Abstract
Cancer, a disease due to uncontrolled cell proliferation is as ancient as multicellular organisms. A 255-million-years-old fossilized forerunner mammal gorgonopsian is probably the oldest evidence of cancer, to date. Cancer seems to have evolved by adapting to the microenvironment occupied by immune sentinel, modulating the cellular behavior from cytotoxic to regulatory, acquiring resistance to chemotherapy and surviving hypoxia. The interaction of genes with environmental carcinogens is central to cancer onset, seen as a spectrum of cancer susceptibility among human population. Cancer occurs in life forms other than human also, although their exposure to environmental carcinogens can be different. Role of genetic etiology in cancer in multiple species can be interesting with regard to not only cancer susceptibility, but also genetic conservation and adaptation in speciation. The widely used model organisms for cancer research are mouse and rat which are short-lived and reproduce rapidly. Research in these cancer prone animal models has been valuable as these have led to cancer therapy. However, another rewarding area of cancer research can be the cancer-resistant animal species. The Peto’s paradox and G-value paradox are evident when natural cancer resistance is observed in large mammals, like elephant and whale, small rodents viz. Naked Mole Rat and Blind Mole Rat, and Bat. The cancer resistance remains to be explored in other small or large and long-living animals like giraffe, camel, rhinoceros, water buffalo, Indian bison, Shire horse, polar bear, manatee, elephant seal, walrus, hippopotamus, turtle and tortoise, sloth, and squirrel. Indeed, understanding the molecular mechanisms of avoiding neoplastic transformation across various life forms can be potentially having translational value for human cancer management.
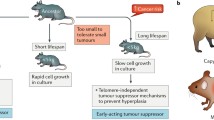
Graphical Abstract
Adapted and Modified from (Hanahan and Weinberg 2011)

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer is a disease of genetic alteration at the somatic level that manifests as uncontrolled cell proliferation and differentiation. The estimated worldwide cancer mortality is 10 million with 19.3 million new cases reported in 2020 (Sung et al. 2021). While some of the targeted anticancer drugs are considerably successful and immunotherapy has shown promising results, there is still a dire need for effective cancer therapy to reduce cancer mortality. Recognizing environmental carcinogens like chemicals, viruses, and radiation did raise hope of preventing cancer by avoiding exposure to them. However, it was soon evident that cancer onset is a result of interplay between individual genetic make-up and external agents, like certain chemicals, light pollution, accidental or intentional wildlife feeding, or reduction of genetic diversity in human-impacted habitats (Giraudeau et al. 2018a; Hochberg and Noble 2017), which can be observed as a spectrum of cancer susceptibility (Dujon et al. 2021d).
Exposure to poisons have a uniform outcome in humans at given concentrations, i.e., toxicity and death. However, the outcome of carcinogenic exposure remains uncertain and unpredictable due to the genetic variability. The optimistic scenario of avoiding cancer by avoiding exposure to environmental agents like UV radiation and chemical carcinogen in genus of Planaria (Kalafatić et al. 2006; Van Roten et al. 2018) and Trichoplax (Fortunato et al. 2021), kindled our interest in prevalence and molecular mechanisms of cancer in life forms other than humans.
The cancer etiology in humans is categorized as follows: (a) genetic, (b) environment, (c) genetic and environment, and (d) none of these, i.e., spontaneous, in the absence of any carcinogen exposure or genetic predisposition. The last category is attributed to baseline mutation rates due to errors in DNA replication and repair in proliferating cells. In the absence of comparable environmental exposure, it can be significant to understand the genetic and molecular mechanisms responsible for cancer risk in these animal species.
Cancer can result from breakdown in five foundations of multicellularity that are central to development, maintenance, and reproduction of multicellular organisms (Aktipis et al. 2015). Every living creature is the result of evolution, humans, and other life forms alike. Nowell P. C. described “clonal development of tumor cell populations” which led to evaluating cancer also in light of evolution (Nowell 1976). The evolutionary basis of cancer is summarized in four principles viz., (1) neoplasms develop in intricate ecosystems, (2) somatic selection drives the evolution of cancer, (3) natural selection can build potent cancer defenses, and (4) those defenses have limitations (Aktipis and Nesse 2013). Most multicellular organisms naturally accumulate oncogenic processes during life. Notably, pregnancy in mammals is known to exacerbate the progression of existing tumors in females (Thomas et al. 2019). The oncogenic processes viz a viz host defenses could play a major role in the selection of post-reproductive life in animals. The post-reproductive life stages, early ceasing of reproduction, &/or menopause may impart females better fitness, i.e., in terms of anticancer mechanism also (Thomas et al. 2019). As cancer evolves, its survival in dynamic microenvironment (Brutovský 2022) and cancer suppression mechanisms may also evolve. The adaptation is affected by age at first reproduction (delayed reproduction), increasing fecundity with age, age at last reproduction, and survival from other causes of mortality (Brown et al. 2015). The cancer suppression as an adaptation increasing with age can be understood using relevant model with varying levels of parental factors in order to predict variation in cancer susceptibility across species (Brown and Aktipis 2015).
In comparison to their wild predecessors, domesticated species exhibit specific health effects depending on the biology of the species and the nature and degree of the domestication process (referred to as domesticated syndrome) (Wilkins et al. 2014). The evolutionary consequences of domestication on cancer development and progression are also discussed (Thomas et al. 2020). The domestic animals with high cancer incidence may show specific traits under positive selection pressure that can favor “anticancer adaptations” in humans and wildlife. Therefore, it would be useful to compare cancer incidence across the tree of life in various multicellular organisms or among similar organisms living under different conditions. Albuquerque Thales A. F. and others have reported cancer across species from human to hydra and show that differential evolutionary pressures acting on cancer onset early in life versus later in life that escapes natural selection. The human cancers are considered to result from environmental exposures as well as from evolutionary accidents (Albuquerque et al. 2018).
Cancer is as ancient as the multicellular organisms. The oldest are fossilized evidence of an odontoma tumor in a 255-million-year-old forerunner mammal gorgonopsian (Whitney et al. 2017) and a case of bone cancer in a 240-million-year-old shell-less turtle Pappochelysrosinae (Haridy et al. 2019). Cancer is also reported in hadrosaurs or duck-billed dinosaurs around 70 million years ago (Rothschild et al. 2003). The incidence in various animals differs significantly (Albuquerque et al. 2018; Madsen et al. 2017; Vincze et al. 2022). Very low incidence of cancer is reported in elephant (Abegglen et al. 2015), no or low evidence of spontaneous tumor in spalacids like blind mole rat (Assi 2017) and naked mole rat (Hadi et al. 2021), and low incidence in hibernating bat (Koh et al. 2019) and marine mammals like orca, dolphin, whale, as well as other wild life animals (Hamede et al. 2020; McAloose and Newton 2009; Pesavento et al. 2018). Whereas, the dog and cat (Vail and Macewen 2000), ferret (Dillberger and Altman 1989), sea turtle (Dujon et al. 2021c), and Tasmanian devil (Hamede et al. 2015) have either comparable or higher cancer incidence than humans. In the context of evolution, larger and more evolved animals can be expected to have higher amount of genetic material, but this is not the case (G-value paradox). British epidemiologist Peto R. reasoned that the cells in large-bodied, long-lived animals undergo more cell divisions than small, short-lived animals. Each cell division carries a small but non-negligible risk of introducing mutations in the daughter cells due to baseline replication and related errors. Thus, there can be higher chances of acquiring necessary and sufficient combination of cancer-causing mutations with increased cell division events. Long-lived and larger animals can be assumed to have a higher cancer risk than small, short-lived animals (Callier 2019; Gaughran et al. 2016). Models suggest that if elephants and whales had the same risk for cancer mutations per cell division as humans, they should die of cancer at a young age and hence should have been extinct (Caulin and Maley 2011). The elephants and whales do not exhibit this phenomenon; however, described as Peto’s paradox (Peto et al. 1975; Vincze et al. 2022). Peto’s paradox and G-value paradox both defy the obvious assumption!
Understanding interspecies differences in terms of cancer vulnerability can be significant for employing better clinical interventions. Cross-species variation in cancer risk have been reported (Boddy et al. 2020; Møller et al. 2017); however, the small sample size, (Abegglen et al. 2015; Cairns 1981, Leroi et al. 2003), lack of information about the age distribution of cancer (Abegglen et al. 2015; Boddy et al. 2020; Møller et al. 2017), data heterogeneity, or lack of control for phylogenetic relatedness among species (Abegglen et al. 2015; Tollis et al. 2021) according to (Vincze et al. 2022). These studies suggest that the risk of developing cancer is independent of body size and adult life expectancy. Universal oncogenesis processes and significant variations in cancer mortality across the mammals can be related to diet in case of carnivorous mammals (Vincze et al. 2022). Apart from elephant and whale, small rodents like naked mole rat and blind mole rat and bat also show resistance to cancer. The novel cancer resistance mechanisms could be attributed to novel evolutionary paths in these species. Plant tumors are mainly pathogen induced and rarely spontaneous.
New cell mass acquired by neoplastic transformation helps evolve novel genes that are expressed in tumor (Makashov et al. 2019). The human cancer genes, i.e., oncogenes and tumor suppressor genes, are considered to be significantly under purifying selection as compared to the other genes; however, these are not found to impart cancer resistance (Thomas et al. 2003).
Antagonistic Pleiotropy and Cancer
Natural selection shapes organisms to maximize reproductive fitness, which also involves trade-offs around which major traits evolve (Harshman and Zera 2007; Roff 1993, 2002; Stearns 1989; Williams 1966). The genes under positive selection that confer fitness during early life (e.g., increased reproductive output) increase the risk of various pathologies later in life, a phenomena known as antagonistic pleiotropy (Mitteldorf 2019). This observation was first proposed by Medawar P. B. (Medawar 1946) and further articulated by Williams G. C. (Williams 2001), Hamilton W. D. (Hamilton 1966), and Wallace D. C. (Wallace 1967), leading to population genetic theory of aging (Byars and Voskarides 2020). Pleiotropy is a mechanism of gene action (Barreiro et al. 2008; Knight et al. 2006), that explains why patterns of positive selection in humans are linked to risks of diseases, like cancer (Nielsen et al. 2005; Vasseur and Quintana‐Murci 2013). Since cancer is reported in all forms of multicellular life, including hydra and whales (Aktipis et al. 2015; Leroi et al. 2003), Frédéric Thomas suggested that cancer could be a key player in mediating life-history trade-offs as a cost of reproduction, where energy is diverted away from maintenance (cell repair and immune function) and toward reproduction and also a contributor to life-history evolution (Dujon et al. 2022a).
The antagonistic pleiotropy is observed in animals, like fish (Fernandez 2010; Summers and Crespi 2010; Voskarides et al. 2022); however, its role in humans is debatable (Austad and Hoffman 2018; Byars and Voskarides 2019; Voskarides 2018). Based on the evidence for a variety of diseases, including neurodegenerative, cancer, and host–pathogen interactions, antagonistic pleiotropy may be a common mechanism significant for understanding various human diseases (Byars and Voskarides 2020). The invasive placentation (placenta development) and metastasis (cancer) could be correlated in terms of antagonistic pleiotropy (Costanzo et al. 2018; D'Souza and Wagner 2014). The selection for mutations that improve male reproductive success, selfish genes, and various genetic conflicts might be explained in terms of antagonistic pleiotropy that shows strong evolutionary forces known as sexual selection. The patterns of gene expression in spermatogenic cells are suggested to be shaped by selection-driven processes (Kleene 2005). Thus the ecological and evolutionary consequences in terms of antagonistic pleiotropy can be exemplified for anticancer defense; p53 and cellular senescence that could paradoxically favor the development of cancer in later life (Boutry et al. 2020).
Research in cancer resistance mechanisms in ‘unconventional’ model organisms related with animal body mass, life span, and cancer provide novel insights (Seluanov et al. 2018). We aim to update and correlate various cancer resisting mechanisms in these animals and also in invertebrates and plants. Unraveling novel cancer resistance mechanisms in animals can be promising candidates for evolving cancer therapy and prevention. We give an overview of current progress on novel cancer resistance mechanisms in vertebrates (elephant, blind mole rat, naked mole rat, whale, and bat) and invertebrates. We also discuss low incidence and carcinogenesis mechanisms in plants.
Animals with the Ability to Resist Cancer
Vertebrates
Longest Living Rodent
Naked Mole Rat
The naked mole rat (Heterocephalus glaber) is the longest living rodent species with around 30 yrs of life expectancy and a small body mass (Tian et al. 2013) that inhabits the subterranean tunnel in East Africa. It is reported that the individual animals that live in captivity (e.g., Zoo) and live longer compared to the animals living in natural habitat. Delaney M. A. first reported cancer in a 20-yr and a 22-yrs-old naked mole rats for the first time in animals living in the Zoo (Delaney et al. 2016) that might be the result of exposure to light and greater temperature ranges than in its natural environment as underground temperature is constant. Loss of contact inhibition is a characteristic of in vitro-transformed cells (Pavel et al. 2018). The naked mole rat fibroblasts are reported to secrete five times larger high-molecular mass hyaluronan (HMM-HA) compared to the human or mouse, a possible mechanism of cancer resistance (Fig. 1). Similar role of HA was observed by knocking down HAS2 o, overexpressing the HA-degrading enzyme HYAL2 affecting increased susceptibility to malignant transformation (Tian et al. 2013). The reprogramming process of induced pluripotent stem cells (Blum and Benvenisty 2008; Folmes et al. 2011; Suvà et al. 2013) resembles malignant transformation that can be explained by stable epigenome of the naked mole rat that can resist reprograming by Yamanaka factors (Oct4, Sox2, Klf4, and Myc) with low reprogramming efficiency, like mouse cells (Toole 2004).
