Abstract
Hypocitraturia, hypokaliuria, and increased oxidative stress are common lithogenic risk factors found in nephrolithiasis patients, especially in Thailand. We previously developed lime powder regimen (LPR), and demonstrated that LPR delivered citraturic, alkalinizing, and antioxidative effects in kidney stone patients. In this study, in vitro anti-lithogenic activity, in vivo acute toxicity, and crossover-designed phase 1 trial (in 13 healthy volunteers) of LPR were investigated. LPR inhibited the growth of calcium oxalate monohydrate (COM) crystals in dose-dependent manner, and inhibited the intracellular production of reactive oxygen species (ROS) in COM-treated HK-2 cells. LPR did not significantly alter viability of HK-2 cells. No acute toxicity was detected in mice orally fed with LPR (10 g/kg). No adverse effect and complaint of LPR ingestion (5 g/dose) were observed in the tested volunteers. Plasma citrate was elevated at 30 min after LPR load, which was higher than the water load control. Plasma potassium was significantly elevated at 30 min after LPR load and remained high for 2 h, and at 2 h, it was significantly higher than the water load. Urinary citrate was significantly increased at 1 h after LPR load and remained high for 2 h, and at 2 h, it was significantly higher than the water load. Urinary potassium was significantly increased at 1 h after LPR load and remained high for 3 h, and its levels at 1, 2, and 3 h were significantly higher than the water load. Urinary total antioxidant status was significantly increased at 2 h after LPR load. In conclusion, LPR had an inhibitory effect on COM growth and exerted as antioxidant to attenuate ROS production in the COM-treated renal tubular cells. LPR provided citraturic, kaliuric, and antioxidative responses in healthy individuals without any adverse events. This suggests that LPR is well tolerated and safe for daily consumption.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Preventing kidney stone recurrence is still a challenging issue in medical management of kidney stone disease. Increase fluid intake is well accepted to be an effective approach to reduce the risk for stone relapse in patients with first episode calcium stone, however in those with multiple past calcium stone, additional pharmaceutical therapy (such as thiazide, alkali citrate, and allopurinol) is required to reduce the recurrent likelihood [1]. Due to the side effect of pharmaceutical treatment, dietary therapy has gained recognition as an alternative for preventing the relapse of urinary calculi, particularly in those patients with hypocitraturic phenotype [1]. In the past decade, development of new medical regimen for treating stone disease is rare, although an effective treatment with no adverse effect is clearly needed [2].
Hypocitraturia is an important risk factor for kidney stone recurrence. In fact, it is a major metabolic abnormality found in the stone patients in Thailand [3, 4]. The well-known pharmaceutical regimen for treating the hypocitraturic stone patients is alkali citrate [5]. Many forms of this alkali salts such as potassium citrate (the most common preparation), potassium–magnesium citrate, and sodium citrate have been prescribed as preventive treatment, and they effectively deliver the citraturic and alkalinizing actions that clinically reduce the risk for recurrence [6]. However, intolerable side effect (for instance, gastrointestinal upset) that reduces the rate of compliance is still a main concern in long-term use of these salts [5, 7]. Additionally, use of potassium or sodium citrate causes increase in urine pH that could potentially increase the risk for calcium phosphate calculi.
Diet plays an important role in kidney stone formation, as it directly influences concentration and composition of urine. Dietary modification has been shown to reduce the risk for recurrent stone formation [8]. Dietary therapy is, therefore, an alternative for treating kidney stone disease. Since increase in urine output and citrate excretion are proved to prevent the formation of recurrent stone, fruit juice rich in citric acid content is considered to be the first-line candidate for dietary therapy of kidney stone disease. A crossover trial in eight healthy men by Wabner and Pak [9] showed that orange juice consumption delivered urine alkalinizing and citraturic effects equivalent to potassium citrate, but it lacked ability to reduce saturation of calcium oxalate. Seltzer et al. demonstrated in 12 stone patients that 6-day consumption of lemonade caused more than twofold increase in urinary citrate excretion, and suggested that lemon juice (containing the highest content of citric acid relative to other citrus juices [10]) was a cheap and well-tolerated source of dietary citrate [11]. In vitro data by Oussama et al. [12] showed that lemon juice inhibited crystallization of calcium oxalate in dose-dependent manner. Lemonade treatment for an average of 44.4 months revealed that the rate of stone formation was significantly decreased from 1.00 to 0.13 stones per patient per year [13]. Additionally, lemon juice efficiently inhibited the intrarenal deposit of calcium oxalate crystals in the ethylene glycol-induced urolithic rat model [14]. We preliminarily treated 13 nephrolithiasis patients with our in-house lime powder regimen (LPR), and demonstrated that LPR effectively delivered citraturic, urine alkalinizing, and antioxidative effects [15].
