Abstract
Several experimental and animal studies have demonstrated that substances rich in antioxidants can reduce the physicochemical and peroxidative risk factors for calcium oxalate (CaOx) renal stone formation in urine and blood. However, there are very few such investigations in humans. In the present pilot study, two varieties of tea, a green one from Japan (JGT) and a herbal one from South Africa (Rooibos) (RT), both rich in antioxidants, were administered to a group of CaOx stone formers (SF) (n = 8) for 30 days. Both teas were analysed for polyphenols by high-performance liquid chromatography and for minerals by plasma atomic and optical emission spectroscopy. 24 h urines (baseline and day 30) were analysed for lithogenic factors. CaOx metastable limits and crystal nucleation and growth kinetics were also determined in each urine sample. Deposited crystals were inspected by scanning electron microscopy. Blood samples were collected (baseline and day 30). Biomarkers of oxidative stress including plasma and urinary thiobarbituric acid reactive substances (TBARS) and urinary N-acetyl-β-D-glucosaminidase (NAG) were also determined. Urinary physicochemical risk factors were also investigated after ingestion of RT for 30 days in two control groups (CG1 and CG2), the latter one of which consisted of habitual JGT drinkers. Statistical analyses were performed using Wilcoxon signed rank tests and Mann–Whitney tests for paired and independent measurements, respectively. Several flavonoids and catechins were quantified in RT and JGT, respectively, confirming that both teas are rich sources of antioxidants. Mineral content was found to be far below dietary reference intakes. There were no significant changes in any of the urinary physicochemical or peroxidative risk factors in the control groups or in SF, except for the supersaturation (SS) of brushite (Bru) which decreased in the latter group after ingestion of JGT. Crystal morphology showed a tendency to change from mixed CaOx mono- and di-hydrate to monohydrate after ingestion of each tea. Since the latter form has a stronger binding affinity for epithelial cells, this effect is not protective. Analysis of the physicochemical and peroxidative risk factors in CG1 and CG2 did not reveal any evidence of a synergistic effect between the two teas. Paradoxically, baseline risk factors in the habitual JGT control group were significantly raised relative to those in CG1. Our preliminary results suggest that ingestion of RT and JGT does not reduce the risk factors for CaOx stone formation in humans, but these findings need to be tested in further studies involving much larger sample sizes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Several in vitro experimental and in vivo animal studies have demonstrated a strong correlation between oxidative stress and the risk of calcium oxalate (CaOx) renal stone formation [1–3]. These and other studies have also shown that administration of substances rich in antioxidants can reduce such risks [4–9]. Unfortunately, there are only a few studies which have investigated the potential prophylactic effects of dietary antioxidants in humans and these have involved only two such substances, vitamin E [10, 11] and pomegranate extract [12]. Vitamin E lowered urinary excretion of oxalate and calcium, and increased excretion of citrate in hyperoxaluric stone formers in the first of the studies involving this substance [10]. In the other study, it reduced lipid peroxidation products, increased plasma antioxidant enzymes and plasma vitamin E and restored the biochemical and kinetic properties of urinary THP, a well-known inhibitor of CaOx crystallization, in hypertensive and hyperoxaluric patients [11]. In the pomegranate study, supersaturation (SS) of CaOx tended towards decreasing values in a group of calcium stone formers who had demonstrated increased antioxidant activity after administration of this substance [12].
Various teas are rich in potent antioxidants such as flavonoids and other polyphenols [13–15]. As such, they may have antilithogenic capacity. Teas are broadly classified as green tea (unfermented), oolong tea (semi-fermented) and black and red tea (fermented) [15]. While all types have been investigated for their beneficial health effects, green tea (GT) has received the most attention [15]. Numerous such benefits have been demonstrated [15]. Despite this, GT’s potential protective effects on CaOx nephrolithiasis have been investigated only in experimental and animal models [6, 7, 16]. These studies have shown that it decreases CaOx crystal deposits [6, 7, 16], decreases urinary oxalate excretion [6, 7] and inhibits free-radical production induced by oxalate [7].
Green tea is widely consumed in China, Japan, Korea and Morocco [15]. A South African herbal tea, rooibos (Aspalathus linearis), indigenous to the Cedarburg Mountain [17], is rich in antioxidants such as flavonoids, polyphenols and phenolic acid [18, 19]. It also contains the unique C–C linked dihydrochalcone glucoside aspalathin and cyclic dihydrochalcone aspalathin, both of which are strong antioxidants [20].
It is apparent that prospective studies for assessing whether teas rich in anti-oxidants have a capacity for reducing the risk of renal stone formation in humans are lacking. Investigations of their antilithogenic potential are, therefore, warranted. Rooibos herbal tea (RT) and a variety of green tea commonly consumed in Japan (JGT) were selected for the present study.
