Abstract
Purpose
The purpose of this study is to compare the surgical and imaging outcome in children who underwent brain tumour surgery with intention of complete tumour resection, prior to and following the start of intra-operative MRI (ioMRI) service.
Methods
ioMRI service for brain tumour resection commenced in October 2009. A cohort of patients operated between June 2007 and September 2009 with a pre-surgical intention of complete tumour resection were selected (Group A). A similar number of consecutive cases were selected from a prospective database of patients undergoing ioMRI (Group B). The demographics, imaging, pathology and surgical outcome of both groups were compared.
Results
Thirty-six of 47 cases from Group A met the inclusion criterion and 36 cases were selected from Group B; 7 of the 36 cases in Group A had unequivocal evidence of residual tumour on the post-operative scan; 5 (14 %) of them underwent repeat resection within 6 months post-surgery. In Group B, ioMRI revealed unequivocal evidence of residual tumour in 11 of the 36 cases following initial resection. In 10 of these 11 cases, repeat resections were performed during the same surgical episode and none of these 11 cases required repeat surgery in the following 6 months. Early repeat resection rate was significantly different between both groups (p = 0.003).
Conclusion
Following the advent of ioMRI at our institution, the need for repeat resection within 6 months has been prevented in cases where ioMRI revealed unequivocal evidence of residual tumour.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Intra-operative magnetic resonance imaging (ioMRI) is increasingly gaining recognition in the management of brain tumour resection. Its feasibility and safety has been well established in adults and children [3, 6, 8, 10, 12]. The extent of brain tumour resection is an important prognostic factor in the paediatric age group. We have demonstrated an increase in the rate of complete resection in our initial experience using a high field strength (3 T) ioMRI facility at our tertiary level paediatric neurosurgical centre [3]. There is however, limited published evidence on its efficacy when compared to conventional brain tumour resection performed without the aid of ioMRI. To date, there is one randomized controlled study by Senft et al. [16] that has demonstrated greater rates of complete tumour resection in adults with gliomas. Randomized controlled trials in the paediatric brain tumour population are not possible, given the ethical and logistical difficulties involved. A retrospective comparative study is an alternative but published evidence remains limited in adults and children [17, 19]. Variability of surgical intent (biopsy, partial resection, complete resection) prior to resection can compromise the accuracy of comparison in assessing the utility of ioMRI. In the current study, we wish to compare the rates of early repeat resection within 6 months in patients with and without ioMRI-aided surgery where complete tumour resection was intended.
Methods
Patient selection and study design
The ioMRI facility has been fully functional at our institution since October 2009. The inclusion criterion was paediatric (0–18 years) brain tumour cases where complete tumour resection was intended. The decision of complete resection was made at the pre-operative neuro-oncology multidisciplinary meeting based on the imaging, clinical findings and tumour histology where available. Cases from the conventional surgery group (Group A) that met the inclusion criterion were selected for the study from a cohort of patients operated upon between 2007 and September 2009. This cohort of patients was selected from an independent audit study evaluating the accuracy of MRI reporting, approved by the audit department of our institution. An identical number of consecutive cases that met the inclusion criterion were selected from a prospective database of ioMRI cases maintained since October 2009 (Group B). This data was collected as part of service evaluation as approved by the research department of our institution. Variables that were reviewed included patient demographics, location of the tumour (supratentorial or infratentorial), pathological diagnosis, MRI scan findings on the intra-operative (Group B), post-operative and early follow-up scans and repeat resections performed within the initial 6-month period.
MR Imaging methods
All the MRI scans in Group A were performed on a Philips Achieva® 1.5-T scanner. The pre-operative scans in Group B were performed either on the 3-T scanner or a Philips Achieva® 1.5-T scanner. The ioMRI and post-operative follow-up scans in Group B were performed on a 3-T scanner. The pre- and post-operative imagings were performed according to the United Kingdom Children’s Cancer and Leukaemia Group (CCLG) radiology guidelines [4]. The ioMRI scan protocol complemented the CCLG protocol with T2-weighted turbo spin echo sequences (T2-TSE) in sagittal and coronal planes in addition to the standard axial plane. T1-weighted isotropic 3-D gradient-echo (T1-TFE) sequences were obtained pre- and post-contrast medium administration instead of the standard T1 spin echo sequences. Details of our ioMRI setup and scan parameters have been published previously [1, 3]. In Group A, the immediate post-operative scan was performed 24 to 48 h following surgery. In Group B, the final ioMRI scan served as the post-operative scan. Timing of the subsequent follow-up MRI in both groups was based on the tumour histology and associated management protocols.