Lime (Citrus aurantifolia, Swingle), but not lemon (Citrus limonum) is usually grown in tropical countries including Thailand. Lime is mainly used as an important ingredient in many types of Thai food. Additionally, lime juice has a medicinal value, and it has been recognized as Thai herbal remedy. One of the medicinal properties of lime juice is to prevent the formation of urinary stones. In many studies, particularly those from western countries, lemon juice and lemonade are suggested to be clinically useful as dietary therapy for urolithiasis [6, 11–14]. The difference between lemon juice and lemonade is that lemon juice is fresh juice squeezed from lemons, but lemonade (usually commercially available) is added with water and sugar to make a flavored taste. On the other side, LPR is made from fresh lime juice, which is produced by our research group [15]. According to literature, oral doses of potassium citrate drug for treating kidney stone patients range 20–80 mEq for potassium content, and 30–110 mEq for citrate content [16–19]. Based on our research experiences and study by Ettinger et al. [16], we opted to use 21 mEq potassium and 63 mEq citrate for treating Thai kidney stone patients [15, 20, 21].
Nephrolithiasis can also be considered as oxidative stress-mediated disease, as increase in oxidative stress is clearly demonstrated in both experimental models and patients [22]. We showed that nephrolithiasis patients had elevated excretion of urinary 8-hydroxy deoxyguanosine (8-OHdG) compared with the healthy subjects [4, 23], and this oxidative DNA lesion was over-expressed in the renal tissues obtained from the patients [24]. Recently, oxidative damage induced by urine oxalate at physiological level was shown in the antioxidant-depleted LLC-PK1 cells [25], and the level and activity of antioxidant enzymes in erythrocytes from kidney and ureteral stone patients were significantly lower than that in the erythrocyte control [26]. Beside oxidative stress, intrarenal inflammation and fibrosis are commonly found in stone-containing kidneys of the Thai patients [27–29], and we hypothesize that urinary lithogenic milieu induces over-generation of reactive oxygen species (ROS) to cause oxidative damage, activate inflammatory response, and finally trigger renal fibrogenesis that eventually leads to kidney dysfunction. Therefore, an effective remedy for treating kidney stone patients should provide pharmacological actions of both reducing the urinary saturation (or crystal-forming potential) and boosting the antioxidant status. As mentioned above, LPR delivers citrituric and antioxidative actions, it is one of the candidate regimens to test in the clinical trials.
In this study, we conducted an in vitro study to investigate the stone-forming inhibitory activity, antioxidative property, and cytotoxicity of LPR. An in vivo acute toxicity of LPR in mice was explored. A crossover-designed phase 1 clinical trial was conducted in healthy volunteers to investigate the pharmacokinetic profile of LPR.
Materials and methods
LPR
A sachet of LPR (5 g lime juice powder) contained 21 mEq potassium, 63 mEq citrate, and significant amounts of natural antioxidants. Manufacture of LPR (from fresh lime juice) and its antioxidant content were clearly described in our previous study [15]. Briefly, after squeezing, lime juice was filtered and processed to reduce bitterness. The juice was subsequently freeze-dried to obtain lime powder. Lime juice contains high citrate content, but its potassium content is relatively low [10, 30]. The citrate level in our lime powder reached the therapeutic dose (63 mEq per 5 g lime powder), but potassium did not. Therefore, we added potassium (pharmaceutical grade) into lime powder LPR to get the potassium concentration of 21 mEq per 5 g lime powder, and called it LPR.
Total antioxidant capacity (TAC) of LPR measured by diphenylpicrylhydrazyl method showed that TAC of LPR was very high and significantly greater than that of potassium citrate drug [15]. Contents of ascorbic acid, total polyphenols, and flavonoids in LPR were of 27.30, 9.20, and 8.80 mg per 5 g LPR, respectively [15]. The quality control of LPR was checked by the certified laboratory of the process and environmental analysis center, pilot plant development and training institute, King Mongkut’s University of Technology Thonburi, Bangkok, Thailand. Potassium and citrate contents per 5 g LPR had to fall in the range of 19–23 and 57–63 mEq, respectively.
For nutritional value, amounts of total carbohydrate, total fat and protein were 3.28, 0.05, and 0.17 mg per sachet, respectively. Total energy was 14.2 kcal per sachet. Based on inductively coupled plasma optical emission spectroscopy data, cadmium, cobalt, and chromium were undetectable in LPR, while arsenic, lead, mercury, and nickel were found at concentrations of 0.07, 0.02, 0.001, and 0.07 ppm, respectively. These metal constituents did not exceed the acceptable limits for daily consumption of heavy metals (US Pharmacopeial), supporting that LPR was safe for a basis of daily consumption.