Subjects and methods
Determination of oxalate release in RT and JGT as a function of brewing time
An enzymatic assay was used for the determination of oxalate in the two teas (Oxalate Kit, Sigma Diagnostics, St. Louis, MO, USA). Samples were prepared for analysis by adding 250 ml of boiling MilliQ water to each teabag. These were removed after brewing times of 5 and 10 min. The samples were stirred to obtain homogenous solutions and were allowed to cool to 25 °C. Aliquots of 20 ml were decanted for the kit analysis.
Determination of polyphenol compounds in aqueous infusions of RT and JGT
Aqueous extracts were prepared by infusion, by pouring 100 ml of hot distilled water (90 °C) on 1 g of tea and steeping it for 5 min. Infusions were filtered prior to analysis. Chromatographic separation was performed at the Oxidative Stress Research Centre (Cape Peninsula University of Technology, Bellville, Cape Town) using an Agilent 1200 Series HPLC System. The column was an YMC Pack pro C-18 column (150 × 4.6 mm id). Eluents for the determination of flavonoids in RT were (A) aqueous 300 µl/L formic acid and (B) methanol containing 300 µl/L formic acid, while for the determinations in JGT, eluents were (A) aqueous 3 % acetic acid and (B) methanol.
Flow rate was 1 ml/min. Acquisition in RT was set at 287 nm for aspalathin and at 360 nm for all other components, while in JGT it was set at 280 nm for all components. Analyses were performed in duplicate.
Determination of minerals in aqueous infusions of RT and JGT
Infusions for mineral analysis were prepared in the same way as for the analysis of polyphenols. These were filtered prior to analyses. Sodium, potassium, calcium and magnesium were analysed using an Agilent 4100 Microwave Plasma Atomic Emission Spectrometer (MP-AES) with an Agilent 4107 nitrogen generator (operating conditions: pump speed 15 rpm, nebulizer pressure 240 kPa, rinse time 30 s, sample uptake 90 s and equilibration 15 s). Phosphorus was analysed by inductively coupled plasma optical emission spectroscopy (ICP-OES) using a Varian 730 Series instrument (operating conditions: plasma power 1.2 kW, plasma flow 15 l/min, auxiliary flow 1.5 l/min, nebuliser flow 0.75 l/min, pump rate 15 rpm, rinse time 10 s and sample uptake 15 s).
Study groups
Healthy white male volunteers between the ages of 18–26 with no chronic illness of any nature (n = 10) were recruited from the student and staff cohorts of the University of Cape Town (control group 1). Another cohort (n = 10) having the same characteristics was recruited at the University of Nagoya (control group 2). In addition, CaOx kidney stone patients (n = 8) (group SF) between the ages of 30–60 were recruited in Cape Town from the Urology Departments at Groote Schuur and Tygerberg Hospitals and from a urologist in private practice.
Trials involving the healthy subjects were independently conducted at the research centres in Cape Town and Nagoya and involved the ingestion of RT only. Trials involving stone formers (SF) were conducted at the University of Cape Town only. These involved the ingestion of RT and JGT. Participants were required to complete a medical questionnaire; those who reported any current medical problems were excluded from participation.
The rationale for studying the response of control subjects to RT ingestion was to allow an informed decision to be made regarding a suitable dosage in the subsequent investigation involving SF patients. The rationale for involving control group 2 (in addition to control group 1) was to investigate whether the effects of RT would be modulated in any way in subjects whose habitual dietary regimen included consumption of JGT.
The study at both universities was conducted in accordance with the principles of the 1964 Helsinki Declaration and its amendments or comparable ethical standards. Informed consent was obtained from all participants. In addition, the study was approved by the Faculty of Human and Health Sciences Research Ethics Committee of the University of Cape Town (Ref number: HREC REF 221/2012). Ethics approval per se was not required by the University of Nagoya.
Protocols
Control groups
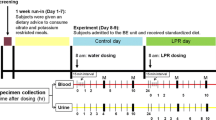
Each subject at both research centres was provided with Freshpak rooibos tea (Entyce Beverages, Rivonia, South Africa). Subjects were instructed to drink 250 ml of a low mineral content water (Cape Town–Ca: 6.1 mg/l, Mg: 1.0 mg/l; Nagoya–Ca: 7.0 mg/l, Mg: 0.9 mg/l) during the 24 h period prior to collection of their baseline urine samples as described below. They drank 2 cups (125 ml per cup) of RT per day (2 tea bags per 125 ml low mineral content water, brewing time 5 min at 90 °C) for 30 days. The tea was taken in two doses of 125 ml each: the first at mid-morning and the second at mid-afternoon.
Stone formers
After consideration of the results obtained in the study involving the control groups as described below, the dosage for RT in this phase of the study (SF) was doubled. Each subject drank 4 cups of tea per day (2 tea bags per 125 ml) for 30 days. The tea was taken in four doses of 125 ml each: morning, mid-morning, mid-afternoon and before retiring. Prior to collecting their baseline urine samples, subjects were required to drink 500 ml of the low mineral content water. A final urine sample was collected on day 30.