Anticancer mechanisms in the naked mole rat. Naked mole rat cells and tissues produce five times larger high-molecular mass hyaluronan (HMM-HA) that interacts with various cell surface receptors like CD44 and trigger early contact inhibition regulated by p16Ink4a or the naked mole rat-specific INK4a/b locus product pALTInk4a/b. Both of the molecules act as a CDK inhibitor that resulted in arresting the cell cycle at low density thus preventing hyperplasia. HMM-HA also act as an antioxidant to protect DNA and protein from reactive oxygen species-induced damage and also protecting from metastasis by maintaining a stronger extracellular matrix. Naked mole rat have a more stable epigenome compared to mouse cells, which can resist reprograming by Yamanaka factors (Oct4, Sox2, Klf4, and Myc) and may similarly resist reprogramming associate with malignant transformation. They also possess unique ability to sense the loss of a single tumor suppressor such as p53, RB, or p19ARF and undergo apoptosis or senescence. Higher expression of SMAD3 product (TGF-β regulator) during aging play a key role in cancer development by slowing down the rate of cell proliferation and may optimize the rate of cell death. Furthermore, activation of alternate lengthening pathway by five protein complex (BML helicase, TOP2A, TRF1, TEP1, and HSP90) altered telomerase function and co-expression of long non-coding RNA (lncRNAs) with potential tumor suppressor might help naked mole rat to resist cancer
Multiple factors for cancer resistance are understood in naked mole rat that are phenotypically unchanged since 30–50 million years ago, suggesting a high level of genomic stability (Bredberg and Schmitz 2019). Genome sequencing revealed the mechanism for longevity and physiology as a set of genes under selection viz., APEX1, RFC1, and proteins TOP2A, TEP1, and TRF1 (Kim et al. 2011) directly bind to BLM helicase along with HSP90 to form a five protein complex playing role in lengthening telomere pathway (Bhattacharyya et al. 2009). They also found elevated expression of SMAD3 in the naked mole rat during aging that may optimize the rate of the cell death and protect against cancer. Together these molecular mechanisms may extend life span and impart cancer resistance of the naked mole rat (Kim et al. 2011). Somatic cells of naked mole rat contain additional p53/pRb-dependent mechanism for early contact inhibition (ECI) in fibroblasts; however, the replicative senescence is missing, which can control cell proliferation and ARF-dependant aging (Petruseva et al. 2017). The SV40 virus is known for its transformation activity. An independent study with RAS activation and SV40 Large-T failed to induce robust anchorage-independent growth in the naked mole rat cells, while it readily transformed mouse fibroblasts (Seluanov et al. 2009).
In human or mouse, the contact inhibition mechanism is mainly regulated by p27Kip1, while naked mole rat shows two-tier contact inhibition, an ECI mechanism which is regulated by p16Ink4a which inhibits cells accumulation. Normally contact inhibition is controlled by p27Kip1; whereas, in case of mutation or silencing of p16Ink4a it may impart cancer resistance (Seluanov et al. 2009). The INK4a/b (Cdkn2a-Cdkn2b) locus is among the most frequently mutated in human cancers (Gu et al. 2015). This locus is a rapidly evolving one which contains three key tumor suppressor genes, encoding cyclin-dependent kinase inhibitors in human and mice p15Ink4b, regulator of RB pathway p16Ink4a, and ARF (alternate reading frame), a regulator for p53 pathway (Gu et al. 2015) (Sharpless and DePinho 2005). A novel transcript pALTInk4a/b is generated as a result of fusion between p15Ink4b exon 1 and p16Ink4a exon 2 and 3 in the process of alternative splicing in naked mole rat and acts as a potent CDK inhibitor, an additional mode of cell cycle control in the naked mole rat cells (Gu et al. 2015).
Blind Mole Rat
The subterranean rodent, blind mole rat lives longer than mouse and rat, with a lifespan of around 21 yrs. (Edrey et al. 2012). It shows various mechanisms for cancer resistance which may hold cellular clues for effective treatments in humans. The underground habitat of blind mole rat is extremely hypoxic (up to 3% O2) compared to on the ground. Adaptation to this environment could be by regression of some tissues, organs, and progression of others (Nevo 2007). The tumor suppressor genes control cellular response to variety of stress conditions like hypoxia and DNA damage leading to cell growth arrest and/or apoptosis. Inactivation of p53 is found in approximately 40–50% of human cancers which leads to tumor progression under hypoxic environment (Ashur-Fabian et al. 2004). Blind moles have evolved to survive under hypoxic conditions. The hypoxia-tolerant human tumors were found to have a TP53 gene mutation Arg174Lys, which is also reported in blind mole rat suggesting more than 40 million yrs. of evolutionary adaptation to underground hypoxic life (Ashur-Fabian et al. 2004).
Heparanase is an endoglycosidase enzyme that degrades heparan sulfate (HS) at the cell surface and in the extracellular matrix (ECM), which is mainly expressed by aggressive cancer cells associated with metastasis and angiogenesis (Vlodavsky and Friedmann 2001). The cloning of a unique splice variant (splice 36 results from skipping part of exon 3, exons 4 and 5, and part of exon 6 and it functions as dominant negative) of heparanase downregulates the tumorigenic potential and inhibits its ability to degrade HS in the ECM (Nasser et al. 2009). The heparanase inhibiting strategies could be employed in the treatment of cancer (Fig. 2). The in vivo and in vitro study reveal resistance to chemical carcinogen that blind mole rat (Spalax). Fibroblasts isolated from normal Spalax have a unique ability to inhibit growth and to kill cancer cells, either through direct fibroblast–cancer cell interaction or via soluble factors, without affecting the normal cells. This was accompanied by decreased cancer cell viability, reduced colony formation in soft agar, disturbed cell cycle progression, chromatin condensation, and mitochondrial fragmentation (Manov et al. 2013).
Anticancer mechanisms in the blind mole rat. Blind mole rat also secretes HMM-HA but did not show early contact inhibition. However, it may contribute to protecting the cells from reactive oxygen species (ROS)-mediated damage. In vivo and in vitro experiments of blind mole rat fibroblast show that fibroblast can kill cancerous cells in two ways either through direct interaction or via secreting soluble factors. Expression of unique dominant splice variant of heparanase, together with HMM-HA, may contribute to stronger ECM and prevent tumor growth and metastasis. In response to hyperplasia cause by carcinogens in vivo or by hyperproliferative cells in vitro, BMR cells secrete IFN-β that activates P53 and Rb pathway; as a result cancer cells die through necrotic and apoptotic mechanisms. Blind mole rat showed overexpression of Short Interspersed Elements (SINEs) which is linked to hypoxia tolerance and resistance to cancer as well as loss of DNA methylation triggers the upregulations of retrotransposable elements due to which cytoplasmic RNA–DNA hybrids occur that leads to activation of cGAS-STING pathway and resulted in cell death. All these together may lead to cancer resistance in BMR
An in vitro study of two different blind mole rat (Spalax judaei and Spalax golani) fibroblast cells showed cancer resistance by massive necrotic response to over proliferation mediated by P53 and Rb pathways triggered by the release of IFN-β (Gorbunova et al. 2012). Blind mole rat (Spalax galili) may have evolved a unique mechanism involving genes playing role in regulation of necrosis and inflammation like Ifnb1, Mx1, Nfkb, Tnfrsf1, Birc3, Fem1b, and Aifm1 which underwent duplication events compared to mouse, rat, and naked mole rat. Genes such as Tnfrsf1a and Nfkb1 are also positively selected for necrosis and immunoinflammatory responses to partly replace apoptosis due to its remarkable hypoxia tolerance, cancer resistance, and aging (Fang et al. 2014). Role of these genes, mainly Ifnb1, is likely to be an effective compensatory mechanism to complement insufficient p53-mediated tumor suppression in blind mole rat. High rates of RNA/DNA editing, reduced chromosome rearrangements, an over-representation of short interspersed elements (SINEs) probably linked to hypoxia & hypercapnia tolerance, degeneration of vision, and progression of photoperiodic perception and resistance to cancer have been reported (Fang et al. 2014).
The mode of cancer resistance in in vitro study of Spalax carmeli fibroblasts was found to be due to various stress responses: 1) oxidative stress treatment (serum free medium containing H2O2); 2) topoisomerase inhibition (medium containing etoposide dissolved in DMSO), and 3) DNA damage using UV-C radiation given at doses from 2000 to 8000 jm−2 using UV-C-500 cross-linker instrument (Domankevich et al. 2018). Their results showed that the DNA repair capacity was five times more active in S. carmeli than in rat, for the H2O2-induced lesions. There was also significant difference in viability following etoposide treatment and repair capacity of UV-C induced lesions, suggesting role of nucleotide excision repair and base excision repair pathways. Other DNA repair pathways, like the DSBs and cross-links, for example, the homologous recombination and Fanconi anemia (FA) pathways may also be involved.
Cytosolic DNA sensing, the cyclic GMP-AMP synthase stimulator of interferon genes (cGAS-STING) pathway has a novel role in the immune system to respond to infection, inflammation, and cancer (Burdette and Vance 2013; Dhanwani et al. 2018). It is a candidate pathway for cancer immunotherapy as many STING agonists were developed with satisfactory results in preclinical study (Jiang et al. 2020). Recent study reported retro-transposable elements (RTEs)-mediated cancer resistance in blind mole rat which showed very low level of DNA methyltransferase-1 and reduced DNA methylation in hyperplasia which activate RTEs. Further, upregulation of RTEs resulted in cytoplasmic RNA–DNA hybrids, which activate the cGAS-STING pathway inducing cell death. Thus, tumor suppressor function of RTEs is an important cancer resistance mechanism in blind mole rat (Zhao et al. 2021).
Largest and Longest Living Mammal on Land: Elephant
The elephant is one of the largest living land mammals and shows cancer resistance. Peto R. speculated a biological mechanism in case of large, long-lived mammals for avoiding cancer-causing somatic mutations protecting them from cancer (Caulin and Maley 2011; Peto et al. 1975; Tollis et al. 2017). Two independent studies report extra copies of the gene TP53 in elephants that might help to avoid cell transformation (Abegglen et al. 2015; Sulak et al. 2016). The gene TP53 rightly called master guardian of the genome is known to play a central role in cancer suppression as it gets activated when DNA damage takes place. P53 protein plays a role in DNA repair, cell cycle arrest, and apoptosis (Abegglen et al. 2015; Callaway 2015). Inactivation or mutation in p53 facilitates three major hallmarks of cancer cells viz., suppression of apoptosis, increased proliferation, and genomic instability (Hanahan 2022; Hanahan and Weinberg 2000, 2011; Lane 1992). Compared with one pair in humans and other mammals, the 19 copies of TP53 retrogenes in elephants originated by segmental duplication and drift serve as a stronger barrier against neoplastic cell proliferation (Fig. 3). The presence of numerous copies of the TP53 retrogene in elephants needs to be further substantiated before being cited as a classic example of tumor suppression in large-bodied animals, according to (Nunney 2022), who notes that study does not indicate a direct (via conserved TP53 activity) or indirect (via supporting canonical TP53 function) role of retrogene sequences.
Anticancer mechanisms in the Elephant. Elephants are the largest living land mammal. They have 19 copies of TP53 gene, likely to prevent cancer formation as in vitro study using elephant cells exposed to various doses of Ionizing radiation and doxorubicin drug shows increased expression of p21 twice as higher sensitivity to DNA damage-induced apoptosis compare to human cells. It was also shown that elephant genome comprises Accelerated regions uniquely enriched at the VRK2-FANCL-BCL11A locus for shaping mammalian mutation and cancer resistance phenotype. Elephant also have 11 copies of LIF among LIF6 which is upregulated by TP53 in response to DNA damage. Together TP53 and LIF might be protecting elephant from acquiring cancer despite the larger size
A study lead by Schiffman J. D. shows that elephant cells exposed to DNA damage had increased p21 expression (a downstream target of p53 activation) and increased cell death which was twice as sensitive to DNA damage-induced apoptosis as compared to human cells. The increased cell death in the elephant may be a p53 driven process, further enhanced by the additional TP53 retrogenes (Abegglen et al. 2015). However, it remains unclear which TP53 retrogene loci code for functional genes and which code for pseudogenes. Another study showed the expression of TP53RTG12, TP53RTG 18/19, and TP53RTG13 in the dermal fibroblasts of the elephant using RNA-Sequencing and RT-PCR/ Sanger sequencing (Sulak et al. 2016). A team lead by (Abegglen et al. 2015) show two distinct transcripts by RT-PCR and Sanger sequencing from elephant PBMC; however, the transcript loci was not assigned. However, Schiffman J. D. et al. when analyzed the chromatograms shown in Abegglen et al. 2015 Fig. 4 found that 185-bp and 201-bp products likely to be from the transcripts TP53RTG14 and TP53RTG5, respectively. However, they did not observe these retrogenes in adipose, placenta, or fibroblasts suggesting tissue-specific expression of some TP53RTG genes. Thus, their combined data suggest that at least five TP53RTG genes are transcribed.
Anticancer mechanisms in the whale. Whales are the largest living marine mammal with longer lifespan. Despite having largest body, very few cases of cancer found in whales and most of them due to environmental factors. Genomic study in bowhead whale identifies several genes under positive selection that are linked with cancer as well as copy number gains and losses in genes associated with cancer and aging that are involved in DNA damage repair. Further, lncRNAs strongly co-expressed with tumor suppressor likely to help Bowhead whale to avoid cancer. Minke whale genomic study reveals cetacean-specific amino acid changes in 7 glutathione metabolism pathway-associated genes and heptoglobin proteins which indicating adaptation to hypoxic condition. Another comparative analysis study of Humpback whale genome with other ten cetaceans identified large segmental duplication of genes associated with apoptosis pathway as well as upregulation of tumor suppressor gene during apoptosis. All these together suggest that whales evolved with various unique characteristics that might help them to resist cancer
A comparative genomic analysis of Accelerated regions (ARs) in species with distinctive traits could facilitate identification of conserved functional elements that play important roles, e.g., adaptations to different environments, acquiring physiological, anatomical, and other biomedically important traits, such as mutations and cancer resistance in elephants (Ferris et al. 2018). The elephant ARs were found to be enriched at the VRK2-FANCL-BCL11A locus and at genes that respond to DNA damage in elephant blood cells. These are important candidate genetic elements affecting mammalian mutation and cancer resistance phenotype. TP53 is not the only factor protecting elephants against cancer as another study in elephant and their extinct relatives resolved Peto’s paradox. They report re-functionalizing of a leukemia inhibitory factor (LIF; 11 extra copies) pseudogene with pro-apoptotic functions (Callier 2019). One of the copies of LIF6 is transcribed at a very low level under basal conditions but is upregulated by TP53 in response to DNA damage (Vazquez et al. 2018). Their experiments suggested that the LIF functions in a manner analogous to the pro-apoptotic BCL-2 family members by inducing the opening of the outer mitochondrial membrane pore through interaction with membrane-bound BAK/BAX protein. These together suggest that extra copies of TP53 and LIF in the elephants may have synergistic role to protect against cancer in an evolutionary process; however, more studies are required.
