Abstract
Purpose
Intraoperative MRI (ioMRI) is a valuable tool aiding paediatric brain tumour resection. There is no published evidence comparing the effectiveness of the final intraoperative MRI and early post-operative (24–72 h) MRI as baseline scans following brain tumour resection. We aimed to evaluate whether the final ioMRI scan could serve as the post-operative baseline scan after paediatric brain tumour resections.
Methods
This prospective study compared the final ioMRI scan with the immediate post-operative MRI scan performed 24–72 h post-surgery. We included 20 patients aged 6.6–21 years undergoing brain tumour resection using ioMRI and were suitable for MRI scan without general anaesthesia. The scans were independently evaluated by experienced local and external paediatric neuroradiologists. Identical sequences in the final ioMRI and the 24–72-h MRI were compared to assess the extent of resection, imaging characteristics of residual tumour, the surgical field, extent of surgically induced contrast enhancement, and diffusion abnormalities.
Results
In 20 patients undergoing intraoperative and early post-operative MRI, there was no difference between ioMRI and 24–72-h post-op scans in identifying residual tumour. Surgically induced contrast enhancement was similar in both groups. There were more abnormalities on diffusion imaging and a greater degree of oedema around the surgical cavity on the 24–72-h scan.
Conclusion
The final 3-T ioMRI scan may be used as a baseline post-operative scan provided standard imaging guidelines are followed and is evaluated jointly by the operating neurosurgeon and neuroradiologist. Advantages of final ioMRI as a baseline scan are identified.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Intraoperative MRI (ioMRI) has been gradually gaining recognition as a useful aid to surgical resection of brain tumours. Over the last two decades, advances in the technology of MRI scanners used in intraoperative assessment have evolved from low-field strength scanners (0.15 T) to high-field strength scanners (3 T), complemented by newer and optimised sequences available for neuroimaging. As a consequence, the quality of imaging obtained on ioMRI is comparable to standard imaging performed in a non-ioMR setting. The number of intraoperative scans per surgery depends on the complexity and aims of surgery. A final ioMRI is considered to be one after which no further resective surgery is performed. In Europe, 3-T MRI is increasingly undertaken as the preferred standard for paediatric neuro-oncology, particularly with the current generation of 3-T scanners. With the improvement in image quality, the final ioMRI is increasingly considered as a suitable baseline study following surgery for determining the radiological extent of resection. This is particularly popular in the paediatric setting as it negates the need for an additional post-operative scan, which, in a significant number of cases, requires repeat general anaesthesia or sedation. Early post-operative MRI is generally obtained between 24 to 72 h after surgery as studies demonstrated surgically induced contrast enhancement after 72 h on 1.5-T scans [1, 2] and sometimes within 24 h [3]. More recent studies on 3-T MRI scans have shown that surgically induced enhancement can occur before 72 h and may occur at all time points following surgery [4, 5]. In our institution, we have used the final ioMRI as the baseline post-operative scan, and we have seldom encountered issues related to suboptimal post-operative baseline scans. Considerable resistance to the use of ioMRI as the baseline scan has been expressed in the past, predominantly related to the use of lower field strength scanners and imaging techniques employed historically, resulting in diagnostically suboptimal quality of studies obtained [6]. However, there is no published evidence comparing the utility of final ioMRI scans and early post-operative MRI scans (24–72 h) as baseline scans following brain tumour resection. With the increasing use of ioMRI in paediatric neurosurgery, there is a need to validate this practice, particularly as new imaging guidelines are being developed for RAPNO (Radiological Assessment in Pediatric Neuro-oncology) as the standard [6,7,8,9]. As far as we are aware, no studies have been reported that have compared final ioMRI with that undertaken in the conventional 24–72 h. This study aims to evaluate the utility of the final ioMRI scan as the standard post-operative baseline scan following paediatric brain tumour resections.
Methods
This prospective study compared the final ioMRI scan with standard immediate post-operative MRI scan performed between 24 and 72 h post-surgery. As the study involved comparison with standard clinical methodology, and patients did not require additional general anaesthesia, it did not require consideration by the regional ethics committee and was registered as an institutional service evaluation study.
