Abstract
To evaluate the potential protective effects of a calcium channel blocker (Verapamil) on the oxidative stress related changes with an emphasis on the antioxidant capacity of the kidneys an experimental study in rats was performed. A total of 44 rats have been included. Hyperoxaluria was induced in Group 1 by continuous administration of ethylene glycol (EG). Animals in Group 2 received Verapamil in addition to EG. Animals in Group 3 constituted the control group. In addition to the evaluation of tissue and serum levels of three scavenging enzymes, NO, MDA and T-AOC; the presence and degree of crystal formation in renal parenchyma were evaluated in all animals after 7 and 28 days. Our data demonstrated that in addition to the lower level of all three scavenging enzymes (SOD, CAT and GSH) particularly during late phase evaluation (4 weeks); the total antioxidant capacity (T-AOC) of these kidneys were also higher when compared with the animals receiving EG only. Tissue and serum levels of both NO and MDA indicated the preventive effect of Verapamil on the oxidative stress induced changes. Very limited or no crystallization in the kidneys treated with verapamil during early and late phase examination was observed when compared with considerable crystal formation in Group 2 animals. Verapamil treatment may preserve the oxidant capacity of the kidneys and subsequently limit the crystal deposition induced by hyperoxaluria. Verapamil could therefore be considered in the management of kidney stone formation particularly in cases with recurrent kidney stone disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Accumulated experience from the well conducted clinical as well as experimental studies indicated that renal tubular epithelium is the main target for oxalate (Ox) induced injury. Prolonged periods of hyperoxaluric phase and subsequent calcium oxalate (CaOx) crystal formation/deposition have been found to be injurious to the epithelial layer of the renal tubular cells [1–3]. Supporting these observations again; invitro cell culture studies focusing on the exposure of renal epithelial cells to Ox and/or CaOx crystals have provided new insights into the mechanisms involved in stone formation by demonstrating that high concentrations of Ox as well as CaOx crystals could injure the renal epithelial cells in a time and dose dependant manner [4–7].
Regarding the underlying mechanism of renal tubular cell injury induced by hyperoxaluria; such studies again demonstrated the involvement of lipid peroxidation in renal tubular cells, which usually leads to the morphological and functional impairment of cellular components by reactive oxygen species (ROS) formation due to oxidative stress [8]. Apart from possible tubular ischemia, which gives rise to ROS production as a result of lipid peroxidation, animal studies as well as studies on LLC-PK1 cells have clearly shown that the direct interaction between oxalate ions with renal epithelial cells can also elicit a programmed sequence of events which in turn may result in renal tubular cell damage and/or dysfunction and express itself as cell apoptosis [8–10]. Moreover, some other studies again revealed that ROS are intimately involved as signaling molecules of injury as well as inflammation during stone formation [11, 12]. Last but not least, oxalate-generated free radicals were found to disrupt the structural integrity of the membranes in renal epithelial cells [13, 14].
It has been well shown that ROS normally occur at steady state levels, generated when needed and then cleared by activities of various antioxidants and scavengers. But, uncontrolled generation of the reactive oxygen or nitrogen species and/or a reduction in the endogenous antioxidant capacity of the involved tissue creates oxidative stress. The oxidants can react with all basic constituents of cells: lipids, carbohydrates, proteins and nucleic acids by severely affecting their structure and function. Pathological changes may result from the damaging effects of ROS and from ROS-mediated changes in gene expression and signal transduction [15–17]. Major cellular ROS include superoxide anion (O2−•), nitric oxide radical (NO•), hydroxyl radical (OH•), and hydrogen peroxide (H2O2), which are generated by several pathways. On the other hand, however, cells are equipped with a number of scavenging systems to control ROS availability including superoxide dismutase (SOD) to eliminate O2−•, and glutathione (GSH) peroxidase (GPx) and catalase (CAT) to detoxify H2O2. However, once a threshold of damages exceeded; the cellular defences are overwhelmed after which a very small additional insult results in severe cellular injury. Thus, the oxidant-antioxidant balance is a critical determinant of cell sensitivity to free-radical injury. Reduction of antioxidant enzyme (SOD, catalase, and GPx) activities in involved renal tissue following hyperoxaluria will further lower the total antioxidant capacity (TAOC) of the kidneys.
The resultant peroxidative tubular damage coupled with antioxidant imbalance will ease the crystal attachment, subsequent aggregation and growth of calcium oxalate kidney stones in these kidneys [18–20]. Related with this subject, oxalate-induced lipid peroxidation in cultured renal tubular epithelial cells has been found to be associated with a greater production of superoxide and hydroxyl free radicals. The production of these free radicals was greater when the cells are exposed to oxalate and calcium oxalate monohydrate crystals indicating that oxalate itself is injurious to cells and formed calcium oxalate crystals may potentiate the extent of toxicity further [21].
Taking the injurious effects of hyperoxaluria induced apoptotic changes in renal tubular epithelium (as a result of ischemia-induced ROS production) and crystal deposition in renal parenchyma into account, studies focused on the possible protective effects of some agents in an attempt to prevent or at least limit ischemia induced oxidative stress formation and related pathologic alterations [22–24]. Among these agents, while verapamil and nifedipine (calcium channel-blocking agents) were found to limit the histologic changes as well as crystal deposition induced by certain renal trauma [25–30], potassium citrate was found to limit stone recurrence after SWL [31, 32], and allopurinol and vitamin E have been used as antioxidant agents to minimize the effects of lipid peroxidation in certain tissues [33, 34].
