Abstract
The presence of phthalates constitutes a risk to the health of aquatic environments and organisms. This work aimed to evaluate the toxic effects of di-iso-pentyl-phthalate (DiPeP) at environmentally relevant concentrations of 5, 25, and 125 µg/L in Danio rerio after subchronic exposure for 14 days. DiPeP altered the antioxidant system in the liver (125 μg/L), intestine (25 μg/L), brain, and gills in all concentrations tested. In animals exposed to 125 μg/L, DNA damage was identified in the gills. In addition, loss of cell boundary of hepatocytes, vascular congestion, necrosis in the liver, and presence of immune cells in the intestinal lumen were observed. Erythrocytic nuclear alterations in the blood occurred in animals exposed to 25 μg/L. DiPeP was quantified in muscle tissue at all exposure concentrations, appearing in a concentration-dependent manner. Contaminants such as DiPeP will still be used for a long time, mainly by industries, being a challenge for industry versus environmental health.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plasticizers can easily contaminate the environment and induce adverse health effects on different organisms, including humans (Schug et al. 2011). Currently, phthalates are a great public health concern since are ubiquitous in the environment become human exposure a real possibility (Souza et al. 2022). Phthalates or phthalic acid esters are short-chain polymeric additives dispersed in a polymer matrix, causing a reduction in viscosity during processing and thus allowing greater flexibility to the product. This is possible due to the reduction of intermolecular forces of the polymeric chains, allowing a greater sliding between the chains (Koch et al. 2012). People use plasticizers all life (Luongo and Ostman 2016) and oral and dermal via becoming the most relevant exposition route (Wensing et al. 2005). This visible exposure, due to the increase in food packaging, indicates that food can be a great potential source of exposure since phthalates migrate from food and beverage packaging (Rudel et al. 2011).
In the environment, the persistence of phthalates is an emerging public health problem, due to the potential effects on reproduction, obesity, and development (Gutiérrez-García et al. 2019). Rocha et al. (2017) reported that one-quarter of Brazilian children had a hazard index of > 1 for phthalate exposures. The authors found a positive association between urinary phthalate concentrations and oxidative stress.
Phthalates can potentially affect different physiological processes in fish species such as reproductive health (Uren-Webster et al. 2010), embryonic development (Liu and Zhao 2009), and antioxidant system (Kang et al. 2010; Mankidy et al. 2013). The associated risk assessments are mainly directed toward certain phthalates, such as dimethyl phthalate (DMP), diethyl phthalate (DEP), and di-2-ethylhexyl phthalate (DEHP) (EFSA Panel on Food Contact, Materials 2019). The toxicological effects caused by other phthalates such as di-iso-pentyl phthalate (DiPeP) have not received considerable attention.
Di-iso-pentyl phthalate, also called diisoamyl phthalate, is the product of a reaction between phthalic anhydride and isoamyl alcohol, a compound that originates from the manufacture of sugarcane in the process to obtain ethanol. It is used in industrial applications as solvents and esters in general and it is found in commonly used products, such as shoes and hoses (Brisco 2011; Petrom 2016). There are already studies that detect this phthalate in landfills in Brazil (Do Nascimento Filho et al. 2003; Ferreira and Morita 2012). Although the toxicity of this phthalate is not well studied yet, the relevance of human exposure should be examined as metabolites have already been detected in the urine of pregnant women (Bertoncello Souza et al. 2018). High doses of antiandrogenic phthalates can lead to Phthalate Syndrome characterized by male reproductive tract abnormalities (reduced anogenital distance, cryptorchidism, hypospadias, and low sperm count Lioy et al. 2015; Bertoncello Souza et al. 2018). Changes in sexual behavior in rat males were also observed (Neubert da Silva et al. 2019). In humans, there is not a clear pattern of association between prenatal phthalate exposures and toxic effects (Radke et al. 2020).
Danio rerio (zebrafish) is considered an excellent model for toxicology studies due to its sensitivity when exposed to chemicals and being able to quickly absorb compounds added to water (Guyon et al. 2007). The use of zebrafish in toxicology assays is recommended by international standards (Bertoletti 2009; OECD 2010).
Based on the above, this study aimed to evaluate the toxic effects of di-iso-pentyl phthalate at concentrations of 5, 25, and 125 µg/L in different organs of Danio rerio after subchronic exposure.
Material and methods
Reagents
Di-iso-pentyl-phthalate (C18C26O4): available in liquid form (oil; CAS: 84777–06-0; 99% purity; Petrom®—Petrochemical Mogi das Cruzes-SP-Brasil).
Assessment of DiPeP absorption
To determine the degradation/absorption of DiPeP, two-liter aquariums were used, one with five fish (forming a pool of 0.300 g of total muscle tissue) and another without fish, with only the phthalate. The concentration used in this experiment was 125 µg/L of DIPeP, starting from 1 mg/mL of DiPeP (10 mg of DiPeP dissolved in 9 mL of acetone and 1 mL of Mili-Q water). Ten milliliters of water was collected, in triplicate, from both aquariums (with and without fish), at times: 0 (immediately after adding the contaminant to the tank water), 24, 48, 72, and 96 h. After the collection of water samples, these were analyzed employing gas chromatography coupled to mass spectrometry (GC–MS) from Thermo Fisher Scientific® (Waltham, MA, USA) to determine the amount of DiPeP that was absorbed by the fish at each time. After analysis in GC–MS, it was observed that fish absorbed 36% of DiPeP every 48 h.
Experimental model and ethical considerations
Danio rerio adult fish (0.246 ± 0.103 g; 3.1 ± 0.04 cm), of both sexes, were acquired from Planeta Aquários store, Curitiba-PR, and acclimatized for 2 weeks in two-liter tanks, four aquariums holding five fish (n = 20 per group). The aquariums were kept at a pH 7.00 ± 0.60, ammonia 0.04 ppm, nitrite 0 ppm, hardness: 3.00 ppm of CaCO3, temperature 26 ± 1 °C, and photoperiod 14:10 h. The fish were fed once a day with commercial feed (Vipagran, 41.3% protein; Brazil). The leftover fish feed as well as the accumulation of excreta resulting from an excess of organic matter accumulated in the bottom of the aquarium were removed by siphoning every 48 h.
The experiment was conducted as provided in ARRIVE guidelines (Percie du Sert et al. 2020) and EU Directive 2010/63/EU (Council of European Union 2010) for animal experiments. The project was approved by the Ethics Committee on the Use of Animals of the Instituto de Pesquisa Pelé Pequeno Príncipe, Curitiba-PR, under the number 051–2020.
After the assessment of DiPeP absorption, phthalate exposure (DiPeP) was conducted under the controlled parameters. The choice of the concentrations of DIPeP used in this work was based on reported data concerning the occurrence of other phthalates (diethyl phthalate, di-2-ethylhexyl phthalate, dibutyl phthalate). In environmental matrices, including rivers, the total phthalates found in water samples ranged from 313 to 1640 ng/L (Selvaraj et al. 2014). In drinking water treatment plants, the range for phthalates was between 0.02 and 6.5 μg/L (Santana et al. 2014).In food, phthalates were found at concentrations between < 50 μg/kg and ≥ 300 μg/kg (Serrano et al. 2014).
