Abstract
Renal tubular epithelium is the major target for oxalate induced injury, and sustained hyperoxaluria together with CaOx crystal formation/deposition may induce renal tubular cell damage and/or dysfunction. This may express itself in cell apoptosis. To evaluate the possible protective effects of certain agents (vitamin E, potassium citrate, allopurinol, verapamil and MgOH) on the presence and the severity of apoptotic changes caused by hyperoxaluria on renal tubular epithelium, an experimental study in rabbits was performed. Seventy rabbits were divided into seven different groups (each group n=10): in group I severe hyperoxaluria was induced by continuous ethylene glycol (0.75%) administration started on day 0 and completed on day 14. Histologic alterations including crystal formation together with apoptotic changes (by using the TUNEL method) were evaluated on days 21 and 42, respectively. In the remaining experimental groups (groups II–VI), animals received some agents in addition to the induction of hyperoxaluria in an attempt to limit apoptotic changes. Group VII) animals constituted the controls. Kidneys were examined histopathologically under light microscopy for the presence and degree of crystal deposition in the tubular lumen. The percentage of apoptotic nuclei in the control group was significantly different from the other group animals (2.9–2.4%) in all study phases (P<0.05). Apart from potassium citrate and allopurinol, the other medications seemed to prevent or limit the formation of apoptotic changes in renal tubular epithelium during the early period (day 21). The percentage of positively stained nuclei in animals undergoing potassium citrate medication ranged from 24.3% to 28.6%, with an average of 27.1%. This was 18.4% in animals receiving allopurinol. On the other hand, animals receiving magnesium hydroxide (MgOH), verapamil and vitamin E demonstrated limited apoptotic changes (11.2, 9.7, 8.7%, respectively) during this phase(P<0.05). In the long-term (day 42), the animals receiving allopurinol and eitamine E showed a decrease in the percentage of the positively stained nuclei (13.5% and 8.3%, respectively). Animals in the other groups showed an increase in the number and percentage of apoptotic cells. Although, there was a significant decrease in the mean values of apoptosis in animals receiving vitamine E (8.7%–8.3%) and allopurinol (18.4%–13.5%) (P<0.05), animals on verapamil, MgOH and potassium citrate medication had an increase in these values or the change was not found to be significant. In the light of our findings and results from the literature, it is clear that that both hyperoxaluria and CaOx crystals may be injurious to renal epithelial cells. Apoptotic changes observed in renal tubular epithelial cells induced by massive hyperoxaluria might result in cell degradation and may play a role in the pathologic course of urolithiasis. Again, as demonstrated in our study, the limitation of both crystal deposition and apoptotic changes might be instituted by some antioxidant agents as well as urinary inhibitors. Clinical application of such agents in the prophylaxis of stone disease might limit the formation of urinary calculi, especially in recurrent stone formers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hyperoxaluria is the main risk factor for human idiopathic calcium oxalate (CaOx) stone formation, and the induction of hyperoxaluria is a powerful driving force in stone formation and the development of CaOx urolithiasis [9, 10, 11, 18]. Both clinical and experimental studies suggest that renal tubular epithelium is the major target for oxalate induced injury and sustained hyperoxaluria, and that CaOx crystal formation/deposition may result in injury to the renal tubular cells [8, 14, 21]. Thus, it is clear that the interaction between renal epithelial cells and CaOx crystals and/or oxalate ions play a critical role in the formation of urinary calculi [8, 14, 21]. Although crystals themselves can cause cell damage, the cause and effect relationship between injury and crystals has not yet been elucidated. However, recent studies on LLC-PK1 cells have shown that oxalate exposure can produce a variety of changes in renal epithelial cell morphology and function, including increased cellular proliferation and, at elevated concentrations, cell death. Again, the induction of hyperoxaluria is associated with necrosis and cell injury in the renal tubular epithelium. These and other studies demonstrate that the interaction of oxalate ions with renal epithelial cells elicits a programmed sequence of events that can lead to cell proliferation or cell death [19, 34, 35, 41]. Thus, a high oxalate concentration together with CaOx crystal formation/deposition may induce renal tubular cell damage and/or dysfunction which may express itself as cell apoptosis.