Largest and Longest Living Marine Mammal: Whale
The largest and longest living aquatic mammal whales differ in length, weight, and lifespan among different species. The Blue whale can grow up to 33 m in length and weigh more than 181 tonnes (McClain et al. 2015). Bowhead whale is the only whale known to live for more than 150 to 200 yrs. Lifespan of 211 yrs. has been reported in a Bowhead whale using Aspartic acid racemization technique (George et al. 1999). A study in 2019 reported age of a living Bowhead whale to be 268 yrs. They hypothesised that aging is associated with epigenetic changes involving DNA methylation mainly at CpG sites in promoters that are targets of DNA methylation associated with lifespan. This study reported accurate lifespan clock in vertebrates based on CpG islands density in selected 42 promoters (Mayne et al. 2019).
The whales can be expected to have increased mutation rates due to thousand times higher number of cells and longer lifespan than any other owing to increased cell division events. However, cancer rates reported in whale have been controversial. A case study in Beluga whale at St. Lawrence Estuary reported 18% death due to cancer in 263 examined Beluga whales. Other cetaceans including Pilot whale, Fin whale, Killer whale, Blue whale, Pigmy sperm whale, and different species of dolphins and porpoise were found to have various forms of cancer which might be due to agricultural and industrial pollution of the estuary; whereas, cancer was rare among beluga whale in the Beaufort Sea (Martineau et al. 1994, 2002). These suggest that cancer incidence does not correlate with the body size, and external factors might be playing role.
The primary cancer can metastasize to a distant site, the specificity of which is explained in seed and soil theory. In larger organisms, longer time can be required for the tumor to reach lethal stage, and in the process hyper-tumor can evolve. The cancer metastasis is also considered an evolutionary adaptation against competition of resources within a growing tumor (Nagy 2005). It was also hypothesized that the natural selection is acting on the competing phenotypes of neoplastic cell population which tend to favor aggressive “cheaters” that grow on their parent tumor, creating a hyper-tumor that outgrows the original neoplasm (Nagy et al. 2007). The in silico hyper-tumor model illustrated this hypothesis which showed that malignant neoplasms in larger organisms should be disproportionately necrotic, aggressive, and vascularized than the similar deadly tumors in small mammals. Deeper studies of metastasis patterns in varied sizes of mammals can be of great interest.
Marine mammals are deep divers spending more time at deeper level of ocean except during the feed time close to the surface where density of prey is higher in photic zone compared to the deeper areas. Their locomotor muscles have a major contributors to total muscle O2 stores due to their high myoglobin concentration and large muscle mass (Arregui et al. 2021). However, deep diving increases the risk of cellular stress such as hypoxia, oxidative, and osmotic stress induced by the level of O-Linked N-acetylglucosaminylation (O-GlcNAcylation) in numerous nucleo-cytoplasmic proteins (Jones et al. 2008; Ngoh et al. 2011; Zachara et al. 2004). Under hypoxic conditions the reactive oxygen species are generated by several cellular mechanisms that can induce cellular damage (Blokhina et al. 2003; Gonchar and Mankovska 2010). Hypoxia and reactive oxygen species can act as dual-edge sword able to serve as a foe as well as a friend. Cancer cells adapt to survive in hypoxic condition as well as against accumulated reactive oxygen species.
The ability to sense and respond to changes in oxygen levels is crucial for the survival of prokaryotic and eukaryotic organisms. It helps to maintain cell and tissue homeostasis, as well as to adapt to the chronic hypoxic conditions, systemically or localized, as in the case of cancer. The proteomic and genomic changes induced by hypoxic conditions within tumor cells can lead to cell cycle arrest, necrosis, and apoptosis (Lee and Lin 2013). The hypoxia-induced proteomic changes may also stimulate tumor growth, invasion, and metastasis by facilitating the acclimatization and survival in unsupportive, nutrient-deprived environment (Vaupel and Harrison 2004). Overall, hypoxia-activating transcriptional programs involving HIF, NFκB, PI3k, and MAPK pathways control each steps of cancer-adaptive processes and contributes to EMT-like cancer cell migration and cancer stem cell-like properties, including resistance to treatment (Muz et al. 2015). The treatment targeting hypoxia might be relevant to overcome hypoxia-associated therapy resistance in cancer (Jing et al. 2019). Thus, hypoxia and the miscoupling between the increased uptake of nutrients triggered by reduced energy efficiency, and cell proliferation signaling induced by increased accumulation of nutrients serve as driving force for the cancer growth (Cui et al. 2012).
Adaptation of tumor cells at a molecular level to hypoxic stress is largely regulated by hypoxia-inducible factor (HIF), a transcription factor which accumulates in response to decreased cellular oxygen levels (Schito and Rey 2017; Wolff et al. 2017). The HIF stimulates neovascularisation to improve oxygen delivery via accumulation of vascular endothelial growth factor (VEGF), hence HIF and VEGF are promising targets for using inhibitors to fight cancer (Akanji et al. 2019; Choueiri and Kaelin 2020). However, it is recently reported that VEGF targeting reduces primary tumor size but leads to intra-tumor hypoxia, resulting in a cell–cell junction upregulation that form circulating tumor cell (CTC) clusters that show intravasation and metastasis. It was concluded that the pro-angiogenic therapy with EpB2 reduces hypoxia and suppresses metastasis formation through prevention of CTC clusters generation (Donato et al. 2020).
The role of ROS generated as a by-product of hypoxic cellular metabolism has long been associated with cancer. A neutral balance in redox processes is a must to maintain physiological homeostasis of normal metabolically active cells. A moderate level of ROS plays a substantial role in signaling cascade to induce tumorigenesis, tumor promulgation, metastasis and survival (Kumari et al. 2018). Whereas, with tumor progression the higher ROS levels induce apoptosis and cellular damage (Assi 2017; Raza et al. 2017). The failure in effective neutralization of cellular ROS can lead to transition of normal cells into cancerous cells through various signaling pathways viz., PI3/Akt/mTOR/PTEN/MAPK/VEGF/VEGFR and MMPs (Aggarwal et al. 2019). The double-edge sword effect by ROS provides an opportunity for cancer therapeutics. Rising evidences for dual role of ROS suggest that antitumor therapy may use dietary antioxidants to reduce ROS formation thus preventing carcinogenesis or use chemical agents like phytochemicals that promote a sudden increase in ROS which can cause oxidative stress in tumor mass (de Sá Junior et al. 2017).
Glutathione is a widely studied antioxidant involved in protection of cells from ROS; both directly and as a cofactor of glutathione peroxidases (Pompella and Corti 2015; Pompella et al. 2003). A study conducted on glutathione metabolism pathway (GMP) showed cetacean-specific amino acid changes in seven GMP (GPX2, ODC1, GSR, GGT6, GGT7, GGLC, and ANPEP) genes and these changes were present in four minke whale, a fin whale and two bottlenose dolphin and porpoise (Yim et al. 2014). It is known that increased expression of GSR increase antioxidant capacity of cells (Foyer et al. 1995). The first deep-sequenced genome of marine mammal as reference, the minke whale revealed expansion of antioxidant-related genes. The whale-specific variations in glutathione-associated and haptoglobin proteins indicate adaptation to hypoxic condition during the deep diving (Yim et al. 2014) and support their hypothesis regarding adaptation for metabolism under low-oxygen and high-salt conditions and development of unique morphological traits (Fig. 4). This might be a reason for low cancer incidence in large marine mammals. More studies are required to support this hypothesis.
The first genome-wide transcriptome of the Bowhead whale based on de novo assembly identified genes under positive selection linked to cancer; mitochondrial tumor suppressor 1 (Mtus1); glycogen synthase kinase 3 alpha (Gsk3a); and prune exopolyphosphatase (Prune), which act in concert to regulate cell migration; cytoplasmic FMR1-interacting protein 1 (Cyfip1); and genes involved in insulin signaling (Seim et al. 2014). Another genomic and transcriptomic study in bowhead whale reports copy number gains and losses in genes associated with cancer and aging and remarkably a duplication in the proliferating cell nuclear antigen (PCNA) and excision repair cross-complementation group 1 (ERCC1) which encodes a DNA repair protein (Keane et al. 2015). Unlike elephant, in spite of having a higher number of cells, the whale does not have extra copies of TP53 gene. The ERCC1 and PCNA1 proteins that are involved in DNA damage repair might be protecting the whale from cancer by lowering mutation rate or by decreasing cell proliferation as in aged rat liver (Tanno et al. 1996).
A comparative analysis of humpback whales de novo genome assembly with the genomes of ten cetacean species identified large segmental duplications (LSDs) in whale genome that contained genes (PRMT2, SLC25A6, and NOX5, specifically in humpback whale) controlling the apoptotic pathway. The study showed that the genes under accelerated evolution and positive selection in cetaceans were enriched for biological processes, like cell cycle checkpoint, cell signaling, and proliferation, in case of tumor suppressor genes. Tumor suppressor genes include SALL4 in the Sei whale, TGM3 and SEMA3B in the orca, UVRAG in the sperm whale, North Atlantic right whale, and bowhead whale; and PDCD5 in humpback whale which is upregulated during apoptosis (Tollis et al. 2019). A single copy of PDCD5 in humpback whale and 3 and 2 annotated copies of SALL4 and UVRAG were observed, respectively, in its skin.
These genomic studies suggest that larger animals have evolved compensatory adaptations to cope up with the negative effects of the huge number of cell divisions and the subsequent cancer risk. Another illustration can be seen in ruminants, the only extant mammalian group possessing bony headgear having extremely rapid growth rate (Davis et al. 2011) and with rapid growth, risk of cancer come handy. According to research from (Wang et al. 2019), fast-growing cervid antlers show expression profiles that are more comparable to those of osteosarcoma than those of healthy bone tissues. On the other hand, cervids have a much lower incidence of cancer than other mammals (Griner 1983; Lombard and Witte 1959), which may be due to effective cancer-defensive mechanisms with positive selection of genes (PML, NMT2, CD2AP, ELOVL6, S100A8, ISG15, and CCDC69) functioning in the p53 pathway reveals the genetic mechanisms underlying the evolutionary, developmental, and histological origins of pecoran headgear and provides insight into the molecular mechanisms of regeneration of deer antler and its relevance to cancer resistance (Wang et al. 2019).
In addition to reports regarding association of protein coding genes with cancer resistance in long-live mammals, the role of epigenetic factors like long non-coding RNAs (lncRNAs) remains largely unknown. A study in longest living mammal like Bowhead whale (BW) and Brandt’s bat (BB) identified lncRNAs to correlate with cancer resistance. Thousands of BB and BW lncRNAs are strongly co-expressed, with potential tumor suppressor function, probably involved in the anticancer regulation in long-lived mammals (Jiang and Kong 2020).
Only Mammal with Flying Ability: Bat
Bats are the sole flying mammals with small size and longer lifespan of 7 to 42 yrs. There are around 1100 species of bats accounting almost one-fourth of all mammal species (Jiang and Kong 2020). However, only a few cases of tumors have ever been described in bats (Bradford et al. 2010; McLelland et al. 2009; Siegal-Willott et al. 2007). Bats require more energy to fly which increase the metabolic rate and fluctuation in body temperature. High metabolic rates tend to generate high ROS levels and this oxidative stress can damage the mitochondria and DNA. However, bat mitochondrial function seems to have evolved the ability to reduce the ROS levels through forming autophagosomes to get rid of the damaged mitochondria for cell survival. This can increase the lifespan as inducing apoptosis signal when high ROS level present for apoptotic necrobiosis. A review comprising last few decades studies on mitochondrial role, it suggests that these organelles might be responsible for tumor resistance as well as pathogen control in the bats (Brook and Dobson 2015). A pioneering study reported that Blood miRNomes and transcriptomes in Myotis myotis (mouse-eared bat) may possess unique regulatory mechanisms for resisting tumorigenesis and repairing cellular damage and to mitigate oxidative stresses (Huang et al. 2016). A genome-wide comparative analyses were conducted between the bat M. myotis and non-bat mammals (human, pig, and cow), in both blood miRNomes and transcriptomes deep sequence, including 246.5 million small RNA reads. In the case of bats, 3 out of 4 upregulated miRNAs (miR-101-3p, miR-16-5p, miR-143-3p) were likely to function as tumor suppressor against various kinds of cancer, while a downregulated miRNA (miR-221-5p) was a tumorigenesis promoter in human breast and pancreatic cancers (Fig. 5).
Anticancer mechanisms in the Bat. Bats comprise the one-fourth part of all the mammal species. Bat like M. davidii and P. alecto minimizing and/or repairing the ROS generated negative effects mediated through TP53, BRCA2, and LIG4, respectively, which may lead to cancer resistance. In vitro study of cells derived from M. davidii and P. alecto to find the drug efflux role of ABCB1 shows that these cells effectively transport the drug out of the cellular system and that may suggest the role of ABCB1 acquiring cancer resistance toward chemicals that are carcinogenic in nature. Long-eared bat (M. myotis) have unique regulatory mechanism which involved upregulation of miRNA likely to function as tumor suppressor. M. myotis also have stable genome and anticancer activity, respectively, maintained by (DZIP3 and PLK4) and (WDR12 and WRAP53). FBXO31 protein in M. brandtii involved in DNA damage-induced growth arrest and maintenance of oxidative stress by genes like OSGIN1 and LYPD3 as well as lncRNAs strongly associated with potential tumor suppressor may lead to cancer resistance. Reduced signaling of GH-IGF1 in four different bats M. brandtii, M. lucifugus, E. fuscus, and T. brasiliensis may be responsible for cancer resistance
Comparative analysis of two bat genomes viz., Myotis davidii and Pteropus alecto suggested that genes in the DNA damage checkpoint DNA repair pathway like ATM, DNA-PKc, RAD50, KU80, and MDM2 were under positive selection. The TP53 and BRCA2 were under positive selection in M. Davidii, and LIG4 was positively selected in P. Alecto. These genes were directly related to minimizing and/or repairing the ROS generated negative effects as a consequence of flight (Zhang et al. 2013). Another comparative study of transcriptomes between M. myotis and 3 other species like Homo sapiens (human), Mus musculus (mouse), and Canis lupus (wolf) revealed a transcriptomic signature of bat aging after network analysis, showing that genes related to DNA repair pathway, similar to the mammalian DNA repair pathway ones. When they conducted longitudinal age-related signatures across mammals, they found that age-correlated genes were mainly related to the maintenance of genome stability (DZIP3 and PLK4) and anticancer activity (WDR12 and WRAP53) in M. myotis and the majority of these have not been associated directly with aging in humans or model species (Huang et al. 2019). From these data it can be suggested that similar kind of studies in another bat species can decipher more about the bat longevity and cancer resistance.
