Abstract
Hyperoxaluria and cell injury are key factors in urolithiasis. Oxalate metabolism may be altered by renal dysfunction and therefore, impact the deposition of calcium oxalate (CaOx) crystals. We investigated the relationship of renal function, oxalate metabolism and CaOx crystal deposition in renal ischemia. One hundred male Sprague–Dawley rats were randomly divided into four groups. Hyperoxaluria model (Group A and B) was established by feeding rats with 0.75 % ethylene glycol (EG). The left renal pedicle was clamped for 30 min to establish renal ischemia Groups (B and C), while Groups A and D underwent sham operation. Then, serum and urine oxalate (Ox), creatinine (Cr) and urea nitrogen (UN) levels were evaluated by liquid chromatography mass spectrometry (LCMS) and ion mass spectrum (IMS) at days 0, 2, 4, 7, and 14. CaOx crystallization was assessed by transmission electron microscope (TEM). A temporal and significant increase of serum Cr and UN levels was observed in Groups B and C compared to values obtained for Groups A and D (P < 0.05). Ox levels in serum and urine were significantly higher in Groups A and B than in the other two groups from day 7 (P < 0.05). In addition, CaOx crystallization was observed in both Groups A and B, but Group B showed earlier and more pronounced crystal deposition in the renal tissue. Our results indicated that renal tubular injury induced by renal ischemia might not affect Ox levels but could promote CaOx crystal retention under hyperoxaluria.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urinary stone formation is a common disease that affects the urinary tract in all age groups [1]. The formation of urinary stones is a multifactorial process that involves precipitation, growth, aggregation and concretion of various lithogenic salts in urine [2]. Existing theories such as nucleation and supersaturation can only partly explain the process, and the underlying etiology of nephrolithiasis has not been properly elucidated to date [3–5].
Calcium oxalate (CaOx) is the most common type of urinary stone, and patients suffering from urolithiasis caused by CaOx stones account for more than 60 % renal stone cases [2]. The subsequent formation of CaOx crystals may account for kidney stone formation. As proposed previously, hyperoxaluria is considered a major risk factor for urinary stone, and experimental induction of hyperoxaluria can lead to crystalluria and CaOx crystal deposition in the kidney [6, 7]. In addition, recurrent idiopathic stone formers always show mild hyperoxaluria; meanwhile, amount and size of CaOx crystals, alongside disease severity, are highly related to urinary excretion of oxalate (Ox) [8]. Many studies suggested a more pronounced role for hyperoxaluria than that of hypercalciuria in the formation of urinary stones [8, 9], although this notion was challenged by others [10] proposing hypercalciuria to be the prime metabolic ailment involved in stone formation.
However, CaOx particle formation does not mean that symptomatic stone disease is present. Indeed, although particles can be retained within the kidney, they need to undergo the size-enhancing process of growth and aggregation to cause symptoms [11]. Crystals can be located at sites where they are accessible, i.e., beneath the urothelium of the papilla or adhered to the tubular lumen wall. With respect to sub-urothelial crystals, injury or degradation of urothelium is considered to play an important role as this leads to crystal exposure to the urinary environment favouring further stone formation. Some scholars speculate that urothelial injury could promote crystal retention and the development of a stone nucleus allowing stone formation on the renal papilla [7, 12]. Furthermore, renal cell injury can also promote crystal–cell interaction and crystal retention in the tubules. Renal interstitium and tubular injury can be caused by several factors such as ischemia. Renal ischemia can lead to changes of oxygen tension in the renal tissue, thus inducing renal cell injury and dysfunction. It was proposed that vascular etiology is involved in primary stone event; meanwhile, kidney stones were shown to be associated with increased cardiovascular risk, including the risk for incident CHD or stroke [13, 14]. Therefore, the present study was conducted to investigate the association of renal tubular injury induced by renal ischemia with oxalate metabolism and CaOx crystal deposition, and further describe the etiology of CaOx stone formation.
Materials and methods
Experimental animals
Adult male Sprague–Dawley rats, weighing 120–130 g, were purchased from Shandong Lu Kang Animal center (China). They were singly housed in plastic cages and acclimated to room temperature (22 °C) with a 12 h light/12 h dark cycle for 1 week, in the vivarium of the Qingdao University Affiliated Hospital. The animals were provided with standard rat chow (Shandong LU Kang Animal center People’s Republic of China) throughout the study and allowed free access to water. The animal care and experimental protocols were in accordance with the guidelines of the Institutional Animal Care and Use Committee.