The etiopathogenesis of cell injury during hyperoxaluric diet involves induced lipid peroxidation in tubular cells, which usually leads to the functional impairment of cellular components by reactive oxygen species (ROS) formation due to oxidative stress [19, 20, 37, 39, 41]. Apart from possible tubular ischemia, which gives rise to ROS production as a result of lipid peroxidation, results of some animal studies suggest that the interaction of oxalate ions with renal epithelial cells may initiate a programmed sequence of events which can lead to cell proliferation or cell death [19, 30, 33, 34, 42].
Taking the injurious effects of hyperoxaluria causing crystal deposition in renal parenchyma and apoptotic changes in renal tubular epithelium (as a result of ROS production) into account, it may be useful to apply protective agents in an attempt to prevent or at least to limit the extent of the pathologic alterations. While verapamil and nifedipine (calcium channel blocking agents) were found to limit the histologic changes as well as crystal deposition induced by certain renal trauma [4, 28, 39, 38], potassium citrate was found to limit stone recurrence after SWL [37], and allopurinol as well as vitamin E have been used as antioxidant agents to minimize the effects of free oxygen radical formation in certain tissues [4, 6, 25, 26, 43, 44].
In this present animal study, it was our main goal to evaluate the possible protective effect of some definite agents (vitamin E, MgOH, allopurinol, potassium citrate and verapamil) on the presence and degree of apoptotic changes in renal tubular epithelial cells induced by an hyperoxaluric diet in a rabbit model.
Materials and methods
TMale, New Zealand white rabbits, each weighing 3–5 kg, were used. All animals were fed standard chow and kept under normal room conditions. Following a complete physical examination, all animals underwent biochemical evaluation including blood and urine analyses and stool examination. Ultrasonographic examination of the kidneys was performed to detect any anatomic abnormalities. No pathologic findings or urinary tract infectionswere found.
The rabbits were then divided into seven groups (each with n=10): group I, the animals were given an hyperoxaluria inducing diet of 0.75% ethylene glycol (EG) in distilled drinking water from day 0 to day 14. Animals in groups II–VI received some protective agents in addition to the induction of hyperoxaluria by EG: group II, vitamin E (50 IU/day); group III, magnesium hydroxide (MgOH, 200 mg/day); group IV, allopurinol (200 mg/kg); group V, potasium citrate (1 g/kg/day);and in group VI, verapamil (1 mg/kg). Lastly, group VII comprised control animals. While the induction of hyperoxaluria was made by EG in distilled drinking water, antioxidant protective agents were given via a feeding catheter. Control group animals received only distilled drinking water during all study phases. During the experimental period, all animals were provided regular rabbit chow ad libitum. Following a 2 week hyperoxaluric diet (days 0–14) in group I and additional protective agents in groups II–VI and a normal diet in group VII, five animals were killed on both day 21 and day 42. Bilateral flank incision was performed and the kidneys were removed for histopathologic evaluation.
Evaluation of renal tissue histology and crystal deposition were performed under light microscopy. Tissue specimens were fixed with 10% formalin solution and then embedded in paraffin blocs. After this procedure, 3–5 μm sections were cut with a microtome for hematoxylin and eosin staining. The tissue sections were examined under a light microscope (10×40, 10×10). By utilizing a specific grading system, tubules demonstrating granular crystallization and/or calcification were counted in a 1 cm2 area and the extent of the crystallization was graded as follows: minimal (+), crystal and/or calcification in 1–3 tubules; moderate (++) same findings in 4–7 tubules; severe (+++) same findings in ≥7tubules.