The experimental groups were 5, 25, and 125 μg/L DiPeP, water control, and solvent control (acetone 0.0056%). According to the results of the absorption evaluation, every 48 h the amount of absorbed DiPeP (36%) was replaced.
The fish were exposed to DiPeP through water exposure for 14 days, changing half of the aquarium water every 48 h and replacing the contaminant to ensure homogeneity of concentrations throughout the bioassay. After 14 days of exposure, the fish were anesthetized with benzocaine (0.001%), euthanized, and the tissues were removed for evaluation of the antioxidant system, quantification of DiPeP, histopathology, genotoxicity, and carbonic anhydrase activity. Due to the small size of zebrafish organs, the bioassays were performed with 20 animals per group so that all analyses could be performed with an n = 10. However, some fish had very small organs, making it impossible to use them in the analyses, so we had n = 7–10.
Water quality parameters
The water used in the tests was monitored during the experiment, checking the parameters of pH, ammonia, nitrite, chlorine, and hardness (commercial kits from the Labcon Test brand: pH tropical, toxic ammonia, nitrite, chlorine test, carbonate hardness KH), and temperature.
Tissue preparation for the evaluation of antioxidant system and acetylcholinesterase
The brain, intestine, liver, and gill samples (n = 7–10 per group) were homogenized in 600 µL of sodium phosphate buffer (0.1 M, pH 7.0) and centrifuged at 4 °C, 12,000 g, for 15 min. Supernatants were aliquoted for the determination of superoxide dismutase (SOD; EC 1.15.1.1), total proteins (P), glutathione peroxidase (GPx; EC 1.11.1.9), glutathione S-transferase (GST; EC 2.5.1.18), and reduced glutathione or non-protein thiols (GSH). Acetylcholinesterase (AChE; EC 3.1.1.18) activity was performed only in the brain.
GSH concentration (non-protein thiols)
GSH concentration was performed by comparing it to the standard curve of GSH (0 μM, 2.5 μM, 5 μM, 10 μM, 20 μM, 40 μM, 80 μM). Trichloroacetic acid (50%) was used for protein precipitation in the samples, after which they were centrifuged at 4 °C, 12,000 g, for 10 min, and the supernatants were used for analysis. In microplate, 50 μL of the sample, 230 μL of Tris-base buffer (0.4 M; pH 8.9), and 20 μL of 2.5 mM 5.5′-dithio bis-2-nitrobenzoic (DTNB) dissolved in methanol and 0.4 M Tris-base buffer (pH 8.9) were added. The GSH concentration was expressed in μg·mg of protein−1 (Sedlak and Lindsay 1968).
GST activity
The methodology for analyzing the GST was described by Keen et al. (1976), with modifications. The samples were diluted 1:2 (v/v) in potassium phosphate buffer (0.1 M, pH 6.5). In 96-well microplates, 20 μL of the sample was added, in triplicate, followed by 180 μL of the reaction medium containing the solutions with 3 mM GSH dissolved in potassium phosphate buffer (0.1 M, pH 6.5) and 3 mM 1-chloro-2,4-dinitrobenzene (CDNB) dissolved in ethanol. The reading was performed immediately at a wavelength of 340 nm with a total time of 5 min, with readings every minute. The gradual increase in absorbance was monitored, and activity was expressed in nmol of thioether formed·min−1·mg of protein−1.
GPx activity
The methodology was described by Paglia and Valentine (1967), using 10 μL of the sample and 130 μL of solution 1 (0.1 M sodium phosphate buffer, pH 7.0; sodium azide to 3.08 mM; NADPH at 0.308 mM; GSH at 3.08 mM; glutathione reductase at 1.54 U/mL). After 2 min, 60 μL of solution 2 (hydrogen peroxide — H2O2 to 5 mM; 0.1 M sodium phosphate buffer, pH 7.0) was added. The absorbance reading was carried out at 340 nm for 5 min with a one-minute interval between each reading. Enzyme activity was expressed in nmol·min−1·mg protein−1.
SOD activity
The analysis of SOD activity was based on the method proposed by Gao et al. (1998), which assesses the ability of SOD to inhibit the auto-oxidation of pyrogallol. In microtubes, 40 µL of the sample was added, followed by 885 µL of Tris 1 M/EDTA 5 mM buffer (pH 8.0) and 50 µL of pyrogallol (15 mM). The solution was incubated, and protected from light, for 30 min and the reaction was stopped with 25 µL of HCl (1 N). In the microplate, 200 µL of the reaction solution was added in triplicate and the reading was carried out in a spectrophotometer at 440 nm. Simultaneously, a control sample was performed with the addition of reagents but without incubation. Enzyme activity was expressed as U·mg protein−1, where 1 U of SOD is responsible for inhibiting by 50% the auto-oxidation of pyrogallol.
AChE activity
The methodology for analyzing the AChE was described by Ellman et al. (1961), with modifications. The brain samples were diluted at 1:2 (v/v) and 25 μL was added to the microplate, followed by 200 μL of DTNB (5.5′-dithio bis-2-nitrobenzoic; 0.75 mM), and 50 μL of acetylthiocholine (10 mM) was added. The reading was carried out in a spectrophotometer at 405 nm. Results were expressed in nmol·min−1·mg of protein−1.
Total protein quantification
The concentration of total protein was determined by the method of Bradford (1976) using a standard curve with bovine serum albumin (0, 125, 250, 500, 1000 µg/mL). For this analysis, brain and gill samples were diluted 1:2 (v/v), while liver and intestine samples did not need to be diluted. On a microplate, 10 µL of the sample was added, followed by 250 µL of Bradford’s reagent (Sigma-Aldrich®), and the reading was performed at 595 nm. Results were expressed in mg of protein.
Histopathology
The liver and intestine (n = 7–10 per group) were fixed in ALFAC (80% ethanol, 10% formalin, and 5% acetic acid) for 16 h and kept in 70% ethanol until the dehydration phase. Samples were dehydrated in a graded series of alcohol concentrations (80%, 90%, 95%, and 100%) and inserted into Paraplast Plus® (Sigma-Aldrich®). Sections of 3 µm were obtained in a microtome (American Optical Manual Rotary Microtome—Model 820—Series 54,637) at an angle of 45 °C and stained with Eosin (3 min) and 2% Floxin (2 min), followed by a histological bath in water at the temperature of 50 °C. Then, the sections were adhered to a glass slide in 10% formalin and placed in an oven at 65 °C for 30 min. After this time, the slides were submerged in a 65 °C xylol solution for 5 min to completely remove the paraffin.
Based on histopathological findings, the lesion index was calculated according to Bernet et al. (1999) modified by Mela et al. (2013b, 2013a). Injuries and tissue changes were classified according to biological importance, such as 1 — minimal, easily reversible; 2 — moderate, reversible in most cases; and 3 — marked, usually irreversible, and severity establishing scores from 0 to 6. The injury index for each group of liver or bowel injuries was calculated using the formula: Iorg. = ∑rp ∑alt (a × w), where org represents the organ (constant), rp the reaction pattern, alt the change, a the score value, and w the injury importance factor (Bernet et al. 1999; Mela et al. 2013b, 2013a).