Image Evaluation
The ioMRI scan findings were based on evaluation performed in consensus by the radiologist and the neurosurgeon during ioMRI acquisition. The post-operative MRI was reviewed by the lead paediatric neuroradiologist (SA) independently on a GE PACS station and the clinical data was reviewed by the lead paediatric neurosurgeon (CM). The foci of signal abnormality or areas of contrast enhancement that were identical to the signal or enhancement characteristics of the tumour as defined by the radiologist and neurosurgeon on the pre-operative imaging were identified as residual tumour. Thin rim of enhancement along the surgical cavity or outside the pre-operative margin of enhancement was regarded as surgically induced contrast enhancement. In cases where it was not possible to distinguish residual tumour from surgically induced change, the scan was regarded as equivocal for residual tumour.
Decision for repeat resection
In Group A, the decision to re-operate was made based on the histology, evidence of residual tumour on the scan performed 24–48 h post-surgery or evidence of disease progression on the subsequent follow-up scan. In Group B, ioMRI was performed to evaluate for evidence of residual tumour. The decision to extend resection was based on the unequivocal evidence of residual tumour on ioMRI. In cases where the ioMRI appearances were equivocal for residual tumour, the surgical site was re-examined after updating neuronavigation.
Statistical analysis
The demographic and clinical data were summarised using standard descriptive statistic methods (mean, standard deviation, percentages). Statistical analysis of the two groups was performed on SPSS software version 20.0. The statistical analysis was performed using student’s t test for continuous variables and chi-square test or Fisher’s exact test for categorical variables where appropriate. A p value < 0.05 was considered to be statistically significant.
Results
Comparison of demographics and pathology
Thirty-six out of 47 cases from the conventional group (Group A) met the inclusion criterion, i.e. complete surgical resection was the intended surgical aim. Thirty-six consecutive cases that met the inclusion criterion were selected from the ioMRI group (Group B). Comparison of the mean ages and sex ratio showed no significant difference between both groups and location of the tumour in relation to the tentorium was not significantly different in either group (Table 1). Surgical resection was carried out by one of five consultant neurosurgeons, with the majority of the surgical procedures carried out by the lead neurosurgical consultant (CM) for neuro-oncology (Group A—23; Group B—25).
The pathology results in both groups are summarised in Table 2. The majority of the tumours in both groups were low-grade tumours (WHO grade I and II). Among the low-grade tumours, pilocytic astrocytoma was the most common pathology with 12 cases in either group. Primitive neuroectodermal tumours were the most common among the high-grade tumours (WHO high-grade III/IV) with four in Group A and three in Group B. One child with a frontal lobe vascular malformation has been included, as the surgery was performed for a suspected brain tumour, based on the pre-operative imaging.
Surgical outcome
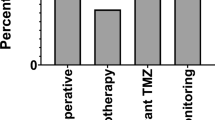
There was evidence of complete resection in 20 (56 %) of the 36 cases in Group B on the first ioMRI scan. This was not statistically different (p = 0.93) from Group A where 24 (66 %) of the 36 cases showed evidence of complete resection on the immediate post-operative scan (Table 3). There was unequivocal evidence of residual tumour in seven cases in Group A and ten cases in Group B. Nine of the 10 patients in Group B underwent further resection following the first ioMRI scan. In one patient, further resection was not performed as no obvious residual tumour was visible on surgical re-exploration following ioMRI (after updating neuronavigation). Follow-up MRI scan in this patient showed gradual reduction at the site of abnormal contrast enhancement over the following 1 year [3]. None of the ten patients required further resection in the following 6 months. Five of the seven patients (14 %) in Group A underwent further surgical resection. Two of these cases (anaplastic astrocytoma and ependymoma) had repeat surgery within 2 months and three cases with pilocytic astrocytomas had repeat surgery within 6 months after follow-up scans that showed evidence of disease progression. The remaining two (of the seven) cases with atypical teratoid rhabdoid tumour and pituitary adenoma received chemotherapy and radiotherapy, respectively, for their residual disease. The difference in early repeat resection rates (within 6 months) between both groups was statistically significant (p = 0.003) in cases with unequivocal residual tumour (Table 3 and Fig. 1). Two case examples from Group A and B, respectively, are shown in Fig. 2.