After completion of the RT protocol, each SF observed a washout period of 7 days. A 24 h urine sample was then collected. This served as the baseline sample for the next phase of the study in which Japanese green tea (JGT) (Ito-en Ltd, Tokyo) was ingested for 30 days. The same dosage (4 × 125 ml per day) and brewing time (5 min) were administered as that used for RT ingestion.
Dietary restrictions
During the trial periods themselves (controls and SF patients), subjects were given a list of foods high in antioxidants and oxalate which they were instructed to avoid [21–23]. However, subjects in control group 2 were advised not to restrict their habitual daily consumption of green tea. After the trial, these subjects were asked to estimate their daily intake of this tea. SF subjects completed 24 h food diaries at baseline and on day 30. On day 30 they were required to follow the same diet as day 0. Diaries were analysed using FoodFinder 2 [24].
Urine collection and analysis
Each subject collected 24 h urine samples in plastic bottles without preservatives, on day 0 (baseline), and on day 30 of the study. Urines were tested for nitrite using urinary test strips. Those testing positive were discarded. Urines were filtered through a 0.75 μm pre-filter followed by a 0.45 μm nitrocellulose filter paper and were then stored at 4 °C prior to analysis. Samples were analysed in duplicate for pH, Na, K, Ca, Mg, phosphate, Ox, citrate, uric acid and chloride as described elsewhere [25, 26]. These parameters were used to calculate the Tiselius risk index (TRI) [27] using in-house software and SS of various stone-forming salts using EQUIL2 [28].
Blood collection and preparation
Subjects were required to fast for 12 h prior to blood collection. Blood was drawn by a qualified phlebotomist from each subject on the mornings of day 0 and 30. Samples were stored on ice until centrifugation. Plasma samples were prepared within an hour of blood collection by centrifuging at 3000 rpm for 15 min (Labofuge 200 Centrifuge, Heraeus Sepatech, Germany).
Determination of urinary and plasma biomarkers of oxidative stress
Identical methods were used in the respective laboratories at the University of Cape Town and at the University of Nagoya for the determination of urinary and plasma biomarkers of oxidative stress.
Plasma and urinary thiobarbituric acid reactive substances (TBARS) were measured as MDA equivalents using an assay kit (OXI-TEK TBARS Assay kit, ZeptoMetrix, Buffalo, New York, USA). The reaction is based on malondialdehyde (MDA) forming a 1:2 adduct with thiobarbituric acid (TBA), producing an MDA-TBA adduct with a pink colour which can be measured by spectrophotometry at 532 nm (Spectronic Unicam Helios, Cambridge, England).
Urinary N-acetyl-β-D-glucosaminidase (NAG) was determined using a NAG assay kit (Roche, Mannheim, Germany). The reaction is based on 3-cresolsulfonphthaleinyl-N-acetyl-β-D-glucosaminide being hydrolysed by N-acetyl-β-D-glucosaminidase, producing 3-cresolsulfonphthalein with an orange colour which can be measured by spectrophotometry at 580 nm.
Calcium oxalate metastable limit (CaOx MSL)
The CaOx MSL was determined in the urines of SF subjects only, using the method described by Ryall and co-workes [29]. Briefly, aliquots (10 ml) of filtered urine were pipetted into small plastic cups. Increasing concentrations (15–195 mM) of sodium oxalate (Na2Ox) were added stepwise to successive cups which were then incubated at 37 °C for 30 min. Absorbance of each sample was measured at 620 nm using a UV–Vis spectrophotometer (AnalytikJena Specord 40, Germany) and was plotted as a function of Na2Ox concentration. The concentration of sodium oxalate (µmol/l) at which a sudden increase in absorbance occurred (corresponding to crystal formation) was taken as a measure of the CaOx MSL.
Simultaneous measurement of CaOx crystal nucleation and aggregation kinetics
CaOx crystallisation kinetics was determined in SF urines only. CaOx crystal nucleation and aggregation were induced according to the procedure described by Hess and co-workers [30]. Briefly, stock solutions of Ca and Ox containing 200 mM sodium chloride, 10 mM sodium acetate, and 8.5 mM calcium chloride and 1.0 mM potassium oxalate, respectively, were prepared. pH was adjusted to 5.7 in both solutions using either hydrochloric acid (HCl) or sodium hydroxide (NaOH). The stock solutions were filtered through a 0.22 μm filter. Aliquots of each stock solution were placed in plastic cups clamped in a circulating water bath at 37 °C. 1 ml of the Ca solution was transferred to a cuvette placed in the spectrophotometer, regulated at 37 °C and constantly stirred at 500 rpm. 20 μl of pre-filtered urine and 1 ml of the Ox solution were added. Absorbance readings at 620 nm were recorded for 30 min. These values were plotted as a function of time and the slopes of the linear increase and decrease in absorbance were measured. The maximum increase in the slope was interpreted as the maximum rate of the formation of new particles (crystal nucleation), while the decrease in the slope was interpreted as the decrease in the rate of particle number caused by crystal aggregation.