Genotoxicity
Comet assay
Comet assay was performed according to the method proposed by Singh et al. (1988) and modified by Ramsdorf et al. (2009). Gills were stored in microtubes containing 1 mL of fetal bovine serum (FBS) and subsequently homogenized at 15,000 rpm with the aid of a microhomogenizer. The preparation of slides was performed using 60 μL of suspension mixed with low melting point agarose. The suspension was placed on a microscope slide previously covered with 1.5% agarose. The slide was covered with a coverslip and placed in the refrigerator at 4 °C for 15 min. After this time, the coverslip was removed and the slides were placed in a lysis solution for 24 h at 4 °C. The slides were transferred to the electrophoresis tank, where they were immersed in electrophoresis buffer (0.3 M NaOH/0.001 M EDTA) with pH > 13 for 25 min. Electrophoresis was performed at 300 mA, 25 V for 25 min, and then, slides were neutralized in Tris buffer (0.4 M, pH 7.5) and fixed in absolute ethanol for 5 min. For the analysis, slides were stained with ethidium bromide (10 μg/mL) and analyzed under an epifluorescence microscope (Leica®) at 400 × magnification. Comets were visually classified according to the migration of DNA fragments into class 0 (no apparent damage), class 1 (small damage), class 2 (medium damage), class 3 (extensive damage), and class 4 (maximum damage) (Collins et al. 1997). Analysis was blinded with coded slides. For each fish, 100 nucleoids were analyzed.
Piscine Micronucleus Test
A blood extension was performed for the Piscine Micronucleus Test following the method proposed by Schmid (1976), Carrasco et al. (1990), and Fenech (2000). Slides were dried at room temperature for 24 h. Then, they were fixed with absolute alcohol for 15 min. Subsequently, the slides were stained with 10% Giemsa for 15 min, followed by washing with distilled water to remove excess dye and drying at room temperature for 24 h. Analysis was blinded with coded slides. For each fish, 2000 erythrocytes were counted per slide under an optical microscope at 1000 × magnification.
Carbonic anhydrase activity
Carbonic anhydrase (EC 4.2.1.1) activity was determined according to the method established by Henry (1996) and described by Vitale et al. (1999). The gills were weighed and homogenized at 10% (w/v) in phosphate buffer (0.01 M, pH 7.4). Then, the homogenate was centrifuged at approximately 2000 × g for 5 min at room temperature. The supernatant was divided into aliquots to measure the concentration of total protein and to quantify the enzyme activity. Distilled water saturated with CO2 and a reaction medium with mannitol (0.225 M), sucrose (0.075 M), and Tris–phosphate buffer (0.01 M, pH 7.4) was added to the supernatant. The drop in pH resulting from the reaction was monitored every 4 s for 20 s. The calculation of carbonic anhydrase activity (CAA) was performed based on the descriptions by Burnett et al. (1981) and Vitale et al. (1999), using the formula: CAA = [TC / (TNC − 1)]/mg of total protein, where TC is the rate of catalyzed reaction and TNC is the rate of the uncatalyzed reaction. Results were expressed as specific CAA·mg of protein−1.
Statistical analysis
Statistical analyses were performed using GraphPad Prism 5.0 statistical program. Data normality was evaluated using the Shapiro–Wilk test. One-way analysis of variance (ANOVA) was applied for parametric data followed by Dunnett’s test and, for non-parametric data, the Kruskal–Wallis test followed by Dunn’s was used. The evaluation of outliers was performed using the Grubbs test. Results were expressed as mean ± standard error of mean or median and interquartile interval. A p-value < 0.05 was considered statistically significant. The bioassay was carried out with two control groups: water and solvent (acetone). As there was no statistical difference between the controls (Student’s t-test; Figs. 1S, 2S, 3S, 4S, and 5S), the groups exposed to DiPeP were compared with the solvent control in which the phthalate was diluted.
Antioxidant system in the liver of Danio rerio after 14 days of exposure to di-iso-pentyl-phthalate (DiPeP). A Superoxide dismutase activity, B glutathione peroxidase activity, C glutathione S-transferase activity, and D reduced glutathione concentration or non-protein thiols. Results are expressed as mean ± standard error. One-way ANOVA was followed by Dunnett’s test. n = 7–10. Asterisk indicates statistical difference compared to the control group (p < 0.05)
Antioxidant system in the intestine of Danio rerio after 14 days of exposure to di-iso-pentyl-phthalate (DiPeP). A Superoxide dismutase activity, B glutathione peroxidase activity, C glutathione S-transferase activity, D and reduced glutathione concentration or non-protein thiols. Results are expressed as mean ± standard error. One-way ANOVA was followed by Dunnett’s test. n = 7–10. Asterisk indicates statistical difference compared to the control group (p < 0.05)
Antioxidant system and osmoregulation in the gills of Danio rerio after 14 days of exposure to di-iso-pentyl-phthalate (DiPeP). A Superoxide dismutase activity, B glutathione peroxidase activity, C glutathione S-transferase activity, D reduced glutathione concentration or non-protein thiols, and E carbonic anhydrase activity. Results are expressed as mean ± standard error. One-way ANOVA was followed by Dunnett’s test. n = 7–10. Asterisk indicates a statistical difference from the control group (p < 0.05)
Antioxidant system and neurotoxicity in the brain of Danio rerio after 14 days of exposure to di-iso-pentyl-phthalate (DiPeP). A Superoxide dismutase activity, B glutathione peroxidase activity, C glutathione S-transferase activity, D reduced glutathione concentration or non-protein thiols, and E acetylcholinesterase activity. Results are expressed as mean ± standard error. One-way ANOVA was followed by Dunnett’s test. n = 7–10. Asterisk indicates a statistical difference from the control group (p < 0.05)
Results
Experimental conditions
There was no mortality during the experiment. The water parameters were as follows: pH 7.00 ± 0.60, ammonia 0.04 ppm, nitrite 0 ppm, hardness: 3.00 ppm of CaCO3, and temperature 26 ± 1 °C. All water parameters were determined in triplicate and values did not differ among groups.
Chemical analysis of DiPeP in water
DiPeP absorption experiment results
The DiPeP absorption demonstrated phthalate stability over 96 h. However, in the presence of fish, it was possible to observe a drop in phthalate concentration over time: 33.3% in 24 h, 35.8% in 48 h, 44.7% in 72 h, and 46.3% in 96 h (Table 1). Based on this result, it was decided to change the water with the replacement of phthalate (36%) every 48 h.
After 96 h, it was observed that there was a deposition of DiPeP in muscle tissue at a concentration of 37.96 μg/L.
Fourteen-day bioassay
Quantification of DiPeP in muscle tissue
DiPeP was quantified in Danio rerio muscle after 14 days of exposure. The values found were control group below the limit of detection (< LLOD), group 5 μg/L = 0.88 ± 0.02 ng/g, 25 μg/L = 4.76 ± 0.03 ng/g, and 125 μg/L = 22.68 ± 0.06 ng/g, demonstrating that there was deposition in muscle tissue.
