of embryonic development (USEPA 1996), and therefore earlier stages of development. In addition to developmental stages, water chemistry parameters (pH, hardness, alkalinity, etc.) also differed across studies. Although not completely understood, previous work has suggested that water chemistry might influence embryo permeability (Alderdice 1988; Finn 2007). This indicates the need to exercise caution when making direct comparisons to other aqueous SeMet exposure studies with different water chemistry parameters, as these factors might mediate SeMet toxicity.
Mean (± SE) percentage of A skeletal deformities (kyphosis, lordosis, and scoliosis) and B other deformities (craniofacial, finfold, and edema) in larval fathead minnows exposed to increasing concentrations of l-selenomethionine (SeMet) via aqueous exposure. Asterisks represent significant differences compared to the control using either a Kruskal–Wallis one-way analysis of variance (ANOVA) by ranks followed by a Dunn’s multiple comparisons test, or a one-way ANOVA followed by a Dunnett’s multiple comparisons test (p < 0.05); n = 3–6 replicates of 20–30 embryos
In addition to frequency, the severity of deformities was also evaluated. Although relatively uncommon in the literature, a total GSI score was calculated to reflect the overall severity of deformities in each treatment. At 810, 2430, and 21,870 µg/L a significant increase in the severity of deformities was observed, with total GSI scores of 2.40 ± 0.51, 2.27 ± 0.73, and 2.85 ± 0.06 (p = 0.0150, 0.0378, and 0.0094, respectively) compared to 0.77 ± 0.14 in the control larvae (Fig. S4). In addition to total GSI, the severity of individual categories was also assessed (Fig. 3). At 810, 2430, and 21,870 µg/L, the edema index increased significantly, with GSI scores of 0.63 ± 0.17, 0.69 ± 0.19, and 0.95 ± 0.07 (p = 0.0431, 0.0229, and 0.0016, respectively) compared to 0.20 ± 0.07 in the control larvae. The severity of fin malformations increased significantly at 810 and 21,870 µg/L, with GSI scores of 0.44 ± 0.11 and 0.55 ± 0.09 (p = 0.0066 and 0.0032, respectively) compared to 0.06 ± 0.04 in the controls. For spinal deformities, the severity of kyphosis increased significantly at 810 µg/L, with a GSI score of 0.31 ± 0.09 (p = 0.036) compared to 0.03 ± 0.02 in the controls. Scoliosis increased significantly in severity at 270, 810, and 2430 µg/L, with GSI scores of 0.27 ± 0.06, 0.34 ± 0.07 and 0.34 ± 0.11 (p = 0.036, 0.0061, and 0.0073, respectively) compared to 0.03 ± 0.01 in the controls. Similar to frequency analysis results, edema and fin/craniofacial malformations appeared more responsive to SeMet concentration than spinal deformities. However, further work would be required to determine why these types of deformities were more common in fathead minnows exposed aqueously to SeMet compared to other fish species.
Mean (± SE) graduated severity index (GSI) scores of A skeletal deformities (kyphosis, lordosis, and scoliosis) and B other deformities (craniofacial, finfold, and edema) in larval fathead minnows exposed to increasing concentrations of l-selenomethionine (SeMet) via aqueous exposure. Asterisks represent significant differences compared to the control using either a Kruskal–Wallis one-way analysis of variance (ANOVA) by ranks followed by a Dunn’s multiple comparisons test, or a one-way ANOVA followed by a Dunnett’s multiple comparisons test (p < 0.05); n = 3–6 replicates of 20–30 embryos
With exception of the greatest test concentrations, SeMet exposure had no significant effect on mean days to hatch or percent hatch of fathead minnow larvae (Figs. S5 and S6). A significant reduction in mean days to hatch (approximately 3d compared to 4d in the controls) was observed at 65,610 µg/L (p = 0.0194; Fig. S5). In addition, a significant reduction in embryo hatchability was observed at 21,870 µg/L and 65,610 µg/L, with percentages of 86.5 ± 3.1% and 21.7 ± 4.4% (p = 0.0389 and p < 0.0001, respectively) compared to 96.6 ± 1.2% in the controls (Fig. S6). Previously, Japanese medaka embryos exposed aqueously to 0.5, 5, and 50 µM (approximately 100, 1000, and 10,000 µg/L) SeMet at six different developmental stages reported a significant reduction in the median days to hatch only in embryos exposed with 5 µM SeMet at stage 34 (Kupsco and Schlenk 2016b); however, significant reductions in hatchability were reported at concentrations ≥ 5 µM SeMet at all stages of development (Kupsco and Schlenk 2016b). Similarly, Japanese medaka exposed to 0.05 mM (approximately 10,000 µg/L) SeMet in freshwater and three different hypersaline conditions reported a significant reduction in hatchability in all treatment groups (Lavado et al. 2012).
In summary, our results indicate that aqueous exposures to SeMet increased the incidence and severity of deformities, as well as led to the bioaccumulation of Se within embryonic tissue.
The production of deformities and adverse effects similar to those observed with other methods of Se exposure (i.e. dietary and yolk microinjections), suggests that aqueous exposures still represent a simplified and rapid method for studying the embryotoxic effects of Se. However, the lack of clear dose–response relationships for both the frequency and severity of larval deformities suggests that the chorion potentially limits uptake of SeMet, and thus reduces exposure. Furthermore, the apparent tolerance of fathead minnow embryos used in this study relative to other aqueously exposed fish species inspires more questions about the role of factors such as developmental pattern and chorion physiology, and how this potentially influences toxicity across fish species.