Experimental protocols
One hundred rats were randomly divided into four groups (N = 25): Group A, Group B, Group C and Group D, respectively. As described in Table 1, rats in Groups A and B received 0.75 % ethylene glycol (EG) in drinking water, while Groups C and D animals were provided regular water. In addition, right nephrectomy was performed on all rats, and left renal ischemia was established by clamping the left renal artery of rats with silver clamper for 30 min in Groups B and C, as described previously [15]. Rats in Groups A and D underwent sham operation (Table 1).
The experiment lasted 14 days. Five rats per group were killed at experimental days 0, 2, 4, 7 and 14, and blood and urine samples collected for evaluating oxalate levels and renal function. After euthanasia by intraperitoneal injection of sodium pentobarbital, left kidney tissues were extracted for crystals deposition assessment.
Evaluation of oxalate metabolism and renal function
Rats were placed in metabolic cages for the collection of 24 h urine. Afterward, urine samples were centrifuged for 10 min at 1000 rpm and filtrated through 0.22 μm filters. Blood was extracted, after the rats were killed,via vena cava, mixed with methyl cyanide, and centrifuged for 10 min at 1000 rpm. The levels of oxalate (Ox) in urine and serum samples as well as creatinine (Cr) and urea nitrogen (UN) in serum specimens were analyzed by liquid chromatography mass spectrometry (LCMS) and ion mass spectrum(IMS) (L-1290, Thermo Electron Corporation, Waltham, MA, USA). Briefly, 10 µL of the sample was injected into a Waters OASIS WAX 2.1 × 50 mm column, and Ox, Cr and UN amounts in the samples were determined by the established standard curve. The column was eluted at a flow rate of 0.6 mL/min for 35 min at ambient temperature. Data acquisition and processing were performed with the Chromeleon version 6.80 software (Dionex, Sunnyvale, CA).
Assessment of CaOx deposition and crystal accumulation in the kidney
Left kidneys were extracted immediately after the rats were killed. Then, renal tissues were cut into 1.0 × 1.0 cm blocks marked with medulla and parenchyma, and immersed in 4 % paraformaldehyde for 24 h. After washing with PBS for 1.5 h, samples were fixed in osmic acid for 45 min. Then, tissue sections were prepared, and CaOx crystals distribution was determined under a transmission electron microscope (TEM, Hitachi H-7650, Japan). For the quantitation of crystal accumulation in the kidney, five transmission electron microscopy (TEM) fields were randomly selected, in which crystals were counted and shown as mean ± standard deviation (SD).
Inflammatory cell assessment
Inflammatory cells in rats’ kidney were detected using the Anti-ICAM1 antibody (ab124760, Abcam, Cambridge, UK) and Anti-Streptavidin antibody (HRP) (ab191338, Abcam) by immunohistochemistry. Also, paraffin-embedded sections were examined under a polarizing optical microscope (OLYMPUS).
Statistical analysis
Data were expressed as mean ± SD. Differences among groups were determined using one-way analysis of variance (ANOVA) followed by Student–Newman–Keuls test (SPSS version 19.0; SPSS Inc., Chicago, IL, USA). A probability value of <0.05 was considered statistically significant.
Results
Detection of Cr and UN levels in serum
Serum Cr and UN amounts were detected to assess renal function changes in the four experimental groups.
As shown in Fig. 1, no significant difference was found in both Cr and UN concentrations among the four groups at day 0 (P > 0.05). However, Cr levels increased overtly on day 2 in both Groups B (0.63 ± 0.05 mg/L) and C (0.62 ± 0.04 mg/L), which showed significantly higher values compared with those obtained in Groups A (0.24 ± 0.02 mg/L) and D (0.22 ± 0.02 mg/L) (P < 0.05). Meanwhile, UN levels in Groups B (171.36 ± 10.5 mg/L) and C (176.48 ± 15.2 mg/L) were also dramatically elevated on day 2, and significantly higher than Group A (106.97 ± 11.7 mg/L) or D (105.72 ± 9.6 mg/L) (P < 0.05) values. From day 4, Cr and UN amounts decreased in Groups B and C and were significantly higher compared to Group A or D levels (P < 0.05) till day 7. The final detection results of Cr and UN levels in serum on day 14 showed no significant difference among the four groups (P > 0.05). During the experiment, Cr and UN levels in Groups A and D changed inconspicuously and remained at relatively low concentrations. In addition, when comparing Cr and UN levels between Groups B and C or between Groups A and D, no significant differences were observed throughout the study (P > 0.05).