Scanning electron microscopy (SEM)
Urines obtained at baseline and after ingestion of both teas for 30 days were subjected to SEM analyses. These were performed in SF samples only. Because of logistical constraints, urines from only 6 subjects were examined. Crystallization was induced in 50 ml urine (SF only) by the addition of a standard oxalate load (30 μmol/100 ml Na2Ox) in excess of the previously determined CaOx MSL [31]. These samples were then incubated for 120 min. Aliquots of 2 ml were then filtered through 0.22 μm Millipore filters. After drying, each filter was glued to an aluminium stub and splutter coated with 3–5 nm of Au/Pd for 10 min (Bio-Rad, SEM Coating System) and then examined with a scanning electron microscope (Nova NanoSEM230). Crystals were viewed at a working distance of 5–7 mm using a low voltage back scatter detector. Photographs were taken at 1000× and 5000× magnification.
Statistical methods
Our small sample sizes precluded a thorough assessment of the assumptions underlying parametric statistical methods. We, therefore, conducted our analyses using non-parametric methods. Where measurements are paired by subject, i.e. before and after the intake of rooibos and/or Japanese green tea, these were compared using Wilcoxon signed rank tests. Where measurements are from independent samples, i.e. stone formers and a control group, these were compared using Mann–Whitney tests. All tests were conducted in R version 3.1.3.
Results
Oxalate content and brewing times of RT and JGT
The oxalate release in RT was 2.35 and 4.52 mg/L after brewing times of 5 and 10 min, respectively (p < 0.01). In JGT, values were 22.10 and 24.12 mg/L, respectively. To minimise the oxalate intake during the trial, subjects were instructed to use a brewing time of 5 min when preparing their tea for ingestion.
The oxalate concentration in RT has not been previously reported. Our values for JGT are similar to some of the teas investigated by Honow and co-workers [32], but comparisons are odious as oxalate content varies widely with regional origin and time of harvest [32].
Polyphenol and mineral content of RT and JGT
The concentration of various polyphenolic compounds in each tea is given in Table 1. These include several flavonoids (in RT) and catechins (in JGT). Their presence in the respective teas has been previously reported by other workers [33, 34]. It is the potential effect of these antioxidants on the risk of kidney stone formation which is the focus of the present study. On the other hand, our values for the concentration of several minerals in each tea (Table 2) indicate that they are present in minute quantities far below dietary reference intakes. This has been reported previously [35]. As such, it is unlikely that they would significantly affect urinary physicochemical risk factors for stone formation.
Control groups 1 and 2
Physicochemical risk factors and peroxidative biomarkers
The mean habitual intake of JGT in control group 2 was 355 ± 187 ml/d (range 200–750 ml/d). Mean 24 h urinary physicochemical risk factors for control groups 1 and 2 at baseline and after ingestion of RT for 30 days are given in Table 3. The only statistically significant change which was observed after the ingestion period was a decrease in urinary chloride (Group 1). This is not regarded as being clinically important. Notably, neither SS nor Tiselius Risk Index values changed in either group.
The mean urinary and plasma biomarkers for oxidative stress for control groups 1 and 2 at baseline and after ingestion of RT for 30 days are given in Table 4. No statistically significant changes were observed.
Stone formers
Dietary intake
The mean dietary intakes at baseline and on day 30 for the RT and JGT protocols are given in Table 5. There were no statistically significant differences in the intakes on day 0 vs 30, nor were there any differences between the respective baseline intakes themselves. The intra- and inter-group consistency in the intake of all nutrients indicates the absence of any dietary conflicts.
Physicochemical risk factors and peroxidative biomarkers
Mean 24 h urinary physicochemical risk factors for stone formers at baseline and after independent ingestion of RT and JGT for 30 days are given in Table 6. The only statistically significant changes achieved by either of the teas were a decrease in urinary volume after RT ingestion and decreases in urinary sodium, potassium and SS brushite after JGT ingestion. Besides the decrease in SS(Bru), none of the other crystallization risk factors (MSL, nucleation rate) changed significantly after ingestion of either tea. Aggregation was not detected in the baseline or 30-day urines.
The mean urinary and plasma biomarkers for oxidative stress for stone formers after ingestion of RT and JGT for 30 days are given in Table 7. There were no statistically significant changes in any of the biomarkers after ingestion of either tea.
Our concentrations for urinary TBARS in normal subjects agree with those reported by others [36–38], but values reported by Huang and co-workers [39] are much higher than ours as well as those of the aforementioned others. Our concentrations for urinary NAG also agree with the literature values [36–39]. Concentrations of plasma TBARS in the present study are 3–12 fold greater than those reported by others [20, 40–43], but this can possibly be attributed to the general inaccuracy in plasma TBARS assays described by Moselhy and co-workers [44]. We were able to find only one study in which the concentration of urinary TBARS was measured in stone formers [39] and two studies in which urinary NAG was measured [38, 39]. We were not able to find any study reporting plasma TBARS in stone formers. The paucity in reference levels for these biomarkers in stone formers precludes us from making meaningful comparisons with our results.