Genome analysis of Myotis brandtii syntenic regions reveals that M. brandtii has five copies of FBXO31 that encode for a protein, which mediates DNA damage-induced growth arrest by targeting CDK1 for ubiquitin-mediated degradation and suggests additional possible 52 copies of FBXO31 in the M. brandtii genome (Seim et al. 2013). They also observed that the genes OSGIN1 and LYPD3 were involved in the maintenance of oxidative stress. The role of OSGIN1 as a tumor suppressor is observable in human kidney (Ong et al. 2004), liver (Liu et al. 2014), airway epithelium (Wang et al. 2017), and astrocytes (Brennan et al. 2017). Insensitivity of growth hormone (GH) was environed by an extent of genomic abnormalities of GH-insulin like growth factor-1 (IGF1). It has been shown that mutations in the single transmembrane receptor GHR, including the transmembrane domain-coding exon 8, resulted in human Laron-type dwarfism (short-stature), a subtype of GH insensitivity (David et al. 2011). It was evident that GHR mutations or GH signaling deficiencies including those associated with Laron-type dwarfism have been associated with increased resistance to cancer and diabetes in humans (Guevara-Aguirre et al. 2011) and mice (Coschigano et al. 2000; Ikeno et al. 2009). Seim I. and team members found that Leu284 in the transmembrane domain of GHR, which is highly conserved in tetrapods, is deleted in M. brandtii, M. lucifugus, as well as in two other echolocating bat species, the big brown bat (Eptesicus fuscus) and the Mexican free-tailed bat (Tadarida brasiliensis) (Seim et al. 2013). Therefore, these data suggest that reduced GH-IGF1 signaling may be responsible for cancer resistance in long-lived bats.
As all living species grow old in terms of cellular division, cells at certain point acquire aging and that can be led by telomere shortening, which resulted in replicative senescence, differentiation, or apoptosis and these can be act as tumor suppressor. However, cancer cells avoid this fate through upregulating of the enzyme of telomerase, which extends telomeres, or by activating a mechanism termed alternative lengthening of telomeres (ALT), which is based on a common method of DNA repair, homologous recombination (Roake and Artandi 2016). It appears as the relationship between telomere length and cancer risk is likely nonlinear with evidence showing that both too long and too short telomeres can be associated with increased cancer risk (Ujvari et al. 2022). Transcriptome study of telomere in various bat blood reveal that telomeres shorten with age in Rhinolophus ferrumequinum (horseshoe bat) and Miniopterus schreibersii (common bent-wing bat), but not in the bat genus with the greatest longevity, Myotis (Foley et al. 2018). They found that 21 telomere maintenance genes that are differentially expressed in Myotis, of which 14 are related to DNA repair and 5 with alternative telomere-lengthening mechanisms. Their data suggest that affected DNA repair, controlled regulation, and maintenance of telomere mediated by the DNA repair genes ATM and SETX contributed to the evolution of exceptional longevity in M. myotis and this may be responsible for bat resistance toward cancer and also for longer life span compare to their size.
ATP-Binding Cassette (ABC) protein like ABCB1 (ABC subfamily B member 1) is well known for regulation of cellular drug efflux (Robey et al. 2018) specially expressed in region of detoxification, excretion, and protective barriers, such as the intestinal epithelium, lumens of the liver, proximal tubule of the kidney, as well as blood-tissue barriers of the brain (Kathawala et al. 2015). It was originally discovered in cancer cells where its high and functional expression promotes resistance to variety of chemotherapeutic drugs, such as paclitaxel, vinblastine, etoposide, and doxorubicin, responsible for one of the major causes of cancer relapse (Kathawala et al. 2015; Robey et al. 2018; Ueda et al. 1987). In a study using a bat-derived cell line (M. myotis and P. alecto) for finding the drug efflux role of ABCB1 protein, in the presence of various genotoxic drugs, reveal that ABCB1 effectively transports the drug out of the cellular system and toxic drugs cause less damage in bat cells compare to human cells (Koh et al. 2019). Also, when they inhibited ABCB1 in bat cells, it triggered an accumulation of doxorubicin, DNA damage, as well as cell death, suggesting a protective role of ABCB1 toward genotoxic drugs that induce DNA damage.
Invertebrates
Previously, it was thought that animals of lower evolutionary scale than fishes, they could not acquire tumors (Teutschlaender 1920), and the reason why invertebrates are incapable of developing cancer was thoroughly explained by (Engel 1930). Indeed, a meticulous review published in the middle of the nineteenth century by (Scharrer and Lochhead 1950) offers insights on the evidence of neoplastic growth studies in a number of invertebrate phyla, and tumors of either epithelia or connective tissue origin have been reported in annelids, sipunculids, arthropods, molluscs, and ascidians. These tumors can be classified as spontaneous growths, hereditary, or of other factors like parasites, endocrine imbalance, and disturbance of innervation, as well as chemical carcinogen and radiation in case of experimental studies on insects and molluscs (Tascedda and Ottaviani 2014). Aktipis C. A. and group members searched for cancer in all lineages containing multicellular organisms and found cancer or cancer-like diseases in all but 5 lineages: Choanoflagellata, Ctenophora and Placozoa, Porifera, and Hemicordata (Aktipis et al. 2015).
According to the study of two distinct species of Hydra revealed that the cellular and molecular characteristics of their tumor cells seem to share some similarities with the previously described hallmarks of cancer cells in vertebrates, and the proposed mechanism for tumorigenesis is the accumulation of stem cells that are not properly removed by programmed cell death (Domazet-Lošo et al. 2014). This indicates that even pre-bilaterian cnidarians can develop naturally occurring tumors. Numerous anthropogenic stressors, including local ones like poor water quality (Baker et al. 2007; Bruno et al. 2003; Kim and Harvell 2002; Kuta and Richardson 2002; Voss and Richardson 2006; Williams et al. 2010), as well as global ones like abnormal sea surface temperatures (Bruno et al. 2007), increased the prevalence of various diseases in coral among cnidarians, which led to coral bleaching (Brandt and McManus 2009; Harvell et al. 2001; Mcclanahan et al. 2009; Muller et al. 2008). Growth anomalies or tumor-like diseases, which were also detected in two coral genera, Acropora and Porites, were widespread throughout the Indo-Pacific, appearing in eleven of the thirteen study regions that is strongly associated with host density and human population size (Aeby et al. 2011).
On the contrary arm, planarians and placozoans have been discovered to be cancer resistant. There have been no reports of placozoan cancer or any that have yet to be found, as radiation (X-ray) study on Trichoplax adhaerens showed tolerating high levels of radiation damage (218.6 Gy), with the aid of overexpression of genes involved in DNA repair and apoptosis (e.g., MDM2) and also able to extrude clusters of inviable cells after X-ray exposures (Fortunato et al. 2021). Planarians are a perfect model to investigate proliferation, mutagenicity, and cancer because of their long-known capacity for regeneration, which hinges on pluripotency. Smed-MmpB is a planarian tumor suppressor gene that prevents stem cells from proliferating and differentiating to create tumors outside the appropriate milieu, according to an investigation employing CDCl2 on Schmidtea mediterranea to explore the function of tumor suppressor genes (Van Roten et al. 2018).
The Drosophila genus of flies, one of the most well-studied groups of invertebrates, serves as a model organism for genetic analysis of many aspects of developmental biology, including cell migration (Jang et al. 2007) as well as in understanding the role of tumor suppressor genes in how cell and tissue growth are coordinated during organismal development and perturbed in disease states, like cancer (Hariharan and Bilder 2006). The most common tumors in this species are those of the lymph glands (which have hematopoietic function in Drosophila) and of heamatocytes (Scharrer and Lochhead 1950). Molluscs and their haematopoietic tumors are another excellent example from the invertebrates (Leavitt et al. 1990; Odintsova et al. 2011). In mussel Mytilus trossulus, cells that make up these malignant heamatocytes overexpress the gene of heat shock protein 70, which codes for a protein that deactivates the tumor suppressor TP53 (Muttray et al. 2010), making the molluscs an emerging animal model for human cancer displaying a mortalin-based phenotype (De Vico and Carella 2015; Walker et al. 2009). The signal transduction pathways that control differentiation and cell proliferation are being revealed by genetic studies in other invertebrates, such as nematodes (Caenorhabditis elegans), Drosophila, and yeast. Important molecules involved in these pathways are homologous to proto-oncogenes, and others are likely to be analogous to the products of tumor suppressor genes (Hoffmann et al. 1992). In fact, the mammalian homologs of genes identified through invertebrate genetics may also offer fresh perspectives and scientific tools to comprehend the molecular underpinnings of cancer.
In terms of vertebrates, transmissible or contagious cancer is uncommon, with the majority of cases being two in Tasmanian Devils (Loh et al. 2006; Pearse et al. 2012; Pye et al. 2016) and one in dogs (Ganguly et al. 2016), whereas there is a wealth of evidence revealing that it may be common in invertebrates (six out of six species of bivalve) (Burioli et al. 2021; Garcia-Souto et al. 2022; Hammel et al. 2022; Metzger and Goff 2016; Metzger et al. 2015, 2016; Skazina et al. 2021; Ujvari et al. 2017; Yonemitsu et al. 2019). A fascinating and understudied host–pathogen system with important ecological and evolutionary implications is the transmission of cancer cells across individuals. According to (Dujon et al. 2020) only nine transmissible cancer lineages in eight host species, from both terrestrial and marine environments (mammals and bivalves; Dujon et al. 2021b; Ujvari et al. 2017)), have been investigated. They discuss the prerequisites and necessary conditions for cancer transmission and give a thorough analysis of the evolutionary dynamics between transmissible cancers and their hosts.
Human activities significantly increased oncogenic pressures on ecosystems (Acevedo-Whitehouse and Duffus 2009; Giraudeau et al. 2018b; Pesavento et al. 2018). Tumor-bearing individuals frequently have amended interactions with other individuals or species present in the ecosystem (e.g., predator–prey interactions, host–parasite interactions, and/or intra- and interspecific competition Boutry et al. 2022a, 2022b; Comte et al. 2020; Cunningham et al. 2018; Hollings et al. 2014). Understanding the effects of cancer on species and ecosystems remains a key question to answer in order to mitigate their consequences on biodiversity and ecosystems functioning (Dujon et al. 2021a; Hamede et al. 2020; Hochberg and Noble 2017; Vittecoq et al. 2013). At last, invertebrate species have the potential to provide novel and unprecedented information on the significance of oncogenic processes in animal evolutionary ecology. Tumors can be induced in invertebrates for studying the effects of cancer on individuals that possess the disease on their movement, reproduction, feeding behaviors, social interactions, holobiont, and predation risk (Dujon et al. 2022b).
Multicellular Eukaryote that Rarely Get Cancer: Plant
Among multicellular eukaryotes, the plants are also not free from tumor although rare as compared to human. Plant tumors have more than a century of research history. There are mainly two types of plants tumors: pathogen-induced tumors (bacteria, viruses, fungi, insects, etc.) and spontaneous form of tumors that are mainly due to altered genotypes (e.g., interspecific hybrids, inbred lines, and mutants) (Dodueva et al. 2020). The first ever documented evidence of a pathogen-induced plant tumor is a crown gall caused by Agrobacterium reported by (Smith and Townsend 1907). The first spontaneous plant tumor was reported in a tobacco interspecific hybrid by (Kostoff 1930). The plant tumor studies have led to many important discoveries including the agrobacterial transformation (Chilton et al. 1977), the ability of certain pathogens to synthesize plant growth regulators (Garfinkel et al. 1981; Wang et al. 2001), and horizontal gene transfer from bacteria to plants (Furner et al. 1986).
The plants are more “resistant” to tumor formation than animals. Excepting pathogen-induced tumors, the spontaneous tumor induction is very rare. The disruption of stem cell maintenance or deregulation in mitogenic signals (Doonan and Sablowski 2010; Ullrich et al. 2019) change in balance of the key component of cell cycle like Indole Acetic Acid and Cytokinin in plants and the upregulation of meristem-specific regulators play a role in plant tumors (Dodueva et al. 2020). There is significant difference in plants and animals’ tumor formation; however, both exhibit similarity in hyperplasia (increased cell numbers), hypertrophy (increased tissue size), reduced cell differentiation, pronounced cellular atypia, and a high level of vascularization (Ullrich and Aloni 2000).
Plant tumors can develop on leaves, stems, roots, and floral organs and may exhibit different degrees of cell differentiation (Ahuja 1998). There are individual guidelines for animal and human tumor grading and staging, while plant tumor can be distinguished in two categories: (i) tumors with indeterminate, i.e., “Calli”-like growth and (ii) tumors with determinate, i.e., “gall”-like growth (Dodueva et al. 2020) and references therein). Both animal and human tumors show metastasis which is not reported in plant tumors. The surgical transplantation of tumor part into a healthy plant show new focus of cell proliferation involving only the transferred material (De Ropp 1948) or only healthy cells of its own (White 1944), but plant tumors are not able to self-metastasize.
The basic principles of animal tumorigenesis are the classical concepts of proto-oncogenes and oncogenes (Todd and Wong 1999), which are poorly applicable to plants. Many animal tumor suppressors and oncogenes are present in higher plants as functional orthologs; however, the mutations in these genes tend to be non-oncogenic for plants (Dodueva et al. 2020). The downregulation of Arabidopsis retinoblastoma-related (RBR) gene lead to altered stem cell division in the meristems but did not cause tumor formation (Borghi et al. 2010). The low incidence of spontaneous tumors in plants may hold an evolutionary significance, which we believe to be due to the insufficient or incompatible multicellular organization that cannot support irregularities of cell proliferation. On the other hand, the role of phytohormones in pathogen-induced plant tumor may give important clues regarding potential medicinal value of phytohormone or similar conserved bio-molecule.
This overview of cellular and molecular mechanisms across species explains the loss of regulation of cell proliferation which may be of translational value for the human cancers.
Discussion
Globally, cancer is one of the leading cause of death, and regardless of its type, the morbidity and mortality rates are escalating annually (Sung et al. 2021). Cancer is an ancient disease that predates multicellular organisms by millions of years, not just a few decades (Whitney et al. 2017). It may have been favored by natural selection at some point during the course of development, becoming a component of nature and dispersing across the chain of life (multicellular organism) (Albuquerque et al. 2018). One of the central mechanisms of evolutionary change is natural selection, which drives the evolution of adaptive traits (Gregory 2009), such as certain physical characteristics, or phenotypes, that are passed on to offspring to make them more adapted to their environment (Fillon 2012). Natural selection has also been applied in parallel for the evolution of cancer (Goymer 2008) as differences between individual cells within the tumor favor the fittest (cell with the mutations that alter their microenvironment for their own benefit, favor uncontrolled growth, by promoting angiogenesis, changing the function of stromal cells, inducing neural damage, neutralizing immune cells as well as promoting an immunosuppressive environment to escape immune surveillance) to survive (Dujon et al. 2021a).