In this present animal study, we aimed to evaluate the presence and degree of hyperoxaluria induced oxidative stress in rat kidneys in terms of reduction in total renal antioxidant capacity (levels of scavenging enzymes as well) of involved kidneys and possible protective effect of a calcium channel blocking agent “verapamil” on these changes.
Materials and methods
A total of 44 Sprague–Dawley rats (350–400 g/each) were included into the study program after obtaining the ethical committee approval from the Pendik Institute of Veterinary Control and Research. All animals were kept in special cages under normal room conditions (with temperatures of 23 ± 1 °C and humidity of 55 % ± 5 %) with 12 h of light and dark periods. Following a complete physical examination, all animals underwent biochemical evaluation including blood and urine analyses and stool examination for parasitic infections which may affect the renal parenchymal alterations induced by hyperoxaluria. No pathologic anatomical findings and/or infections of the urinary tract were found. Apart from the study protocol, no specific treatment has been applied. Animals (n: 44) were then divided into three main groups as follows: Group 1 (EG only, n:16) Hyperoxaluria was induced by 0.75 % EG containing drinking water. Group 2 (EG + Verapamil, n:16) In addition to hyperoxaluria induction, animals in this group were given verapamil (1 mg/kg, through feeding tube). Group 3 (Control, n:12) Animals were fed with standard chaw and water without any specific medication. Subgroup animals in all study groups were evaluated during early (7 days) and late (28 days) follow-up period (Table 1).
Animals were euthanized and bilateral flank incision was performed to remove both kidneys for histopathologic evaluation under anesthesia. To evaluate the effect of hyperoxaluria induced lipid peroxidation on the actual status of oxidant/antioxidant system both serum and kidney tissue specimens were evaluated from three different aspects; in addition to the assessment of the levels of antioxidant enzymes [catalase (CAT), superoxide dismutase (SOD), total glutathione (GSH); the total antioxidant capacity (T-AOC)] was also evaluated in these specimens (by using the ELISA kits for catalase, superoxide dismutase, total glutathione, nitric oxide, malonil dialdehid, total anti-oxidant capacity) on days 7 (short-term) and 28 (long-term), respectively. Additionally, as a non-radical oxidant agent nitric oxide (NO) levels and as an end-product of lipid peroxidation, an oxidative injury bio-marker malonyl dialdehyde (MDA) levels were well evaluated. Last but not least, renal crystal formation and deposition was evaluated under light microscopy in all renal tissue specimens obtained on days 7 (short term) and 28 (late term), respectively.
Evaluation of tubular crystallization
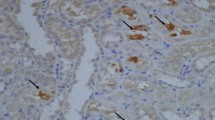
Evaluation of renal crystal deposition were performed under light microscopy. The tissues were fixed in 10 % formalin for 24 h. After routine tissue processing, the tissues were embedded in paraffin. 4-ml-thick sections were stained with hematoxylin and eosin for histopathological evaluation. Detection of crystallization in frozen kidney sections from male Sprague–Dawley rats treated with EG alone or ethylene glycol and the drug verapamil (EG + V), were assessed with histopathological evaluation. Crystal deposition and calcification were evaluated under light microscopy by calculating the percentage of crystal granules and/or calcification in tubules of cortical and medullar area, respectively.
Experimental design
Kidney tissue samples were obtained after cervical decapitation of animals. Tissues were grinded with liquid nitrogen using a ceramic mortar and pestle. Then homogenized with tissue raptor (Qiagen, Netherlands). After the homogenization tissue samples were weighed and equilibrated to 1 mg tissue/1 ml PBS (Phosphate Buffered Saline, Biochrome). Blood samples were collected before the decapitation process and were centrifuged in 3000 rpm for 5 min.
ELISA
ELISA tests of catalase (CAT), superoxide dismutase (SOD), total glutathione (GSH), nitric oxide (NO), malonil dialdehyde (MDA), total anti-oxidant capacity (T-AOC) have been used to evaluate oxidant/antioxidant status of tissue and serum samples. The experiments were performed according to the manufacturer’s protocols (YH-Biosearch, China).
Statistical analysis
The statistical analysis were performed by using GraphPad Prism 6.0 software. The data were analyzed by multiple t-tests and statistical significance determined using the Sidak-Bonferroni method, with alpha = 1.000 % for finding the differences between the control and treated groups.
Results
Renal oxidant/antioxidant status
Evaluation of the tissue levels of scavenging enzymes
Comparative evaluation of tissue catalase (CAT) levels between animals treated with EG only and EG + verapamil demonstrated that while enzyme levels were relatively higher during early phase; significantly lower levels were noted in animals receiving verapamil in addition to EG application (p < 0.0001) during the late (4 weeks) period (Fig. 1a).
Regarding the tissue levels of SOD again, although there was no statistically significant difference between two groups during early follow-up evaluation; a statistically significant difference with respect to renal tissue SOD levels was noted (p < 0.0049) during late follow-up with significantly lower enzyme levels in animals receiving verapamil (Fig.1c).
Lastly, regarding the tissue levels of GSH, however, despite the relatively lower level of this enzyme in animals receiving Verapamil during early follow-up evaluation (p = 0.27); unlike to the first two scavenging enzymes; tissue levels of GSH was significantly higher in animals receiving verapamil during long-term evaluation (Fig. 1e).