In situ detection of apoptosis
The in situ detection of renal tubular cells with DNA strand breaks in paraffin-embedded parenchymal sections was achieved by the terminal deoxynucleotidyl transferase (TdT)-mediated dUTP in situ nick and-labeling (TUNEL) method using an ApopTag kit (Oncor, Gaithersburg, Md.). Briefly, after deparaiffinization and rehydration, tissue sections were incubated with proteinase K (200 μg/ml, Oncor) for 20 min at room temperature, washed in distilled water and then treated with 3% hydrogen peroxide in PBS for 10 min at room temperature to quench endogenous peroxidase activity. Sections were incubated with terminal deoxynucleotidyl transferase (TdT) and dioxigenin-1, l-dUTP in a humidified chamber at 37°C for 1 h and then treated with antidioxigenin-peroxidase at room temperature for 30 min. Subsequently, sections were exposed to 0.05% substrate for 7 min, washed with distilled water and PBS, and then counterstained for 10 min. Sections were then dehydrated in 100% butanol, cleared in xylene and mounted with Entellan (Merck Scientific, Fairlacon, N.J.) Negative controls were obtained by omitting the TdT enzyme, and the same volume of distilled water was used. The Apoptag kit used during the study contained the positive controls.
Quantification of apoptosis in tubular cells
To quantitate the incidence of apoptosis at each time point, the percentage of the number of TUNEL-positive cell nuclei within a renal tubule cross section was been calculated after counting 1,000 tubular cells in each sample. Scoring the degree of apoptosis was defined as; + (limited) apoptosis less than 5 %; >++ (moderate) apoptosis ranging from 5 to 10%; +++ (evident) apoptosis >10%
Results
Light microscopy findings
Examination of renal parenchymal tissue specimens from rabbits on an hyperoxaluric diet for 2 weeks revealed various degrees of crystal deposition, mainly in the tubular lumen. Apart from a slight degree of crystal deposition, no notable histologic alteration could be demonstrated in the interstitial areas. Crystal deposition was widespread in two rabbits, moderate in two and minimal or slight in one rabbit during the early phase (day 21), and tended to be self limiting in the long-term (day 42) evaluation. At this time, there were two moderately and one severely affected rabbit. Different degrees of crystal deposition were present in the other specimens obtained from animals given protective agents. The degree of crystal deposition in all groups is given in Table 1.
Evaluation of tissue specimens from the control group revealed no detectable crystal formation/deposition.
Evaluation of apoptotic changes during early follow-up (day 21)
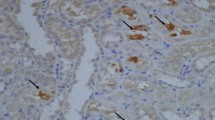
More apoptotic changes were present close to crystal deposits. Thus, in addition to hyperoxaluria itself, crystal formation and deposition also affected the degree of apoptosis in tubular cells. Apoptotic changes were found to be more prominent in tubular cells surrounding the crystals, however some degree of apoptotic change could also be found in tubular cells, even when no crystal formation was seen. On the other hand, apoptotic changes were prominent in distal tubular as well as collecting duct cells. The degree of apoptotic change was less prominent in proximal tubular cells.
A larger number of positively stained nuclei were found on evaluation during the early phase (day 21) follow-up in animals receiving potassium citrate medication (Fig. 1), and to some extend in animals receiving allopurinol. The percentage of positively stained nuclei in animals undergoing potassium citrate medication ranged from 24.3% to 28.6% with an average value of 27.1%. This value was 18.4% in animals receiving allopurinol. Animals receiving MgOH, verapamil and vitamin E showed limited apoptotic changes during this early phase examination (Fig. 2). The percentage of positively stained nuclei in the animals receiving different protective agents during the early phase are listed in Table 2. Verapamil and vitamine E seemed to prevent or limit the formation of apoptotic changes in renal tubular epithelium during the early period. Although the protective effect was found to be meaningful in these animals, it was limited in animals receiving other medications. There was a significant difference between the control group and the all other groups with respect to the apoptotic changes during early-term evaluation (Table 2).