Patients undergoing brain tumour resection using ioMRI and suitable for MRI without general anaesthesia 24 to 72 h following surgery were included. Since the commencement of ioMRI in our institution, the final intraoperative MRI was regarded as the baseline post-operative MRI scan following surgery. For this study, the patients who could tolerate an MRI scan without general anaesthesia during regular working hours (between Monday and Friday) underwent a second MRI scan between 24 and 72 h, as per national imaging guidelines [10]. In 5 cases, it was possible to perform the second MRI scan over the weekend, and they were also included in the study. The patients included were required to have undergone the pre-operative, intraoperative, and post-operative imaging on the same 3-T MRI scanner (Philips Achieva® 3-T scanner, Philips Healthcare, Best, Netherlands), located alongside the neurosurgical theatre. The study period was between October 2012 and January 2016. From October 2015, the scans were performed on a Philips Ingenia® 3-T scanner (Philips Healthcare, Best, Netherlands).
The MRI scan protocol was based on the UK Children’s Cancer and Leukaemia Group (CCLG) imaging guidelines [10]. The sequences used for intraoperative assessment were T2-weighted TSE in three planes, T1-weighted 1-mm isotropic 3-D gradient-echo (T1-TFE) pre and post-contrast medium administration, T1-weighted spin-echo (T1-SE) post-contrast in the axial plane, and coronal T2-weighted FLAIR sequence. Diffusion imaging was performed as DWI in the axial plane (b values 0 and 1000 mm2/s) or DTI in the axial plane (b values 0 and 800 mm2/s, 16 or 32 directions). On the Philips Ingenia® 3-T scanner, diffusion imaging was performed as a DWI-TSE sequence in the axial plane (b values 0 and 1000 mm2/s) in cases where significant air was noted in the intracranial space during intraoperative imaging to reduce the degree of susceptibility artefact. The sequences and amount of contrast used on the pre-operative MRI, ioMRI, and 24–72-h scans were identical. Subtle differences in image quality between the ioMRI and non-ioMRI scans were due to differences in coils used and did not affect the scans’ diagnostic quality. A NORAS head holder (NORAS MRI products, Hoechberg, Germany) with an 8 channel coil was used for ioMRI, and for the non-ioMRI scan, a 16 channel head coil was used. If patients had external scans, additional sequences to facilitate ioMRI comparison were performed on our 3-T MR scanner. All newly diagnosed brain tumour patients underwent spine imaging pre-operatively to exclude spinal dissemination.
At our institution, intraoperative MR imaging was introduced in 2009, and almost all brain tumour resections have been performed with the aid of ioMRI. The scans are evaluated during the surgical procedure by the local neuroradiologist in consensus with the neurosurgeon performing the surgery. When the surgical aim (complete/partial resection) is achieved, the final ioMRI scan (employing the full tumour imaging protocol) has been used as the immediate post-operative scan. For the study, the pre-operative, final intraoperative MRI and 24–72-h MRI scans were anonymised and independently evaluated by 2 experienced neuroradiologists. Both neuroradiologists were aware of the histopathology and the aim of the surgery (complete or partial resection). The local neuroradiologist (SA) had 7-year experience in paediatric neuroimaging with 4-year experience in intraoperative MR imaging at the start of the study. The second neuroradiologist (TJ) based at a different tertiary level neurosurgical centre had more than 20 years of paediatric neuroimaging experience. Both readers were familiar with the type of scanner, field strength, and sequences used. Qualitative analysis of the final intraoperative MRI and 24–72-h post-operative MRI was performed, and the scans were compared for the following variables: (1) evidence of gross total resection (GTR) or presence of residual tumour on the individual sequences (unequivocal versus equivocal), (2) evidence of surgically induced contrast enhancement (meningeal enhancement unrelated to adjacent tumour resection was excluded), and (3) evidence of diffusion abnormality (not related to the tumour, haemorrhage, or air). The final ioMRI and 24–72-h MRI were compared for the degree of surgically induced contrast enhancement, T2/T2 FLAIR signal change, and the presence or absence of diffusion restriction. This was qualitatively assessed based on the subjective comparison of the extent of abnormalities, as these abnormalities were widespread, diffuse, and unmeasurable by conventional radiological methods. Additional relevant imaging findings in each case were also recorded. This included the presence of artefacts and the overall quality of the scans. For this study, GTR was defined by the absence of any residual tissue which resembled the tumour seen on the pre-operative MRI based on its position, signal, and enhancement characteristics. Independent evaluations by both neuroradiologists were compared, and the final observation for each variable was based on consensus between the two observers. Cohen’s Kappa statistic was used to assess the inter-rater agreement using IBM SPSS Statistics software (version 25).