Antioxidant system, osmoregulation, and neurotoxicity
In the liver, GPx enzyme activity and GSH concentration had a significant increase at 25 μg/L of DiPeP (p < 0.05) compared to the control group (Fig. 1 B and D, respectively). GST activity was reduced at the 5 and 125 μg/L of DiPeP (p < 0.05) (Fig. 1C). Statistical analysis did not show a difference in SOD activity in fish exposed to DiPeP (Fig. 1A).
In the intestine, GPx activity had a significant increase at 25 μg/L of DiPeP (p < 0.05) (Fig. 2B), and GST activity was reduced at the higher concentration of DiPeP (p < 0.05), compared to the control group (Fig. 2C). Statistical analysis did not show a difference in SOD activity and GSH concentration at different concentrations of DiPeP (Fig. 2 A and D).
In the gills, there was a significant increase in SOD enzyme activity at the concentrations of 25 and 125 μg/L of DiPeP (p < 0.05) (Fig. 3A). GPx and GST activity and GSH concentration did not show a significant difference in fish exposed to DiPeP (Fig. 3 B, C, and D).
Carbonic anhydrase activity showed no significant change in groups exposed to DIPeP when compared to the control group (Fig. 3E).
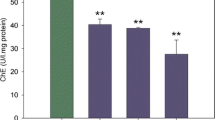
In the brain, SOD activity had a significant increase at concentrations of 5 and 25 μg/L of DiPeP (p < 0.05) compared to the control group (Fig. 4A), as well as an increase in GPx activity in the group exposed to 25 μg/L of DiPeP (p < 0.05) (Fig. 4B). GST activity was reduced in all groups exposed to DiPeP (p < 0.05) (Fig. 4C). GSH concentration did not show a statistical difference in the DiPeP-exposed groups (Fig. 4D).
AChE enzyme activity showed no significant difference in DiPeP-exposed groups when compared to the control group (Fig. 4E).
Histopathology
Histologically the intestine of Danio rerio is consisting of four layers: the mucosa, submucosa, muscular, and serosa. The mucosa was folded into long and relatively narrow branched villi. Two cell types were identified in the epithelial layer: the absorptive or columnar and the goblet cells. In fish exposed to 125 µg/L of DiPeP we observed the presence of cells of the immune system; in the lumen of the organ, organ could cause inflammation of the intestinal wall. There was an increase in the injury index in animals exposed to DiPeP, and at the highest concentration, this increase was statistically significant (p < 0.05) (Fig. 5).
The histopathological evaluation of the liver showed hepatocytes with dense and compactly organized cytoplasm and a nucleus located centrally between sinusoids (Fig. 6A). In the DiPeP-exposed groups the observed changes were vascular congestion (Fig. 6B), necrosis (Fig. 6C), and loss of cell limits (Fig. 6D). The higher concentration of DiPeP (125 µg/L) caused morphological changes statistically significant (p < 0.05) (Fig. 7).
Structural organization of the liver of Danio rerio stained with hematoxylin and eosin. A Liver of control individual. Sinusoids in the liver parenchyma (). B Liver of an individual from the group was exposed to 125 µg/L of DiPeP. Vascular congestion (►). C Liver from an individual from the group exposed to DiPeP 125 µg/L. Presence of necrotic area (N). D Liver from an individual from the group exposed to DiPeP 125 µg/L. Loss of hepatocyte cell limits (⇨). n = 7–10
Genotoxicity: comet assay
The comet assay was used to determine DNA damage in the gills of Danio rerio at three different concentrations of DiPeP and the control (Fig. 8). The data showed that, compared to the control group, the concentration of 125 µg/L of DiPeP caused a significant increase in the score of DNA damage in the gills (p < 0.05).
Genotoxicity: Piscine Micronucleus Test
In waterborne exposure to DiPeP, no micronuclei occurred in any of the groups. The erythrocytic nuclear alterations (ENAs) observed were Binuclei (double nucleus), Blebbed (evagination), Notched (invagination), and Vacuolated (presence of vacuole). The least observed alteration was binucleus and the most observed was Blebbed. The group exposed to 25 µg/L of DiPeP showed a significant increase in Blebbed, Vacuolated, and Total AEN changes (p < 0.05) (Table 2).
Discussion
Our study provides important information on the deposition of DiPeP in fish tissue, not yet reported in the literature, which may contribute to establishing safe threshold levels concerning animal and human health. Lu et al. (2021) evaluated the presence of phthalates and other endocrine disruptors in fish consumed by the Taiwanese population and the predominant compounds were diethyl phthalate, di-2-ethylhexyl phthalate, and di-iso-nonyl phthalate with mean concentrations of 42.5–52.9 ng/g dry weight. These results corroborate the findings in this paper that show the ability to accumulate phthalates, which, in the long term, can cause risks of intoxication for humans through biomagnification processes, that is, a progressive accumulation of DIPeP along with trophic levels.
It is already known that the bioaccumulation of toxic substances can generate free radicals, especially intracellular ROS. In the intracellular environment, a dynamic balance is maintained between the production and elimination of ROS, and these can become reactive, attacking cellular targets via oxidative damage and thus causing cell death (Van der Oost et al. 2003; Lushachak 2011).
Therefore, the antioxidant system acts to prevent such oxidative damage (Velisek et al. 2012). As an antioxidant enzyme of great importance, GPx acts by catalyzing the reduction of hydroperoxides and consequently assisting in the maintenance of antioxidants in aquatic organisms (Zhou et al. 2011). In this study, there was an increase in GPx activity and GSH concentration in liver tissue when exposed to a concentration of 25 μg/L of DiPeP. GSH is a non-enzymatic antioxidant that is also used by other enzymes of the glutathione group such as GPx and GST. GPx catalyzes the reaction of GSH with hydrogen peroxide or lipid peroxides performing a cellular detoxification function (Sarikaya and Doğan 2020). Therefore, this increase in GPx activity to combat reactive species may have caused an augmentation in GSH consumption, which led to the elevated synthesis of the molecule and, consequently, a higher concentration compared to the control group.
In the intestine and brain of Danio rerio exposed to DiPeP, there was also an increase in GPx at the concentration of 25 μg/L; thus, a pattern of increase in the response of GPx against a concentration of 25 μg/L of DiPeP was observed. DEP phthalate at a dose of 900 mg/kg also induced GPx activity in the liver of Paralichthys olivaceus fish after parenteral exposure for 3 days (Kang et al. 2010).
DiPeP was responsible for reducing the activity of GST in the liver and intestine, and there was also a reduction in the activity of the enzyme in the brain after exposure to all concentrations of DiPeP. This demonstrates similar effects on the antioxidant system of these organs, with the brain being the most affected. No data were found about the effects of phthalates on the antioxidant system of fish intestines. GST is considered an important phase II enzyme in organisms, acting to facilitate the excretion of xenobiotics in addition to its antioxidant capacity (Regoli and Giuliani 2014; Allocatti et al. 2018). Therefore, a reduction in GST can increase the toxicity of compounds such as phthalates since their metabolism will be reduced. In a study carried out with annelids of the Eisenia fetida species exposed to doses (0.1, 1, 10, and 50 mg/kg) of butyl benzyl phthalate (BBP) at different times (7, 14, 21, and 28 days), a reduction in GST activity was also observed after 28 days of exposure (Song et al. 2018).