Case examples from Group A (a–c) and Group B (d–g). Contrast-enhanced T1-weighted scan reveals a heterogeneously enhancing tumour involving the right temporal lobe (a). Post-operative scan 24 h following surgery (b) revealed a residual enhancing component (white arrow). The lesion was confirmed as an anaplastic astrocytoma on pathological analysis and a repeat resection was performed 2 weeks later (c). Sagittal contrast-enhanced T1-weighted (d) and axial T2-weighted (e) scans reveal a lesion in the inferior vermis with two enhancing components. T2-weighted scan on the first ioMRI revealed residual tumour along the posterior wall of the surgical cavity (black arrow). The residual abnormality was subsequently resected (open arrow) as shown on the second ioMRI (g). Both resection samples showed tumour tissue compatible with pilocytic astrocytoma on pathological analysis
Among five cases in Group A and six cases in Group B, the scans were equivocal for the presence of residual tumour as it was not possible to differentiate oedema or surgically induced contrast enhancement from residual tumour. The number of scans equivocal for residual tumour on the post-operative MRI in Group A and first ioMRI in Group B were not statistically different (p = 0.107). In Group B, two cases with equivocal scans required repeat surgery within 6 months. One 16-month-old boy with anaplastic ependymoma involving the right temporal lobe demonstrated equivocal findings on ioMRI as the tumour was non-enhancing and almost isointense to the adjacent brain parenchyma. Further immediate resection was performed following the first ioMRI. The second scan remained equivocal for residual tumour. The patient was started on chemotherapy and follow-up MRI performed after 10 weeks showed evidence of progression requiring repeat surgery (Fig. 3). The second case was a 4-year-old boy with a pilocytic astrocytoma whose ioMRI showed minimal enhancement along the surgical margin that was thought to represent post-surgical contrast enhancement as there was no evidence of tumour on direct visualisation. Follow-up imaging at 3 months revealed an enhancing mass at the primary site suggesting progression of residual tumour requiring repeat tumour resection. The cases in Group A with equivocal scans showed stable or resolving post-operative findings on follow-up imaging.
A 16-month-old boy with a solid/cystic tumour involving the right temporal lobe. The solid component appears isointense to the cortex on the axial T2-weighted (a), T1-weighted (b) scans and demonstrates mild contrast enhancement (white arrow). It also appeared isointense to the cortex on the FLAIR (not shown here). The first ioMRI revealed residual tumour (black arrow) on the T2-weighted scan (d). Following immediate repeat resection, there was no convincing evidence of residual tumour on the second ioMRI (e,f) and evidence of linear contrast enhancement along the surgical cavity (small arrows). The appearances were equivocal for the presence of residual tumour. Chemotherapy was commenced after a diagnosis of anaplastic ependymoma was made and follow-up MRI 10 weeks later revealed enhancing tumour tissue (open arrow) compatible with disease progression
Discussion
Value of ioMRI in paediatric brain tumour resection
It is well established that extent of resection is an important prognostic factor in children with high-grade glioma, medulloblastoma and ependymoma [2, 15, 21] and low-grade tumours such as pilocytic astrocytomas [5]. ioMRI can influence the extent of tumour resection by helping identify residual tumour and providing imaging data to update neuronavigation, compensating for inaccuracies caused by brain shift. In our previously reported initial experience, ioMRI has modified the surgical strategy in 32 % of the tumour resections [22]. The reported rates of extended surgical resection after ioMRI vary between 27.5 and 60 % [8–10, 12, 14, 18]. There are however, a limited number of published studies that compare the efficacy of ioMRI-guided neurosurgery to that of conventional surgery. A randomized controlled study will be an ideal method in establishing the effectiveness of ioMRI but would pose a number of ethical and logistical challenges especially in the paediatric brain tumour population. In a retrospective comparison study of adults with glioblastoma multiforme, Senft et al. showed significantly higher complete tumour resection rates in the ioMRI group when compared to conventional surgery [17]. A subsequent randomized control study by Senft et al. [16] evaluating glioma resections in the adult population showed increased complete resection rates in the ioMRI group compared to conventional surgery (96 vs. 68 %, p = 0.023). The only paediatric comparison study to date by Shah et al. [19] comparing 42 ioMRI-guided resections with 103 conventional resections in children showed greater number of early resection rates (within 2 weeks) in the conventional group (7.7 vs. 0 %, p = 0.06, one-tailed test). Our study has shown higher early resection rates (within 6 months) in the conventional group compared to the ioMRI group (14 vs. 0 %, p = 0.003) in patients where there was unequivocal evidence of residual tumour on the immediate post-operative imaging or the first ioMRI. This study therefore suggests that ioMRI is beneficial in preventing early repeat surgery in cases where there is clear evidence of residual tumour after initial surgical resection. The comparison study by Shah et al. excluded patients less than 18 months of age and included surgical procedures in non-neoplastic disorders such as epilepsy [19]. The current study is specific to brain tumour surgery and includes the entire paediatric age range (2 months to 19 years).