Oxalate depletion assay
Spectrophotometric oxalate depletion assay was carried out to assess the crystal growth inhibitory activity of LPR according to procedure described elsewhere [31]. In brief, 2 mM calcium chloride (0.5 mL) was mixed with 2 mM sodium oxalate (0.5 mL). Calcium oxalate monohydrate (COM) seed crystals (150 μg) were added into the mixture. Various concentrations of LPR (0 as control, 2, 10, 20 100, and 200 mg%, 100 μL) were added, and absorption at 214 nm (for detecting free oxalate) was kinetically recorded starting from 0 to 60, 5 min interval. Percent remaining oxalate was calculated as followed: (OD ti /OD t0) × 100, where t 0 was time at 0 min, and t i was time at each time point. A higher percentage remaining oxalate indicates a higher inhibition of the COM crystal growth.
Cytotoxicity test by methylthiazolyldiphenyl-tetrazolium bromide (MTT) assay
Proximal tubule human kidney cells (HK2-cells) purchased from ATCC was used for the experiment. The cell was cultured in DMEM/high glucose, pH 7.25, 5 % CO2 at 37 °C in 96-well plate. The confluent cells (70–80 %) were treated with various concentrations of LPR (0 as control, 2.5, 5, 10, 50, and 100 mg%), and incubated at 37 °C, 5 % CO2 for 24 h. After washing, MTT solution (0.5 mg/mL) was added, and incubated at 37 °C, 5 % CO2 for 4 h. Dimethyl sulfoxide was added. The absorbance of the solution at 570 nm was measured. A higher absorbance value indicates a higher viability. Cell viability (%) was calculated from (ODtest/ODcontrol) × 100.
Intracellular ROS production by dichloro-dihydro-fluorescein diacetate (DCFH-DA) assay
The HK-2 cells were seeded in 96-well black plate. After washing, the confluent cells were added with serum-free DMEM containing 0.1 mM DCFH-DA and incubated at 37 °C, 5 % CO2 for 30 min. COM crystals at 100, 500, and 1,000 μg/cm2 with or without LPR (50 mg %) were added. Fluorescent intensity at 0 and 60 min were measured (485 nm for excitation and 535 nm for emission). The arbitrary fluorescent unit (ratio), which reflects intracellular ROS production, was calculated from fluorescent intensity at 60 min divided by fluorescent intensity at 0 min.
Acute oral toxicity of LPR in mice
To test the acute toxicity of LPR in animal model, LPR was sent to the Medicinal Plant Research Institute, Department of Medical Sciences, Ministry of Public Health, Nonthaburi, Thailand. LPR was reconstituted with distilled water to reach the concentration of 0.5 g/mL. The LPR solution was orally fed (10 mL/kg) to mice (n = 10, 5 males and 5 females) twice (4 h interval) to get the final loading dose of 10 g/kg. In control group, mice (n = 10, 5 males and 5 females) were fed with the same amount of water. Mice of both groups were observed for 14 days prior to necropsy. H&E staining of liver and kidney tissues was performed to assess the pathological change in the fed mice.
Phase 1 trial of LPR in healthy volunteers
The crossover design of clinical phase 1 study is shown in Fig. 1. Sample size for a crossover trial is generally calculated from the equation that is used for comparing means in the t test for unpaired samples [32, 33]. To compute the sample size in this trial, power, allowable difference, variance, and superiority were assumed at 80%, 50 %, 1, and 0.1, respectively (free online computation at: http://www.cct.cuhk.edu.hk/stat/mean/tsmc_sup.htm). Considering a dropout rate of 15 %, 15 subjects were required for the trial. Therefore, 15 volunteers were initially screened for healthy status, and recruited for the trial. Of 15, 13 subjects completed the study, and 2 subjects dropped out before the experiment began. There were 8 (61.5 %) men and 5 (38.5 %) women. Their mean age was 34 ± 4 years. The blood chemistry profile to ensure their healthy condition is shown in Table 1. All women were neither pregnant nor having menstrual period. One week (days 1–7) before the experiment, called run-in week, all subjected were instructed to consume citrate and potassium-restricted diets. On day 8, subjects admitted to the Chula Clinical Research Center, Faculty of Medicine, Chulalongkorn University. All subjects received the same standardized diets. In a control day, subjects were orally loaded with 200 mL water at 8 am. Timed blood and urine specimens were collected from all participants. Heparinized blood at 0 min, 15 min, 30 min, 45 min, 1 h, 2 h, 5 h, and 10 h, and urine samples at 0, 1, 2, 3, 4, 6, 8, 10, and 24 h were collected after loading. In a LPR day, subjects were orally loaded with LPR solution (dissolving one sachet of LPR in 200 mL water). The subjects were asked to drink all LPR solution up within 10 min. Timed blood and urine specimens were collected similar to the control day (Fig. 1). Smoking was not allowed throughout the experimental trial.