Serum creatinine (Cr) and urea nitrogen (UN) levels in different groups of rats. Cr and UN levels were measured in serum samples obtained from Groups A (EG diet + Sham operation), B (EG diet + renal ischemia), C (normal water + renal ischemia) and D (normal water + Sham operation) rats, at several time points after the operation. *P < 0.05 vs. Group A, # P < 0.05 vs. Group D. A creatinine; B Urea nitrogen
Detection of Ox levels in urine and serum
Ox concentrations in serum and urine samples were assessed, and the results are reported in Fig. 2.
Urine and serum Ox levels in different groups of rats. Ox levels were assessed in serum and urine samples obtained from Groups A (EG diet + Sham operation), B (EG diet + renal ischemia), C (normal water + renal ischemia) and D (normal water + Sham operation) rats, at several time points after the operation. *P < 0.05 vs. Group C, # P < 0.05 vs. Group D. Serum and urine levels are presented in A and B, respectively
There was no difference in Ox levels among the four groups in either serum or urine samples at experimental days 0, 2 and 4 (P > 0.05). However, a significant increasing trend in urine and serum Ox levels could be seen from day 7 in Groups A and B. On days 7 and 14, serum Ox levels in Group A (day 7: 0.61 ± 0.01 mg/L; day 14: 0.64 ± 0.03 mg/L) or B (day 7: 0.59 ± 0.02 mg/L; day 14: 0.65 ± 0.04 mg/L) were significantly higher than values obtained for both Groups C (day 7: 0.19 ± 0.01 mg/L; day 14: 0.15 ± 0.01 mg/L) and D (day 7: 0.14 ± 0.01 mg/L; day 14: 0.15 ± 0.01 mg/L) (P < 0.05). Meanwhile, compared with urine Ox amounts in Group C (day 7: 4.26 ± 0.56 mg/L; day 14: 4.00 ± 0.67 mg/L) or Group D (day 7: 3.89 ± 0.43 mg/L; day 14: 3.77 ± 0.57 mg/L) on days 7 and 14, those of both Group A (day 7: 17.42 ± 2.4 mg/L; day 14: 19.40 ± 3.6 mg/L) and Group B (day 7: 16.28 ± 3.0 mg/L; day 14: 18.26 ± 5.0 mg/L) were overtly higher (P < 0.05). However, no significant differences in Ox levels were found in both serum and urine samples when comparing Groups A and B or Groups C and D throughout the experiment (P > 0.05).
Determination of CaOx crystals deposition
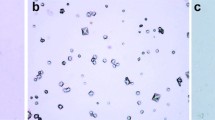
CaOx crystal deposition and pathological changes in the kidney were evaluated through examination of paraffin-embedded sections by TEM and H&E. The renal tissues of rats in Groups A and B had a speckled appearance, which indicated the presence of crystals (Figs. 3 and 4).
Crystal deposition in kidney samples from Group A rats. Crystal deposition was evaluated by H&E staining (×200) and TEM (×3000). Crystal deposition in the kidney was found from day 7 (H&E) staining and day 4 (TEM). More crystals were observed in collection ducts and the interstitial at 7 and 14 days. Renal tubular cell death or tissue disorganization could be seen. White arrows indicate crystals; black arrows indicate glomerulus; blue arrows indicate tubules; red arrows indicate renal interstitium
Crystal deposition in kidney samples from Group B rats. Crystal deposition was evaluated by H&E staining (×200) and TEM (×3000). Crystal deposition in renal papillary was observed from day 4 by HE staining, and day 2 by TEM. More crystallization could be found at 7 and 14 days. Arrows indicate crystals
TEM showed earlier detection of crystals deposition than H&E. As shown in Fig. 4, crystal depositions were observed as early as day 2 in Group B by TEM but only at day 4 by H&E. Gray and black crystal deposits could be found in interstitial tissues and papillary ducts. Moreover, more crystal deposits were observed in renal tissues at days 7 and 14. Crystal deposition was observed from day 4 in Group A as shown in Fig. 3. There were more crystal deposits in Group B than in Group A for the same time point. Accompanied with crystal deposition, renal cell death and tissue necrosis were also examined in both Group A and Group B; these pathological changes were more pronounced in renal tissues from Group B than those of Group A. Furthermore, most crystal deposits in renal tissues adhered to the cell membrane and were surrounded by inflammatory cells, which revealed the relationship between crystallization and renal cell injury.