Has ioMRI influenced surgical strategy?
In our earlier publication, we have shown that ioMRI had led to extension of tumour resection and had altered surgical strategy in 32 % of the patients [22]. The wide variation in the reported rates of extended resection after ioMRI creates an impression that intra-operative imaging may be undertaken earlier than intended simply due to its availability. To evaluate this, we compared the rates of complete resection on the post-operative MRI in Group A and on the first ioMRI scan in Group B (performed after neurosurgical impression of complete resection)and found no statistically significant difference between both groups (Table 3) suggesting that ioMRI did not influence the initial complete resection rates in our institution. We intentionally excluded cases where partial tumour resection was intended as it is often difficult to quantify the surgical aim in this group. In our initial experience, 41 % of cases where partial resection was intended underwent further resection following ioMRI. These were often large central chiasmatic/hypothalamic lesions where a staged procedure with a limited ioMRI was used to reassess the residual tumour tissue and update neuronavigation [3, 22]. ioMRI has thus played varying roles in the surgical management of brain tumours depending on the size, location and the surgical intent. With increasing use of ioMRI, it is possible that its use can vary further depending on tumour characteristics, neurosurgical and radiological expertise at different institutions. The overall impact on paediatric brain tumour surgery is likely to be safer and complete or adequate tumour resections that can improve prognosis.
Challenges involving ioMRI and need for further research
In this study, we classified scans as equivocal for the presence of residual tumour when it was difficult to differentiate surgically induced contrast enhancement or peri-tumoural signal abnormality from residual tumour. The number of equivocal scans was similar in both groups (Table 3). Two children from the ioMRI group required repeat surgery due to disease progression on follow-up imaging. In one case of pilocytic astrocytoma, contrast enhancement lining the surgical cavity was thought to represent post-surgical enhancement. The phenomenon of surgically induced contrast enhancement is well recognized [7]. In our practice, the abnormal region is re explored after updating neuronavigation and further excision or biopsy is performed if there is evidence of residual tumour on direct visualisation. With the exception of one case, the equivocal areas of enhancement resolved or remained stable. In another child with an anaplastic ependymoma, the absence of tumour enhancement and similarity of signal characteristics of the tumour and surrounding parenchyma made it difficult to exclude small foci of residual tumour. These are rare tumours that can pose challenges in interpretation. Advanced MRI techniques can help in some situations and the 3-T MRI scanner is particularly helpful in these situations. Where feasible, we have used MR spectroscopy to evaluate the region of signal abnormality for evidence of abnormal metabolites such as choline (Fig. 4). Dynamic susceptibility contrast perfusion imaging has also shown to be useful in interpretation of ioMRI [20]. Diffusion-weighted imaging has to be interpreted with caution, as diffusion abnormalities can also be contributed by haemorrhage and surgery related ischemia.
An 18-month-old girl with an atypical teratoid rhabdoid tumour. A T2 hyperintense tumour with minimal contrast enhancement (inset) is noted within the posterior fossa (a). Single voxel spectroscopy (echo time = 144) reveals a raised choline peak (Cho) in relation to the creatine (Cr) peak in keeping with increased cell membrane turnover. ioMRI (b) showed T2 hyperintensity around the surgical cavity (arrows). The absence of elevated choline in relation to creatine in the region of interest suggested the absence of residual tumour. Follow-up imaging following chemotherapy showed no evidence of residual/progressive tumour at the primary site
The impact of costs vs. benefits on ioMRI
The cost of setting up and utilisation of an ioMRI facility is substantial and can be difficult to justify particularly in a paediatric neurosurgical centre. This can influence the design and equipment of an ioMRI set up, including the strength of the magnet (high- vs. low field strength) type of scanner (mobile ceiling mounted vs. fixed) and the workflow (single vs. dual room). We had adopted a dual room solution with a 3-T MRI scanner (fixed) which enabled independent use of the two rooms. Innovative use of equipment and workflow, adapted to the needs of the individual institution can help offset the costs incurred from an ioMRI facility. The benefits of the dual room solution in our institution have been discussed in detail in our previous publications [1, 3]. Briefly, the independent use of the 3-T scanner has helped generate income by adding clinical and research capacity to our radiology department which was equipped with a 1.5-T scanner. It has improved the quality of advanced MRI imaging which include diffusion tensor imaging, spectroscopy, functional MRI and arterial spin labelling. We have adopted a flexible working pattern that helps accommodate urgent scans and outpatient scans on the day of an ioMRI-aided surgery. Good communication between the surgical, anaestheic and radiology teams is vital in avoiding delays in patient transfer and preparation of the scanner. There are cost benefits in avoiding early repeat surgery and early post-operative scans traditionally performed 24–48 h post-surgery, sometimes requiring general anaesthesia. The dual room solution also enables the use of standard surgical equipment making it cost-effective. Similar benefits with a dual room solution has been reported by other neurosurgical centres [11, 13] making this a more cost-effective option in the current economic climate.