Schematic of crossover experimental design for phase 1 clinical trial. Timed heparinized blood samples were collected 0 min, 15 min, 30 min, 45 min, 1 h, 2 h, 5 h, and 10 h after dosing, and timed urine samples were collected at 0, 1, 2, 3, 4, 6, 8, 10, and 24 h after dosing for both control and LPR days. BE bioequivalent, LPR lime powder regimen, M meal
We skipped the wash-out period in the study design because the subjects received only water in the control day. The main purpose of water loading was to explore the baselines of citrate, potassium, and antioxidants in plasma and urine samples.
The research protocol was approved by the Ethics committee, Faculty of Medicine, Chulalongkorn University. Informed consents were received from all participants. The committee also approved the use of LPR in healthy volunteers.
Plasma citrate was determined using high-performance liquid chromatography [34]. Plasma samples were sent to the routine laboratory of Nephrology Unit, King Chulalongkorn Memorial Hospital for measurement of potassium using atomic absorption spectrometry. Urine samples were analyzed for citrate, potassium, pH, and total antioxidant status (TAS). Citrate and potassium measurements in urine were performed similar to the plasma samples. Urinary TAS was determined by 2,2-dipheny-2-picryl hydrazyl (DPPH) method [35].
Statistical analysis
Data presented as mean and standard error (SE). One-way ANOVA was employed for testing the mean difference of cell viability and ROS production among the groups. One-way repeated measures ANOVA was used to test the difference of % remaining oxalate between time points, and the difference plasma/urine parameters between time points in each load in the crossover trial. Two-way repeated measures ANOVA was used for testing the difference of plasma/urine parameters in each time point between the control and LPR loads. GraphPad Prism 5.0 and Stata version 10 were used for graphs and statistical computation. P value <0.05 was considered as statistically significant.
Results
Inhibition of crystal growth and intracellular ROS generation by LPR
Oxalate depletion assay was performed to test the inhibitory effect of LPR on the growth of COM crystals. The result clearly showed that % free remaining oxalate was gradually increased when the concentrations of LPR was increased (Fig. 2). We concluded that LPR retarded the COM crystal growth in dose-dependent manner.
Based on MTT assay, treatment with LPR up to 100 mg % for 24 h did not alter the viability of HK-2 cells relative to the untreated cells (Fig. 3a). This indicated that the LPR, at least at the tested concentrations, had no cytotoxicity on the human proximal renal tubular cells.
Effects of LPR on HK-2 cell viability and intracellular ROS production. a HK-2 cells exposed to LPR concentrations up to 100 mg % caused no significant change of cell viability compared with the untreated control (0 %). b Intracellular ROS generation was significantly increased in COM-treated HK-2 cells at concentrations of 500 and 1,000 μg/cm2 compared with the untreated cells (control). In the COM-treated cells, co-treatment with 50 mg % LPR caused a significant reduction of ROS generation. Cells treated with LPR alone also caused significant decrease in ROS production compared with the untreated control. *P < 0.05 vs. control. § P < 0.05 vs. corresponded COM-treated condition. NS not significant
We also investigated whether LPR exerted the antioxidative action to inhibit ROS production in the COM-treated HK-2 cells. LPR (50 mg %) significantly inhibited ROS production in HK-2 cells compared with the untreated cells (Fig. 3b). In COM-treated cells (500 and 1,000 μg/cm2), the ROS generation was significantly greater than the untreated control. Interestingly, LPR was drastically reduced the ROS production in the COM-treated cells. This data indicated that LPR acted as antioxidant to restrain the production of ROS in the renal tubular cells exposed to lithogenic crystals.
No acute toxic effect of LPR in mice
Approximately 3–5 min post-administration, nausea-like symptom was observed in mice orally fed with LPR (10 g/kg). However, this adverse effect disappeared within 24 h. No deaths and other adverse effects were observed throughout the 14 days of trial. Based on gross anatomy, all visceral organs and brain appeared to be normal in both LPR-fed and control groups. H&E staining showed no pathological changes in the liver and kidney tissues of the LPR-fed mice (data not shown). We concluded that there was no acute toxicity found in the mice fed with high dose LPR.