Evaluation of the serum levels of scavenging enzymes
Regarding the serum levels of three scavenging enzymes; although there was no significant difference between two group of animals during early period (7 day); serum CAT levels were significantly lower in animals undergoing verapamil treatment in addition to EG application during 4 weeks evaluation period (p < 0.0003) (Fig. 1b). Evaluation of serum SOD levels did not show any statistically significant differences between groups (Fig. 1d). Lastly, evaluation of serum levels of GSH levels did not show any statistically significant difference between two groups. However, verapamil seemed to be effective on serum GSH levels during early but more prominently during late phase evaluation (4 weeks) to some extend (p < 0.029) (Fig. 1f).
Evaluation of NO levels
Our findings indicated that although there was no statistically significant difference between the groups during early follow-up (Fig. 2a) evaluation; a statistically significant difference in favor of verapamil applied group has been noted during late phase evaluation (p < 0.001) (Fig. 2a) Taking the fact into account that NO is a molecule being metabolized in a short period of time after formation; the possible explanation for early phase findings could be related with the rapid removal of NO from the system effectively by antioxidant enzymes during this period.
Moreover, depending on the well known antiapoptotic and ant fibrotic effects of NO particularly during the chronic phase of such events; our data support the preservation of tissue NO levels by verapamil during the chronic (late) phase of our study.
On the other hand evaluation of serum levels of NO demonstrated that although levels in verapamil receiving animals demonstrated elevation (Fig. 2b); there was no statistically significant difference between the groups on this aspect (p < 0.33).
Evaluation of T-AOC in both groups
Evaluation of T-AOC levels demonstrated that although TAOC levels in verapamil receiving animals demonstrated elevation (Fig. 3a); there was no statistically significant difference between the groups on this aspect (p < 0.1). It is well known that T-AOC reflects the cumulative effect of all antioxidant systems in the body and its specificity may be lower when compared with each of these components that form T-AOC as a whole. For that reason its depletion during early follow-up may not constitute a specific meaning when compared with its components evaluated separately in our study. Regarding the serum T-AOC levels again, our data demonstrated that although not statistically significant (p < 0.12), the levels tended to be elevated in animals receiving verapamil in addition to EG (Fig. 3b).
Evaluation of MDA levels
Evaluation of tissue MDA levels demonstrated that while Verapamil treated animals had lower levels (p = 0.21, p = 0.10 (Fig. 4a, b) than the animals receiving EG only during early follow-up; elevated levels of MDA have been noted during late phase evaluation in these animals when compared with EG only group (Fig. 4a). Lastly evaluation of serum MDA levels demonstrated that despite elevated levels of serum MDA in animals receiving verapamil during early follow-up; a statistically significant lowered levels of serum MDA were noted in animals receiving verapamil during long-term evaluation (p < 0.0001) (Fig. 4b). Regarding this discordance again, although the levels of MDA may be higher due to its increased production as a result of oxidative stress during early phase, its levels will be lowered with the potent antioxidant effects of increasing scavenging enzymes during late phase follow-up.
Evaluation of tubular crystal deposition
Although evident crystallization could be demonstrated in animals receiving EG only (44 % in renal cortex) no crystal deposition was found in animals receiving Verapamil during early phase evaluation (7 days) (Table 2); Verapamil was found to be effective enough to limit crystal formation at this particular level during early-term evaluation. During long-term evaluation again, while statistically significant crystallization (up to 77–51 % in cortex and medulla, respectively) was present in EG group (Fig. 5); verapamil did tend to limit the degree of crystal formation during this period (Fig. 6) (up to 65–32 % in cortex and medulla, respectively) (Table 2).
Discussion
Both clinical as well as animal studies have clearly shown that hyperoxaluria is the main risk factor for idiopathic calcium oxalate (CaOx) crystal formation [2–4] where the interaction between renal tubular epithelial cells and oxalate ions and/or CaOx crystals plays a critical role in the formation of urinary stones. Induction of hyperoxaluria has been found to be associated with cellular injury and necrosis in renal tubular epithelial cells and the presence of the injury even in the absence of crystalluria have clearly suggested that the oxalate-induced damage was not due solely to the injury produced by CaOx crystals themselves [4, 5].
The progression from hyperoxaluria to nephrolithiasis is a chain of staged events and renal tubular injury is thought to be central to the process [3–5]. Data derived from animal studies focusing on the hyperoxaluria induced oxidative stress originating from tubular ischemia have led the authors to propose that the interaction between oxalate ions and renal epithelial cells may initiate a programmed sequence of events [6]. Related with this subject, studies on LLC-PK1 have clearly demonstrated that oxalate exposure may produce a variety of changes in renal epithelial cell morphology and function, including increased cellular proliferation and, at elevated concentrations, cell death in other words cell apoptosis [6, 7]. To support this proposal, we were able to demonstrate apoptotic changes in rabbit renal tubular cells, the degree and extent of which were found to be time dependent [8].
Regarding the main underlying mechanism for tubular cell injury and subsequent apoptotic alterations; studies performed with several models of CaOx stone disease suggest that the generation of reactive oxygen species (ROS) and subsequent lipid peroxidation is a crucial part of this specific process [4, 5, 7, 35, 36]. Furthermore, the relationship among oxalate-induced renal injury, oxidative stress and lipid peroxidation has also been well demonstrated in humans [37].Under physiologic conditions, the cells are endowed with several antioxidant systems, including enzymatic (superoxide dismutase, SOD, catalase and glutathione peroxidase, GPx) and non-enzymatic, e.g., reduced glutathione (GSH), vitamins E, A, and C to limit the extent of lipid peroxidation. Up to a certain limit, they are able to control the damage with GPx, catalase, SOD or other antioxidative mechanisms. However, once the threshold level of damage is exceeded; the cellular defences are overwhelmed and a very small additional insult results in severe cellular injury. Thus, the oxidant/antioxidant balance is a critical determinant of cell sensitivity to free-radical induced injury [38] where an increased production of ROS in response to hyperoxaluria triggers the kidney to adapt to this insult by up-regulating the antioxidant defense systems such as superoxide dismutase (SOD), catalase, glutathione peroxidase (GP) and glutathione (GSH). Related with this issue, if the production of ROS overwhelms the antioxidant response or when the antioxidant levels are depleted due to a chronic pro-oxidant insult; the cells face with “oxidative stress” [13, 39] with ischemic reperfusion injury which may result in a significant depression of antioxidant enzyme levels (SOD, CAT and GPX) [40].