The significant difference (Table 7) in the NAG baselines for the RT and JGT phases of the SF study (0.64 ± 0.08 vs 1.04 ± 0.14, p = 0.015 U/g creatinine), despite all other peroxidative risk factors remaining stable (Table 7), demonstrates that this biomarker may vary over a relatively short period of time (37 days) in the same group of subjects. Notwithstanding that such a difference prevailed, findings in the present study were not compromised, since independent protocols, with independently determined baselines, were followed for the two teas under investigation.
Scanning electron microscopy
SEM revealed the presence at baseline of mainly COM crystals and a few sparse COD deposits (Fig. 1). COM crystals tended to dominate to a greater extent after ingestion of RT (Fig. 2) and JGT (Fig. 3). Aggregated crystals were rarely observed in any of the urines, thereby supporting the result of the aggregation assay mentioned above.
Discussion
It is apparent from previous experimental, animal and human studies that two different stone-forming pathways can be modulated by antioxidants. Firstly, the physicochemical risk factors for CaOx crystallisation can be reduced, manifesting themselves in decreases in the excretion of lithogenic urinary components, such as calcium and oxalate, decreases in SS (CaOx) and decreases in CaOx crystal deposition. Although these effects have been reported, researchers have not attempted to explain the mechanisms by which antioxidants are able to achieve such results. Indeed, an explanatory mechanism is not obvious and remains undeclared.
Despite this, studies such as those described in the present paper which investigate whether substances containing antioxidants are able to induce decreases in the physicochemical risk factors for CaOx renal stone formation are warranted. In the present study, the only statistically significant change which occurred was a decrease in SS(Bru) in SF after ingestion of JGT. It is not possible to identify which urinary components per se were affected by JGT to have induced this change, but the latter can be speculatively noted as a favourable effect since a link between brushite and CaOx stone formation has been previously mooted [45]. However, because of the small sample sizes which we used in the present study, this result, and others, can only be regarded as a preliminary finding.
The second stone-forming pathway which might be modulated by antioxidants is that in which chemical and physical injury to renal cells is attenuated, thereby reducing the process in which CaOx crystals become attached or anchored at the injury site [1, 5]. While detection and measurement of this process in renal cells can be achieved in experimental and animal models, it cannot be done in humans. Instead, an indirect approach is required in which levels of blood [12, 20] and urinary [12, 39] biomarkers of oxidative injury are measured prior to and after administration of potential therapeutic or prophylactic antioxidants. In the present study, no statistically significant change in any of the biomarkers was observed. As mentioned earlier, we regard this as a preliminary finding only.
In the Oshaki National Health Insurance Cohort Study involving over 40,000 Japanese adults, 74 % of the cohort consumed more that 1 cup/d JGT while 30 % consumed 5 or more cups/d [46]. In the present study, 100 % of our subjects in control group 2 consumed more than 1 cup/d during the baseline and rooibos phases of the trial (mean intake 2.4 ± 1.3 cups/d) confirming that they may be regarded as habitual drinkers of this tea. Despite this protocol, we did not find any evidence of synergistic effects arising from the dual consumption of the teas.
Our finding that oxalate release is dependent on the brewing time in RT but not in JGT is probably due to the different matrices which are likely to exist in the two tea species. Of interest is the significantly higher oxalate content in the latter tea. There is no unanimous agreement whether this should be of concern to CaOx stone formers or not. Charrier and co-workers suggest that the oxalate intake arising from a daily consumption of 6 cups of GT per day is modest compared to some common foods and hence does not pose a risk for increasing urinary oxalate concentration [47]. On the other hand, Honow and co-workers advise that patients at risk for recurrent stone formation should take into account the oxalate content of GT [32], while Jeong and co-workers claim that GT should not be consumed by SF [7]. In total contrast to this point of view, Curhan and co-workers have suggested that tea consumption decreases the risk of developing kidney stones [48]. We have calculated that an average consumption of 6 cups of RT per day (i.e. 6 × 250 ml) equates to an intake of 3.5 and 6.8 mg soluble oxalate/day brewed for 5 min and 10 min, respectively, while an equivalent daily volume of JGT equates to 33.2 and 36.2 mg soluble oxalate/day. Since these values lie below the estimated normal dietary intake of oxalate of 50 mg/day [49], we conclude that daily consumption of RT and JGT would not increase the risk of CaOx renal stone formation.
Our finding that crystal morphology showed a tendency to change from mixed COM/COD to COM is also not encouraging; a shift to COD formation would have been preferable, since these crystals have a lower binding affinity for epithelial cells, and as such they will have a decreased crystal retention capacity [50].