Zhang et al. (2014) described in their study that GST activity also decreased in the liver of carp exposed to a concentration of 125 μg/L of DEP for 20 days, demonstrating a similar effect to that found in our study. This reduction can impair the protection of cells against antioxidant substances and thus cause cellular damage (Aikten and Roman 2008; Liu et al. 2014). Many enzymes involved in antioxidant defenses can be inactivated by the excess of oxidants (Modesto and Martinez 2010). The reduction of enzymes such as GST was verified in Chrironomus riparius larvae exposed to BBP at concentrations from 1 to 1000 μg/L (Santos Morais et al. 2020).
SOD is considered the main defense mechanism against oxidative damage derived from the increase in ROS being responsible for catalyzing the dismutation of the superoxide anion (Velisek et al. 2011) in hydrogen peroxide. In our study, a significant increase in brain SOD activity at concentrations of 5 and 25 μg/L of DiPeP was observed. Other studies reported similar results in the brain of exposed fish to synthetic organic pollutants and biotoxins (da Silva et al. 2011; Xing et al. 2012). In the present study, the increase in SOD activity suggests that it is an organism’s response to environmental stress, aiming to reduce cell damage in the Danio rerio brain. Also, in the gills, there was an increase in SOD activity after 14 days of exposure to DiPeP at concentrations of 25 and 125 μg/L, which demonstrates a defense mechanism of the gills in trying to remove the free radicals formed. Different from gills and brain, there was no change in intestinal and hepatic SOD in the groups exposed to DiPeP. Other studies have reported changes in hepatic SOD in Danio rerio (Lu et al. 2016), Dicentrarchus labrax (Espinosa et al. 2019), and Clarias gariepinus (Iheanacho and Odo 2020) fish exposed to polyvinyl chloride (PVC) microparticles and microplastics.
Regarding the neurotoxicity evaluated through acetylcholinesterase in the brain, there were no significant changes compared to the control group, but it is not excluded that there is an effect of DiPeP in the brain of Danio rerio, as there was a change in antioxidant enzymes. Although in our study with DiPeP there were no changes in AChE activity, an increase of this enzyme has already been observed in Pseudobagrus fulvidraco chronically exposed to 100, 500, and 1000 mg/kg of dibutyl phthalate (DBP) for 8 weeks (Jee et al. 2009). As an enzyme that degrades the neurotransmitter acetylcholine (ACh), AChE is important because when ACh is released by presynaptic neurons, it regulates the concentration of this neurotransmitter (Soreqh and Seidman 2001).
In the histopathological evaluation of the intestine, the group exposed to 125 µg/L of DiPeP showed the presence of macrophages in the lumen. These cells moved from the connective tissue to the epithelium and thus reached the lumen. The intestine is one of the major organ systems of fish that interact with the environment and plays a critical role in survival under stressful environments. Although we did not observe morphological changes in the intestinal epithelium after 14 days, the contact of the pollutant with this tissue probably triggered an inflammatory process, being the first line of defense to act in this organ, before morphological lesions are established.
Marinsek et al. (2018) also observed a strong inflammatory response in the intestine, reinforcing the influence of polluted environments on the gastrointestinal tract of fish. According to Brierley and Linden (2014), the environmental contaminants may be responsible for the recruitment of cells from the intestinal immune system, causing an increase in the inflammatory process.
In the hepatic tissue, severe alterations were observed such as loss of cell limit that can precede necrosis, blood congestion, and necrotic areas. All these findings were significant at exposure to 125 μg/L of DiPeP. Blood congestion is liver dysfunction caused by venous congestion, usually as a result of heart dysfunction, also known as congestive heart failure (Barja-Fernández et al. 2013). The fish liver is especially susceptible to environmental pollutants thanks to slow blood flow relative to cardiac output (Leão-Buchir et al. 2021).
Confirming the hepatotoxic effects induced by phthalates, Clarias gariepinus exposed to different concentrations (30 μg/L, 40 μg/L, 60 μg/L, and 80 μg/L) of DEP showed congestion, necrosis, and degeneration of hepatocytes (Obiezue et al. 2014). In Channa striatus fish exposed to 0.4 mg/L, 4 mg/L, and 40 mg/L of DEP for 7, 14, and 21 days, hepatic necrosis was also observed in addition to cytoplasmic vacuolization (George et al. 2017). These data demonstrate the toxicological potential of phthalates for the liver tissue of fish.
The gills are an organ of great importance for responding effectively to environmental variations in aquatic organisms, ensuring that the animal’s physiological functions remain preserved (Virgens et al. 2015). As they are the first organs to have contact with contaminants, they may change due to defense responses and injuries caused by contaminants (Gomes et al. 2012).
Regarding the analysis of genotoxicity in Danio rerio gills, there was a significant increase in DNA breaks at the concentration of 125 μg/L of DiPeP. According to our knowledge, no study has evaluated the genotoxic effects of DiPeP in Danio rerio; however, genotoxicity by other phthalates has been observed. Oreochromis niloticus exposed to 0.59 and 1.18 mg/L of DBP had an increase in DNA damage in gills (Zeid et al. 2014). Sublethal concentrations of DBP (1/2 LC50 96 h and 1/3 LC50 96 h) were also genotoxic to fish gills (Khalil et al. 2016). In these two studies, the authors evaluated the effect of high concentrations of DBP. Our study showed DNA damage even when Danio rerio were exposed to concentrations a thousand times lower.
In the blood, there was an increase in blebbed (evagination), vacuolated (vacuole in the middle of the nucleus), and total erythrocytic nuclear alterations in the group exposed to 25 µg/L of DiPeP, demonstrating a mutagenic potential of DiPeP. Environmental contaminants can induce damage to DNA and proteins involved in the cell division process through several mechanisms that cause the loss of genome integrity and the formation of micronuclei and nuclear morphological changes (Iheanacho et al. 2021). Possible damages will depend on whether the contaminant is clastogenic (micronuclei originate from acentric chromosomal fragments) and/or aneugenic (whole chromosomes that do not complete the anaphasic migration of cell division), according to the stage of the cell cycle affected (Canedo et al. 2021). Mutagenic effects of phthalates have already been demonstrated in the exposure of Nile tilapia (Oreochromis niloticus) to the sublethal concentration of 10 mg/L of DBP, obtaining a greater difference in the Notched-type abnormality (invagination) after 96-h exposure (Benli et al. 2016). The type of abnormality observed may vary depending on the exposure time and via and the contaminant class.
In the gills, carbonic anhydrase activity did not show any significant alteration at the concentrations tested. Carbonic anhydrase regulates acid–base respiration and ion uptake in fish such as Danio rerio, revealing itself to be sensitive to the presence of contaminants of different classes (Lionetto et al. 2012) but it was not affected by DiPeP. Unlike what happened in our study, other contaminants such as drugs and metals can alter the activity of this enzyme (Saravanan et al. 2011; Mela et al. 2013b; Perussolo et al. 2019).