To support the above mentioned mechanisms; higher levels of renal enzymes namely gamma-glutamyl transpeptidase (GGTP), angiotensin 1 converting enzyme (ACE), β-galactosidase (GAL) and N-acetyl- β-glucoseaminidase (NAG) were found in the urine of idiopathic CaOx stone formers [41] indicative of renal proximal tubular injury. Their urine contained significantly increased levels of NAG, β-GAL, α-glutathione S-transferase (α-GST), malondialdehyde (MDA) and thio barbituric acid-reactive substances (TBARS) [37], suggesting that stone-associated injury was most likely caused by the production of ROS. Urinary 8-hydroxydeoxy guanosine (8-OHdG), a marker of oxidative damage of DNA, was increased in stone forming patients which was positively correlated with tubular damage as assessed by urinary excretion of NAG [42]. Moreover, tissue culture studies have also provided adequate evidence for the involvement of free radicals in the production of various crystallization modulators and anti-inflammatory macromolecules [13, 43]. It has also been shown that the renal cells exposed to CaOx crystals secrete superoxide ions [44] where cellular injury could be ameliorated by antioxidants and free radical scavengers [13, 43]. Free radical scavengers, CAT and SOD provided protection from oxalate induced injury to LLC-PK1 and MDCK cells [43].
Lastly, regarding the possible effect of verapamil in preserving or maintaining NO levels; recently published studies clearly indicate the protective and restorative effects of verapamil (calcium antagonist agents) on serum as well as tissue NO levels which have been disturbed as a result of lipid peroxidation induced by different toxic agents. These findings indicate well that these agents are effective in limiting the ischemia–reperfusion induced alterations in serum and tissue as demonstrated by the levels of antioxidant enzymes and NO [45–47].
In addition to the evaluation of scavenging enzymes; to assess the oxidant/antioxidant balance in biological tissues, in the beginning of 1990s, Miller et al. had created a new test to measure the total antioxidant status, which has been designated as total antioxidant capacity [47]. The major advantage of this test is to measure the antioxidant capacity of all anti-oxidants in a biological sample. TAC evaluation, used with other oxidative stress and antioxidant defense biomarkers, was accepted to form the first step in search for a healthy body status. The total-antioxidant-activity assay offers many advantages and is considered to be a useful tool for detecting the presence as well as degree of oxidative stress in bodily fluids and tissues. Additionally, it may also serve as an appropriate tool for the evaluation of antioxidant therapy [48, 49].
After observing the injurious effects of hyperoxaluria induced crystal deposition in renal parenchyma and apoptotic changes in renal tubular epithelium; physicians began to search for protective agents to prevent or at least minimize the extent of these pathologic alterations. Limitation of the hyperoxaluria-induced renal injury with the application of potent antioxidant agents in hyperoxaluric rats has once again indicated the importance of lipid peroxidation in renal tubular cells with the involvement of reactive oxygen species [10, 11].
Among the agents used, while verapamil and nifedipine (calcium channel-blocking agents) were found to limit histologic changes and crystal deposition, possibly induced by blunt renal trauma [12–15] after SWL; as a very potent antioxidant, vitamin E has been applied to minimize free oxygen radical-induced deleterious effects in parenchymatous organs [19–21]. In addition to their possible protective effects on hyperoxaluria induced renal tubular injury; in one of our previously published study, during an at least 1-year follow-up period, we were able to demonstrate that calcium antagonists may decrease the excretion of stone forming risk factors in recurrent stone formers among which the excretion of oxalate has been significantly influenced by these agents (30). Although the mechanism action is not clear, based on the available data in the literature, we may say that the limitation of internal calcium shift by these agents may also well affect the tubular process related to oxalate handling which ultimately limits its excretion in urine. Additionally, in their original study Iguchi et al. were able to show that the calcium oxalate risk index of hypercalciuric and hyperoxaluric patients was significantly reduced after the administration of verapamil, which led them to conclude that verapamil is effective in reducing urinary oxalate excretion in hyperoxaluric patients [50]. Regarding the possible mechanisms in addition to the reduced reabsorption of calcium in the tubulus, which could possibly be due to the lower calcium influx into the tubular cells [25–27]; the authors stated that renal tubular cell activity on calcium shift by verapamil may work well at the intestinal epithelial cell level, which could ultimately affect the uptake of oxalate at this level [50–53].
Regarding the mechanism of potential protective effects of calcium antagonists on ischemia induced alterations in parenchymatous organs; the ischemia-induced alterations in renal tubular cells could be achieved by lowering the blood pressure mainly through vasodilatation and reduction of peripheral resistance to increase renal blood flow for the maintenance of normal renal physiology [54–61]. In other words, verapamil has been found to be effective in preserving renal integrity by lowering blood pressure, improving renal capacity and hemodynamics [62, 63].