Thus, the present study has not revealed any compelling evidence to support the notion that RT and JGT have a capacity for reducing the physicochemical or peroxidative risk factors for CaOx renal stone formation. However, as we alluded to earlier in this paper, we recognise that owing to our small sample sizes, our statistical tests have limited power to reject the null hypothesis. As such, the absence of significant p values can only be interpreted as an absence of large effects. It cannot be interpreted as being indicative of no effects. The present study therefore presents preliminary findings only. Future studies with greater statistical power may indeed detect smaller effects if they are present.
Despite this, our preliminary findings are disappointing, as RT (and GT) has strong antioxidant properties and might have been expected to have demonstrated such activity even in our small samples. This is particularly true for RT which contains a unique polyphenol, Aspalathin. The latter contributes approximately 43 % of the total antioxidant activity of RT [51, 52] and mimics superoxide dismutase for its antioxidant activity [53]. Moreover, numerous health promoting properties, attributed to RT’s antioxidant activity, have been demonstrated in in vitro assays and in animal models [20].
Further evidence indicating that green tea may not reduce the risk factors for stone formation is provided by comparison of baseline urinary physicochemical and peroxidative risk factors in the two control groups (Tables 1 and 2, respectively). Paradoxically, this shows significantly higher levels of calcium, sodium, potassium, SS(CaOx), TBARS and NAG in Group 2, despite habitual consumption of JGT in this group. However, all values were within their respective normal ranges and are most likely due to variations in the free diets of the two groups, notwithstanding that these were restricted with respect to intake of oxalate and antioxidants.
Besides the limitation of small sample sizes in the present study, another possible explanation for the apparent absence of any anti-lithogenic effects following the consumption of RT and JGT is that the present study may have been too conservative in terms of duration and dosage of the administered teas. In a study on the effects of pomegranate extract, which is rich in multiple antioxidants, the authors observed that much more pronounced effects occurred after 1 year of administration than after 90 days [12]. They suggest that long periods of administration (undefined) may be required for antioxidants to have an inhibitory effect on stone formation, because there may be differences among individuals in the absorption of polyphenols [12]. In the study mentioned earlier, in which the authors observed a decrease in plasma TBARS after ingestion of RT, the trial period was 6 weeks [20], whereas in the present study the period was 30 days.
Arguments have also been advanced to suggest that patients with greater baseline abnormalities (especially severe hyperoxaluria or hypercalciuria) may respond more dramatically to antioxidant supplementation [12]. Neither of these clinical conditions applied to the subjects in the present study, thereby culminating in the absence of any effects.
Since our results are not sufficiently robust to pronounce definitively on whether RT and JGT are able to decrease the physicochemical and peroxidative risk factors for CaOx stone formation in humans, further studies are warranted. In this regard, we believe that our study provides a template for future investigations in which the size of the test groups is increased to achieve statistical power and experimental conditions relating to dosage, duration and patient pathologies are altered. However, as with many other potential anti-stone therapies, the ultimate test of efficacy would be to monitor stone recurrence in a group of stone-forming patients who ingest rooibos and other green teas over a prolonged period of time.
References
Selvam R (2002) Calcium oxalate stone disease; role of lipid peroxidation and antioxidants. Urol Res 30:35–47
Khan S (2012) Is oxidative stress a link between nephrolithiasis and obesity, hypertension, diabetes, chronic kidney disease, metabolic syndrome? Urol Res 40:95–112
Khan S (2013) Reactive oxygen species as the molecular modulators of calcium oxalate kidney stone formation: evidence from clinical and experimental investigations. J Urol 189:803–811
Thamilselvan S, Byer K, Hackett R, Khan S (2000) Free radical scavengers, catalase and superoxide dismutase provide protection from oxalate-associated injury to LLC-PK1 and MDCK cells. J Urol 164:2240229
Thamilselvan S, Khan S (2003) Oxalate and calcium oxalate mediated free radical toxicity in renal epithelial cells: effect of antioxidants. Urol Res 31:3–9