Evaluation of apoptotic changes during long-term follow-up (day 42)
During long-term follow-up (day 42), among the groups receiving medications apart from those receiving allopurinol and vitamin E, a considerable percent increase in the number of positively stained apoptotic nuclei was noted. While the animals receiving allopurinol and vitamin E showed a decrease in the percent positively stained nuclei (Fig. 3), all the other animals showed an increase in the number and percentage of apoptotic cells. While there was a decrease in the mean percentage of apoptotic nuclei in animals receiving vitamin E (from 8.7% to 8.3%) and allopurinol (from 18.4% to 13.5%); in animals undergoing verapamil, MgOH and potassium citrate medication there was an increase in the mean value or the change was not found to be significant (Table 2). Thus, apart from allopurinol and vitamin E, all other medications failed to exhibit a protective effect on the presence and degree of apoptotic changes in renal tubular epithelial cells with a persistence and/or increase of apoptotic changes. Evaluation of tissue sections obtained from control animals did not demonstrate any significant evidence of apoptotic changes in tubular epithelial cells. The percentage of TUNEL-positive nuclei varied from 2.4% to 3.2% (mean 2.9%) at day 21 and 1.7%–2.6% (mean 2.4%) at day 42 (during the long-term phase), and positive staining of cell nuclei was present in a very limited number of tubules. However, as seen in Table 2, there was a significant difference between the control group and the all other groups with respect to the apoptotic changes during long-term evaluation.
The degree of crystal deposition in all groups is given in Table 1.
Discussion
Hyperoxaluria is the main risk factor for idiopathic calcium oxalate (CaOx) stone formation [7, 9, 12, 18], and experimental studies have demonstrated that the interaction between renal epithelial cells and CaOx crystals and/or oxalate ions plays a critical role in the formation of urinary stones. Induction of hyperoxaluria has been found to be associated with cellular injury and necrosis in tubular epithelial cells [9, 10, 12, 13, 21]. Demonstration of enzymuria, proteinuria as well as membranuria following an hyperoxaluric diet in animal models also supports the proposal that either hyperoxaluria itself or in combination with CaOx crystal formation could be injurious to renal tubular epithelial cells [14].
Moreover, injury to the tubular cells was observed even in the absence of crystalluria, suggesting that the oxalate induced damage was not due solely to injury produced by CaOx crystals [15, 18, 22].
Apart from the original study by Khan etal. [12], other groups were also able to demonstrate the functional impairment of cellular components by ROS [19, 20, 37, 39, 41]. Scheid et al. demonstrated that of the various mono-and dicarboxylates examined in their study, oxalate was the most potent at increasing free radical production and cell death [35]. Apart from possible tubular ischemia which gives rise to ROS production, results of some animal studies led the authors to propose that the interaction of oxalate ions with renal epithelial cells may initiate a programmed sequence of events which can lead to cell proliferation or death, in other words [19, 30, 33, 34, 42]. Koul et al. were able to show that there may be a causal link between the oxalate-induced increase in C-myc gene expression and oxalate-induced increase in cellular proliferation [19]. Taking the results of recent studies on LLC-PK1 cells into account, oxalate exposure has been found to produce a variety of changes in renal epithelial cell morphology and function, including increased cellular proliferation and, at elevated concentrations, cell death. These and other studies demonstrate that oxalate interactions with renal epithelial cells may elicit a programmed sequence of events that can lead to cell proliferation or cell death [19, 20, 23, 33, 34, 42]. Thus, as a distinctive form of cell death, apoptosis could also be responsible for the injury induced by hyperoxaluria. To support this proposal, we were able to demonstrate apoptotic changes in rabbit renal tubular cells, the degree and extent of which were found to be time dependent [32].
After observing the injurious effects of hyperoxaluria causing crystal deposition in renal parenchyma and apoptotic changes in renal tubular epithelium, physicians began to search for protective agents to prevent or at least minimize the extent of these pathologic alterations. Among the agents used, verapamil and nifedipine (calcium channel blocking agents) were found to limit histologic changes and crystal deposition, possibly induced by blunt renal trauma [2, 27, 28, 31, 38, 39]. Potassium citrate was found to limit stone recurrence after SWL [37] and allopurinol as well as vitamin E have been used as antioxidant agents in an attempt to minimize free oxygen radical induced alterations in certain tissues [1, 4, 5, 6, 25, 26, 36, 43, 44]. Magnesium is a potent inhibitor of calcium oxalate crystallization in vitro and accounts for about 20% of urine’s total calcium phosphate crystallization inhibitory activity. However, studies dealing of its potential inhibitory effects on crystallization as well as subsequent stone formation could not demonstrate a discrete, positive benefit although there were some limited, contradictory results [16, 17, 24, 40]. As a very potent antioxidant, vitamin E appears to be an effective scavenger, especially in preventing the deleterious effects of free radicals in parenchymateous organs [5, 25, 29, 36, 44]. It has been shown that a deficiency of vitamin E, which protects well against free radical formation, leads to parenchymateous organ damage, for example in testes or kidneys. Antioxidant agents such as vitamin E and allopurinol have been regarded and applied as protective agents in order to prevent parenchymal damage by inhibiting ROS formation. In the pharmacological activity of allopurinol and its metabolites, the main mechanism of action was found to be the inhibition of xanthine oxidase.