Calcium antagonists (a heterogeneous group which includes three main classes-phenyl alkylamines, benzo thiazepines and dihydro pyridines) have been found to be successful in limiting the ischemia-induced alterations in target organs, such as the heart and kidney, by maintaining blood flow. Although they differ in molecular structure, sites and modes of action as well as the effects on various other cardiovascular functions; they effectively lower blood pressure mainly through vasodilation and reduction of peripheral resistance. In this way, these agents can successfully increase renal blood flow to maintain normal renal physiology. Taking these specific protective effects on parenchymatous organs into account, studies dealing with the adverse effects of high energy shock waves (HESW) on renal tissue integrity have demonstrated well the protective effects of verapamil in terms of tissue alterations as well as crystal deposition in traumatized tissue [57, 62]. Due to their beneficial physiological and protective effects on the parenchymatous organs such as heart, liver and kidneys, calcium entry blocking agents have been successfully used in the management of ischemia induced injury in these organs.
Because of their specific effects on renal hemodynamy and inhibition of calcium-mediated injury, the CAs have been found to be promising to attenuate the extent of kidney damage during certain types of traumas, including radio-contrast-induced nephropathy and hypoperfusion ischemic injury during cardiac surgery [57, 62]. These agents have specific effects on blood pressure control and this has been attributed largely to preferential vasodilatory action of CCBs on the afferent arterioles. As a consequence of these microcirculatory regulations, CCBs reduce intra glomerular pressure to preserve GFR and renal blood flow [63–65].
Related with the underlying mechanisms through which Verapamil may be effective in limiting tubular alterations and crystal deposition; published data so far supported the hypothesis that both tubular cell apoptosis and crystal deposition were more prominent at papillary tip level (compared with cortical and medullar tubules) indicating the high likelihood of such specific changes due to the relatively ischemic (high CO2 and low O2 content) nature of this region. Being mostly supplied by end arteries, low O2 and high CO2 pressure in papillary region of the kidney influence the metabolism of interstitial and tubular cells, and cell death may occur more commonly in these specific portions of the kidneys which promote subsequent crystal deposition and possible stone formation [25].
As stated above the underlying mechanism of cell injury during hyperoxaluria involves lipid peroxidation in tubular cells as well as functional impairment of cellular components by reactive oxygen species (ROS) formation due to oxidative stress. On the other hand, published data clearly demonstrated that calcium antagonist agents could limit the ischemia-induced alterations in target organs such as the heart and kidney mainly by lowering the blood pressure mainly through vasodilatation and reduction of peripheral resistance to increase renal blood flow for the maintenance of normal renal physiology [58, 59, 64, 65].
Thus, concerning the hyperoxaluria-induced tubular ischemia as the main responsible factor initiating the programed sequence of events leading to cell injury and death [6–9, 62] verapamil could also limit the specific alterations following hyperoxaluria induction and protect renal tubular cell integrity. Related with this subject again, use of verapamil has been found to be further effective in preserving renal integrity by lowering blood pressure, improving renal capacity and hemodynamics [58, 59, 62, 63] Verapamil decreases renal vasoconstriction and inhibits intracellular calcium toxicity (74).
On the other hand again, with respect to the possible effects of calcium antagonists on urinary stone forming risk factors; evaluation of the published data so far has clearly demonstrated that in addition to its specific renal tissue protection and its protective effects on crystal deposition; the possible effects of verapamil on urinary stone-forming risk factors, namely calcium and oxalate excretion, have also been evaluated. The calcium oxalate risk index of hypercalciuric and hyperoxaluric patients has been found to be significantly reduced after the administration of verapamil.
Related with this subject again, in their original study Iguchi et al. focused on the effect of this agent on urinary calcium and oxalate excretion and they were able to show that the calcium oxalate risk index of hypercalciuric and hyperoxaluric patients was significantly reduced after the administration of verapamil, which led them to conclude that verapamil is effective in reducing urinary oxalate excretion in hyperoxaluric patients [50]. Concerning the possible pathophysiological mechanisms underlying the effect of calcium antagonists on the excretion of the urine parameters, the authors stated that hypercalciuria may be the result of a reduced reabsorption of calcium in the tubulus, which could possibly be due to the lower calcium influx into the tubular cells [51–53].
However, hypoxaluric activity of calcium antagonists may not sufficiently be explained by this effect and taking the absorption process in the intestinal lumen (which is also a strong determinator of urinary oxalate levels) into account, we believe that the renal tubular cell activity on calcium shift by verapamil may work well at the intestinal epithelial cell level, which could ultimately affect the uptake of oxalate at this level [66, 67].
Last but not least, in one of our recently published study; during an at least 1-year follow-up period, we were able to demonstrate that verapamil may decrease the excretion of stone forming risk factors in recurrent stone formers among which the excretion of oxalate has been significantly influenced by this agent. In addition to urinary oxalate levels, verapamil application did slightly affect the excretion of the other risk factors, mechanism of action is not clear; depending on the literature data we may claim that the limitation of internal calcium shift by these agents may also well affect the tubular process related to oxalate handling which ultimately limits its excretion in urine. As demonstrated above although sufficient data could be derived related with urinary risk factors; there is extremely limited data in the literature regarding the possible effects of these agents on serum risk factors [30].
In this present animal study, following hyperoxaluria induction, we aimed to evaluate the oxidant/antioxidant capacity as well as crystal formation at different regions (cortical and medullar) of the kidney and examine the possible preventive effects of verapamil on the presence and degree of these changes.