Itoh Y, Yasui T, Okada A, TozawaK Hayashi Y, Kohri K (2005) Preventative effects of green tea on renal stone formation and the role of oxidative stress in nephrolithiasis. J Urol 173:271–275
Jeong B, Kim B, Kim J, Kim H (2006) Effects of green tea on urinary stone formation: an in vivo and in vitro study. J Endourol 20:356–361
Jyothilakshmi V, Thellamudhu G, Chinta R, Anil K, Debadatta N (2014) Beneficial effect of homeopathic preparation Berberis vulgaris in alleviation oxidative stress in experimental urolithiasis. Forschende Komlementarmedizin 21:7–12
Tugcu V, Kemahli E, Ozbek E, Annci Y, Uhri M et al (2008) Protective effect of a potent antioxidant, pomegranate juice, in the kidney of rats with nephrolithiasis induced by ethylene glycol. J Endourol 22:2723–2731
Abbazhagan M, Hariprasad C, Samudram P, Latha E, Latha M, Selvam R (1999) Effect of supplementation of vitamin E on urinary risk factors in patients with hyperoxaluria. J Clin Biochem Nutr 27:37–47
Sumitra K, Pragasam V, Sakthivel R, Kalaiselvi P, Varalakshmi P (2005) Beneficial effect of vitamin E supplementation on the biochemical and kinetic properties of Tamm-Horsfall glycoprotein in hypertensive and hyperoxaluric patients. Nephrol Dial Transplant 20:1407–1415
Tracy C, Henning J, Newton M, Aviram M, Zimmerman M (2014) Oxidative stress and nephrolitiasis: a comparative pilot study evaluating the effect of pomegranate extract on stone risk factors and elevated oxidative stress levels of recurrent stone formers and controls. Urolithiasis 42:401–408
Balentine D, Wiseman S, Bouwens L (1997) The chemistry of tea flavonoids. Clin Rev Food Sci Nutr 37:693–704
du Toit R, Volsteedt Y, Apostolides Z (2001) Comparison of the antioxidant content of fruits, vegetables and teas measured as vitamin C equivalents. Toxicology 166:63–69
Cabrera C, Artacho R, Gimenez R (2006) Beneficial effects of green tea—a review. J Am Coll Nutr 25:79–99
Kim J, Choi J, Yoon G, Yang E, Kim D (2005) Effect of green tea on calcium oxalate stone formation and excretion in ethylene glycol-treated rats. Korean J Urol 46:299–305
Wilson N (2005) Cape natural tea products and the US market: rooibos rebels ready to raid. Rev Agric Econ 27:139–148
Dos A, Ayhan Z, Sumnu G (2005) Effects of different factors on the sensory attributes, overall acceptance and preferences of rooibos (Aspalathus linearis) tea. J Sens Stud 20:228–242
Huang M, Plessis J, Preez J, Hamman J, Viljoen A (2008) Transport of Aspalathin, a rooibos tea flavonoid, across the skin and intestinal epithelium. Phytother Res 22:699–704
Marnewick JL, Rautenbach F, Venter I, Neethling H, Blackhurst D et al (2011) Effects of rooibos (Aspalathus linearis) on oxidative stress and biochemical parameters in adults at risk for cardiovascular disease. J Ethnopharm 133:46–52
Holmes R, Kennedy M (2000) Estimation of the oxalate content of foods and daily oxalate intake. Kidney Int 57:1662–1667
Halvorsen B, Carlsen M, Phillips K, Bohn S, Holte K et al (2006) Contents of redox-active compounds (i e antioxidants) in foods consumed in the United States. Am J Clin Nutr 84:95–135
Grases F, Costa-Bauza A, Prieto R (2006) Renal lithiasis and nutrition. Nutr J 5:1–7
Wolmarans P, Humphreys J, Sayed N (2001) Foodfinder™ 2. Nutritional Intervention Unit, South African Medical Research Council, Cape Town
Allie-Hamdulay S, Rodgers A (2005) Prophylactic and therapeutic properties of a sodium citrate preparation in the management of calcium oxalate urolthiasis: randomized, placebo-controlled trial. Urol Res 33:116–124
Yasui T, Itoh Y, Okada A, Hamamoto S, Hirose M, Kobayashi T, Tozawa K, Kohri K (2009) Alendronate reduces the excretion of risk factors for calcium phosphate stone formation in postmenopausal women with osteoporosis. Urol Int 83:226–229
Tiselius H (1982) An improved method for the routine biochemical evaluation of patients with recurrent calcium oxalate stone disease. Clin Chim Acta 122:409–418
Werness PG, Brown CM, Smith LH et al (1985) EQUIL2: a basic computer program for the calculation of urinary saturation. J Urol 134:1242–1244
Ryall R, Hibberd C, Marshall V (1985) A method for studying inhibitory acrivity in whole urine. Urol Res 12:285–289
Hess B, Mienhardt U, Zipperle L, Giovanoli R, Jaeger P (1995) Simultaneous measurements of calcium oxalate crystal nucleation and aggregation: impact of various modifiers. Urol Res 23:231–238
Ryall R, Grover P, Stapleton A et al (1995) The urinary F1 activation peptide of human prothrombin is a potent inhibitor of calcium oxalate crystallisation in undiluted human urine in vitro. Clin Sci 89:533–541
Honow R, Gu K, Hesse A, Siener R (2010) Oxalate content of green tea of different origin, quality, preparation and time of harvest. Urol Res 38:377–381