Our results demonstrate significant crystal formation/deposition together with apparent TUNEL-positive cell apoptosis in the early phase of follow-up (day 21). Apoptotic changes tended to be widespread and evident in animals, forming a higher degree of CaOx crystals in tubules. Quantitative examination of cell apoptosis also demonstrated the predominance of tubular cell apoptosis in early follow-up. However a number of animals showed that both apoptotic changes and crystal deposition tended to decrease during late follow-up examination (day 42). Although apoptotic changes disappeared and were self limiting to a certain proportion of tubules; crystal deposition tended to persist to some extent in some tissue sections after 6 weeks, although the hyperoxaluric diet has been discontinued. No crystal deposition with even insignificant apoptotic changes could be demonstrated in control group animals.
While animals receiving EG alone showed an evident percentage of apoptotic changes in renal tubular cells during the early hyperoxaluric period, both crystal deposition and/or apoptotic changes were found to be limited in animals receiving protective agents. Of these agents, vitamin E and allopurinol pre-treatment were more effective in limiting the apoptotic changes. In this way, the presence and degree of such changes in tubular epithelial cells were limited to a considerable extent when compared with other groups. MgOH, potassium citrate and verapamil pre-treatment were found to be less effective. Although pre-treatment with MgOH revealed limited apoptotic changes during the early phase, the percentage of apoptotic changes increased during long-term follow-up under this medication. In summary, pre-treatment of the animals with vitamin E and allopurinol provided a significant protection from calcium oxalate deposition and apoptosis in tubular cells. However, the doses of the agents used in the animal model in this study do not match those used clinically in humans.
In the light of our findings and those in the literature, it is clear that both hyperoxaluria itself and CaOx crystal formation are injurious to renal epithelial cells. These findings supported the hypothesis that apoptotic changes do occur during the hyperoxaluric phase, and that these alterations may result from free radical formation causing lipid peroxydation. Apoptotic changes observed in renal tubular epithelial cells damaged by massive hyperoxaluria might result in cell degradation and could be responsible for the pathologic course of urolithiasis. Again, as demonstrated in our study, limitation of both crystal deposition and apoptotic changes might well be instituted by some antioxidant agents as well as urinary inhibitors. Clinical application of such agents (especially vitamin E and allopurinol) in the prophylaxis of stone disease might limit the formation of urinary calculi, especially in recurrent stone formers.