Our data clearly demonstrated that in addition to the scavenging enzymes; the total T-AOC of the kidney obtained from animals undergoing verapamil treatment was well preserved when compared with the ones receiving EG only. In addition to the lower levels of all three scavenging enzymes (SOD, CAT and GSH); the T-AOC of the kidneys were also significantly lower in animals receiving EG only.
On the other hand again, as a further support to our findings; evaluation of the tissue and serum levels of NO coupled with MDA levels indicated the preventive effect of verapamil on the oxidative stress induced changes after hypreoxaluria induction. Taking the well known antiapoptotic and antifibrotic effects of NO particularly during chronic phase of ischemic events into account; our data demonstrated well preservation of tissue NO levels by verapamil during late phase of our study.
Again as an end-product of lipid peroxidation, an oxidative injury bio-marker, evaluation of tissue as well as serum MDA levels did clearly show that although relatively higher levels of this important marker has been observed during early phase evaluation (due to increased production originating from oxidative stress); its levels were reasonably low with the potent antioxidant effects of increasing scavenging enzymes during late phase follow-up. Last but not least, our findings revealed no crystallization in the sections of the kidneys treated with verapamil during early phase examination and limited crystal formation was present during late phase evaluation. Thus, verapamil application was also found to be effective in limiting crystal formation during both early as well as late follow-up.
In the light of our findings and published data in the literature as well, it is clear that both hyperoxaluria and CaOx crystal formation may cause injury in renal tubular epithelial cells as a result of free radical formation causing lipid peroxidation. These findings supported the hypothesis that antioxidant capacity of the kidneys may change during the hyperoxaluric phase, a condition that may further worsen the pathologic alterations in tubular cells. Alterations in scavenging enzyme levels, decrease in the T-AOC of the kidney and evident crystal deposition in animals receiving EG only indicated the high likelihood of hyperoxaluria induced ischemic insult in renal tubular cells resulting in with these specific changes.
Moreover, studies indicated that prominent apoptotic changes originating from hyperoxaluria-induced ischemia in the papillary renal tubular epithelial cells might result in cell degradation and could be responsible for the pathologic course of urolithiasis. Again, as demonstrated in our study, evident crystal deposition could be limited by these blood flow regulating agents which may increase renal blood flow and limit ischemia induced oxidative stress formation. Clinical application of such agents in the prophylaxis of stone disease might be promising and may result in limited new stone formation particularly in recurrent stone formers.
Conclusions
These findings present novel and direct evidence in vivo that hyperoxaluria-induced peroxidative damage to the renal tubular membrane surface provides a favourable environment for individual calcium oxalate crystal attachment and subsequent development of kidney stones. Verapamil treatment limited the calcium oxalate crystal deposition in the kidney, by preventing hyperoxaluria induced lipid peroxidation and tissue antioxidant imbalance. From these findings, verapamil could therefore be considered in the therapy of hyperoxaluria-induced kidney stone formation, and this could benefit individuals with recurrent kidney stone disease.
References
Coe FL, Evan AP, Worcester EM, Lingeman JE (2010) Three pathways for human kidney stone formation. Urol Res 38(3):147–160
Hackett RL, Shevock PN, Khan SR (1994) Madin-Darby canine kidney cells are injured by exposure to oxalate and to calcium oxalate crystals. Urol Res 22:197
Thamilselvan S, Khan SR (1998) Oxalate and calcium oxalate crystals are injurious to renal epithelial cells: results of in vivo and in vitro studies. J Nephrol 11:66
Khan SR (1995) Calcium oxalate crystal interaction with renal tubular epithelium, mechanism of crystal adhesion and its impact on stone development. Urol Res 23:71
Khaskhali MH, Byer KJ, Khan SR (2009) The effect of calcium on calcium oxalate monohydrate crystal induced renal epithelial injury. Urol Res 37(1):1–6
Koul H, Kennington L, Honeyman T et al (1996) Activation of c-myc gene mediates the mitogenic effects of oxalate in LLC-PK1 cells, a line of renal epithelial cells. Kidney Int 50:1525–1530
Scheid C, Koul H, Hill WA et al (1996) Oxalate toxicity in LLC-PK1 cells, a line of renal epithelial cells. J Urol 155:1112–1116
Sarica K, Yagcı F, Bakır K, Erturhan S, Ucak R (2001) Renal tubular injury induced by hyperoxaluria: evaluation of apoptotic changes. Urol Res 29:34
Hackett RL, Shevock PN, Khan SR (1995) Alterations in MDCK and LLC-PK1 cells exposed to oxalate and calcium oxalate monohydrate crystals. Scanning Micorosc 9:587