Results
Twenty patients underwent intraoperative and early post-operative MRI during the study period. The pre-operative planned surgical aim was for complete resection in 11 patients and partial resection in 9 cases. The age of the patients ranged from 6.6 years to 21 years (mean 13.1 years). Of the 20 children, 13 were boys. The 24–72-h scans were performed between 24 and 36 h in 14 cases, between 36 and 48 h in 5 cases (40, 46,46, 48, 50 h), and between 48 and 72 h in one case (66 h post-surgery). In 12 cases, the tumours were located in the supratentorial compartment and 8 in the infratentorial compartment. The histological diagnoses were medulloblastoma in 4, pilocytic astrocytoma in 5 (3 posterior fossae, 1 optic pathway, and 1 thalamic), fibrillary astrocytoma in 2, ganglioglioma in 2, craniopharyngioma in 2, and one each of high-grade glioma, pleomorphic xanthoastrocytoma, pilomyxoid astrocytoma, subependymal giant cell astrocytoma, and pituitary adenoma.
Unequivocal evidence of GTR was noted in 11 cases on both the ioMRI and the 24–72-h scans, with 100% independent agreement between both readers. The surgical aim in all the 11 cases was complete tumour resection.
In 9 cases, where the surgical aim was partial tumour resection, unequivocal evidence of residual tumour was identified on both the final ioMRI and 24–72-h scans in all cases. There was 100% independent agreement between both readers for the presence of residual tumour on both scans based on the combined interpretation of the sequences in each scan. In 6 of the cases, residual tumour was seen on all sequences (T1W, T2W, T2 FLAIR, DWI, and T1 W post-contrast). In 1 case, the residual tumour could not be seen on DWI, and in 2 cases, it could not be seen on DWI and T2 FLAIR. The inter-rater agreement between both observers for individual sequences is provided in Table 1.
Six of the 20 cases showed no evidence of surgically induced contrast enhancement on both the ioMRI and the 24–72-h scans. Ten of the 20 cases revealed an equal degree of surgically induced enhancement (Fig. 1). In 3 cases, the ioMRI showed a greater degree of enhancement, and in 1 case, the 24–72-h scan showed a greater degree of enhancement. Independent evaluation for surgically induced contrast enhancement yielded an agreement in 15 of the 20 cases (75%); an agreement was achieved following consensus in the remaining 5 cases.
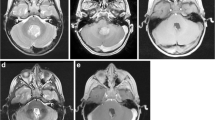
Axial post-contrast T1 volumetric images following resection of a right cerebellar pilocytic astrocytoma show equal surgically induced contrast enhancement (circled area) on the ioMRI (a) and scan performed following 24 h (b). Post-contrast images following resection of a pilomyxoid astrocytoma in a different case show increased enhancement related to the choroid plexus (white arrow) on the ioMRI scan (c) when compared to the scan performed following 24 h (d)
Evaluation of diffusion abnormalities around the surgical cavity revealed a greater extent of diffusion restriction on the 24–72-h scan in 11 cases (Fig. 5) and a greater extent of diffusion restriction in ioMRI scan in 2 cases. In 4 cases, the extent of diffusion restriction was equal. The DWI scan was suboptimal for assessment in 2 ioMRI cases due to susceptibility artefact from intracranial air on ioMRI, and in 1 case, a DWI sequence was not performed on ioMRI. Independent agreement on the diffusion abnormalities was achieved in 13 of the 17 cases (72%), and an agreement was achieved following consensus on the remaining 5 cases.
An additional observation made by the reviewers was that in 8 of the 20 cases, there was increased T2 signal abnormality on the T2 and T2 FLAIR sequences around the surgical cavity on the 24–72-h scans. This was thought to represent evolving oedema and/or ischemia (when associated with diffusion restriction). As a general observation by both readers, the T1 hyperintensity of blood was more prominent on the 24–72-h scans. There were smaller foci of marked hyperintensity on both the ioMRI and 24–72-h scans, which were thought to represent areas of electrocoagulation.