References
Agarwal A, İkemoto I, Loughlin KR (1997) Prevention of testicular damage by free radical scavengers. Urology 50: 759
Akbay C, Sayın C, Sarıca K, Soygür T, Sabuncuoğlu B (1998) Effect of verapamil on rabbit renal tissue after shock wave lithotripsy: an ultrastructural approach. Electron Microsc 4: 493
Anuradha CV, Selvam R (1989) Increased lipid peroxidation in the erythrocytes of kidney stone formers. Indian J Biochem Biophys 26: 39
Benyi L, Weilherg Z, Puyun L (1995) Protective effects of nifedipine and allopurinol on high energy shock wave induced acute changes of renal function. J Urol 153: 596
Biri H, Öztürk HS, Büyükkoçak S, Kaçmaz M (1998) Antioxidant defense potential of rabbit renal tissues after ESWL: protective effect of antioxidant vitamins Nephron 79: 181
Cohen PJ (1992) Allopurinol administered prior to hepatic ischemia in the rat prevents chemiluminescence folloin restoration of circulation. Can J Anesth 39: 1090
De Water R, Boeve ER, Van Miert PP, Vermaire CP, Van Run PR, Cao LC, De Bruijn WC, Schroder FH (1996) Pathological and immunocytochemical changes in chronic calcium oxalate neprolithiasis in the rat. Scanning Microsc 10: 577
Finlayson B (1978) Physicochemical aspects of urolithiasis. Kidney Int 13: 344
Hackett RL, Shevock PN, Khan SR (1990) Cell injury associated with calcium oxalate crystalluria. J Urol 144: 1535
Hackett RL, Shevock PN, Khan SR (1994) Madin-Darby canine kidney cells are injured by exposure to oxalate and to calcium oxalate crystals. Urol Res 22: 197
Hackett RL, Shevock PN, Khan SR (1995) Alterations in MDCK and LLC-PK1 cells exposed to oxalate and calcium oxalate monohydrate crystals. Scanning Micorosc 9: 587
Khan SR (1995) Calcium oxalate crystal interaction with renal tubular epithelium, mechanism of crystal adhesion and its impact on stone development. Urol Res 23: 71
Khan SR, Hackett RL (1993) Hyperoxaluria, enzymuria and nephrolithiasis. Contrib Nephrol 101: 190
Khan SR, Shevock PN, Hackett RL (1989) Urinary enzymes and calcium oxalate urolithiasis. J Urol 142: 846
Khan SR, Shevock PN, Hackett RL (1992) Acute hyperoxaluria, renal injury and calcium oxalete urolithiasis. J Urol 147: 226
Khan SR, Shevock PN, Hackett RL (1993) Magnesium oxide administration and prevention of calcium oxalate nephrolithiasis. J Urol 149: 412
Kohri K, Garside J, Blacklock NJ (1988)The role of magnesium in calcium oxalate urolithiasis. Br J Urol 61: 107
Koul H, Kennington L, Honeyman T, Jonassen J, Menon M, Scheid C(1996) Activation of c-myc gene mediates the mitogenic effects of oxalate in LLC-PK1 cells, a line of renal epithelil cells. Kidney Int 50: 1525
Koul H, Kennington L, Nair G, Honeyman T, Menon M, Scheid C (1994) Oxalate-induced initiation of DNA synthesis in LLC-PK1 cells, a line of renal epithelial cells. Biochem Biophys Res Commun 205: 1632
Koul S, Fu S, Menon M, Koul H(2000) Oxalate exposure induces apoptosis in renal proximal tubular epithelial cells (LLC-PK1 and HK-2 cells) in culture. Urolithiasis 2000, 9th International Symposium on Urolithiasis, Proceedings, p 247
Lieske JC, Norris R, Swift H, Toback FG(1997) Adhesion, internalization and metabolism of calcium oxalate monohydrate crystals by renal epithelial cells. Kidney Int 52: 1291
Mandel N (1994) Crystal-membrane interaction in kidney stone disease. J Am Soc Nephrol 5: 37
Miyazawa K, Suzuki K, Ueda Y, Katsuda S (2000) Demonstration of apoptosis and its related genes in rat tubular epithelium of calcium oxalate crystal formation. Urolithiasis 2000, 9th International Symposium on Urolithiasis, Proceedings, p 253
Ogawa Y, Yamaguchi K, Morozumi M (1990) Effects of magnesium salts in preventing experimental oxalate urolithiasis. J Urol, 144: 385