Davalos M, Konno S, Eshghi M, Choudhury M (2010) Oxidative renal cell injury induced by calcium oxalate crystal and reno protection with antioxidants: a possible role of oxidative stress in nephrolithiasis. J Endourol 24(3):339–345
Khan SR (2004) Crystal-induced inflammation of the kidneys: results from human studies, animal models and tissue-culture studies. Clin Exp Nephrol 8:75–88
Khan SR (2006) Renal tubular damage/dysfunction: key to the formation of kidney stones. Urol Res 34:86–91
Thamilselvan S, Khan SR, Menon M (2003) Oxalate and calcium oxalate mediated free radical toxicity in renal epithelial cells: effect of antioxidants. Urol Res 31:3–9
Bhandari A, Koul S, Sekhon A et al (2002) Effects of oxalate on HK-2 cells, a line of proximal tubular epithelial cells from normal human kidney. J Urol 168:253–259
Khan SR (2014) Reactive oxygen species, inflammation and calcium oxalate nephrolithiasis. Transl Androl Urol 3(3):256–276
Kamata H, Hirata H (1999) Redox regulation of cellular signalling. Cell Signal 11:1–14
Silva JP, Coutinho OP (2010) Free radicals in the regulation of damage and cell death – basicmechanisms and prevention. Drug Discov Ther 4(3):144–167
Wilcox JK, Ash SL, Catignani GL (2004) Antioxidants and prevention of chronic disease. Crit Rev Food Sci Nutr 44:275–295
Halliwell B, Aeschbach R, Loliger J, Aruoma OI (1995) The characterization of antioxidants. Food Chem Toxicol 33:601–617
Biri H, Ozturk HS, Buyukkocak S, Kacmaz M (1998) Antioxidant defense potential of rabbit renal tissues after ESWL: protective effect of antioxidant vitamins. Nephron 79:181
Thamilselvan S, Byer KJ, Hackett RL, Khan SR (2000) Free radical scavengers, catalase and superoxide dismutase provide protection from oxalate associated injury to LLC-PK1 and MDCK cells. J Urol 164:224–229
Park HK, Jeong BC, Sung MK et al (2008) Reduction of oxidative stress in cultured renal tubular cells and preventive effects on renal stone formation by the bioflavonoid quercetin. J Urol 179(4):1620–1626
Khan SR, Shevock PN, Hackett RL (1993) Magnesium oxide administration and prevention of calcium oxalate nephrolithiasis. J Urol 149:412
Sahin C, Sarikaya S, Sarica K et al (2015) Limitation of apoptotic changes and crystal deposition by Tutukon following hyperoxaluria-induced tubular cell injury in rat model. Urolithiasis 43(4):313–322
Tanriverdi O, Telci D, Aydin M, Ekici ID, Miroglu C, Sarıca K (2012) Hyperoxaluria-induced tubular ischemia: the effects of verapamil and vitamin E on apoptotic changes with an emphasis on renal papilla in rat model. Urol Res 40(1):17–25
Benyi L, Weilherg Z, Puyun L (1995) Protective effects of nifedipine and allopurinol on high energy shock wave induced acute changes of renal function. J Urol 153:596
Sarica K, Bakır K, Yagcı F, Topcu O, Akbay C, Sayın N, Korkmaz C (1999) Limitation of possible enhanced crystal deposition by verapamil in renal parenchyma after shock wave application in rabbit model. J Endourol 13:343
Strohmaier WL, Abelius A, Billes J, Grossmann T, Wilbert DM, Bichler KH (1994) Verapamil limits shock wave-induced renal tubular damage in vivo. J Endourol 8:269
Yencilek F, Sarica K, Eryildirim B, Erturhan S, Karakok M, Kuyumcuoglu U (2010) Hyperoxaluria-induced tubular ischemia: the effect of verapamil on the limitation of tissue HIF-1 alpha levels in renal parenchyma. Int Urol Nephrol 42(2):361–367
Sarica K, Erturhan S, Altay B (2007) Effect of verapamil on urinary stone-forming risk factors. Urol Res 35(1):23–27
Byer K, Khan SR (2005) Citrate provides protection against oxalate and calcium oxalate crystal induced oxidative damage to renal epithelium. J Urol 173(2):640–646
Tracy CR, Pearle MS (2009) Update on the medical management of stone disease. Curr Opin Urol 19(2):200–204
Huang HS, Chen J, Chen CF et al (2006) Vitamin E attenuates crystal formation in rat kidneys: roles of renal tubular cell death and crystallization inhibitors. Kidney Int 70:699–710
Huang HS, Chen J, Chen CF, Ma MC (2006) Vitamin E attenuates crystal formation in rat kidneys: roles of renal tubular cell death and crystallization inhibitors. Kidney Int 70(4):699–710 (erratum (2007) 71(7):712)
Thamilselvan S, Hackett RL, Khan SR (1997) Lipid peroxidation in ethylene glycol induced hyperoxaluria and calcium Oxalate nephrolithiasis. J Urol 157:1059–1063
Muhtukumar A, Selvam R (1997) Renal injury mediated calcium oxalate nephrolithiasis: role of lipid peroxidation. Ren Fail 19:401–408
Huang HS, Ma MC, Chen CF et al (2003) Lipid peroxidation and its correlations with urinary levels of oxalate, citric acid, and osteopontin in patients with renal calcium oxalate stones. Urology 62:1123–1128
Strohmaier WL, Bichler KH, Koch J, Balk KN, Wilbert DM (1993) Protective effect of verapamil on shock wave-induced renal tubular dysfunction. J Urol 150:27
Huang HS, Ma MC, Chen J et al (2002) Changes in the oxidant antioxidant balance in the kidney of rats with nephrolithiasis induced by ethylene glycol. J Urol 167:2584–2593
Dobashi K, Ghosh B, Orak JK, Singh I, Singh AK (2000) Kidney ischemia-reperfusion: modulation of antioxidant defenses. Mol Cell Biochem 205(1–2):1–11
Baggio B, Gambaro G, Ossi E et al (1983) Increased urinary excretion of renal enzymes in idiopathic calcium oxalate nephrolithiasis. J Urol 129:1161–1162