Citratemic, kalemic, citraturic, kaliuric, and antioxidative effects of LPR in healthy volunteers
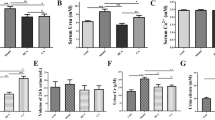
Plasma citrate was significantly increased at 30 min post-LPR load compared with the pre-LPR load, and it was significantly higher than the post-water load control (Fig. 4a; Table 2). After 30 min post-load, plasma citrate levels between LPR and water loads were not significantly different. Plasma potassium was significantly increased at 30 min post-LPR load compared with the pre-LPR load baseline and remained significantly high for 2 h (Fig. 4b). At 2 h post-load, plasma potassium in LPR load group was significantly greater than in the water load group. Thereafter, plasma potassium levels between LPR and water loads were not significantly different. Line graphs of plasma citrate and potassium showing each individual response are shown in Supplementary Fig. 1. Our current data indicated that LPR rapidly and transiently delivered the citratemic and kalemic effects in the healthy volunteers.
Plasma citrate and potassium pharmacokinetic profiles in 13 healthy volunteers loaded with water (control) or LPR. Plasma citrate significantly increased at 30 min after LPR load (a) whereas significant increase in plasma potassium was found at 30 min to 2 h after LPR load (b). At 30 min after dosing, plasma citrate in LPR day was significantly higher than that in control day (a). Plasma potassium at 2 h after LPR dosing was significantly greater than that at 2 h after water dosing (b). No significant change of plasma citrate and potassium in the subjects loaded with water. *P < 0.05 vs. Baseline (0 h). $ P < 0.05 vs. control at the same time point
Urinary level of citrate was significantly increased at 1 h post-LPR load and remained elevated for 2 h, compared with the pre-LPR load baseline (Fig. 5a; Table 3). The significant difference of urinary citrate between LPR and water loads was found at 1 h after loading. Level of urinary potassium was significantly increased at 1, 2, and 3 h post-LPR load relative to the pre-LPR load baseline (Fig. 5b), and at these three time points, plasma potassium in LPR load group were significantly higher than that in water load group.
Profiles of urinary citrate, potassium, pH, and TAS in 13 healthy volunteers loaded with water (control) and LPR. Significant excretion of urinary citrate in LPR-loaded subjects was found at between 1 and 2 h after dosing (a). At 1 h after LPR load, urinary citrate was significantly higher than that after water load. Urinary potassium was significantly increased at 1, 2, and 3 h after LPR load compared with the baseline, and these levels were significantly higher than in water load control at the same time points (b). Both water and LPR loads caused significant increase in urine pH at 1, 2, 3, 4, 6, 8, and 10 h after dosing, but no significant change was observed between the two loads (c). Urinary TAS was exclusively increased at 2 h after LPR load (d), and significant change between the two loads was not revealed. *P < 0.05 vs. baseline (0 h). $ P < 0.05 vs. control at the same time point
Both LPR and water loads showed a significant increase in urine pH relative to their respective baselines (Fig. 5c). Significant difference of urine pH between LPR and water loads was not observed. Urinary TAS was significantly increased at 2 h post-LPR load compared with the pre-LPR load baseline (Fig. 5d). Thereafter, level of urinary TAS trended to be increased in the LPR load, but still there was no statistical significance between LPR and water loads. Line graphs of urinary citrate, potassium, pH, and TAS displaying each individual response are shown in Supplementary Fig. 2.
All spot urine samples of each subject in each day were pooled, and regarded as 24-h urine sample. Average 24-h urine volume (mean ± SD) in control day was 2,171 ± 875 mL, and in LPR day was 2,436 ± 710 mL (P > 0.05). Average values of urine pH in control and LPR days were 6.77 ± 0.59 and 6.76 ± 0.51, respectively (P > 0.05). Mean of urinary citrate in control and LPR days were 731 ± 307 and 1,065 ± 322 mg/day, respectively (P < 0.05). Unfortunately, we did not have data of urinary potassium in 24-h urine samples.
None of adverse effects was found in the trialed subjects. Complaints about sour taste and smell of the LPR solution were not observed either.
Discussion
Kidney stone, particularly idiopathic calcium oxalate stone, is largely contributed by diets, and it can be considered as a diet-related disease [36]. Nutraceutical is, therefore, an appealing inexpensive and safe alternative for prevention and treatment of kidney stones. The major metabolic risk factors found in kidney stone patients in Thailand were hypocitraturia and hypokaliuria [3]. In addition, oxidative stress is increased in the patients compared with the healthy controls [23, 24]. We have developed a nutraceutical lime-based regimen called LPR to test if it has a health benefit to treat kidney stone disease [15]. Our previous pilot study in kidney stone patients showed that daily consumption of LPR for 3 months caused increases in urinary citrate, urine pH, and antioxidant capacity, and the citraturic and alkalinizing actions of LPR were comparable to the potassium citrate drug [15]. We propose that these medicinal properties of LPR may clinically help to reduce the risk for stone formation and prevent the relapse of calculi. Clinical studies of LPR in phase 1, phase 2, and phase 3 have been planned to conduct in the development pipeline. In this study, phase 1 trial in healthy volunteers was conducted to explore the safety profile of LPR as well as pharmacokinetic profiles of its stone-combating ingredients, viz. citrate, potassium, and antioxidants.