Parekh MH, Lobel R, O’Connor L, Leggett RE, Levin RM (2001) Protective effect of Vitamin E on the response of the rabbit bladder to partial outlet obstruction. J Urol 166: 341
Reilly P, Schiller HJ, Bulkley GB (1992) Pharmacologic approach to tissue injury mediated by free radicals and other reactive oxygen metabolites. Am J Surg 161: 488
Sarıca K, Bakır K, Yağcı F, Erbağcı A, Topçu O, Uysal O (2000) Unilateral testicular torsion; protective effect of verapamil on contralateral testicular histology. Urol Int 62: 159
Sarıca K, Bakır K, Yağcı F, Topçu O, Akbay C, Sayın N, Korkmaz C (1999) Limitation of possible enhanced crystal deposition by verapamil in renal parenchyma after shock wave application in rabbit model. J Endourol 13: 343
Sarıca K, Koşar A, Yaman Ö, Bedük Y, Durak İ, Göğüş O, Kavukçu M (1996) Evaluation of ischemia after ESWL: detection of free oxygen radical scavenger enzymes in renal parenchyma subjected to high energy shock waves. Urol Int 57: 221
Sarıca K, Küpeli B, Budak M, Koşar A, Kavukçu M, Durak İ, Göğüş O (1997) Influence of experimental spermatic cord torsion on the contralateral testes in rats: evaluation of tissue free oxygen scavenger enzyme levels. Urol Int 58: 208
Sarıca K, Özer G, Soygür T, Yaman Ö, Özer E, Üstün H, Yaman LS, Göğüş O (1997) Preservation of shock-wave-induced renal histologic changes by Dermatan sulphate. Urology 49: 145
Sarıca K, Yağcı F, Bakır K, Erturhan S, Uçak R (2001) Renal tubular injury induced by hyperoxaluria: evaluation of apoptotic changes. Urol Res 29: 34
Scheid C, Koul H, Hill WA, Luber-Narod J, Kennington L, Honeyman T, Jonassen J, Menon M (1996) Oxalate toxicity in LLC-PK1 cells:role of free radicals. Kidney Int 49: 413
Scheid CR, Koul H, Hill WA, Luber-Narod J, Jonassen J, Honeyman T, Kennington L, Kohli R, Hodapp J, Ayvazian P, Menon M (1996) Oxalate toxicity in in LLC-PK1 cells, a line of renal epithelial cells. J Urol 155: 1112
Scheid CR, Koul H, Kennington L, Hill WA, Luber-Narod J, Jonassen J, Honeyman T, Menon M (1995) Oxalate-induced damage to renal tubular cells. Scanning Microsc 9: 1097
Selvam R, Adhirai M (1997) Vitamin E pretreatment prevents Cyclosporin A induced crystal deposition in hyperoxaluric rats. Nephron 75: 77
Selvam R, Bijikurien T (1992) Effect of citrate feeding on free radical induced changes in experimental urolithiasis. Indian J Exp Biol 30: 705
Strohmaier WL, Abelius A, Billes J, Grossmann T, Wilbert DM, Bichler KH (1994) Verapamil limits shock wave-induced renal tubular damage in vivo. J Endourol 8: 269
Strohmaier WL, Bichler KH, Koch J, Balk KN, Wilbert DM (1993) Protective effect of verapamil on shock wave-induced renal tubular dysfunction. J Urol 150: 27
Su CJ, Shevock PN, Khan SR, Hackett RL (1991) Effect of magnesium on calcium oxalate urolithiasis. J Urol, 45: 1092
Thamilselvan S, Hackett RL, Khan SR (1997)Lipid peroxidation in ethylene glycol induced hyperoxaluria and calcium oxalate nephrolithiasis. J Urol 157: 1059
Thamilselvan S, Khan SR (1998) Oxalate and calcium oxalate crystals are injurious to renal epithelial cells: results of in vivo and in vitro studies. J Nephrol 11: 66
Vaughan WG, Horton JW, Walker PB (1992) Allopurinol prevents intestinal permeability changes after ischemia-reperfusion injury. J Pediatr Surg 27: 968
Wu SH, Oldfield JE, Whanger PD, Weswig PH (1973) Effect of selenium, vitamin E and antioxidants on testicular function in rats. Biol Reprod 8: 625
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sarica, K., Erbagci, A., Yağci, F. et al. Limitation of apoptotic changes in renal tubular cell injury induced by hyperoxaluria. Urol Res 32, 271–277 (2004). https://doi.org/10.1007/s00240-003-0393-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00240-003-0393-3