In addition to favoring cancer throughout the tree of life, natural selection also incorporates anticancer defenses evolved during the evolutionary process in various multicellular species at the expense of fitness and other elements of life, leading to ecological and evolutionary repercussions (Boutry et al. 2020). Boutry et al. 2020 discuss these effects in detail and highlight several areas of the evolutionary ecology of multicellular organisms that have been significantly constrained at the cell, individual, population, species, and ecosystem levels by the evolution of anticancer adaptations and offer some avenues for further study (Boutry et al. 2020). When it comes to natural defenses against cancer, they can be divided into two groups: non-immune defenses against cancer (anti-carcinogenic substances present in foods, molecules that cleanse cells of cancer-causing substances, repair of DNA damage, tumor suppressor genes, cellular senescence, natural inhibitors of angiogenesis, and natural defenses at the invasion and metastasis stages) and immune defenses against cancer (comprising various sentinels of innate and adaptive immune surveillances) (Jakóbisiak et al. 2003).
However, cancer defense mechanisms vary across species (comprising physical, molecular and microenvironmental, and immune mediated), especially in the long lived or large bodied which have evolved different cancer defense mechanisms, such as high-molecular mass hyaluronan in the naked mole rat and p53 amplification in elephants (Harris et al. 2017). Indeed, known as peto’s paradox and the evolution is solution for it (Nunney 2013), as understanding of evolutionary perspectives, an increasing frequency of prereproductive cancer death results in selection for enhanced cancer suppression. It has been supported by the work of (Nunney 2013) for the evolutionary model of cancer suppression. It is believed that the existence of cancer indicates that it must be in some sense of beneficial; otherwise, natural selection would surely have eliminated it (Garcia-Garcia 2009; Lichtenstein 2005), which is supported with the action of natural selection acting against a detrimental pathology: prereproductive cancer is rare, while the incidence of post-reproductive cancer is much higher (Nunney 2013). Numerous questions remain unanswered regarding the effects of cancer development on biotic interactions and the dynamics of entire ecosystems, as well as the impact of altered biotic interactions on the evolution of cancer resistance in the context of community ecology (Perret et al. 2020). According to the novel viewpoints through theoretical investigation in predator–prey model system, their evolutionary analysis clarified how biotic interactions can result in various levels of resistance in predator populations and showed that cancer in wildlife is an important ecological and evolutionary force to take into account (Perret et al. 2020).
Cancer develops resistance and survival tactics inside the host systems in spite of the defense or protection mechanisms against it. This is a main hallmark of cancer (Hanahan 2022). The development of defense mechanisms by some multicellular organisms, while the vulnerability of others to cancer is an intriguing question to investigate from an evolutionary perspective. In our opinion, examining the prevalence of cancer and potential protective mechanisms in other wild and domesticated animals, whether they are free-ranging or kept in captivity, it can help to clarify the Peto’s paradox, the idea that certain species are vulnerable to cancer while others are not. In this article, we highlighted possible cancer defense mechanisms in well-studied vertebrates (mammals and rodents), invertebrates, and plants that may be beneficial in future for developing cancer therapies for humans.
Conclusion
-
(1)
The chances of cancer are not directly correlated with body mass, number of cells, and number of cell divisions, as this is explained by Peto’s paradox.
-
(2)
The antagonistic pleiotropy, mutation accumulation, and other factors of natural selection toward oncogenes and tumor suppressor genes seem to play a role in protecting or predisposing the animals against cancer, although all the genetic players are still to be identified for their developmental stage-specific roles.
-
(3)
Organisms have various mechanisms to avoid cancer by altered cell characteristics (Figs. 1–5)
-
(4)
It remains to see if cancer therapy in humans can be evolved based on the cancer resistance mechanisms observed in various animal species.
Abbreviations
- ARs:
-
Accelerated regions
- BB:
-
Brandt’s bat
- BW:
-
Bowhead whale
- cGAS-Sting:
-
Cyclic GMP-AMP synthase stimulator of interferon genes
- CTC:
-
Circulating tumor cell
- ECI:
-
Early contact inhibition
- ECM:
-
Extracellular matrix
- GH:
-
Growth hormone
- HIF:
-
Hypoxia-inducible factor
- HS:
-
Heparan sulfate
- IGF1:
-
Insulin-like growth factor-1
- LIF:
-
Leukemia inhibitory factor
- ROS:
-
Reactive oxygen species
- RTEs:
-
Retrotransposable elements
References
Abegglen LM, Caulin AF, Chan A, Lee K, Robinson R, Campbell MS, Kiso WK, Schmitt DL, Waddell PJ, Bhaskara S, Jensen ST, Maley CC, Schiffman JD (2015) Potential mechanisms for cancer resistance in elephants and comparative cellular response to DNA damage in humans. J Jama 314:1850–1860
Acevedo-Whitehouse K, Duffus ALJ (2009) Effects of environmental change on wildlife health. J Philos Trans R Soc Lond B Biol Sci 364:3429–3438
Aeby GS, Williams GJ, Franklin EC, Haapkyla J, Harvell CD, Neale S, Page CA, Raymundo L, Vargas-Angel B, Willis BL, Work TM, Davy SK (2011) Growth anomalies on the coral genera Acropora and Porites are strongly associated with host density and human population size across the Indo-Pacific. J PloS One 6:e16887
Aggarwal V, Tuli HS, Varol A, Thakral F, Yerer MB, Sak K, Varol M, Jain A, Khan M, Sethi G (2019) Role of reactive oxygen species in cancer progression: molecular mechanisms and recent advancements. J Biomolecules 9:735
Ahuja MJ (1998) Genetic tumors in Nicotiana and other plants. J the Quarterly Review of Biology 73:439–462
Akanji MA, Rotimi D, Adeyemi OS (2019) Hypoxia-inducible factors as an alternative source of treatment strategy for cancer. J Oxi Med Cell Longev 2019:1
Aktipis CA, Boddy AM, Jansen G, Hibner U, Hochberg ME, Maley CC, Wilkinson GS (2015) Cancer across the tree of life: cooperation and cheating in multicellularity. J Philos Trans R Soc Lond B Biol Sci 370:20140219
Aktipis CA, Nesse RM (2013) Evolutionary foundations for cancer biology. J Evol Appl 6:144–159
Albuquerque TA, do Drummond L, Doherty A, de Magalhaes JP (2018) From humans to hydra: patterns of cancer across the tree of life. J Biol Rev Camb Philos Soc 93:1715–1734
Arregui M, Singleton EM, Saavedra P, Pabst DA, Moore MJ, Sierra E, Rivero MA, Câmara N, Niemeyer M, Fahlman A, McLellan AW, de Bernaldo QY (2021) Myoglobin concentration and oxygen stores in different functional muscle groups from three small cetacean species. J Anim (basel) 11:451
Ashur-Fabian O, Avivi A, Trakhtenbrot L, Adamsky K, Cohen M, Kajakaro G, Joel A, Amariglio N, Nevo E, Rechavi G (2004) Evolution of p53 in hypoxia-stressed Spalax mimics human tumor mutation. J Proc Natl Acad Sci USA 101:12236–12241
Assi M (2017) The differential role of reactive oxygen species in early and late stages of cancer. J Am J Physiol Regul Integr Comp Physiol 313:R646–R653
Austad SN, Hoffman JM (2018) Is antagonistic pleiotropy ubiquitous in aging biology? J Evol Med Public Health 2018:287–294
Baker DM, MacAvoy SE, Kim K (2007) Relationship between water quality, δ15N, and aspergillosis of Caribbean sea fan corals. J Mar Ecol Prog Ser 343:123–130
Barreiro LB, Laval G, Quach H, Patin E, Quintana-Murci L (2008) Natural selection has driven population differentiation in modern humans. J Nat Genet 40:340–345
Bhattacharyya S, Keirsey J, Russell B, Kavecansky J, Lillard-Wetherell K, Tahmaseb K, Turchi JJ, Groden J (2009) Telomerase-associated protein 1, HSP90, and topoisomerase IIα associate directly with the BLM helicase in immortalized cells using ALT and modulate its helicase activity using telomeric DNA substrates. J Biol Chem 284:14966–14977
Blokhina O, Virolainen E, Fagerstedt KV (2003) Antioxidants, oxidative damage and oxygen deprivation stress: a review. J Ann Bot 91:179–194
Blum B, Benvenisty N (2008) The tumorigenicity of human embryonic stem cells. J Adv Cancer Res 100:133–158
Boddy AM, Abegglen LM, Pessier AP, Aktipis A, Schiffman JD, Maley CC, Witte C (2020) Lifetime cancer prevalence and life history traits in mammals. J Evol Med Public Health 2020:187–195
Borghi L, Gutzat R, Fütterer J, Laizet Yh, Hennig L, Gruissem W (2010) Arabidopsis RETINOBLASTOMA-RELATED is required for stem cell maintenance, cell differentiation, and lateral organ production. J Plant Cell 22:1792–1811
Boutry J, Dujon AM, Gerard A-L, Tissot S, Macdonald N, Schultz A, Biro PA, Beckmann C, Hamede R, Hamilton DG, Giraudeau M, Ujvari B, Thomas F (2020) Ecological and evolutionary consequences of anticancer adaptations. J iSci 23:101716
Boutry J, Mistral J, Berlioz L, Klimovich A, Tökölyi J, Fontenille L, Ujvari B, Dujon AM, Giraudeau M, Thomas F (2022a) Tumors (re) shape biotic interactions within ecosystems: experimental evidence from the freshwater cnidarian Hydra. J Sci Total Environ 803:149923
Boutry J, Tissot S, Ujvari B, Capp J-P, Giraudeau M, Nedelcu AM, Thomas F (2022b) The evolution and ecology of benign tumors. J Biochim Biophys Acta Rev Cancer 1877:188643
Bradford C, Jennings R, Ramos-Vara J (2010) Gastrointestinal leiomyosarcoma in an Egyptian fruit bat (Rousettus aegyptiacus). J Vet Diagn Invest 22:462–465
Brandt ME, McManus JW (2009) Disease incidence is related to bleaching extent in reef-building corals. J Ecology 90:2859–2867
Bredberg A, Schmitz B (2019) Human cancer, the naked mole rat and faunal turnovers. J Cancer Med 8:1652–1654
Brennan MS, Matos MF, Richter KE, Li B, Scannevin RH (2017) The NRF2 transcriptional target, OSGIN1, contributes to monomethyl fumarate-mediated cytoprotection in human astrocytes. J Sci Rep 7:1–15
Brook CE, Dobson AP (2015) Bats as ‘special’reservoirs for emerging zoonotic pathogens. J Trends Microbiol 23:172–180
Brown JS, Aktipis CA (2015) Inclusive fitness effects can select for cancer suppression into old age. J Philos Trans R Soc Lond B Biol Sci 370:20150160
Brown JS, Cunningham JJ, Gatenby RA (2015) The multiple facets of Peto’s paradox: a life-history model for the evolution of cancer suppression. J Philos Trans R Soc Lond B Biol Sci 370:20140221
Bruno JF, Petes LE, Drew Harvell C, Hettinger A (2003) Nutrient enrichment can increase the severity of coral diseases. J Ecology Letters 6:1056–1061
Bruno JF, Selig ER, Casey KS, Page CA, Willis BL, Harvell CD, Sweatman H, Melendy AM (2007) Thermal stress and coral cover as drivers of coral disease outbreaks. J PLoS Biol 5:e124
Brutovský B (2022) Scales of cancer evolution: selfish genome or cooperating cells? J Cancers (basel) 14:3253
Burdette DL, Vance R (2013) STING and the innate immune response to nucleic acids in the cytosol. Nat Immunol 14:19–26
Burioli E, Hammel M, Bierne N, Thomas F, Houssin M, Destoumieux-Garzón D, Charriere G (2021) Traits of a mussel transmissible cancer are reminiscent of a parasitic life style. J Sci Rep 11:1–11
Byars SG, Voskarides K (2019) Genes that improved fitness also cost modern humans: evidence for genes with antagonistic effects on longevity and disease. J Evol Med Public Health 2019:4–6
Byars SG, Voskarides K (2020) Antagonistic pleiotropy in human disease. J Mol Evol 88:12–25
Cairns J (1981) The origin of human cancers. J Nature 289:353–357
Callaway E (2015) How elephants avoid cancer. J Nat News. https://doi.org/10.1038/nature.2015.18534
Callier V (2019) Core concept: solving Peto’s paradox to better understand cancer. J Proc Natl Acad Sci USA 116:1825–1828
Caulin AF, Maley CC (2011) Peto’s Paradox: evolution’s prescription for cancer prevention. J Trends Ecol Evol 26:175–182
Chilton M-D, Drummond MH, Merlo DJ, Sciaky D, Montoya AL, Gordon MP, Nester EW (1977) Stable incorporation of plasmid DNA into higher plant cells: the molecular basis of crown gall tumorigenesis. J Cell 11:263–271
Choueiri TK, Kaelin WG (2020) Targeting the HIF2–VEGF axis in renal cell carcinoma. J Nat Med 26:1519–1530