Discussion
The utility and safety of ioMRI-assisted neurosurgery for paediatric brain tumours have been well-established [11,12,13,14]. Interpretation of intraoperative MRI scans can pose challenges, but the imaging appearances and pitfalls have been well documented in the published literature [15,16,17,18]. The main advantages of ioMRI in brain tumour surgery are facilitating safe intraoperative extension of surgical resection and neuro-navigation update to compensate for brain shift during surgery. Among other advantages is that the final intraoperative scan could serve as the definitive immediate post-operative baseline scan (which currently is usually performed between 24 and 72 h following surgery, requiring general anaesthesia/sedation in a number of these children). An “awake” post-operative scan can sometimes be delayed due to the patient’s inability to cooperate during MRI scanning or due to post-surgical morbidity. In some centres, if the operating days are during the latter part of the week, the 24–72-h scan may not be logistically possible during the weekends. The ability to use the final intraoperative MRI scan as the immediate post-operative scan can serve as an additional incentive to invest in an intraoperative MRI scanner both from the perspective of quality of care and cost-benefit of avoiding additional scans/general anaesthesia.
At our tertiary level paediatric neurosurgical centre, the final intraoperative MRI has served as the baseline post-operative scan, provided the following guidelines are maintained: (a) the standard post-operative brain tumour protocol (based on the UK CCLG brain tumour imaging guidelines) is followed for the final intraoperative MRI scan to justify its use as a baseline scan, (b) there is full awareness of the imaging appearances and potential pitfalls related to ioMRI, and (c) the interpretation of the intraoperative imaging is performed in “real time” by the neuroradiologist in conjunction with the operating neurosurgeon. Employing these principles, in our experience, the final ioMRI has been as effective as a post-operative baseline MRI.
The rationale for performing post-operative MRI scan before 72 h post-surgery is due to the evidence of abnormal enhancement related to post-surgical changes that increase after 72 h [1, 2]. Surgically induced contrast enhancement (SICE) has, however, also been recognised in intraoperative MRI scans. Knauth and colleagues described four types of SICE on ioMRI scans: (1) meningeal enhancement, (2) increased enhancement of the choroid plexus, (3) enhancement at the resection margins, and (4) intra-parenchymal enhancement immediately deep to the surgical margin [15]. The latter 2 forms of SICE can pose challenges during intraoperative MR image interpretation as they can resemble residual enhancing tumour tissue. SICE can also create challenges in the assessment of standard post-operative imaging performed between 24 and 72 h [4, 5]. In our study, surgically induced contrast enhancement was not seen in 6 cases on both ioMRI and 24–72-h scans, and the degree of enhancement was equal in 10 cases. Among 3 cases, SICE was greater on the ioMRI scans. In one case, there was an increased enhancement of the choroid plexus (Fig. 1c and d). This is a well-recognised phenomenon described by Knauth et al., and careful assessment of the enhancement and its continuity with the choroid plexus either within the lateral ventricle or the fourth ventricle can help differentiate this from pathological enhancement. The second case demonstrated increased enhancement immediately deep to the surgical margin (Fig. 2). This has also been described previously [15]. In our experience, we have encountered this form of enhancement deep to an area of haemorrhage seen as T1 hyperintensity on the non-contrast scan. Careful comparison to the pre-operative imaging will help in differentiating pathological enhancement within residual tumour tissue from SICE. In the third case, there was minimal linear enhancement along the surgical margin (maximum depth 3mm) on the ioMRI that was not seen on the 24–72-h scan.
Axial images following resection of a right thalamic low-grade glioma. The pre-contrast ioMRI T1 image demonstrates haemorrhage lining the surgical cavity, best seen on the magnified image (a). The post-contrast sequence on ioMRI (b) demonstrates curvilinear enhancement deep to the linear area of haemorrhage lining the surgical cavity (white arrow). The enhancement is absent on the scan performed 24 h later (c). There is evidence of diffusion abnormality (dotted arrows) on the b1000 (e) and ADC (f) images on the 24-h scan but less prominent on the b1000 image of the ioMRI (d)
In one case following partial resection of a high-grade glioma, post-surgical enhancement was seen only on the 24–72-h scan (Fig. 3). As a general rule, SICE appears as an area of thin linear enhancement along/deep to the surgical margin. In our 10-year experience, it has not measured more than 3 mm in depth. Foci of enhancement that are nodular, measuring greater than 3 mm in depth, or appear similar to that seen on the pre-operative imaging should raise the suspicion of residual tumour tissue. In our practice, the neurosurgeon re-explores these areas of abnormal enhancement with the updated neuro-navigation using the new volume dataset. If there is residual resectable tumour visible on re-exploration and further resection is performed, a final ioMRI is obtained. If there is further resection on re-exploration, histological assessment of the specimen is performed. In this study cohort, none of the patients required re-exploration following the final ioMRI.