The retrospective nature of the study and the limited number of patients are acknowledged. It can be difficult to exactly match the case and control groups in a single neurosurgical centre, given the relative rarity of brain tumours in children but the two groups show similarity in demographic features, location and grade of tumours as shown in Tables 1 and 2. All surgeons were of the consultant grade and majority of the surgical resections were performed by one lead neurosurgeon. Our study demonstrates a significant change in surgical outcome in a single tertiary neurosurgical centre after the advent of ioMRI. A multicenter study or a meta-analysis in the future will throw more light on the impact of ioMRI on paediatric brain tumour surgery and the long-term outcome in these children.
Conclusion
We have demonstrated a change in the surgical outcome after the introduction of ioMRI with a significant reduction in the number of early repeat surgeries in children undergoing brain tumour surgery. Avoiding early repeat surgery has clinical and cost benefits as well as emotional and psychological implications on the patients and carers. There are challenges involved in the interpretation of ioMRI and advanced MRI techniques that are possible with high field strength scanners can be helpful in appropriate situations. Further research demonstrating the role of advanced MRI techniques in ioMRI will be beneficial.
References
Abernethy LJ, Avula S, Hughes GM, Wright EJ, Mallucci CL (2012) Intra-operative 3-T MRI for paediatric brain tumours: challenges and perspectives. Pediatr Radiol 42(2):147–157. doi:10.1007/s00247-011-2280-3
Albright AL, Wisoff JH, Zeltzer PM, Boyett JM, Rorke LB, Stanley P (1996) Effects of medulloblastoma resections on outcome in children: a report from the Children's Cancer Group. Neurosurgery 38(2):265–271
Avula S, Mallucci CL, Pizer B, Garlick D, Crooks D, Abernethy LJ (2012) Intraoperative 3-Tesla MRI in the management of paediatric cranial tumours—initial experience. Pediatr Radiol 42(2):158–167. doi:10.1007/s00247-011-2261-6
Craig E, Connolly DJ, Griffiths PD, Raghavan A, Lee V, Batty R (2012) MRI protocols for imaging paediatric brain tumours. Clin Radiol. doi:10.1016/j.crad.2012.03.018
Fernandez C, Figarella-Branger D, Girard N, Bouvier-Labit C, Gouvernet J, Paz Paredes A, Lena G (2003) Pilocytic astrocytomas in children: prognostic factors—a retrospective study of 80 cases. Neurosurgery 53(3):544–553, discussion 554–545
Hall WA, Martin AJ, Liu H, Pozza CH, Casey SO, Michel E, Nussbaum ES, Maxwell RE, Truwit CL (1998) High-field strength interventional magnetic resonance imaging for pediatric neurosurgery. Pediatr Neurosurg 29(5):253–259
Knauth M, Aras N, Wirtz CR, Dorfler A, Engelhorn T, Sartor K (1999) Surgically induced intracranial contrast enhancement: potential source of diagnostic error in intraoperative MR imaging. AJNR Am J Neuroradiol 20(8):1547–1553
Knauth M, Wirtz CR, Tronnier VM, Aras N, Kunze S, Sartor K (1999) Intraoperative MR imaging increases the extent of tumor resection in patients with high-grade gliomas. AJNR Am J Neuroradiol 20(9):1642–1646
Kremer P, Tronnier V, Steiner HH, Metzner R, Ebinger F, Rating D, Hartmann M, Seitz A, Unterberg A, Wirtz CR (2006) Intraoperative MRI for interventional neurosurgical procedures and tumor resection control in children. Child's nervous system: ChNS: Off J Int Soc Pediatr Neurosurg 22(7):674–678. doi:10.1007/s00381-005-0030-2