Comte S, Carver S, Hamede R, Jones M (2020) Changes in spatial organization following an acute epizootic: tasmanian devils and their transmissible cancer. J Glob Ecol Conserv 22:e00993
Coschigano KT, Clemmons D, Bellush LL, Kopchick JJ (2000) Assessment of growth parameters and life span of GHR/BP gene-disrupted mice. J Endocrinology 141:2608–2613
Costanzo V, Bardelli A, Siena S, Abrignani S (2018) Exploring the links between cancer and placenta development. J Open Biol 8:180081
Cui J, Mao X, Olman V, Hastings P, Xu Y (2012) Hypoxia and miscoupling between reduced energy efficiency and signaling to cell proliferation drive cancer to grow increasingly faster. J J Mol Cell Biol 4:174–176
Cunningham CX, Johnson CN, Barmuta LA, Hollings T, Woehler EJ, Jones ME (2018) Top carnivore decline has cascading effects on scavengers and carrion persistence. J Proc Biol Sci 285:20181582
D’Souza AW, Wagner GP (2014) Malignant cancer and invasive placentation: a case for positive pleiotropy between endometrial and malignancy phenotypes. J Evol Med Public Health 2014:136–145
David A, Hwa V, Metherell LA, Netchine I, Camacho-Hübner C, Clark AJ, Rosenfeld RG, Savage MO (2011) Evidence for a continuum of genetic, phenotypic, and biochemical abnormalities in children with growth hormone insensitivity. J Endocr Rev 32:472–497
Davis EB, Brakora KA, Lee AH (2011) Evolution of ruminant headgear: a review. J Proc Biol Sci 278:2857–2865
De Ropp R (1948) The interaction of normal and crown-gall tumor tissue in vitro grafts. J Am J Bot 35:372–377
de Sá Junior PL, Câmara DAD, Porcacchia AS, Fonseca PMM, Jorge SD, Araldi RP, Ferreira AK (2017) The roles of ROS in cancer heterogeneity and therapy. J Oxid Med Cell Longev 2017:2467940
De Vico G, Carella F (2015) Tumors in invertebrates: molluscs as an emerging animal model for human cancer. J Invertebr Surviv J 12:19–21
Delaney M, Ward J, Walsh TF, Chinnadurai S, Kerns K, Kinsel M, Treuting P (2016) Initial case reports of cancer in naked mole-rats (Heterocephalus glaber). J Vet Pathol 53:691–696
Dhanwani R, Takahashi M, Sharma S (2018) Cytosolic sensing of immuno-stimulatory DNA, the enemy within. Curr Opin Immunol 50:82–87
Dillberger J, Altman N (1989) Neoplasia in ferrets: eleven cases with a review. J Comp Pathol 100:161–176
Dodueva IE, Lebedeva MA, Kuznetsova KA, Gancheva MS, Paponova SS, Lutova LL (2020) Plant tumors: a hundred years of study. J Planta 251:1–28
Domankevich V, Eddini H, Odeh A, Shams I (2018) Resistance to DNA damage and enhanced DNA repair capacity in the hypoxia-tolerant blind mole rat Spalax carmeli. J Exp Biol 221:jeb174540
Domazet-Lošo T, Klimovich A, Anokhin B, Anton-Erxleben F, Hamm MJ, Lange C, Bosch TC (2014) Naturally occurring tumours in the basal metazoan Hydra. J Nat Commun 5:1–8
Donato C, Kunz L, Castro-Giner F, Paasinen-Sohns A, Strittmatter K, Szczerba BM, Scherrer R, Di Maggio N, Heusermann W, Biehlmaier O, Beisel C, Vetter M, Rochlitz C, Weber WP, Banfi A, Schroeder T, Aceto N (2020) Hypoxia triggers the intravasation of clustered circulating tumor cells. J Cell Rep 32:108105
Doonan JH, Sablowski R (2010) Walls around tumours—why plants do not develop cancer. J Nat Rev Cancer 10:794–802
Dujon A, Boutry J, Tissot S, Lemaître JF, Boddy A, Gérard A-L, Alvergne A, Arnal A, Vincze O, Nicolas D, Giraudeau M, Telonis-Scott M, Schultz A, Pujol P, Biro PA, Beckmann C, Hamede R, Roche B, Ujvari B, Thomas F (2022a) Cancer Susceptibility as a cost of reproduction and contributor to life history evolution. J Front Ecol Evolu 10:861103
Dujon AM, Boutry J, Tissot S, Meliani J, Guimard L, Rieu O, Ujvari B, Thomas F (2022b) A review of the methods used to induce cancer in invertebrates to study its effects on the evolution of species and ecosystem functioning. J Methods Ecol Evolu 13:1885–1898
Dujon AM, Aktipis A, Alix-Panabières C, Amend SR, Boddy AM, Brown JS, Jp C, DeGregori J, Ewald P, Gatenby R, Gerlinger M, Giraudeau M, Hamede RK, Hansen E, Kareva I, Maley CC, Marusyk A, McGranahan N, Metzger MJ, Nedelcu AM, Noble R, Nunney L, Pienta KJ, Polyak K, Pujol P, Read AF, Roche B, Sebens S, Solary E, Staňková K, Swain Ewald H, Thomas F, Ujvari B (2021a) Identifying key questions in the ecology and evolution of cancer. J Evol Appl 14:877–892
Dujon AM, Bramwell G, Roche B, Thomas F, Ujvari B (2021b) Transmissible cancers in mammals and bivalves: how many examples are there? predictions indicate widespread occurrence. J Bioessays 43:2000222
Dujon AM, Schofield G, Venegas RM, Thomas F, Ujvari B (2021c) Sea turtles in the cancer risk landscape: a global meta-analysis of fibropapillomatosis prevalence and associated risk factors. J Pathogens 10:1295
Dujon AM, Ujvari B, Thomas F (2021d) Cancer risk landscapes: a framework to study cancer in ecosystems. J Sci Total Environ 763:142955
Dujon AM, Gatenby RA, Bramwell G, MacDonald N, Dohrmann E, Raven N, Schultz A, Hamede R, Gérard A-L, Giraudeau M, Thomas F, Ujvari B (2020) Transmissible cancers in an evolutionary perspective. J iScience 23:101269
Edrey YH, Casper D, Huchon D, Mele J, Gelfond JA, Kristan DM, Nevo E, Buffenstein R (2012) Sustained high levels of neuregulin-1 in the longest-lived rodents; a key determinant of rodent longevity. J Aging Cell 11:213–222
Engel C (1930) Warum erkranken wirbellose Tiere nicht an Krebs? Z Krebs-Forsch 32:531–543
Fang X, Nevo E, Han L, Levanon EY, Zhao J, Avivi A, Larkin D, Jiang X, Feranchuk S, Zhu Y, Fishman A, Feng Y, Sher N, Xiong Z, Hankeln T, Huang Z, Gorbunova V, Zhang L, Zhao W, Wildman DE, Xiong Y, Gudkov A, Zheng Q, Rechavi G, Liu S, Bazak L, Chen J, Knisbacher BA, Lu Y, Shams I, Gajda K, Farré M, Kim J, Lewin HA, Ma J, Band M, Bicker A, Kranz V, Mattheus T, Schmidt H, Seluanov A, Azpurua J, McGowen MR, Ben Jacob E, Li K, Peng S, Zhu X, Liao X, Li S, Krogh A, Zhou X, Brodsky L, Jun W (2014) Genome-wide adaptive complexes to underground stresses in blind mole rats Spalax. J Nat Commun 5:1–11
Fernandez AA (2010) A cancer-causing gene is positively correlated with male aggression in Xiphophorus cortezi. J J Evol Biol 23:386–396
Ferris E, Abegglen LM, Schiffman JD, Gregg C (2018) Accelerated evolution in distinctive species reveals candidate elements for clinically relevant traits, including mutation and cancer resistance. J Cell Rep 22:2742–2755
Fillon M (2012) Cancer and natural selection. J Natl Cancer Inst 104:1773–1774
Foley NM, Hughes GM, Huang Z, Clarke M, Jebb D, Whelan CV, Petit EJ, Touzalin F, Farcy O, Jones G, Ransome RD, Kacprzyk J, O’Connell MJ, Kerth G, Rebelo H, Rodrigues L, Puechmaille SJ, Teeling EC (2018) Growing old, yet staying young: the role of telomeres in bats’ exceptional longevity. J Sci Adv 4:eaao0926
Folmes CD, Nelson TJ, Martinez-Fernandez A, Arrell DK, Lindor JZ, Dzeja PP, Ikeda Y, Perez-Terzic C, Terzic A (2011) Somatic oxidative bioenergetics transitions into pluripotency-dependent glycolysis to facilitate nuclear reprogramming. J Cell Metab 14:264–271
Fortunato A, Fleming A, Aktipis A, Maley CC (2021) Upregulation of DNA repair genes and cell extrusion underpin the remarkable radiation resistance of Trichoplax adhaerens. J PLoS Biol 19:e3001471
Foyer CH, Souriau N, Perret S, Lelandais M, Kunert K-J, Pruvost C, Jouanin L (1995) Overexpression of glutathione reductase but not glutathione synthetase leads to increases in antioxidant capacity and resistance to photoinhibition in poplar trees. J Plant Physiol 109:1047–1057
Furner IJ, Huffman GA, Amasino RM, Garfinkel DJ, Gordon MP, Nester EW (1986) An Agrobacterium transformation in the evolution of the genus Nicotiana. J Nature 319:422–427
Ganguly B, Das U, Das A (2016) Canine transmissible venereal tumour: a review. J Vet Comp Oncol 14:1–12
Garcia-Garcia A (2009) Is cancer a genetic program with an unknown function? J Med Hypotheses 72:407–408
Garcia-Souto D, Bruzos AL, Diaz S, Rocha S, Pequeño-Valtierra A, Roman-Lewis CF, Alonso J, Rodriguez R, Costas D, Rodriguez-Castro J, Villanueva A, Silva L, Valencia JM, Annona G, Tarallo A, Ricardo F, Bratoš Cetinić A, Posada D, Pasantes JJ, Tubio JM (2022) Mitochondrial genome sequencing of marine leukaemias reveals cancer contagion between clam species in the Seas of Southern Europe. J Elife 11:e66946
Garfinkel DJ, Simpson RB, Ream LW, White FF, Gordon MP, Nester EW (1981) Genetic analysis of crown gall: fine structure map of the T-DNA by site-directed mutagenesis. J Cell 27:143–153
Gaughran SJ, Pless E, Stearns S (2016) Evolutionary biology: how elephants beat cancer. J Elife 5:e21864
George JC, Bada J, Zeh J, Scott L, Brown SE, O’Hara T, Suydam R (1999) Age and growth estimates of bowhead whales (Balaena mysticetus) via aspartic acid racemization. J Can J Zool 77:571–580
Giraudeau M, Sepp T, Ujvari B, Ewald PW, Thomas F (2018a) Human activities might influence oncogenic processes in wild animal populations. J Nat Ecol Evol 2:1065–1070
Giraudeau M, Sepp T, Ujvari B, Ewald PW, Thomas FJNE (2018b) Human activities might influence oncogenic processes in wild animal populations. Nat Ecol Evolu 2:1065–1070
Gonchar O, Mankovska I (2010) Antioxidant system in adaptation to intermittent hypoxia. J Biol Sci 10:545–554
Gorbunova V, Hine C, Tian X, Ablaeva J, Gudkov AV, Nevo E, Seluanov A (2012) Cancer resistance in the blind mole rat is mediated by concerted necrotic cell death mechanism. J Proc Natl Acad Sci USA 109:19392–19396
Goymer P (2008) The evolution of cancer: cancer cells vary; they compete; the fittest survive. Patrick Goymer reports on how evolutionary biology can be applied to cancer–and what good it might do. J Nature 454:1046–1049
Gregory TR (2009) Understanding natural selection: essential concepts and common misconceptions. J Evolu: Educ Outreach 2:156–175
Griner LA (1983) Pathology of zoo animals. A review of necropsies conducted over a fourteen-year period at the San Diego Zoo and San Diego Wild Animal Park. Zoological Society, PO Box 551
Gu X-B, Tian T, Tian X-J, Zhang X-J (2015) Prognostic significance of neutrophil-to-lymphocyte ratio in non-small cell lung cancer: a meta-analysis. J Sci Rep 5:1–9
Guevara-Aguirre J, Balasubramanian P, Guevara-Aguirre M, Wei M, Madia F, Cheng C-W, Hwang D, Martin-Montalvo A, Saavedra J, Ingles S, de Cabo R, Cohen P, Longo VD (2011) Growth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humans. J Sci Transl Med. 3:70ra13-70ra13
Hadi F, Smith ESJ, Khaled WT (2021) Naked Mole-Rats: Resistant to Developing Cancer or Good at Avoiding It? The Extraordinary Biology of the Naked Mole-Rat. Springer, Cham, pp 341–352
Hamede R, Owen R, Siddle H, Peck S, Jones M, Dujon AM, Giraudeau M, Roche B, Ujvari B, Thomas F (2020) The ecology and evolution of wildlife cancers: applications for management and conservation. J Evol Appl 13:1719–1732
Hamede RK, Pearse A-M, Swift K, Barmuta LA, Murchison EP, Jones ME (2015) Transmissible cancer in Tasmanian devils: localized lineage replacement and host population response. J Proc Biol Sci 282:20151468
Hamilton WD (1966) The moulding of senescence by natural selection. J Theor Biol 12:12–45
Hammel M, Simon A, Arbiol C, Villalba A, Burioli EA, Jf P, Jb L, Benabdelmouna A, Bernard I, Houssin M, Charrière GM, Destoumieux-Garzon D, Welch JJ, Metzger MJ, Bierne N (2022) Prevalence and polymorphism of a mussel transmissible cancer in Europe. J Mol Ecol 31:736–751
Hanahan D (2022) Hallmarks of cancer: new dimensions. J Cell 12:31–46
Hanahan D, Weinberg RA (2000) The hallmarks of cancer. J Cell 100:57–70
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. J Cell 144:646–674
Haridy Y, Witzmann F, Asbach P, Schoch RR, Fröbisch N, Rothschild BM (2019) Triassic cancer—osteosarcoma in a 240-million-year-old stem-turtle. J JAMA Oncol 5:425–426
Hariharan IK, Bilder D (2006) Regulation of imaginal disc growth by tumor-suppressor genes in Drosophila. J Annu Rev Genet 40:335–361
Harris VK, Schiffman JD, Boddy AM (2017) Evolution of cancer defense mechanisms across species Ecology and evolution of cancer. Elsevier, Netherlands, pp 99–110
Harshman LG, Zera AJ (2007) The cost of reproduction: the devil in the details. J Trends Ecol Evol 22:80–86
Harvell D, Kim K, Quirolo C, Weir J, Smith G (2001) Coral bleaching and disease: contributors to 1998 mass mortality in Briareum asbestinum (Octocorallia, Gorgonacea). J Hydrobiologia 460:97–104
Hochberg ME, Noble RJ (2017) A framework for how environment contributes to cancer risk. J Ecol Lett 20:117–134