Conclusion
In the present study, phthalate deposition occurred in muscle tissue of Danio rerio at all concentrations tested. Therefore, our data suggest that in the long term DiPeP can cause risks to humans via bioaccumulation processes. The antioxidant system was altered in all evaluated organs (liver, intestine, gills, and brain), demonstrating to be sensitive even to exposure to low concentrations of this phthalate. DiPeP caused major tissue damage in the liver, including necrosis. In the intestine, it was possible to observe cells of the immune system in the lumen of the organ as a response to exposure to phthalate. The genotoxic potential of DiPeP was observed in the gills after exposure to the highest concentration, and, in the blood, there were nuclear morphological changes in erythrocytes in fish exposed to 25 µg/L of DiPeP.
DiPeP did not change the neurotoxicity for zebrafish or caused osmoregulatory change (inferred from carbonic anhydrase activity) at the concentrations tested. However, the occurrence of neurotoxicity should not be excluded, since the antioxidant system was altered in the zebrafish brain, proving it to be sensitive even to exposure to low concentrations of this phthalate.
Future studies evaluating the effects of chronic exposure, exposure in developmental periods, behavior, and analysis of the gonads and/or hormones, would help to clarify the toxicological potential of this phthalate.
Data availability
Not applicable.
Code availability
Not applicable.
References
Aikten RJ, Roman S (2008) Antioxidant systems and oxidative stress in the testes. Adv Exp Med Biol 636:154–171
Allocatti N, Masulli M, Di Ilio C, Federici L (2018) Glutathione transferases: substrates, inhibitors and pro-drugs in cancer and neurodegenerative diseases. Oncogenesis 7:8
Barja-Fernández S, Míguez JM, Álvarez-Otero R (2013) Histopathological effects of 2,2′,4,4′-tetrabromodiphenyl ether (BDE-47) in the gills, intestine and liver of turbot (Psetta maxima). Ecotoxicol Environ Saf 95:60–68. https://doi.org/10.1016/j.ecoenv.2013.05.028
Benli ACK, Erkmen B, Erkoc F (2016) Genotoxicity of sub-lethal di-n-butyl phthalate (DBP) in Nile tilapia (Oreochromis niloticus). Arh Hig Rada Toksikol 67:25–30. https://doi.org/10.1515/aiht-2016-67-2723
Bernet D, Schmidt H, Meier W et al (1999) Histopathology in fish: proposal for a protocol to assess aquatic pollution. J Fish Dis 22:25–34. https://doi.org/10.1046/j.1365-2761.1999.00134.x
Bertoletti E (2009) Determinação da Ecotoxicidade Crônica para Danio rerio. J Braz Soc Ecotoxicol 4:1–7
Bertoncello Souza M, Passoni MT, Pälmke C et al (2018) Unexpected, ubiquitous exposure of pregnant Brazilian women to diisopentyl phthalate, one of the most potent antiandrogenic phthalates. Environ Int 119:447–454. https://doi.org/10.1016/j.envint.2018.06.042
Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brierley SM, Linden DR (2014) Neuroplasticity and disfunction after gastrointestinal inflamation. Nat Rev Gastroenterol Hepatol 10:611–627. https://doi.org/10.1038/nrgastro.2014.103
Brisco (2011) Diap (Diisoamil Ftalato). https://www.brisco.com.br/fichas-tecnicas/diap-diisoamil-ftalato.pdf
Canedo A, De Jesus LWO, Bailão EFLC, Rocha TL (2021) Micronucleus test and nuclear abnormality assay in zebrafish (Danio rerio): past, present, and future trends. Env Pollut 290:118019
Burnett LE, Woodson PB, Rietow MG, Vilicich VC (1981) Crab gill intra-epithelial carbonic anhydrase plays a major role in haemolymph CO2 and chloride ion regulation. J Exp Biol 92:243–254
Carrasco KR, Tilbury KL, Myers MS (1990) Assessment of the piscine micronucleus test as an in situ biological indicator of chemical contaminant effects. J Fish Aquat Sci 47:2123–2136
Collins A, Dušinská M, Franklin M et al (1997) Comet assay in human biomonitoring studies: reliability, validation, and applications. Environ Mol Mutagen 30:139–146. https://doi.org/10.1002/(SICI)1098-2280(1997)30:2%3c139::AID-EM6%3e3.0.CO;2-I
Council of European Union (2010) Directive 2010/63/EU of the European Parliament and of the Council on the protection of animals used for scientific purposes. Off J Eur Union. https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2010:276:0033:0079:en:PDF
da Silva CA, Oba ET, Ramsdorf WA et al (2011) First report about saxitoxins in freshwater fish Hoplias malabaricus through trophic exposure. Toxicon 57:141–147. https://doi.org/10.1016/j.toxicon.2010.10.015
Do Nascimento Filho I, Von Mühlen C, Schossler P, Bastos Caramão E (2003) Identification of some plasticizers compounds in landfill leachate. Chemosphere 50:657–663. https://doi.org/10.1016/S0045-6535(02)00581-7
EFSA Panel on Food Contact, Materials (2019) Update of the risk assessment of di-butylphthalate (DBP), butyl-benzyl-phthalate (BBP), bis (2-ethylhexyl) phthalate (DEHP), di-isononylphthalate (DINP) and di-isodecylphthalate (DIDP) for use in food contact materials. EFSA J 17:e05838
Ellman GL, Courtney KD, Andres V, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95. https://doi.org/10.1016/0006-2952(61)90145-9
Espinosa C, Esteban MÁ, Cuesta A (2019) Dietary administration of PVC and PE microplastics produces histological damage, oxidative stress and immunoregulation in European sea bass (Dicentrarchus labrax L.). Fish Shellfish Immunol 95:574–583. https://doi.org/10.1016/j.fsi.2019.10.072
Fenech M (2000) The in vitro micronucleus technique. Mutat Res Mol Mech Mutagen 455:81–95
Ferreira ID, Morita DM (2012) Ex-situ bioremediation of Brazilian soil contaminated with plasticizers process wastes. Brazilian J Chem Eng 29:77–86. https://doi.org/10.1590/S0104-66322012000100009
Gao R, Yuan Z, Zhao Z, Gao X (1998) Mechanism of pyrogallol autoxidation and determination of superoxide dismutase enzyme activity. Bioelectrochemistry Bioenerg 45:41–45. https://doi.org/10.1016/S0302-4598(98)00072-5
George KR, Gokul GN, Malini NA (2017) Effects of different sub-lethal concentrations of plasticizer-diethyl phthalate on freshwater murrel, Channa striatus (Bloch). J Appl Nat Sci 9:476481
Gomes I, Nascimento AA, Sales A, Gerson F (2012) Can fish gill anomalies be used to assess water qualityin freshwater Neotropical systems? Env Monit Assessm 184:5523–31. https://doi.org/10.1007/s10661-011-2358-2
Gutiérrez-García AK, Flores-Kelly JM, Ortiz-Rodríguez T et al (2019) Phthalates affect the in vitro expansion of human hematopoietic stem cell. Cytotechnology 7:553–561