We found that consumption of LPR (63 mEq citrate) was capable of boosting urinary citrate in healthy subjects. Plasma citrate was significantly increased at 30 min after LPR load similar to the study by Sakhaee et al. [37] that investigated in six normal subjects loaded with a single dose of citric acid (40 mEq). The discrepancy was that the serum citrate concentration remained elevated for 3 h after citric acid load, but in this study plasma citrate was rapidly dropped after it reached the peak (Fig. 4a). Although precise explanation for this discrepant result is not known, source of citrate (synthetic vs. natural) might be the case. Concentration of urinary citrate was maximized at 2 h after citric acid load [37]. We found the peak of urinary citrate concentration at 1 h after LPR load, and it persistently elevated for 2 h. Harvey et al. [38] investigated the bioavailability of citrate from potassium citrate drugs in 18 normal volunteers and found that urinary citrate excretion was significantly increased at 1 h after dosing (60 mEq) and remained significantly high for 4–5 h. Although it cannot be directly compared, it is a clue that our LPR has a slightly lower bioavailability of citrate relative to the potassium citrate preparation. The actual reason remains to be elucidated. However, the main advantage of our LPR over the potassium citrate drug is that LPR is a naturally derived remedy, which is relatively inexpensive and has no disfavor side effect.
Potassium level in plasma was significantly increased at 30 min after LPR load, and persisted longer than plasma citrate (Fig. 4b). However, none of the studied subjects developed hyperkalemia, as a normal level of potassium in plasma is between 3.5 and 5.0 mM (mEq/L). A significantly elevated level of urinary potassium was found between 1 and 3 h after LPR load. Our data suggested that both citrate and potassium traveled rapidly from blood circulation to urine. Perhaps, they were directly excreted into the urine without any metabolic reactions [37]. This speculation, however, needs further investigation. Harvey et al. [38] demonstrated that potassium citrate load caused a significant increase in urinary potassium up to 6 h, and also caused significant increase in urine pH. As a significant difference of increase in urine pH between LPR and water loads was not observed, an increase in urine pH after LPR and water loads that was found in this study may be due to the provided meals rather than water or LPR. We speculate that a lesser alkalinizing effect of LPR relative to potassium citrate might be due to a lower bioavailability of citrate and potassium from LPR relative to from potassium citrate.
It is well recognized that oxidative stress mediates the pathogenesis of kidney stone [22–24, 26]. Antioxidant intervention has been proved to inhibit the development of kidney stone at least in the animal model [39]. In this study, we provided an evidence of antioxidant property of LPR for inhibiting ROS generation in HK-2 cells challenged with lithogenic COM crystals. Also, in healthy subjects LPR ingestion was able to increase urinary level of TAS significantly. This antioxidative action was not observed in the treatment with potassium citrate [15]. We hypothesize that this antioxidative response of LPR will act in concert with the citraturic action to prevent the formation of recurrent calculi.
The most important finding in this study was that neither side effect of LPR nor complaint of LPR ingestion was observed in the trialed healthy volunteers. Acute toxicity of LPR in mice was not detected at 10 g/kg oral load. Although a nausea-like symptom or disgust reaction (e.g., gaping response [40]) was observed in mice after administration, it completely vanished within 24 h. LPR up to 100 mg % exposed to HK-2 cells did not change cell viability. These suggest that LPR is well tolerated and safe for a daily consumption basis. For a long-term use of LPR, daily consumption of LPR for 3 months in nephrolithiasis patients showed no adverse event [15]. Furthermore, we had finished 6 months trial of LPR in our nephrolithiasis patients, and no adverse effect was observed (unpublished data).
Limitations of the present study should be mentioned. Although sample size required in the crossover trial is generally lower than that in the parallel group trial, the number of the trialed volunteers in this study was rather small. Standard 24-h urine specimen was not collected to investigate the change of stone modulating substances in healthy volunteers after LPR consumption. Pharmacokinetic profile of LPR in kidney stone patients was not explored. Drug–drug interaction of LPR was not investigated in this study. Perhaps, potassium-containing drugs might somehow interact with LPR.