In our study, the diffusion abnormalities were greater on the 24–72-h MRI scans in 11 of the 16 cases where the DWI could be assessed. The diffusion restriction abnormalities were noted mainly along the surgical cavity wall and along the surgical tract and thought to represent tissue injury (Figs. 2 and 5). This is a well-recognised phenomenon. Smith et al. noted reduced diffusion in 28 of 44 adults (64%) who underwent resective surgery for brain tumours [19]. Complete resolution was noted by 90 days in 86% of the cases, and the diffusion abnormality was replaced by contrast enhancement as early as day 15, appearing as encephalomalacia on long-term follow-up. Ozturk et al. evaluated post-operative (<24 h) scans of patients following surgery for brain tumours and epilepsy [20]. 32.7% of the patients who had tumour surgery and 15.4 % of patients with epilepsy surgery demonstrated restricted diffusion along the resection cavity. The difference in diffusion abnormality between the two scans in our study can be explained by the time required for the diffusion abnormalities related to cytotoxic oedema to be established. In our study, the late scans did not show any area of large vascular territory infarction. Although ioMRI identified diffusion abnormality related to tissue injury to a lesser degree than 24–72-h scan, we believe that this does not significantly change patient management (and may confer an interpretive advantage). In our practice, we have occasionally encountered children who have presented with vascular territory ischaemia related to vasospasm. These children have presented with altered neurology necessitating repeat imaging.
In two cases, the intraoperative DWI was suboptimal for assessment due to susceptibility artefact related to air (Fig. 4 a and b). This was a major limitation during our early intraoperative practice. This was reduced in most cases by irrigation of the surgical cavity with saline/irrigation fluid prior to the scan, use of titanium pins for the head holder, and placement of the pins away from the region of interest [17]. Other measures are to avoid surgical materials (such as haemostatic packing) placed within the resection cavity and positioning the patient’s head in the scanner’s isocentre [18]. During the latter part of the study, after a scanner software upgrade, we were able to perform a turbo spin-echo diffusion-weighted sequence (DWI-TSE). This has significantly reduced susceptibility artefact related to air (Fig. 4c and d) but does require an additional 3 min of scan time.
Axial T2 W image on ioMRI (a) demonstrating foci of air (white arrows) in the surgical field, causing significant susceptibility artefact (dashed arrows) on echo-planar imaging DWI sequence (b). Imaging using a turbo spin-echo DWI sequence (d) shows no evidence of susceptibility artefact despite the presence of air (white arrows) in the ventricles and the extra-axial spaces on the T2 W image (c)
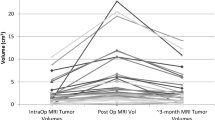
In 8 of the 20 cases, there was evidence of increased T2/T2 FLAIR hyperintensity within the brain parenchyma adjacent to the surgical cavity on the 24–72-h scan. In some cases, these changes were accompanied by foci of diffusion restriction. The corresponding ioMRI scans showed no evidence of signal abnormality, clearly indicating that the changes were related to evolving tissue injury or oedema (Fig. 5). This, in particular, could be an advantage of ioMRI during resection of non-enhancing tumours as the signal abnormalities noted on the 24–72-h scan could be spuriously interpreted as possible residual tumour tissue, often requiring unnecessary early repeat imaging or, in some instances, surgical re-exploration depending on the clinical scenario. Measurement of non-enhancing tumour post-resection has been a well-known challenge in the adult brain tumour population. Pala et al. reviewed the T2 and T2 FLAIR images on ioMRI, early post-operative MRI (<48 h), and late post-operative MRI (3–4 months) of 33 adults following surgery for low-grade gliomas [21]. They found a significant difference of T2 FLAIR/T2 abnormality between the early (<48 h) and late post-operative residual tumour volumes. They concluded that the ioMRI or ultra-early post-operative MRI (directly after surgery) reflects the residual tumour’s actual volume, as there were no significant differences in tumour volumes on T2 and T2 FLAIR.