Hoffmann FM, Sternberg PW, Herskowitz I (1992) Learning about cancer genes through invertebrate genetics. J Curr Opin Genet Dev 2:45–52
Hollings T, Jones M, Mooney N, Mccallum H (2014) Trophic cascades following the disease-induced decline of an apex predator, the Tasmanian devil. J Conserv Biol 28:63–75
Huang Z, Jebb D, Teeling EC (2016) Blood miRNomes and transcriptomes reveal novel longevity mechanisms in the long-lived bat, Myotis myotis. J BMC Genomics 17:1–15
Huang Z, Whelan CV, Foley NM, Jebb D, Touzalin F, Petit EJ, Puechmaille SJ, Teeling EC (2019) Longitudinal comparative transcriptomics reveals unique mechanisms underlying extended healthspan in bats. J Nat Ecol Evol 3:1110–1120
Ikeno Y, Hubbard GB, Lee S, Cortez LA, Lew CM, Webb CR, Berryman DE, List EO, Kopchick JJ, Bartke A (2009) Reduced incidence and delayed occurrence of fatal neoplastic diseases in growth hormone receptor/binding protein knockout mice. J J Gerontol A Biol Sci Med Sci 64:522–529
Jakóbisiak M, Lasek W, Gołąb J (2003) Natural mechanisms protecting against cancer. J Immunol Lett 90:103–122
Jang AC-C, Starz-Gaiano M, Montell DJJJomgb, neoplasia, (2007) Modeling migration and metastasis in Drosophila. J Mammary Gland Biol Neoplasia 12:103–114
Jiang J-J, Kong Q-P (2020) Comparative analysis of long noncoding RNAs in long-lived mammals provides insights into natural cancer-resistance. J RNA Biol 17:1657–1665
Jiang M, Chen P, Wang L, Li W, Chen B, Liu Y, Wang H, Zhao S, Ye L, He Y (2020) cGAS-STING, an important pathway in cancer immunotherapy. J Hematol Oncol 13:1–11
Jing X, Yang F, Shao C, Wei K, Xie M, Shen H, Shu Y (2019) Role of hypoxia in cancer therapy by regulating the tumor microenvironment. J Mol Cancer 18:1–15
Jones SP, Zachara NE, Ngoh GA, Hill BG, Teshima Y, Bhatnagar A, Hart GW, Marbán E (2008) Cardioprotection by N-acetylglucosamine linkage to cellular proteins. J Circulation 117:1172–1182
Kalafatić M, Kovačević G, Franjević D (2006) Resistance of two planarian species to UV-irradiation. J Folia Biol (krakow) 54:103–108
Kathawala RJ, Gupta P, Ashby CR Jr, Chen Z-S (2015) The modulation of ABC transporter-mediated multidrug resistance in cancer: a review of the past decade. J Drug Resist Updat 18:1–17
Keane M, Semeiks J, Webb AE, Li YI, Quesada V, Craig T, Madsen LB, van Dam S, Brawand D, Marques PI, Michalak P, Kang L, Bhak J, Yim H-S, Grishin NV, Nielsen NH, Heide-Jørgensen MP, Oziolor EM, Matson CW, Church GM, Stuart GW, Patton JC, George JC, Suydam R, Larsen K, López-Otín C, O’Connell MJ, Bickham JW, Thomsen B, de Magalhães JP (2015) Insights into the evolution of longevity from the bowhead whale genome. J Cell Rep 10:112–122
Kim EB, Fang X, Fushan AA, Huang Z, Lobanov AV, Han L, Marino SM, Sun X, Turanov AA, Yang P, Yim SH, Zhao X, Kasaikina MV, Stoletzki N, Peng C, Polak V, Xiong Z, Kiezun A, Zhu Y, Chen Y, Kryukov V, Zhang Q, Peshkin L, Yang L, Bronson V, Buffenstein V, Wang B, Han C, Li Q, Chen L, Zhao W, Sunyaev SR, Park TJ, Zhang G, Wang J, Gladyshev VN (2011) Genome sequencing reveals insights into physiology and longevity of the naked mole rat. J Nature 479:223–227
Kim K, Harvell C (2002) Aspergillosis of sea fan corals: dynamics in the Florida keys. In: The everglades, florida bay, and coral reefs of the Florida keys: an ecosystem sourcebook. CRC, Boca Raton, p 813824
Kleene KC (2005) Sexual selection, genetic conflict, selfish genes, and the atypical patterns of gene expression in spermatogenic cells. J Dev Biol 277:16–26
Knight CG, Zitzmann N, Prabhakar S, Antrobus R, Dwek R, Hebestreit H, Rainey PB (2006) Unraveling adaptive evolution: how a single point mutation affects the protein coregulation network. J Nat Genet 38:1015–1022
Koh J, Itahana Y, Mendenhall IH, Low D, Soh EXY, Guo AK, Chionh YT, Wang L-F, Itahana K (2019) ABCB1 protects bat cells from DNA damage induced by genotoxic compounds. J Nat Commun 10:1–14
Kostoff D (1930) Tumours and other malformations on certain Nicotiana hybrids. J Zentralbl. Fur Bakt., Ab 2:81
Kumari S, Badana AK, Malla R (2018) Reactive oxygen species: a key constituent in cancer survival. J Biomark Insights 13:1177271918755391
Kuta K, Richardson LJCR (2002) Ecological aspects of black band disease of corals: relationships between disease incidence and environmental factors. J Coral Reefs 21:393–398
Lane DP (1992) p53, guardian of the genome. J Nature 358:15–16
Leavitt D, McDowell Capuzzo J, Smolowitz R, Miosky D, Lancaster B, Reinisch C (1990) Hematopoietic neoplasia inMya arenaria: Prevalence and indices of physiological condition. J Marine Biology 105:313–321
Lee R-m, Lin P-s (2013) Induction of tumor hypoxia for cancer therapy. U.S. Patent No. 8,591,921. 26 Nov 2013
Leroi AM, Koufopanou V, Burt A (2003) Cancer selection. J Nat Rev Cancer 3:226–231
Lichtenstein AV (2005) On evolutionary origin of cancer. J Cancer Cell Int 5:1–9
Liu M, Li Y, Chen L, Chan THM, Song Y, Fu L, Zeng TT, Dai YD, Zhu YH, Chen J (2014) Allele-specific imbalance of oxidative stress-induced growth inhibitor 1 associates with progression of hepatocellular carcinoma. J Gastroenterology 146:1084–1096
Loh R, Bergfeld J, Hayes D, O’hara A, Pyecroft S, Raidal S, Sharpe R (2006) The pathology of devil facial tumor disease (DFTD) in Tasmanian devils (Sarcophilus harrisii). J Vet Pathol 43:890–895
Lombard LS, Witte EJ (1959) Frequency and types of tumors in mammals and birds of the Philadelphia Zoological Garden. J Cancer Res 19:127–141
Madsen T, Arnal A, Vittecoq M, Bernex F, Abadie J, Labrut S, Garcia D, Faugère D, Lemberger K, Beckmann C (2017) Cancer prevalence and etiology in wild and captive animals Ecology and evolution of cancer. Elsevier, Netherlands, pp 11–46
Makashov A, Malov SV, Kozlov AP (2019) Oncogenes, tumor suppressor and differentiation genes represent the oldest human gene classes and evolve concurrently. J Sci Rep 9:1–12
Manov I, Hirsh M, Iancu TC, Malik A, Sotnichenko N, Band M, Avivi A, Shams I (2013) Pronounced cancer resistance in a subterranean rodent, the blind mole-rat, Spalax: in vivo and in vitro evidence. J BMC Biol 11:1–18
Martineau D, De Guise S, Fournier M, Shugart L, Girard C, Lagace A, Beland P (1994) Pathology and toxicology of beluga whales from the St. Lawrence Estuary, Quebec, Canada. Past, present and future. J Sci Total Environ 154:201–215
Martineau D, Lemberger K, Dallaire A, Labelle P, Lipscomb TP, Michel P, Mikaelian I (2002) Cancer in wildlife, a case study: beluga from the St. Lawrence estuary, Québec Canada. J Environ Health Perspect 110:285–292
Mayne B, Berry O, Davies C, Farley J, Jarman S (2019) A genomic predictor of lifespan in vertebrates. J Sci Rep 9:1–10
McAloose D, Newton AL (2009) Wildlife cancer: a conservation perspective. J Nat Rev Cancer 9:517–526
McClain CR, Balk MA, Benfield MC, Branch TA, Chen C, Cosgrove J, Dove AD, Gaskins L, Helm RR, Hochberg FG, Lee FB, Marshall A, McMurray SE, Schanche C, Stone SN, Thaler AD (2015) Sizing ocean giants: patterns of intraspecific size variation in marine megafauna. J PeerJ 3:e715
Mcclanahan TR, Weil E, Maina J (2009) Strong relationship between coral bleaching and growth anomalies in massive Porites. J Glob Chang Biol 15:1804–1816
McLelland DJ, Dutton CJ, Barker IK (2009) Sarcomatoid carcinoma in the lung of an Egyptian fruit bat (Rousettus aegyptiacus). J Vet Diagn Invest 21:160–163
Medawar PBJMQ (1946) Old age and natural death. J Mod Quart 2:30–49
Metzger MJ, Goff SP (2016) A sixth modality of infectious disease: contagious cancer from devils to clams and beyond. J PLoS Pathog 12:e1005904
Metzger MJ, Reinisch C, Sherry J, Goff SP (2015) Horizontal transmission of clonal cancer cells causes leukemia in soft-shell clams. J Cell 161:255–263
Metzger MJ, Villalba A, Carballal MJ, Iglesias D, Sherry J, Reinisch C, Muttray AF, Baldwin SA, Goff SP (2016) Widespread transmission of independent cancer lineages within multiple bivalve species. J Nature 534:705–709
Mitteldorf J (2019) What is antagonistic pleiotropy? J Biochemistry (mosc) 84:1458–1468
Møller AP, Erritzøe J, Soler JJ (2017) Life history, immunity, Peto’s paradox and tumours in birds. J Evol Biol 30:960–967
Muller E, Rogers CS, Spitzack AS, Van Woesik R (2008) Bleaching increases likelihood of disease on Acropora palmata (Lamarck) in Hawksnest Bay, St John, US virgin islands. J Coral Reefs 27:191–195
Muttray AF, O’Toole TF, Morrill W, Van Beneden RJ, Baldwin SA (2010) An invertebrate mdm homolog interacts with p53 and is differentially expressed together with p53 and ras in neoplastic Mytilus trossulus haemocytes. J Comp Biochem Physiol B Biochem Mol Biol 156:298–308
Muz B, de la Puente P, Azab F, Azab AK (2015) The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. J Hypoxia (auckl) 3:83
Nagy JD (2005) The ecology and evolutionary biology of cancer: a review of mathematical models of necrosis and tumor cell diversity. Math Biosci Eng 2:381
Nagy JD, Victor EM, Cropper JH (2007) Why don’t all whales have cancer? a novel hypothesis resolving Peto’s paradox. J Integr Comp Biol 47:317–328
Nasser NJ, Avivi A, Shafat I, Edovitsky E, Zcharia E, Ilan N, Vlodavsky I, Nevo E (2009) Alternatively spliced Spalax heparanase inhibits extracellular matrix degradation, tumor growth, and metastasis. J Proc Natl Acad Sci USA 106:2253–2258
Nevo E (2007) Mosaic evolution of subterranean mammals: tinkering, regression, progression, and global convergence Subterranean Rodents. Springer, Germany, pp 375–388
Ngoh GA, Watson LJ, Facundo HT, Jones SP (2011) Augmented O-GlcNAc signaling attenuates oxidative stress and calcium overload in cardiomyocytes. J Amino Acids 40:895–911
Nielsen R, Bustamante C, Clark AG, Glanowski S, Sackton TB, Hubisz MJ, Fledel-Alon A, Tanenbaum DM, Civello D, White TJ, Sninsky JJ, Adams MD, Cargill M (2005) A scan for positively selected genes in the genomes of humans and chimpanzees. J PLoS Biol 3:e170
Nowell PC (1976) The clonal evolution of tumor cell populations: acquired genetic lability permits stepwise selection of variant sublines and underlies tumor progression. J Science 194:23–28
Nunney L (2013) The real war on cancer: the evolutionary dynamics of cancer suppression. J Evol Appl 6:11–19
Nunney L (2022) Cancer suppression and the evolution of multiple retrogene copies of TP53 in elephants: a re-evaluation. J Evol Appl 15:891–901
Odintsova NA, Usheva LN, Yakovlev KV, Kiselev KV (2011) Naturally occurring and artificially induced tumor-like formations in marine invertebrates: a search for permanent cell lines. J Exp Mar Biol Ecol 407:241–249
Ong CK, Ng CY, Leong C, Ng CP, Foo KT, Tan PH, Huynh H (2004) Genomic structure of human OKL38 gene and its differential expression in kidney carcinogenesis. J Biol Chem 279:743–754
Pavel M, Renna M, Park SJ, Menzies FM, Ricketts T, Füllgrabe J, Ashkenazi A, Frake RA, Lombarte AC, Bento CF, Franze K, Rubinsztein DC (2018) Contact inhibition controls cell survival and proliferation via YAP/TAZ-autophagy axis. J Nat Commun 9:1–18
Pearse A-M, Swift K, Hodson P, Hua B, McCallum H, Pyecroft S, Taylor R, Eldridge MD, Belov K (2012) Evolution in a transmissible cancer: a study of the chromosomal changes in devil facial tumor (DFT) as it spreads through the wild Tasmanian devil population. J Cancer Genet 205:101–112
Perret C, Gidoin C, Ujvari B, Thomas F, Roche B (2020) Predation shapes the impact of cancer on population dynamics and the evolution of cancer resistance. J Evol Appl 13:1733–1744
Pesavento PA, Agnew D, Keel MK, Woolard KD (2018) Cancer in wildlife: patterns of emergence. J Nat Rev Cancer 18:646–661
Peto R, Roe F, Lee P, Levy L, Clack J (1975) Cancer and ageing in mice and men. J Br J Cancer 32:411–426
Petruseva I, Evdokimov A, Lavrik O (2017) Genome stability maintenance in naked mole-rat. J Acta Nat 9:31
Pompella A, Corti A (2015) The changing faces of glutathione, a cellular protagonist. J Front Pharmacol 6:98
Pompella A, Visvikis A, Paolicchi A, De Tata V, Casini AF (2003) The changing faces of glutathione, a cellular protagonist. J Biochem Pharmacol 66:1499–1503
Pye RJ, Pemberton D, Tovar C, Tubio JM, Dun KA, Fox S, Darby J, Hayes D, Knowles GW, Kreiss A, Siddle HVT, Swift K, Lyons AB, Murchison EP, Woods GM (2016) A second transmissible cancer in Tasmanian devils. J Proc Natl Acad Sci USA 113:374–379
Raza MH, Siraj S, Arshad A, Waheed U, Aldakheel F, Alduraywish S, Arshad M (2017) ROS-modulated therapeutic approaches in cancer treatment. J Cancer Res Clin Oncol 143:1789–1809