Levy R, Cox RG, Hader WJ, Myles T, Sutherland GR, Hamilton MG (2009) Application of intraoperative high-field magnetic resonance imaging in pediatric neurosurgery. J Neurosurg Pediatr 4(5):467–474. doi:10.3171/2009.4.PEDS08464
Martin XP, Vaz G, Fomekong E, Cosnard G, Raftopoulos C (2011) Intra-operative 3.0 T magnetic resonance imaging using a dual-independent room: long-term evaluation of time–cost, problems, and learning-curve effect. Acta Neurochir Suppl 109:139–144. doi:10.1007/978-3-211-99651-5_21
Nimsky C, Ganslandt O, Von Keller B, Romstock J, Fahlbusch R (2004) Intraoperative high-field-strength MR imaging: implementation and experience in 200 patients. Radiology 233(1):67–78. doi:10.1148/radiol.2331031352
Pamir MN (2011) 3 T ioMRI: the Istanbul experience. Acta Neurochir Suppl 109:131–137. doi:10.1007/978-3-211-99651-5_20
Pamir MN, Ozduman K, Dincer A, Yildiz E, Peker S, Ozek MM (2010) First intraoperative, shared-resource, ultrahigh-field 3-Tesla magnetic resonance imaging system and its application in low-grade glioma resection. J Neurosurg 112(1):57–69. doi:10.3171/2009.3.JNS081139
Rodriguez D, Cheung MC, Housri N, Quinones-Hinojosa A, Camphausen K, Koniaris LG (2009) Outcomes of malignant CNS ependymomas: an examination of 2408 cases through the Surveillance, Epidemiology, and End Results (SEER) database (1973–2005). J Surg Res 156(2):340–351. doi:10.1016/j.jss.2009.04.024
Senft C, Bink A, Franz K, Vatter H, Gasser T, Seifert V (2011) Intraoperative MRI guidance and extent of resection in glioma surgery: a randomised, controlled trial. Lancet Oncol 12(11):997–1003. doi:10.1016/S1470-2045(11)70196-6
Senft C, Franz K, Blasel S, Oszvald A, Rathert J, Seifert V, Gasser T (2010) Influence of iMRI-guidance on the extent of resection and survival of patients with glioblastoma multiforme. Technol Cancer Res Treat 9(4):339–346
Senft C, Seifert V, Hermann E, Franz K, Gasser T (2008) Usefulness of intraoperative ultra low-field magnetic resonance imaging in glioma surgery. Neurosurgery 63(4 Suppl 2):257–266. doi:10.1227/01.NEU.0000313624.77452.3C, discussion 266–257
Shah MN, Leonard JR, Inder G, Gao F, Geske M, Haydon DH, Omodon ME, Evans J, Morales D, Dacey RG, Smyth MD, Chicoine MR, Limbrick DD (2012) Intraoperative magnetic resonance imaging to reduce the rate of early reoperation for lesion resection in pediatric neurosurgery. J Neurosurg Pediatr 9(3):259–264. doi:10.3171/2011.12.PEDS11227
Ulmer S, Hartwigsen G, Riedel C, Jansen O, Mehdorn HM, Nabavi A (2010) Intraoperative dynamic susceptibility contrast MRI (iDSC-MRI) is as reliable as preoperatively acquired perfusion mapping. NeuroImage 49(3):2158–2162. doi:10.1016/j.neuroimage.2009.10.084
Wisoff JH, Boyett JM, Berger MS, Brant C, Li H, Yates AJ, McGuire-Cullen P, Turski PA, Sutton LN, Allen JC, Packer RJ, Finlay JL (1998) Current neurosurgical management and the impact of the extent of resection in the treatment of malignant gliomas of childhood: a report of the Children's Cancer Group trial no. CCG-945. J Neurosurg 89(1):52–59. doi:10.3171/jns.1998.89.1.0052
Yousaf J, Avula S, Abernethy LJ, Mallucci CL (2012) Importance of intraoperative magnetic resonance imaging for pediatric brain tumor surgery. Surg Neurol Int 3(Suppl 2):S65–S72. doi:10.4103/2152-7806.95417
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Avula, S., Pettorini, B., Abernethy, L. et al. High field strength magnetic resonance imaging in paediatric brain tumour surgery—its role in prevention of early repeat resections. Childs Nerv Syst 29, 1843–1850 (2013). https://doi.org/10.1007/s00381-013-2106-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-013-2106-8