IoMRI (a, b, c) and 24-h post-operative scans (d, e, f) following resection of a fourth ventricular medulloblastoma. The axial T2 W (d), coronal T2 FLAIR (e) images on the 24-h scan demonstrate abnormal hyperintensity along the right surgical margin (dotted arrows) suggestive of oedema. The corresponding T2 (a) and T2 FLAIR (b) images show no abnormality (white arrow) along the surgical margin on the ioMRI. The diffusion-weighted b1000 image from the 24-h scan (f) shows diffusion abnormality (open arrow) that was not seen on the ioMRI (c). The 24-h ADC image (not shown) confirmed evidence of diffusion restriction, likely reflecting tissue injury
Foci of T1 hyperintensity related to blood were more prominent on the 24–72-h scans (Fig. 6). This is likely related to the signal characteristics of methaemoglobin at the later stage compared to that of oxy-/deoxyhaemoglobin at the time of the ioMRI. The reduced visibility of blood on T1 on ioMRI is not a significant issue as haemorrhagic areas can be identified on the T2-weighted sequences as foci of T2 hypointensity. Some centres perform an SWI sequence to identify blood. The reduced T1 signal of blood on ioMRI can occasionally have an advantage over 24–72-h scans when there are complex post-surgical changes with a mixture of haemorrhage and enhancement that can pose challenges in teasing out haemorrhage from enhancement (despite comparing with T1 non-contrast images). The radiologists in this study also noted focal areas of marked T1 hyperintensity on the ioMRI which they believe are related to areas of electrocoagulation (Fig. 6).
Axial T1 W images of ioMRI (a) and 24-h (b) scans following resection of a fourth ventricular medulloblastoma. The T1 signal abnormality corresponding to haemorrhage (white arrow) appears more hyperintense on the 24-h scan (b) when compared to the ioMRI scan (a). There is a focus of marked hyperintensity (dashed arrows) on both images that is thought to represent abnormality related to electrocoagulation
An important limitation of the study is the small patient population. There were several contributing factors to this. At our institution, brain tumour surgery with ioMRI is usually performed on a Thursday, and obtaining a post-operative scan during working weekday hours proved a challenge. Fifteen cases had the 24–72-h scans 24 h following surgery, and 5 had the scans over the weekend. Many children were not fit for undergoing a scan on the following day, and it was not possible to obtain scans over the weekend. This is a well-known practical difficulty in a number of neurosurgical centres who have, by default, chosen to use the final ioMRI as the baseline scan. Although this study describes a relatively small number of cases, we believe that there is a good representation of children from both the complete and partial tumour resection groups. To our knowledge, this is the first study evaluating the validity of ioMRI scan as a baseline scan following brain tumour resection, which is of importance with the increasing prevalence of ioMRI and the need for updating national and international imaging standards in this respect. This study only tests the validity of a 3-T ioMRI, and further similar studies, including 1.5-T ioMRI, will help strengthen the evidence in this area.
Conclusion
This prospective study demonstrates the utility of 3-T final ioMRI as a baseline scan following brain tumour resection. The ioMRI and 24–72-h MRI scans equally demonstrated evidence of GTR and residual tumour. There are patterns of surgically induced contrast enhancement that the radiologist should be aware of when reporting ioMRI. IoMRI has an advantage over 24–72-h scans with a lesser amount of T2 and T2 FLAIR-related abnormality that is surgically induced. The final ioMRI scan can serve as a valid post-operative baseline scan provided the full post-operative tumour protocol is followed and is reported by a neuroradiologist in consensus with the operating neurosurgeon during the procedure. With the recent development of response assessment in paediatric neuro-oncology (RAPNO) guidelines, this study may also provide supportive evidence for the inclusion of ioMRI in place of 24–72-h scanning, in the appropriate clinical context, in the surgical management of paediatric brain tumours.