Guyon J, Steffen L, Howell M et al (2007) Modeling human muscle disease in zebrafish. Biochim Biophys Acta 1772:205–215
Henry R (1996) Multiple roles of carbonic anhydrase in cellular transport and metabolism. Annu Rev Physiol 58:523–538
Iheanacho SC, Odo GE (2020) Neurotoxicity, oxidative stress biomarkers and haematological responses in African catfish (Clarias gariepinus) exposed to polyvinyl chloride microparticles. Comp Biochem Physiol Part - C Toxicol Pharmacol 232:108741. https://doi.org/10.1016/j.cbpc.2020.108741
Iheanacho SC, Adeolu A, Nwose R et al (2021) Genotoxicity, oxidative stress and lysozyme induction in Clarias gariepinus chronically exposed to water-soluble fraction of burnt tire ash. Ecotoxicology 30:1983–1996. https://doi.org/10.1007/s10646-021-02474-7
Jee JH, Koo JG, Keum YH et al (2009) Effects of dibutyl phthalate and di-ethylhexyl phthalate on acetylcholinesterase activity in bagrid catfish, Pseudobagrus fulvidraco (Richardson). J Appl Ichthyol 25:771–775
Kang JC, Jee JH, Koo JG et al (2010) Anti-oxidative status and hepatic enzymes following acute administration of diethyl phthalate in olive flounder Paralichthys olivaceus, a marine culture fish. Ecotoxicol Environ Saf 73:1449–1455. https://doi.org/10.1016/j.ecoenv.2010.07.025
Keen JH, Habig WH, Jakoby WB (1976) Mechanism for the several activities of the glutathione S-transferases. J Biol Chem 251:6183–6188
Khalil SR, Elhakim YA, El-Nurr AE (2016) Sublethal concentrations of di-n-butyl phthalate promote biochemical changes and DNA damage in juvenile Nile tilapia (Oreochromis niloticus). Jpn J Vet Res 64:67–80
Koch HM, Christensen KLY, Harth V et al (2012) Di-n-butyl phthalate (DnBP) and diisobutyl phthalate (DiBP) metabolism in a human volunteer after single oral doses. Arch Toxicol 86:1829–1839
Leão-Buchir J, Folle NM, De Souza TL et al (2021) Effects of trophic 2,2′, 4,4′-tetrabromodiphenyl ether (BDE-47) exposure in Oreochromis niloticus: a multiple biomarkers analysis. Env Toxicol Pharmacol 87:103693. https://doi.org/10.1016/j.etap.2021.103693
Lionetto MG, Caricato R, Giordano ME et al (2012) Carbonic anhydrase as pollution biomarker: 2012. An ancient enzyme with a new use. Int J Environ Res 9:3965–3977
Lioy PJ, Hauser R, Gennings C et al (2015) Assessment of phthalates/phthalate alternatives in children’s toys and childcare articles: review of the report including conclusions and recommendation of the Chronic Hazard Advisory Panel of the Consumer Product Safety Commission. J Expo Sci Env Epidemiol 25:343–353
Liu C, Zhao X (2009) MicroRNAs in adult and embryonic neurogenesis. Neuromolecular Med 11:141–152
Liu D, Pan L, Ii Z et al (2014) Metabolites analysis, metabolic enzyme activities and bioaccumulation in the clam Ruditapes philippinarum exposed to benzo[a]pyrene. Ecotoxicol Environ Saf 107:251–259
Lu Y, Zhang Y, Deng Y et al (2016) Uptake and accumulation of polystyrene microplastics in zebrafish (Danio rerio) and toxic effects in liver. Environ Sci Technol 50:4054–4060. https://doi.org/10.1021/acs.est.6b00183
Lu I, Chao HR, Mansor WNW et al (2021) Levels of phthalates, bisphenol-A, nonylphenol, and microplastics in fish in the estuaries of northern Taiwan and the impact on human health. Toxics 9:246
Luongo G, Ostman C (2016) Organophosphate and phthalate esters in settled dust from apartment buildings in Stockholm. Indoor Air 26:414–425
Lushachak VI (2011) Environmentally induced oxidative stress in aquatic animals. Aquat Toxicol 101:13–30
Mankidy R, Wiseman S, Ma H, Giesy JP (2013) Biological impact of phthalates. Toxicol Lett 217:50–58. https://doi.org/10.1016/j.toxlet.2012.11.025
Marinsek GP, Abessa DMS, Gusso-Choueri PK et al (2018) Enteric nervous system analyses: new biomarkers for environmental quality assessment. Mar Pollut Bull 137:711–722
Mela M, Guiloski IC, Doria HB et al (2013a) Effects of the herbicide atrazine in neotropical catfish (Rhamdia quelen). Ecotoxicol Environ Saf 93:13–21. https://doi.org/10.1016/j.ecoenv.2013.03.026
Mela M, Guiloski ICC, Doria HBB et al (2013b) Risks of waterborne copper exposure to a cultivated freshwater Neotropical catfish (Rhamdia quelen). Ecotoxicol Environ Saf 88:108–116. https://doi.org/10.1016/j.ecoenv.2012.11.002
Modesto KA, Martinez CBR (2010) Effects of Roundup Transorb on fish: hematology, antioxidant defenses and acetylcholinesterase activity. Chemosphere 78:294–299. https://doi.org/10.1016/j.chemosphere.2010.07.005
Neubert da Silva G, Curi T, Tolouei S et al (2019) Effects of diisopentyl phthalate exposure during gestation and lactation on hormone-dependent behaviors and hormone receptor expression in rats. J Neuroendocr 31:e12816
Obiezue RN, Ikele CB, Mgbenka BO et al (2014) Toxicity study of diethyl phthalate on Clarias gariepinus fingerlings. Afr J Biotechnol 13:884–896
OECD (2012) OECD guideline for testing of chemicals. https://www.oecd.org/env/ehs/testing/Draft%20revised%20TG210-Clean%20version_04-Sept.pdf
Paglia DE, Valentine W (1967) Studies on the quantitative and qualitative characterization oferythrocyte glutathione peroxidase. J Lab Clin Med 70:1958–1969
Percie du Sert N, V H, A A, et al (2020) The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. PLoS Biol 18(7):e3000410
Perussolo MC, Guiloski IC, Lirola JR et al (2019) Integrated biomarker response index to assess toxic effects of environmentally relevant concentrations of paracetamol in a neotropical catfish (Rhamdia quelen). Ecotoxicol Environ Saf 182:109438. https://doi.org/10.1016/j.ecoenv.2019.109438
PETROM (2016) DIAP (Diisoamil Ftalato). https://petrom.com.br/produtos/diap-diisoamil-ftalato/
Radke EG, Braun JM, Nachman RM, Cooper GS (2020) Phthalate exposure and neurodevelopment: a systematic review and meta-analysis of human epidemiological evidence. Environ Int 137:105408
Ramsdorf WA, de Guimarães FSF, Ferraro MVM et al (2009) Establishment of experimental conditions for preserving samples of fish blood for analysis with both comet assay and flow cytometry. Mutat Res - Genet Toxicol Environ Mutagen 673:78–81. https://doi.org/10.1016/j.mrgentox.2008.11.010
Regoli F, Giuliani ME (2014) Oxidative pathways of chemical toxicity and oxidative stress biomarkers in marine organisms. Mar Environ Res 93:106–117
Rocha BA, Asimakopoulos AG, Barbosa F, Kannan K (2017) Urinary concentrations of 25 phthalate metabolites in Brazilian children and their association with oxidative DNA damage. Sci Total Environ 586:152–162. https://doi.org/10.1016/j.scitotenv.2017.01.193