References
Alderdice D (1988) Osmotic and ionic regulation in teleost eggs and larvae. In: Hoar WS, Randall DJ (eds) Fish physiology: the physiology of developing fish eggs and larvae, 11A. Academic Press, San Diego, pp 163–251
Arnold MC, Forte JE, Osterberg JS, Di Giulio RT (2016) Antioxidant rescue of selenomethionine-induced teratogenesis in zebrafish embryos. Arch Environ Contam Toxicol 70(2):311–320
Cold Spring Harbor Protocols (2012) E3 embryo medium (60x). Cold Spring Harbor Protoc https://doi.org/10.1101/pdb.rec070540
Finn RN (2007) The physiology and toxicology of salmonid eggs and larvae in relation to water quality criteria. Aquat Toxicol 81(4):337–354
Holm J, Palace VP, Wautier K, Evans R, Baron CL, Podemski C, Siwik P, Sterling G (2003) An assessment of the development and survival of wild rainbow trout (Oncorhynchus mykiss) and brook trout (Salvelinus fontinalis) exposed to elevated selenium in an area of active coal mining. The Big Fish Bang, Proceedings of the 26th Annual Larval Fish Conference, pp 257–273
Holm J, Palace V, Siwik P, Sterling G, Evans R, Baron C, Werner J, Wautier K (2005) Developmental effects of bioaccumulated selenium in eggs and larvae of two salmonid species. Environ Toxicol Chem 24(9):2373–2381
Iwamatsu T (2004) Stages of normal development in the medaka Oryzias latipes. Mech Dev 121(7):605–618
Janz DM (2012) Selenium. In: Wood CM, Farrell AP, Brauner CJ (eds) Fish physiology: homeostasis and toxicology of essential metals, 31A. Elsevier, San Diego, pp 327–373
Janz DM, DeForest DK, Brooks ML, Chapman PM, Gilron G, Hoff D, Hopkins WD, McIntyre DO, Mebane CA, Palace VP, Skorupa JP, Wayland M (2010) Selenium toxicity to aquatic organisms. In: Chapman PM, Adams WJ, Brooks ML, Delos CG, Luoma SN, Maher WA, Ohlendorf HM, Presser TS, Shaw DP (eds) Ecological assessment of selenium in the aquatic environment. CRC Press Pensacola, FL, pp 141–231
Kupsco A, Schlenk D (2016a) Molecular mechanisms of selenium-induced spinal deformities in fish. Aquat Toxicol 179:143–150
Kupsco A, Schlenk D (2016b) Stage susceptibility of Japanese medaka (Oryzias latipes) to selenomethionine and hypersaline developmental toxicity. Environ Toxicol Chem 35(5):1247–1256
Lane T, Janz DM, Liber K, Green D, Bluhm K, Raes K, Doig LE, Hecker M (2017) Developmental effects of maternally transferred selenium to early life stage fathead minnow (Pimephales promelas). Poster WP031 at SETAC North America 38th Annual Meeting
Lavado R, Shi D, Schlenk D (2012) Effects of salinity on the toxicity and biotransformation of l-selenomethionine in Japanese medaka (Oryzias latipes) embryos: mechanisms of oxidative stress. Aquat Toxicol 108:18–22
Lemly DA (1997) A teratogenic deformity index for evaluating impacts of selenium on fish populations. Ecotoxicol Environ Saf 37(3):259–266
Lemly DA (2002) Symptoms and implications of selenium toxicity in fish: the Belews Lake case example. Aquat Toxicol 57(1):39–49
Lemly DA (2004) Aquatic selenium pollution is a global environmental safety issue. Ecotoxicol Environ Saf 59(1):44–56
McDonald B, Chapman P (2009) The need for adequate quality assurance/quality control measures for selenium larval deformity assessments: Implications for tissue residue guidelines. Integr Environ Assess Manag 5(3):470–475
Muscatello JR, Bennett PM, Belknap AM, Himbeault KT, Janz DM (2006) Larval deformities associated with selenium accumulation in northern pike (Esox lucius) exposed to metal mining effluent. Environ Sci Technol 40(20):6506–6512
Rudolph B-L, Andreller I, Kennedy CJ (2008) Reproductive success, early life stage development, and survival of westslope cutthroat trout (Oncorhynchus clarki lewisi) exposed to elevated selenium in an area of active coal mining. Environ Sci Technol 42(8):3109–3114
Thomas JK, Janz DM (2016) Embryo microinjection of selenomethionine reduces hatchability and modifies oxidant responsive gene expression in zebrafish. Sci Rep 6:26520
USEPA (1996) Prehatching development of the fathead minnow Pimephales promelas Rafinesque. USEPA, Duluth, EPA/600/R-96/079
Young TF, Finley K, Adams WJ, Besser J, Hopkins WD, Jolley D, McNaughton E, Presser TS, Shaw DP, Unrine J (2010) What you need to know about selenium. In: Chapman PM, Adams WJ, Brooks ML, Delos CG, Luoma SN, Maher WA, Ohlendorf HM, Presser TS, Shaw DP (eds) Ecological assessment of selenium in the aquatic environment. CRC Press, Boca Raton, pp 7–45
Acknowledgements
This study was supported by a Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant (RGPIN-2016-05131) to DMJ and a Toxicology Graduate program scholarship to AKG. The authors thank L. Kapronczai, T. Lane and C. Grimard for their laboratory assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gerhart, A.K., Hecker, M. & Janz, D.M. Toxicity of Aqueous l-Selenomethionine Exposure to Early Life-Stages of the Fathead Minnow (Pimephales promelas). Bull Environ Contam Toxicol 102, 323–328 (2019). https://doi.org/10.1007/s00128-018-02537-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-018-02537-2