References
Albert FK, Forsting M, Sartor K, Adams HP, Kunze S (1994) Early postoperative magnetic resonance imaging after resection of malignant glioma: objective evaluation of residual tumor and its influence on regrowth and prognosis. Neurosurgery 34(1):45–60; discussion 60-41. https://doi.org/10.1097/00006123-199401000-00008
Forsyth PA, Petrov E, Mahallati H, Cairncross JG, Brasher P, MacRae ME, Hagen NA, Barnes P, Sevick RJ (1997) Prospective study of postoperative magnetic resonance imaging in patients with malignant gliomas. J Clin Oncol 15(5):2076–2081. https://doi.org/10.1200/JCO.1997.15.5.2076
Oser AB, Moran CJ, Kaufman BA, Park TS (1997) Intracranial tumor in children: MR imaging findings within 24 hours of craniotomy. Radiology 205(3):807–812. https://doi.org/10.1148/radiology.205.3.9393539
Lescher S, Schniewindt S, Jurcoane A, Senft C, Hattingen E (2014) Time window for postoperative reactive enhancement after resection of brain tumors: less than 72 hours. Neurosurg Focus 37(6):E3. https://doi.org/10.3171/2014.9.FOCUS14479
Bette S, Gempt J, Huber T, Boeckh-Behrens T, Ringel F, Meyer B, Zimmer C, Kirschke JS (2016) Patterns and time dependence of unspecific enhancement in postoperative magnetic resonance imaging after glioblastoma resection. World Neurosurg 90:440–447. https://doi.org/10.1016/j.wneu.2016.03.031
Warren KE, Vezina G, Poussaint TY, Warmuth-Metz M, Chamberlain MC, Packer RJ, Brandes AA, Reiss M, Goldman S, Fisher MJ, Pollack IF, Prados MD, Wen PY, Chang SM, Dufour C, Zurakowski D, Kortmann RD, Kieran MW (2018) Response assessment in medulloblastoma and leptomeningeal seeding tumors: recommendations from the Response Assessment in Pediatric Neuro-Oncology committee. Neuro-Oncology 20(1):13–23. https://doi.org/10.1093/neuonc/nox087
Cooney TM, Cohen KJ, Guimaraes CV, Dhall G, Leach J, Massimino M, Erbetta A, Chiapparini L, Malbari F, Kramer K, Pollack IF, Baxter P, Laughlin S, Patay Z, Young Poussaint T, Warren KE (2020) Response assessment in diffuse intrinsic pontine glioma: recommendations from the Response Assessment in Pediatric Neuro-Oncology (RAPNO) working group. Lancet Oncol 21(6):e330–e336. https://doi.org/10.1016/S1470-2045(20)30166-2
Fangusaro J, Witt O, Hernaiz Driever P, Bag AK, de Blank P, Kadom N, Kilburn L, Lober RM, Robison NJ, Fisher MJ, Packer RJ, Young Poussaint T, Papusha L, Avula S, Brandes AA, Bouffet E, Bowers D, Artemov A, Chintagumpala M, Zurakowski D, van den Bent M, Bison B, Yeom KW, Taal W, Warren KE (2020) Response assessment in paediatric low-grade glioma: recommendations from the Response Assessment in Pediatric Neuro-Oncology (RAPNO) working group. Lancet Oncol 21(6):e305–e316. https://doi.org/10.1016/S1470-2045(20)30064-4
Erker C, Tamrazi B, Poussaint TY, Mueller S, Mata-Mbemba D, Franceschi E, Brandes AA, Rao A, Haworth KB, Wen PY, Goldman S, Vezina G, MacDonald TJ, Dunkel IJ, Morgan PS, Jaspan T, Prados MD, Warren KE (2020) Response assessment in paediatric high-grade glioma: recommendations from the Response Assessment in Pediatric Neuro-Oncology (RAPNO) working group. Lancet Oncol 21(6):e317–e329. https://doi.org/10.1016/S1470-2045(20)30173-X
Craig E, Connolly DJ, Griffiths PD, Raghavan A, Lee V, Batty R (2012) MRI protocols for imaging paediatric brain tumours. Clin Radiol 67(9):829–832. https://doi.org/10.1016/j.crad.2012.03.018
Tejada S, Avula S, Pettorini B, Henningan D, Abernethy L, Mallucci C (2018) The impact of intraoperative magnetic resonance in routine pediatric neurosurgical practice-a 6-year appraisal. Childs Nerv Syst 34(4):617–626. https://doi.org/10.1007/s00381-018-3751-8
Choudhri AF, Klimo P Jr, Auschwitz TS, Whitehead MT, Boop FA (2014) 3T intraoperative MRI for management of pediatric CNS neoplasms. AJNR Am J Neuroradiol 35(12):2382–2387. https://doi.org/10.3174/ajnr.A4040