No crystal deposition was observed in both Group C and Group D (Figs. 5 and 6). However, similar to Group B, early cell death, collecting duct’s cell dislocation and cell shrinking (caused by renal ischemia) could be also found in Group C. Normal renal structure in Group D was observed, while altered renal papillary structure was observed in Group C.
Ox crystals quantitation in the kidney
As shown in Fig. 7, CaOx crystals were distributed in both papillary interstitium and tubular lumens variably. Group A (EG diet + Sham operation) showed more CaOx crystals (day 2: 0; day 4: 3.0 ± 0.8; day 7: 7.8 ± 1.6; day 14: 11.4 ± 1.7) compared with saline treated animals, whether renal ischemic (Group C) or sham (Group D) rats treated with saline, which were devoid of CaOx crystals. Furthermore, Group B rats (EG diet + Renal ischemia) showed significantly higher CaOx crystal numbers (day 2: 3.0 ± 0.8; day 4: 8.6 ± 2.7; day 7: 12.2 ± 2.2; day 14: 15.4 ± 2.7) compared with Group A. (P < 0.05).
Degree of crystal accumulation in the kidney. Five transmission electron microscopy (TEM) fields were randomly selected, in which crystals were counted. Crystal numbers are mean ± SD. Groups A (EG diet + Sham operation), B (EG diet + renal ischemia), C (normal water + renal ischemia) and D (normal water + Sham operation) samples were assessed. *P < 0.05
Inflammatory cells in kidney tissues
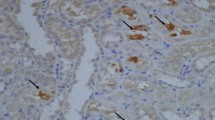
Inflammatory cells were assessed in various tissues using the specific marker ICAM1 by immunohistochemistry. As shown in Fig. 8, Groups B and C showed markedly increased ICAM1 positive cell numbers, especially at days 4, 7 and 14, confirming that ischemia increases inflammation.
Inflammatory cells in different groups of rats inflammatory cells were assessed in various tissues using the specific marker ICAM1 by immunohistochemistry. Groups A (EG diet + Sham operation), B (EG diet + renal ischemia), C (normal water + renal ischemia) and D (normal water + Sham operation) samples were assessed
Discussion
Kidney stone formation is a complex and multifactorial process that involves a series of events such as precipitation, growth, aggregation and concretion of various lithogenic salts in urine. Existing theories such as nucleation and supersaturation can only partly explain the formation of CaOx stones. However, both in vitro and animal studies have demonstrated that high levels of oxalate and/or calcium oxalate monohydrate (COM) is a major risk factor for the formation of stone nuclei [16–18]. In addition, previous studies indicated that renal tubular damage is tightly associated with the formation of kidney stones [2]. Interestingly, studies have revealed the presence of two distinct types of renal microscopical crystal deposition processes, including intratubular and interstitial nephrocalcinosis, and the regeneration of renal epithelial cells may also contribute to the formation of nephrocalcinosis [19]. We performed the present study to investigate the relationship of renal tubular injury, oxalate metabolism and CaOx crystal deposition.
According to a previous study, we established the hyperoxaluria model in rats by given EG, a precursor of Ox, to the rats in drinking water [20]. Animals develop hyperoxaluria [21], which leads to crystalluria and CaOx crystal deposition in the kidneys as shown in our study (Groups A and B). In addition, we detected the Ox levels in both serum and urine samples by LCMS and IMS, which provides a more accurate method to measure low concentrations of molecules in a minimal specimen volume [22]. Ox, a small dicarboxylate ion, is freely filtered at the glomerulus, and its active secretion by renal proximal tubule cells contributes to the overall urinary excretion of Ox [17]. Detecting Ox amounts in urine is a simple way to evaluate the Ox metabolism [18]. In the present study, significant increase of Ox levels in Groups A and B was observed from experimental day 7. In addition, the renal tubular injury was induced by renal ischemia in Groups B and C in the present study. Detection of Cr and BUN levels was performed to evaluate renal function damage. Cr and BUN levels both increased significantly in Groups B and C as early as experimental day 2; this damage was reversible since Cr and BUN levels decreased to basal levels at day 14.