In conclusion, we clearly demonstrated that LPR had an inhibitory effect on calcium oxalate crystal growth, and acted as antioxidant to diminish ROS production in COM-treated renal tubular cells. In phase 1 trial, single oral dose of LPR delivered citraturic and antioxidative effects in healthy subjects. These effects may be beneficial for reducing a risk for kidney stone formation. No toxic response of LPR was found in the tested renal tubular cells, mice as well as healthy individuals. Our current data warrant LPR as a safe remedy for conducting further a phase 2 clinical trial in a large population of patients with kidney calculi.
References
Fink HA, Wilt TJ, Eidman KE, Garimella PS, MacDonald R, Rutks IR, Brasure M, Kane RL, Ouellette J, Monga M (2013) Medical management to prevent recurrent nephrolithiasis in adults: a systematic review for an American college of physicians clinical guideline. Ann Intern Med 158:535–543
Xu H, Zisman AL, Coe FL, Worcester EM (2013) Kidney stones: an update on current pharmacological management and future directions. Expert Opin Pharmacother 14:435–447
Tosukhowong P, Borvonpadungkitti S, Prasongwatana V, Tungsanga K, Jutuporn S, Dissayabutr T, Reungjui S, Sriboonlue P (2002) Urinary citrate excretion in patients with renal stone: roles of leucocyte ATP citrate lyase activity and potassium salts therapy. Clin Chim Acta 325:71–78
Boonla C, Youngjermchan P, Pumpaisanchai S, Tungsanga K, Tosukhowong P (2011) Lithogenic activity and clinical relevance of lipids extracted from urines and stones of nephrolithiasis patients. Urol Res 39:9–19
Mattle D, Hess B (2005) Preventive treatment of nephrolithiasis with alkali citrate––a critical review. Urol Res 33:73–79
Kurtz MP, Eisner BH (2011) Dietary therapy for patients with hypocitraturic nephrolithiasis. Nat Rev Urol 8:146–152
Fazil Marickar YM, Salim A, Vijay A (2010) Effect of blind treatment on stone disease. Urol Res 38:205–209
Taylor EN, Curhan GC (2006) Diet and fluid prescription in stone disease. Kidney Int 70:835–839
Wabner CL, Pak CY (1993) Effect of orange juice consumption on urinary stone risk factors. J Urol 149:1405–1408
Penniston KL, Nakada SY, Holmes RP, Assimos DG (2008) Quantitative assessment of citric acid in lemon juice, lime juice, and commercially-available fruit juice products. J Endourol 22:567–570
Seltzer MA, Low RK, McDonald M, Shami GS, Stoller ML (1996) Dietary manipulation with lemonade to treat hypocitraturic calcium nephrolithiasis. J Urol 156:907–909
Oussama A, Touhami M, Mbarki M (2005) In vitro and in vivo study of effect of lemon juice on urinary lithogenesis. Arch Esp Urol 58:1087–1092
Kang DE, Sur RL, Haleblian GE, Fitzsimons NJ, Borawski KM, Preminger GM (2007) Long-term lemonade based dietary manipulation in patients with hypocitraturic nephrolithiasis. J Urol 177:1358–1362 (discussion 1362; quiz 1591)
Touhami M, Laroubi A, Elhabazi K, Loubna F, Zrara I, Eljahiri Y, Oussama A, Grases F, Chait A (2007) Lemon juice has protective activity in a rat urolithiasis model. BMC Urol 7:18
Tosukhowong P, Yachantha C, Sasivongsbhakdi T, Ratchanon S, Chaisawasdi S, Boonla C, Tungsanga K (2008) Citraturic, alkalinizing and antioxidative effects of limeade-based regimen in nephrolithiasis patients. Urol Res 36:149–155
Ettinger B, Pak CY, Citron JT, Thomas C, Adams-Huet B, Vangessel A (1997) Potassium-magnesium citrate is an effective prophylaxis against recurrent calcium oxalate nephrolithiasis. J Urol 158:2069–2073
Goldberg H, Grass L, Vogl R, Rapoport A, Oreopoulos DG (1989) Urine citrate and renal stone disease. CMAJ 141:217–221
Lipkin ME, Preminger GM (2011) Demystifying the medical management of nephrolithiasis. Rev Urol 13:34–38
Pak CY (2004) Medical management of urinary stone disease. Nephron Clin Pract 98:c49–c53
Tosukhowong P, Tungsanga K, Phongudom S, Sriboonlue P (2005) Effects of potassium-magnesium citrate supplementation on cytosolic ATP citrate lyase and mitochondrial aconitase activity in leukocytes: a window on renal citrate metabolism. Int J Urol 12:140–144
Tungsanga K, Sriboonlue P, Futrakul P, Yachantha C, Tosukhowong P (2005) Renal tubular cell damage and oxidative stress in renal stone patients and the effect of potassium citrate treatment. Urol Res 33:65–69