Avula S, Pettorini B, Abernethy L, Pizer B, Williams D, Mallucci C (2013) High field strength magnetic resonance imaging in paediatric brain tumour surgery--its role in prevention of early repeat resections. Childs Nerv Syst 29(10):1843–1850. https://doi.org/10.1007/s00381-013-2106-8
Roder C, Breitkopf M, Ms BS, Freitas Rda S, Dimostheni A, Ebinger M, Wolff M, Tatagiba M, Schuhmann MU (2016) Beneficial impact of high-field intraoperative magnetic resonance imaging on the efficacy of pediatric low-grade glioma surgery. Neurosurg Focus 40(3):E13. https://doi.org/10.3171/2015.11.focus15530
Knauth M, Aras N, Wirtz CR, Dorfler A, Engelhorn T, Sartor K (1999) Surgically induced intracranial contrast enhancement: potential source of diagnostic error in intraoperative MR imaging. AJNR Am J Neuroradiol 20(8):1547–1553
Avula S, Mallucci CL, Pizer B, Garlick D, Crooks D, Abernethy LJ (2012) Intraoperative 3-Tesla MRI in the management of paediatric cranial tumours--initial experience. Pediatr Radiol 42(2):158–167. https://doi.org/10.1007/s00247-011-2261-6
Yousaf J, Avula S, Abernethy LJ, Mallucci CL (2012) Importance of intraoperative magnetic resonance imaging for pediatric brain tumor surgery. Surg Neurol Int 3(Suppl 2):S65–S72. https://doi.org/10.4103/2152-7806.95417
Roder C, Haas P, Tatagiba M, Ernemann U, Bender B (2019) Technical limitations and pitfalls of diffusion-weighted imaging in intraoperative high-field MRI. Neurosurg Rev 44:327–334. https://doi.org/10.1007/s10143-019-01206-0
Smith JS, Cha S, Mayo MC, McDermott MW, Parsa AT, Chang SM, Dillon WP, Berger MS (2005) Serial diffusion-weighted magnetic resonance imaging in cases of glioma: distinguishing tumor recurrence from postresection injury. J Neurosurg 103(3):428–438. https://doi.org/10.3171/jns.2005.103.3.0428
Ozturk A, Oguz KK, Akalan N, Geyik PO, Cila A (2006) Evaluation of parenchymal changes at the operation site with early postoperative brain diffusion-weighted magnetic resonance imaging. Diagn Interv Radiol 12(3):115–120
Pala A, Brand C, Kapapa T, Hlavac M, König R, Schmitz B, Wirtz CR, Coburger J (2016) The value of intraoperative and early postoperative magnetic resonance imaging in low-grade glioma surgery: a retrospective study. World Neurosurg 93:191–197. https://doi.org/10.1016/j.wneu.2016.04.120
Funding
No funding was received for this study.
Author information
Authors and Affiliations
Contributions
Shivaram Avula: Conceptualization, methodology, implementation, analysis, and writing—original draft
Tim Jaspan: Methodology, implementation, analysis, and writing—review and editing
Barry Pizer: Methodology, implementation, and writing—review and editing
Benedetta Pettorini: Methodology, implementation, and writing—review and editing
Deborah Garlick: Implementation and writing—review and editing
Dawn Hennigan: Implementation and writing—review and editing
Conor Mallucci: Methodology, implementation, and writing—review and editing
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
As the study involved comparison with standard clinical methodology and patients did not require additional general anaesthesia, it did not require consideration by the regional ethics committee and was registered as an institutional service evaluation study.
Informed consent
Patients/carers had consented for the anonymised scans to be used for the purpose of research and audit.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Avula, S., Jaspan, T., Pizer, B. et al. Comparison of intraoperative and post-operative 3-T MRI performed at 24–72 h following brain tumour resection in children. Neuroradiology 63, 1367–1376 (2021). https://doi.org/10.1007/s00234-021-02671-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-021-02671-5