Bramati L, Minoggio M, Gardana C, Simonetti P, Mauri P, Pietta P (2002) Quantitative characterization of flavonoid compounds in rooibos tea (Aspalathus linearis) by LC-UV/DAD. J Agric Food Chem 50:5513–5519
Cabrera C, Gimenez R, Lopez M (2003) determination of tea components with antioxidant activity. J Agric Food Chem 51:4427–4435
Olivier J, Symington EA, Jonker CZ, Rampedi IT, Van Eeden TS (2012) Comparison of the mineral composition of leaves and infusions of traditional and herbal teas. S Afr J Sci 108:1–7
Nelson G, Morris V, Schmidt P, Levander O (1993) The urinary excretion of thiobarbituric acid reactive substances and malondialdehyde by normal adult males after consuming a diet containing salmon. Lipids 28:757–761
Srikrishna K, Kanagasabapathy A, John L (1994) N-acetyl-β-D-glucosaminidase, alanine aminopeptidase and protein: creatinine ratio as early indicators of diabetic microanlopathy. Indian J Clin Biochem 9:5–8
Boonla C, Wunsuwan R, Tungsanga R, Tosukhowong P (2007) Urinary 8-hydroxyguanosine is elevated in patients with nephrolithiasis. Urol Res 35:185–191
Huang H-S, Ma M-C, Chen C-F, Chen J (2003) Lipid peroxidation and its correlations with urinary levels of oxalate, citric acid and osteopontin in patients with renal calcium oxalate stones. Urology 62:1123–1128
Sakuma N, Iwata S, Hibino T, Tamai N, Sasai K et al (1997) Effects of vitamin C and vitamin E on plasma levelsof lipid hydroperoxides and thiobarbituric acid reactive substances in humans. Curr Therapeutic Res 58:317–322
Mol M, de Rijke Y, Demacker P, Stalenhoef A (1997) Plasma levels of lipid and cholesterol oxidation products and cytokines in diabetes mellitus and cigarette smoking: effects of vitamin E treatment. Atherosclerosis 129:169–176
Ide T, Tsutsui H, Ohashi N, Hayashidani S, Suematsu N et al (2002) Greater oxidative stress in healthy young men compared with premenopausal women. Arterioscler Thromb Vasc Biol 22:438–442
Goraca A, Skibska B (2005) Plasma antioxidantstatus in healthy smoking and non-smoking men. Bratisl Lek Listy 106:301–306
Moselhy H, Reid R, Yousef S, Boyle S (2013) A specific, accurate and sensitive measure of total plasma malondialdehyde by HPLC. J Lipid Res 54:852–858
Tang R, Nancollas GH, Giocondi JL, Hoyer JR, Orme CA (2006) Dual roles of brushite crystals in calcium oxalate crystallization provide physicochemical mechanisms underlying renal stone formation. Kidney Int 70:71–78
Kuriyama S, Shimazu T, Ohmori K, Kikuchi N, Nakaya N et al (2006) Green tea consumption and mortality due to cardiovascular disease, cancer and all causes in Japan. JAMA 296:1255–1265
Charrier M, Savage G, Vanhanen L (2002) Oxalate content and calcium binding capacity of tea and herbal teas. Asia Pacific J Clin Nutr 11:298–301
Curhan G, Willett W, Rimm E, Spiegelman D, Stampfer M (1996) Prospective study of beverage use and the risk of kidney stones. J Epidemiol 143:240–247
Siener R, Honow R, Seidler A, Voss S, Hesse A (2006) Oxalate contents of species of the Polygonaceae, Amaranthaceae and Chenopodiaceae families. Food Chem 98:220–224
Wesson J, Worcester E, Weissner J, Mandel N, Kleinman J (1998) Control of calcium oxalate crystal structure and cell adherence by urinary macromolecules. Kidney Int 33:952–957
Joubert E (1996) HPLC quantification of the dihydrochalcones, aspalathin and nothofagin in rooibos tea (Aspalathus linearis). Food Chem 55:403–411
Shulz H, Joubert E, Schutze W (2003) Quantification of quality parameters for reliable evaluation of green rooibos (Aspalathus linearis). Eur Food Res Technol 216:539–543
Ferreira D, Marais C, Steenkamp J (1995). Rooibos as a likely health food supplement. In: Fundamental Foods for Health, University of the Orange Free State, p 73–88
Acknowledgments
The authors wish to thank the South African National Research Foundation (NRF) and the Japan Society for the Promotion of Science (JSPS) for the award of a joint research grant. One of us (AR) also extends thanks to the University of Cape Town for the award of research funding. The Nagoya authors would like to thank Ms N Kasuga and Ms M Noda for their experimental assistance and the NCU Stone Research Team for their participation in the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Conflict of interest
This study was funded by the South African National Research Foundation (NRF) and the Japanese Society for the Promotion of Science (JSPS), (Grant number UID 85106). The authors declare that that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Rodgers, A., Mokoena, M., Durbach, I. et al. Do teas rich in antioxidants reduce the physicochemical and peroxidative risk factors for calcium oxalate nephrolithiasis in humans? Pilot studies with Rooibos herbal tea and Japanese green tea. Urolithiasis 44, 299–310 (2016). https://doi.org/10.1007/s00240-015-0855-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00240-015-0855-4