Khan SR (2013) Reactive oxygen species as the molecular modulators of calcium oxalate kidney stone formation: evidence from clinical and experimental investigations. J Urol 189:803–811
Boonla C, Wunsuwan R, Tungsanga K, Tosukhowong P (2007) Urinary 8-hydroxydeoxyguanosine is elevated in patients with nephrolithiasis. Urol Res 35:185–191
Kittikowit W, Waiwijit U, Boonla C, Ruangvejvorachai P, Pimratana C, Predanon C, Ratchanon S, Tosukhowong P (2014) Increased oxidative DNA damage seen in renal biopsies adjacent stones in patients with nephrolithiasis. Urolithiasis 42:387–394
Thamilselvan V, Menon M, Thamilselvan S (2014) Oxalate at physiological urine concentrations induces oxidative injury in renal epithelial cells: effect of alpha-tocopherol and ascorbic acid. BJU Int 114:140–150
Ma MC, Chen YS, Huang HS (2014) Erythrocyte oxidative stress in patients with calcium oxalate stones correlates with stone size and renal tubular damage. Urology 83(510):e9–e17
Boonla C, Hunapathed C, Bovornpadungkitti S, Poonpirome K, Tungsanga K, Sampatanukul P, Tosukhowong P (2008) Messenger RNA expression of monocyte chemoattractant protein-1 and interleukin-6 in stone-containing kidneys. BJU Int 101:1170–1177
Boonla C, Krieglstein K, Bovornpadungkitti S, Strutz F, Spittau B, Predanon C, Tosukhowong P (2011) Fibrosis and evidence for epithelial-mesenchymal transition in the kidneys of patients with staghorn calculi. BJU Int 108:1336–1345
Boonla C, Tosukhowong P, Spittau B, Schlosser A, Pimratana C, Krieglstein K (2014) Inflammatory and fibrotic proteins proteomic ally identified as key protein constituents in urine and stone matrix of patients with kidney calculi. Clin Chim Acta 429:81–89
Pak CY (2008) Medical stone management: 35 years of advances. J Urol 180:813–819
Chutipongtanate S, Thongboonkerd V (2010) Red blood cell membrane fragments but not intact red blood cells promote calcium oxalate monohydrate crystal growth and aggregation. J Urol 184:743–749
Chow SC, Shao J, Wang H (2003) Sample size calculations in clinical research. Taylor & Francis, Boca Raton
Wellek S, Blettner M (2012) On the proper use of the crossover design in clinical trials: part 18 of a series on evaluation of scientific publications. Dtsch Arztebl Int 109:276–281
Khaskhali MH, Bhanger MI, Khand FD (1996) Simultaneous determination of oxalic and citric acids in urine by high-performance liquid chromatography. J Chromatogr B Biomed Appl 675:147–151
Patchsung M, Boonla C, Amnattrakul P, Dissayabutra T, Mutirangura A, Tosukhowong P (2012) Long interspersed nuclear element-1 hypomethylation and oxidative stress: correlation and bladder cancer diagnostic potential. PLoS One 7:e37009
Heilberg IP, Goldfarb DS (2013) Optimum nutrition for kidney stone disease. Adv Chronic Kidney Dis 20:165–174
Sakhaee K, Alpern R, Poindexter J, Pak CY (1992) Citraturic response to oral citric acid load. J Urol 147:975–976
Harvey JA, Zobitz MM, Pak CY (1989) Bioavailability of citrate from two different preparations of potassium citrate. J Clin Pharmacol 29:338–341
Naghii MR, Mofid M, Hedayati M, Khalagi K (2013) Antioxidants inhibition of high plasma androgenic markers in the pathogenesis of ethylene glycol (EG)-induced nephrolithiasis in Wistar rats. Urolithiasis 42:97–103
Parker LA, Rock EM, Limebeer CL (2011) Regulation of nausea and vomiting by cannabinoids. Br J Pharmacol 163:1411–1422
Acknowledgments
The study was financially supported by the National Research Council of Thailand (NRCT). Thanks to the Biochemistry and Molecular Biology of Metabolic Diseases Research Unit. Poonsin Poungpairoj was a M.Sc. Student in Medical Biochemistry Program, Faculty of Medicine, Chulalongkorn University, and he did all key experiments.
Conflict of interest
The authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chariyavilaskul, P., Poungpairoj, P., Chaisawadi, S. et al. In vitro anti-lithogenic activity of lime powder regimen (LPR) and the effect of LPR on urinary risk factors for kidney stone formation in healthy volunteers. Urolithiasis 43, 125–134 (2015). https://doi.org/10.1007/s00240-015-0751-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00240-015-0751-y