Boonla C, Wunsuwan R, Tungsanga K et al (2007) Urinary 8-hydroxy-deoxyguanosine is elevated in patients with nephrolithiasis. Urol Res 35:185–191
Thamilselvan S, Byer KJ, Hackett RL et al (2000) Free radical scavengers, catalase and superoxide dismutase provide protection from oxalate-associated injury to LLC-PK1 and MDCK cells. J Urol 164:224–229
Gáspár S, Niculiţe C, Cucu D et al (2010) Effect of calcium oxalate on renal cells as revealed by real-time measurement of extracellular oxidative burst. Biosens Bioelectron 25:1729–1734
Messiha BA, Abo-Youssef AM (2015) Protective effects of fish oil, allopurinol, and verapamil on hepatic ischemia-reperfusion injury in rats. J Nat Sci Biol Med. Jul-Dec 6(2):351–355
Zhang J, Cao H, Zhang Y, Zhang Y et al (2013) Nephroprotective effect of calcium channel blockers against toxicity of lead exposure in mice. Toxicol Lett 218(3):273–280
Bhattacharya SK, Rathi N, Mahajan P et al (2009) Effect of Ocimum sanctum, ascorbic acid, and verapamil on macrophage function and oxidative stress in mice exposed to cocaine. Indian J Pharmacol 41(3):134–9
Miller NJ, Rice-Evans C, Davies MJ, Gopinathan V, Milner A (1993) A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin Sci 84:407–412
Kusano C, Ferrari B (2008) Total antioxidant capacity: a biomarker in biomedical and nutritional studies. J. Cel. Mol. Biol. 7(1):1–15
Iguchi M, Ikegami M, Kiwamoto H et al (1993) Effect of verapamil on urinary calcium and oxalate excretion in renal stone formers. Hinyokika Kiyo 39(5):425–431
Jan CR, Chen WC, Wu SN et al (1998) Nifedipine, verapamil and diltiazem block shock-wave-induced rises in cytosolic calcium in MDCK cells. Chin J Physiol 41(4):181–188
Tanaka T, Nangaku M, Miyata T et al (2004) Blockade of calcium influx through L-type calcium channels attenuates mitochondrial injury and apoptosis in hypoxic renal tubular cells. J Am Soc Nephrol 15(9):2320–2333
Ogawa Y, Hayashi K, Nakahama T et al (2001) Role of T-type calcium channels in renal microcirculation: studies in the isolated perfused hydronephrotic kidney. Hypertension 38:343–347
Epstein M (2002) Calcium antagonists in the mamagement of hypertension. Calcium antagonists in clinical medicine. Hanley and Belfus, Philadelphia, pp 293–313
Goaling P (1986) Analytical reviews in clinical biochemistry: calcium measurement. Ann Clin Bichem 23:146
Krieg M, Gunsser KJ, Steinhagen-Tessen E, Becker H (1986) Comparative quantitative clinico-chemical analyses of the characteristics of 24-hour urine and morning urine. J Clin Chem Clin Biochem 24:863
Frishman WH (1994) Current status of calcium channel blockers. Curr Probl Cardiol 19:637–688 (50)
Carmines PK, Fowler BC, Bell PD (1993) Segmentally distinct effects of depolarization on intracellular [Ca2+] in renal arterioles. Am J Physiol 265(5 Pt 2):677–685
Hayashi K, Nagahama T, Oka K, Epstein M, Saruta T (1996) Disparate effects of calcium antagonists on renal microcirculation. Hypertens Res 19(1):31–36
Grossman E, Messerli FH (1998) Effect of calcium antagonists on sympathetic activity. Eur Heart J 19(Suppl F):F27–F31
Mason RP, Marche P, Hintze TH (2003) Novel vascular biology of third-generation L-type calcium channel antagonists: ancillary actions of amlodipine. Arterioscler Thromb Vasc Biol 23(12):2155–2163
Epstein M (1992) Calcium antagonists and renal protection: current status and future perspectives. Arch Intern Med 152:1573–1584
Epstein M (2002) Calcium antagonists and the kidney: implications for renal protection. Calcium antagonists in clinical medicine. Hanley and Belfus, Philadelphia, pp 629–664
Epstein M (2002) Calcium antagonists in the management of hypertension. In: Calcium antagonists in clinical medicine. Hanley and Belfus, Philadelphia, pp 293–313
Frishman WH (1994) Current status of calcium channel blockers. Curr Probl Cardiol 19:637–688
Danon A, Zenser TV, Thomasson DL et al (1990) Effect of verapamil on prostaglandin 2 synthesis by hydronephrotic rabbit cortical interstitial cells in primary culture. J Pharmacol Exp Ther 238:125–130
Iguchi M, Ikegami M, Kiwamoto H et al (1993) Effect of verapamil on urinary calcium and oxalate excretion in renal stone formers. 39(5):425–431
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was not funded by any institution.
Conflict of interest
Author Alper Kafkasli declares that he has no conflict of interest; Author Fehmi Narter declares that he has no conflict of interest; Author Oguz Ozturk declares that he has no conflict of interest; Author Ozgur Yazici declares that she has no conflict of interest; Author Cahit Sahin declares that he/she has no conflict of interest; Author Bilal Hamarat declares that he/she has no conflict of interest; Author Bilal Eryildirim declares that he/she has no conflict of interest; Author Kemal Sarica has received a speaker honorarium from Company Olympus.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Rights and permissions
About this article
Cite this article
Sarica, K., Kafkasli, A., Narter, F. et al. Hyperoxaluria-induced tubular ischemia: the effects of verapamil on the antioxidant capacity of the affected kidneys. Urolithiasis 44, 509–519 (2016). https://doi.org/10.1007/s00240-016-0894-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00240-016-0894-5