Rudel RA, Gray JM, Engel CL et al (2011) Food packaging and bisphenol A and bis (2-ethyhexyl) phthalate exposure: findings from a dietary intervention. Environ Health Perspect 119:914–920
Santana J, Giraudi C, Marengo E et al (2014) Preliminary toxicological assessment of phthalate esters from drinking water consumed in Portugal. Environ Sci Pollut Res 21:1380–1390. https://doi.org/10.1007/s11356-013-2020-3
Santos Morais G, Vieira TB, Santos GS et al (2020) Genotoxic, metabolic, and biological responses of Chironomus sancticaroli Strixino & Strixino, 1981 (Diptera: Chironomidae) after exposure to BBP. Sci Total Environ 715:136937
Saravanan M, Karthika S, Malarvizhi A, Ramesh M (2011) Ecotoxicological impacts of clofibric acid and diclofenac in common carp (Cyprinus carpio) fingerlings: hematological, biochemical, ionoregulatory and enzymological responses. J Hazard Mater 195:188–194. https://doi.org/10.1016/j.jhazmat.2011.08.029
Sarikaya E, Doğan S (2020) Glutathione peroxidase in health and diseases. In: In (Ed.), Glutathione System and Oxidative Stress in Health and Disease. IntechOpen. https://doi.org/10.5772/intechopen.91009
Schmid W (1976) The micronucleus test for cytogenetic analysis. In: Chemical Mutagens 4:31–53
Schug TT, Janesick A, Blumberg B, Heindel JJ (2011) Endocrine-disrupting chemicals and disease susceptibility. J Steroid Biochem Mol Biol 127:204–215
Sedlak J, Lindsay RH (1968) Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem 25:192–205. https://doi.org/10.1016/0003-2697(68)90092-4
Selvaraj KK, Sundaramoorthy G, Ravichandran PK et al (2014) Phthalate esters in water and sediments of the Kaveri River, India: environmental levels and ecotoxicological evaluations. Environ Geochem Health 37:83–96. https://doi.org/10.1007/s10653-014-9632-5
Serrano SE, Karr CJ, Seixas NS et al (2014) Dietary phthalate exposure in pregnant women and the impact of consumer practices. Int J Environ Res Public Health 11:6193–6215. https://doi.org/10.3390/ijerph110606193
Singh N, MT M, RR T, EL. S (1988) A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. https://doi.org/10.1016/0014-4827(88)90265-0
Song E, Song PP, Ping LW et al (2018) Ecotoxicological effects of fertilizers made from pulping waste liquor on earthworm Eisenia fetida. Ecotoxicol Environ Saf 166:237–241
Soreq H, Seidman S (2001) Acetylcholinesterase — new roles for an old actor. Nat Rev Neurosci 2:294–302
Souza JMO, Souza MCO, Rocha BN et al (2022) Levels of phthalates and bisphenol in toys from Brazilian markets: migration rate into children’s saliva and daily exposure. Sci Total Environ 828:154486. https://doi.org/10.1016/j.scitotenv.2022.154486
Uren-Webster TM, Lewis C, Filby AL et al (2010) Effect of chronic exposure to simazine on oxidative stress and antioxidant response in common carp (Cyprinus carpio L.). Aquat Toxicol 99:360–369
Van der Oost R, Beyer J, Vermeulen NPE (2003) Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environ Toxicol Pharmacol 13:57–149
Velisek J, Stara A, Marchova J, Svobodova Z (2012) Effects of long-term exposure to simazine in real concentration on common carp (Cyprinus carpio L.). Ecotoxicol Environ Saf 76:79–86
Velisek J, Stara A, Svobodova Z (2011) The effects of pyrethroid and triazine pesticides on fish physiology. In: In (Ed.), Pesticides in the Modern World - Pests Control and Pesticides Exposure and Toxicity Assessment. IntechOpen.https://doi.org/10.5772/17289
Virgens A, Castro R, Cruz Z (2015) Alterações histológicas em brânquias de Orechromis niloticus (Tilapia-do-Nilo) expostas o Acefato, Difenoconazol e Sulfluramida. Nat Line 13:26–31
Vitale AM, Monserrat JM, Castilho P, Rodriguez EM (1999) Inhibitory effects of cadmium on carbonic anhydrase activity and ionic regulation of the estuarine crab Chasmagnathus granulata (decapoda, grapsidae). Comp Biochem Physiol - C Pharmacol Toxicol Endocrinol 122:121–129. https://doi.org/10.1016/S0742-8413(98)10094-4
Wensing M, Uhde E, Salthammer T (2005) Plastics additives in the indoor environment — flame retardants and plasticizers. Sci Total Environ 339:19–40
Xing HJ, Ii S, Wang ZL et al (2012) Histopathological changes and antioxidant response in brain and kidney of common carp exposed to atrazine and chlorpyrifos. Chemosphere 88:377–383
Zeid E, A. K, Zoubair BENKHASSI (2014) Toxicological consequences of di-n-butyl-phthalate (DBP) on health of Nile Tilapia fingerlings. Am J Anim Vet Sci 9:269–276
Zhang Y, X M, L C, et al (2014) Age and sex-specific relationships between phthalate exposures and obesity in Chinese children at puberty. PLoS One 9:e104852
Zhou D, Wang H, Zhang J (2011) Di-n-butyl phthalate (DBP) exposure induces oxidative stress in epididymis of adult rats. Toxicol Ind Health 27:65–71. https://doi.org/10.1177/0748233710381895
Acknowledgements
We would like to thank the Instituto de Pesquisa Pelé Pequeno Príncipe for the equipment and materials used for the development of the experiment.
Funding
This study was financed in part by Instituto de Pesquisa Pelé Pequeno Príncipe (IPPPP, Curitiba, Brazil). The funders were not involved in the design of the study; data collection, analysis, and interpretation of the data; and the writing of the manuscript.
Author information
Authors and Affiliations
Contributions
Sheila Gabriel dos Santos: methodology, data curation, and writing — original draft. Marília Cristina Oliveira de Souza: investigation and writing — reviewing. Fernando Barbosa Junior: investigation and writing — reviewing. Maritana Mela Prodocimo: investigation and writing — reviewing. Fellip Rodrigues Marcondes: investigation and writing — reviewing. William de Almeida: investigation and writing — reviewing. Marta Margarete Cestari: investigation and writing — reviewing. Luciana Rodrigues de Souza-Bastos: investigation and writing — reviewing. Anderson Joel Martino Andrade: writing — reviewing. Izonete Cristina Guiloski: conceptualization, supervision, and writing — reviewing and editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Bruno Nunes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Santos, S.G., Souza, M.C.O., Barbosa-Junior, F. et al. Evaluation of the toxicity of di-iso-pentyl-phthalate (DiPeP) using the fish Danio rerio as an experimental model. Environ Sci Pollut Res 30, 27996–28009 (2023). https://doi.org/10.1007/s11356-022-24071-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-24071-9