Roake CM, Artandi SE (2016) DNA-repair: Telomere-lengthening mechanism revealed. J Nature 539:35–36
Robey RW, Pluchino KM, Hall MD, Fojo AT, Bates SE, Gottesman MM (2018) Revisiting the role of ABC transporters in multidrug-resistant cancer. J Nat Rev Cancer 18:452–464
Roff D (1993) Evolution of life histories: theory and analysis. Springer Science & Business Media, Germany
Roff DA (2002) Life history evolution. Sinauer Associates Sunderland, MA
Rothschild BM, Tanke D, Helbling M, Martin L (2003) Epidemiologic study of tumors in dinosaurs. J Naturwissenschaften 90:495–500
Scharrer B, Lochhead MS (1950) Tumors in the invertebrates: a review. J Cancer Res 10:403–419
Schito L, Rey S (2017) Hypoxic pathobiology of breast cancer metastasis. Biochim Biophys Acta Rev Cancer 1868:239–245
Seim I, Fang X, Xiong Z, Lobanov AV, Huang Z, Ma S, Feng Y, Turanov AA, Zhu Y, Lenz TL, Gerashchenk MV, Fan D, Yim SH, Yao X, Jordan D, Xiong Y, Ma Y, Lyapunov AN, Chen G, Kulakova OI, Sun Y, Lee S-G, Bronson RT, Moskalev AA, Sunyaev SR, Zhang G, Krogh A, Wang J, Gladyshev VN (2013) Genome analysis reveals insights into physiology and longevity of the Brandt’s bat Myotis brandtii. J Nat Commun 4:1–8
Seim I, Ma S, Zhou X, Gerashchenko MV, Lee S-G, Suydam R, George JC, Bickham JW, Gladyshev VN (2014) The transcriptome of the bowhead whale Balaena mysticetus reveals adaptations of the longest-lived mammal. Aging (albany NY) 6:879
Seluanov A, Gladyshev VN, Vijg J, Gorbunova V (2018) Mechanisms of cancer resistance in long-lived mammals. Nat Rev Cancer 18:433–441
Seluanov A, Hine C, Azpurua J, Feigenson M, Bozzella M, Mao Z, Catania KC, Gorbunova V (2009) Hypersensitivity to contact inhibition provides a clue to cancer resistance of naked mole-rat. Proc Natl Acad Sci USA 106:19352–19357
Sharpless NE, DePinho RA (2005) INK4a/ARF: a multifunctional tumor suppressor locus. J Mutat Res 576:22–38
Siegal-Willott J, Heard D, Sliess N, Naydan D, Roberts J, Medicine W (2007) Microchip-associated leiomyosarcoma in an Egyptian fruit bat (Rousettus aegyptiacus). J Zoo Wildl Med 38:352–356
Skazina M, Odintsova N, Maiorova M, Ivanova A, Väinölä R, Strelkov P (2021) First description of a widespread Mytilus trossulus-derived bivalve transmissible cancer lineage in M. trossulus itself. J Sci Rep 11:1–13
Smith EF, Townsend CO (1907) A plant-tumor of bacterial origin. J Science 25:671–673
Stearns SC (1989) Trade-offs in life-history evolution. J Funct Ecol 3:259–268
Sulak M, Fong L, Mika K, Chigurupati S, Yon L, Mongan NP, Emes RD, Lynch VJ (2016) TP53 copy number expansion is associated with the evolution of increased body size and an enhanced DNA damage response in elephants. J Elife 5:e11994
Summers K, Crespi BJ (2010) Xmrks the spot: life history tradeoffs, sexual selection and the evolutionary ecology of oncogenesis. Mol Ecol 19:3022–3024
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. J CA Cancer J Clin 71:209–249
Suvà ML, Riggi N, Bernstein BE (2013) Epigenetic reprogramming in cancer. J Science 339:1567–1570
Tanno M, Ogihara M, Taguchi T (1996) Age-related changes in proliferating cell nuclear antigen levels. J Mech Ageing Dev 92:53–66
Tascedda F, Ottaviani E (2014) Tumors in invertebrates. J Invertebr Surviv J 11:197–203
Teutschlaender O (1920) Beiträge zur vergleichenden Onkologie mit Berücksichtigung der Identitätsfrage. Z Krebs-Forsch 17:285–407
Thomas F, Giraudeau M, Dheilly NM, Gouzerh F, Boutry J, Beckmann C, Biro PA, Hamede R, Abadie J, Labrut S, Bieuville M, Misse D, Bramwell G, Schultz A, Le Loc’h G, Vincze O, Roche B, Renaud F, Russell T, Ujvari B (2020) Rare and unique adaptations to cancer in domesticated species: an untapped resource? J Evol Appl 13:1605–1614
Thomas F, Giraudeau M, Renaud F, Ujvari B, Roche B, Pujol P, Raymond M, Lemaitre J-F, Alvergne A (2019) Can postfertile life stages evolve as an anticancer mechanism? J PLoS Biol 17:e3000565
Thomas MA, Weston B, Joseph M, Wu W, Nekrutenko A, Tonellato PJ (2003) Evolutionary dynamics of oncogenes and tumor suppressor genes: higher intensities of purifying selection than other genes. J Mol Biol Evol 20:964–968
Tian X, Azpurua J, Hine C, Vaidya A, Myakishev-Rempel M, Ablaeva J, Mao Z, Nevo E, Gorbunova V, Seluanov A (2013) High-molecular-mass hyaluronan mediates the cancer resistance of the naked mole rat. J Nature 499:346–349
Todd R, Wong DT (1999) Oncogenes. J Anticancer Res. 19(6A):4729–4746
Tollis M, Ferris E, Campbell MS, Harris VK, Rupp SM, Harrison TM, Kiso WK, Schmitt DL, Garner MM, Aktipis CA, Maley CC, Boddy AM, Yandell M, Gregg C, Schiffman JD, Abegglen LM (2021) Elephant genomes reveal accelerated evolution in mechanisms underlying disease defenses. J Mol Biol Evol 38:3606–3620
Tollis M, Robbins J, Webb AE, Kuderna LF, Caulin AF, Garcia JD, Bèrubè M, Pourmand N, Marques-Bonet T, O’Connell MJ, Maley CC (2019) Return to the sea, get huge, beat cancer: an analysis of cetacean genomes including an assembly for the humpback whale (Megaptera novaeangliae). J Mol Biol Evol 36:1746–1763
Tollis M, Schiffman JD, Boddy AM (2017) Evolution of cancer suppression as revealed by mammalian comparative genomics. J Curr Opin Genet Dev 42:40–47
Toole BP (2004) Hyaluronan: from extracellular glue to pericellular cue. J Nat Rev Cancer 4:528–539
Ueda K, Cardarelli C, Gottesman MM, Pastan I (1987) Expression of a full-length cDNA for the human" MDR1" gene confers resistance to colchicine, doxorubicin, and vinblastine. J Proc Natl Acad Sci USA 84:3004–3008
Ujvari B, Gatenby RA, Thomas F (2017) Transmissible cancer: the evolution of interindividual metastasis Ecology and evolution of cancer. Elsevier, Netherlands, pp 167–179
Ujvari B, Raven N, Madsen T, Klaassen M, Dujon AM, Schultz AG, Nunney L, Lemaître JF, Giraudeau M, Thomas F (2022) Telomeres, the loop tying cancer to organismal life-histories. J Mol Ecol 31:6273–6285
Ullrich CI, Aloni R (2000) Vascularization is a general requirement for growth of plant and animal tumours. J Exp Bot 51:1951–1960
Ullrich CI, Aloni R, Saeed ME, Ullrich W, Efferth T (2019) Comparison between tumors in plants and human beings: Mechanisms of tumor development and therapy with secondary plant metabolites. J Phytomedicine 64:153081
Vail DM, Macewen EG (2000) Spontaneously occurring tumors of companion animals as models for human cancer. J Cancer Invest 18:781–792
Van Roten A, Barakat AZA-Z, Wouters A, Tran TA, Mouton S, Noben J-P, Gentile L, Smeets K (2018) A carcinogenic trigger to study the function of tumor suppressor genes in Schmidtea mediterranea. J Dis Model Mech 11:dmm032573
Vasseur E, Quintana-Murci L (2013) The impact of natural selection on health and disease: uses of the population genetics approach in humans. J Evol Appl 6:596–607
Vaupel P, Harrison L (2004) Tumor hypoxia: causative factors, compensatory mechanisms, and cellular response. J Oncologist 9:4–9
Vazquez JM, Sulak M, Chigurupati S, Lynch VJ (2018) A zombie LIF gene in elephants is upregulated by TP53 to induce apoptosis in response to DNA damage. J Cell Rep 24:1765–1776
Vincze O, Colchero F, Lemaître J-F, Conde DA, Pavard S, Bieuville M, Urrutia AO, Ujvari B, Boddy AM, Maley CC, Thomas F, Giraudeau M (2022) Cancer risk across mammals. J Nature 601:263–267
Vittecoq M, Roche B, Daoust SP, Ducasse H, Missé D, Abadie J, Labrut S, Renaud F, Gauthier-Clerc M, Thomas F (2013) Cancer: a missing link in ecosystem functioning? J Trends Ecol Evol 28:628–635
Vlodavsky I, Friedmann Y (2001) Molecular properties and involvement of heparanase in cancer metastasis and angiogenesis. J Clin Invest 108:341–347
Voskarides K (2018) Combination of 247 genome-wide association studies reveals high cancer risk as a result of evolutionary adaptation. Mol Biol Evol 35:473–485
Voskarides K, Koutsofti C, Pozova M (2022) TP53 Mutant versus wild-type zebrafish larvae under starvation stress: larvae can live up to 17 days post-fertilization without food. J Zebrafish 19:49–55
Voss JD, Richardson LL (2006) Nutrient enrichment enhances black band disease progression in corals. J Coral Reefs 25:569–576
Walker C, Bottger SA, Mulkern J, Jerszyk E, Litvaitis M, Lesser M (2009) Mass culture and characterization of tumor cells from a naturally occurring invertebrate cancer model: applications for human and animal disease and environmental health. J Biol Bull 216:23–39
Wallace DC (1967) The inevitability of growing old. J Chronic Dis 20:475–486
Wang G, Zhou H, Strulovici-Barel Y, Al-Hijji M, Ou X, Salit J, Walters MS, Staudt MR, Kaner RJ, Crystal RG (2017) Role of OSGIN1 in mediating smoking-induced autophagy in the human airway epithelium. J Autophagy 13:1205–1220
Wang X, Allen R, Ding X, Goellner M, Maier T, de Boer JM, Baum TJ, Hussey RS, Davis EL (2001) Signal peptide-selection of cDNA cloned directly from the esophageal gland cells of the soybean cyst nematode Heterodera glycines. J Mol Plant Microbe Interact 14:536–544
Wang Y, Zhang C, Wang N, Li Z, Heller R, Liu R, Zhao Y, Han J, Pan X, Zheng Z, Dai X, Chen C, Mingle D, Peng S, Chen X, Liu J, Li M, Wang K, Liu C, Lin Z, Chen L, Hao F, Zhu W, Song C, Zhao C, Zheng C, Wang J, Hu S, Li C, Yang H, Jiang L, Li G, Liu M, Sonstegard TS, Zhang G, Jiang Y, Wang W, Qiu Q (2019) Genetic basis of ruminant headgear and rapid antler regeneration. J Science 364:eaav6335
White PR (1944) Transplantation of plant tumors of genetic origin. J Cancer Res 4:791–794
Whitney MR, Mose L, Sidor CA (2017) Odontoma in a 255-million-year-old mammalian forebear. J JAMA Oncol 3:998–1000
Wilkins AS, Wrangham RW, Fitch WT (2014) The “domestication syndrome” in mammals: a unified explanation based on neural crest cell behavior and genetics. J Genetics 197:795–808
Williams GC (1966) Natural selection, the costs of reproduction, and a refinement of Lack’s principle. J Am Nat 100:687–690
Williams GC (2001) Pleiotropy, Natural selection, and the evolution of senescence. J Sci Aging Knowl Environ 2001:398–411
Williams GJ, Aeby GS, Cowie RO, Davy SK (2010) Predictive modeling of coral disease distribution within a reef system. J PLoS One 5:e9264
Wolff M, Kosyna FK, Dunst J, Jelkmann W, Depping R (2017) Impact of hypoxia inducible factors on estrogen receptor expression in breast cancer cells. J Arch Biochem Biophys 613:23–30
Yim H-S, Cho YS, Guang X, Kang SG, Jeong J-Y, Cha S-S, Oh H-M, Lee J-H, Yang EC, Kwon KK, Kim YJ, Kim TW, Kim W, Jeon JH, Kim S-J, Choi DH, Jho S, Kim H-M, Ko J, Kim H, Shin Y-A, Jung H-J, Zheng Y, Wang Z, Chen Y, Chen M, Jiang A, Li E, Zhang S, Hou H, Kim TH, Yu L, Liu S, Ahn K, Cooper J, Park S-G, Hong CP, Jin W, Kim H-S, Park C, Lee K, Chun S, Morin PA, O’Brien SJ, Lee H, Kimura J, Moon DY, Manica A, Edwards J, Kim BC, Kim S, Wang J, Bhak J, Lee HS, Lee J-H (2014) Minke whale genome and aquatic adaptation in cetaceans. J Nat Genet 46:88–92
Yonemitsu MA, Giersch RM, Polo-Prieto M, Hammel M, Simon A, Cremonte F, Avilés FT, Merino-Véliz N, Burioli EA, Muttray AF, Sherry J, Reinisch C, Baldwin SA, Goff SP, Houssin M, Arriagada G, Vázquez N, Bierne N, Metzger MJ (2019) A single clonal lineage of transmissible cancer identified in two marine mussel species in South America and Europe. J Elife 8:e47788
Zachara NE, O’Donnell N, Cheung WD, Mercer JJ, Marth JD, Hart GW (2004) Dynamic O-GlcNAc modification of nucleocytoplasmic proteins in response to stress: a survival response of mammalian cells. J J Biol Chem 279:30133–30142
Zhang G, Cowled C, Shi Z, Huang Z, Bishop-Lilly KA, Fang X, Wynne JW, Xiong Z, Baker ML, Zhao W, Tachedjian M, Zhu Y, Zhou P, Jiang X, Ng J, Yang L, Wu L, Xiao J, Feng Y, Chen Y, Sun X, Zhang Y, Marsh GA, Crameri G, Broder CC, Frey KG, Wang L-F, Wang J (2013) Comparative analysis of bat genomes provides insight into the evolution of flight and immunity. J Science 339:456–460
Zhao Y, Oreskovic E, Zhang Q, Lu Q, Gilman A, Lin YS, He J, Zheng Z, Lu JY, Lee JJNI (2021) Transposon-triggered innate immune response confers cancer resistance to the blind mole rat. Nat Immunol 22:1219–1230
Acknowledgements
The authors acknowledge Nirma University for fellowship to Devangkumar Trivedi.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Handling editor: Konstantinos Voskarides.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Trivedi, D.D., Dalai, S.K. & Bakshi, S.R. The Mystery of Cancer Resistance: A Revelation Within Nature. J Mol Evol 91, 133–155 (2023). https://doi.org/10.1007/s00239-023-10092-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00239-023-10092-6