Stroller hypothesized that stone formation is related to vascular system disorders such as obesity, hypertension and coronary heart disease [13]. Renal ischemia is the result of either acute or chronic vascular diseases such as hypertension or coronary heart disease [13, 14]. Microvascular impairments result in chronic tissue hypoxia, interstitial inflammation and fibrosis [18]. In addition, reversible or irreversible renal failure can be determined by the severity and duration of hypoxia and oxidative stress. Therefore, the metabolic alterations induced by vascular diseases may promote crystal deposition. However, our results showed that renal dysfunction induced by ischemia did not impact oxalate excretion, either in EG or normal diet groups. Indeed, oxalate levels showed no significant differences between EG Groups (A and B) either in serum or in urine (P > 0.05). Only low and stable oxalate levels were seen in Groups C and D, which were not exposed to ethylene glycol. Oxalate measurements were only performed on urine supernatants, with no urinary crystals taken into consideration. Therefore, only a partial assessment of oxalate excretion was carried out, ignoring excreted crystalline oxalate. This was a limitation of this study.
After detection of CaOx crystal deposition by TEM, most crystallization was observed in necrotic areas of the interstitial tissue and papillary duct, attached to the surface of injured or necrotic cells in rats with hyperoxaluria. In addition, compared to rats only fed EG, Group B animals with extra treatment of renal ischemia injury showed earlier and more pronounced crystal deposition in renal papilla. According to our results, renal tubular injury by acute renal ischemia could promote crystal retention and the development of a stone nidus on the renal papillary surface, further enhancing crystal nucleation, corroborating previous findings. In addition, crystal deposition was located at the interstitial tissue and papillary duct as shown above.
Furthermore, exposure to high Ox or CaOx levels can also cause cell death and renal tissue injury as shown in Groups A and B that can further promote crystal deposition. Interaction between CaOx and the renal epithelium and interstitium is considered a key process in the pathogenesis of CaOx stones [23, 24]. Over the past decades, increasing evidence has indicated that production of reactive oxygen species (ROS), such as superoxide radical, hydrogen peroxide and hydroxyl radicals, accounts for renal ischemia induced renal dysfunction [25]. We propose that Ox and/or CaOx crystals may induce oxidative stress in kidneys and lead to an increased production of free radical, resulting in cell damage or cell death in renal tissue; however, this hypothesis should be tested by further studies. Then, crystal retention can be promoted due to increased exposure of crystal attachment sites as a result of tubular cell detachment or membrane structure alteration after oxalate and/or crystal injury, as proposed previously [26–28].
Conclusion
Our findings demonstrated that oxalate metabolism is not influenced by temporary renal dysfunction induced by acute ischemia even under hyperoxaluria, while renal tubular injury induced by renal ischemia could promote CaOx crystal deposition. The interaction of injured cell and CaOx crystals could be a key process in the formation of urinary stones.
References
Mehmet NM, Ender O (2015) Effect of urinary stone disease and its treatment on renal function. World J Nephrol 4(2):271–276
Tsujihata M (2008) Mechanism of calcium oxalate renal stone formation and renal tubular cell injury. Int J Urol 15:115–120
Lieske J, Deganello S (1999) Nucleation, adhesion, and internalization of calcium-containing urinary crystals by renal cells. JAm Soc Nephrol 10:S422–429
Coe FL, Evan A, Worcester E (2005) Kidney stone disease. J Clin Invest 115:2598–2608
Pak CY (1991) Etiology and treatment of urolithiasis. Am J Kidney Dis 18:624–637
Sasikumar P, Gomathi S, Anbazhagan K, Abhishek A, Paul E, Vasudevan V, Sasikumar S, Selvam GS (2014) Recombinant Lactobacillus plantarum expressing and secreting heterologous oxalate decarboxylase prevents renal calcium oxalate stone deposition in experimental rats. J Biomed Sci 30(21):86. doi:10.1186/s12929-014-0086-y
Letavernier E, Vandermeersch S, Traxer O, Tligui M, Baud L, Ronco P, Haymann JP, Daudon M (2015) Demographics and characterization of 10,282 Randall plaque-related kidney stones: a new epidemic? Medicine (Baltimore) 94(10):e566. doi:10.1097/MD.0000000000000566
Verkoelen C, Romijn J (1996) Oxalate transport and calcium oxalate renal stone disease. Urol Res 24(4):183–191
Robertson W (1986) Pathophysiology of stone formation. Urol Int 41(5):329–333
Heilberg IP, Schor N (2006) Renal stone disease: causes, evaluation and medical treatment. Arq Bras Endocrinol Metabol 50(4):823–831
Ferraro PM, Taylor EN, Eisner BH, Gambaro G, Rimm EB, Mukamal KJ, Curhan GC (2013) History of kidney stones and the risk of coronary heart disease. JAMA 310(4):408–415
Fasano JM, Khan SR (2001) Intratubular crystallization of calcium oxalate in the presence of membrane vesicles: an in vitro study. Kidney int 59(1):169–178
Stoller ML, Meng MV, Abrahams HM, Kane JP (2004) The primary stone event: a new hypothesis involving a vascular etiology. J Urol 171(5):1920–1924
Liu Y, Zeng Z, Wang J, Xie L, Li T, He Y, Qin X, Zhao J (2014) Kidney stones and cardiovascular risk: a meta-analysis of cohort studies. Am J Kidney Dis 64(3):402–410
Impellizzeri D, Bruschetta G, Ahmad A, Crupi R, Siracusa R, Di Paola R, Paterniti I, Prosdocimi M, Esposito E, Cuzzocrea S (2015) Effects of palmitoylethanolamide and silymarin combination treatment in an animal model of kidney ischemia and reperfusion. Eur J Pharmacol 762:136–149
Li CY, Deng YL, Sun BH (2009) Taurine protected kidney from oxidative injury through mitochondrial-linked pathway in a rat model of nephrolithiasis. Urol Res 37:211–220
Saw NK, Rao PN, Kavanagh JP (2008) A nidus, crystalluria and aggregation: key ingredients for stone enlargement. Urol Res 36(1):11–15
Hirose M, Tozawa K, Okada A, Hamamoto S, Shimizu H, Kubota Y, Itoh Y, Yasui T, Kohri K (2008) Glyoxylate induces renal tubular cell injury and microstructural changes in experimental mouse. Urol Res 36(3–4):139–147
Vervaet BA, Verhulst A, D’Haese PC, De Broe ME (2009) Nephrocalcinosis: new insights into mechanisms and consequences. Nephrol Dial Transplant 24(7):2030–2035
De Water R, Boevé ER, van Miert PP, Deng G, Cao LC, Stijnen T, de Bruijn WC, Schröder FH (1996) Experimental nephrolithiasis in rats: the effect of ethylene glycol and vitamin D3 on the induction of renal calcium oxalate crystals. Scanning Microsc 10(2):591–601
Khan S (1997) Animal models of kidney stone formation: an analysis. World J Urol 15(4):236–243
Anderson LC, English AM, Wang W, Bai DL, Shabanowitz J, Hunt DF (2015) Protein derivatization and sequential ion/ion reactions to enhance sequence coverage produced by electron transfer dissociation mass spectrometry. Int J Mass Spectrom 377:617–624
De Water R, Noordermeer C, Houtsmuller AB, Nigg AL, Stijnen T, Schröder FH, Kok DJ (2000) Role of macrophages in nephrolithiasis in rats: an analysis of the renal interstitium. Am J Kidney Dis 36(3):615–625
Khan SR (2004) Role of renal epithelial cells in the initiation of calcium oxalate stones. Nephron Exp Nephrol 98(2):e55–60
Yamanobe T, Okada F, Iuchi Y, Onuma K, Tomita Y, Fujii J (2007) Deterioration of ischemia/reperfusion-induced acute renal failure in SOD1-deficient mice. Free Radic Res 41(2):200–207
Rashed T, Menon M, Thamilselvan S (2004) Molecular mechanism of oxalate-induced free radical production and glutathione redox imbalance in renal epithelial cells: effect of antioxidants. Am J Nephrol 24(5):557–568
Thamilselvan S, Khan SR, Menon M (2003) Oxalate and calcium oxalate mediated free radical toxicity in renal epithelial cells: effect of antioxidants. Urol Res 31(1):3–9
Umekawa T, Byer K, Uemura H, Khan SR (2005) Diphenyleneiodium (DPI) reduces oxalate ion-and calcium oxalate monohydrate and brushite crystal-induced upregulation of MCP-1 in NRK 52E cells. Nephrol Dial Transplant 20(5):870–878
Acknowledgments
This work was supported by Chinese Shandong Province Natural Foundation No. ZR1014HM059.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guidelines of Qingdao University for the care and use of animals were followed
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Y. Cao and W. Liu equally contributed to this work.
Rights and permissions
About this article
Cite this article
Cao, Y., Liu, W., Hui, L. et al. Renal tubular injury induced by ischemia promotes the formation of calcium oxalate crystals in rats with hyperoxaluria. Urolithiasis 44, 389–397 (2016). https://doi.org/10.1007/s00240-016-0876-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00240-016-0876-7