Abstract
Purpose
Extent of resection of low grade glioma (LGG) is an important prognostic variable, and may influence decisions regarding adjuvant therapy in certain patient populations. Immediate postoperative magnetic resonance image (MRI) is the mainstay for assessing residual tumor. However, previous studies have suggested that early postoperative MRI fluid-attenuated inversion recovery (FLAIR) (within 48 h) may overestimate residual tumor volume in LGG. Intraoperative magnetic resonance imaging (iMRI) without subsequent resection may more accurately assess residual tumor. Consistency in MRI techniques and utilization of higher magnet strengths may further improve both comparisons between MRI studies performed at different time points as well as the specificity of MRI findings to identify residual tumor. To evaluate the utility of 3 T iMRI in the imaging of LGG, we volumetrically analyzed intraoperative, early, and late (~ 3 months after surgery) postoperative MRIs after resection of LGG.
Methods
A total of 32 patients with LGG were assessed retrospectively. Residual tumor was defined as hyperintense T2 signal on FLAIR. Volumetric assessment was performed with intraoperative, early, and late postoperative FLAIR via TeraRecon iNtuition.
Results
Perilesional FLAIR parenchymal abnormality volumes were significantly different comparing intraoperative and early postoperative MRI (2.17 ± 0.45 cm3 vs. 5.47 ± 1.07 cm3, respectively (p = 0.0002)). A significant difference of perilesional FLAIR parenchymal abnormality volumes was also found comparing early and late postoperative MRI (5.47 ± 1.07 cm3 vs. 3.22 ± 0.64 cm3, respectively (p = 0.0001)). There was no significant difference between intraoperative and late postoperative Perilesional FLAIR parenchymal abnormality volumes.
Conclusions
Intraoperative 3 T MRI without further resection appears to better reflect the volume of residual tumor in LGG compared with early postoperative 3 T MRI. Early postoperative MRI may overestimate residual tumor. As such, intraoperative MRI performed after completion of tumor resection may be more useful for making decisions regarding adjuvant therapy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A World Health Organization (WHO) grade II glioma, or diffuse low-grade glioma (LGG), is a slow-growing, infiltrative primary brain tumor that confers a median overall survival (OS) of 7.8 to 15 years [1,2,3]. Ultimately, LGGs may undergo malignant transformation to a higher grade, including anaplastic glioma (WHO III) or glioblastoma (WHO IV), with poor subsequent prognosis [1, 4, 5]. Early and aggressive management of LGG with maximal safe surgical resection is critical to optimizing overall survival and time to malignant transformation, or malignant progression free survival (PFS), in addition to maintaining and preserving acceptable quality of life [6].
The prognostic benefit of maximal extent of resection (EOR), particularly when achieving > 90% EOR, has been well-described by previous LGG studies [7,8,9]. Advancements in intra-operative imaging, including ultrasound and MRI (iMRI), as well as intracranial mapping techniques, have substantially improved the ability of the neurosurgeon to achieve maximal EOR while minimizing neurologic morbidity [7]. However, despite these advances, gross total resection is not always achieved, even when possible from the perspective of potential neurologic morbidity.
While EOR is a significant prognostic factor in LGG, the accurate determination of residual tumor volume also impacts the clinical decision to pursue adjuvant therapies, including radiotherapy and/or systemic therapies. In a recent study confirming the role of EOR in low grade gliomas including IDH-wildtype astrocytomas, residual volumes as little as 1 cm3 lead to significantly worse OS [9]. Thus the importance of accurately assessing residual tumor volume after surgery is crucial in determining the need for additional surgery and adjuvant therapies [9, 10].
Timing of the imaging study and the particular MRI sequences obtained have important roles in distinguishing residual tumor from postoperative changes that may obscure tumor margins. Intraoperative MRI can be performed to aid in assessing extent of resection during surgery. Several studies have shown improved EOR and improved OS with use of iMRI [10,11,12,13]. However, iMRI sequences are often incomplete relative to formal postoperative imaging, and utilization of iMRI sequences as a post-resection imaging baseline is not well described.
Pala et al. evaluated patients with LGG who underwent surgical resection with 1.5 T iMRI guidance, followed by an early postoperative MRI (< 48-h) and late postoperative MRI (3–4 months) [14]. This study concluded that iMRI residual tumor volumes, evaluated by conventional T2-weighting and Fluid Attenuated Inversion Recovery (FLAIR), were most similar to late postoperative MRI, which was previously described to most accurately depict residual tumor volume [14, 15]. However this study utilized a different strength magnet (1.5 T) for intraoperative MR imaging than post-operative images (3 T), and included patient with LGGs that did not have gross total resection (GTR). Additionally, studies have shown that that 3 T FLAIR sequences more accurately quantitate the volume of low grade gliomas compared to 1.5 T MRI [16,17,18]. Thus, existing data utilizing 1.5 T intraoperative MRI may not quantitate residual tumor volumes as accurately as 3 T intraoperative MRI. 3 T MRI technology has improved signal-to-noise ratio (SNR) compared to 1.5 T, which leads to higher spatial resolution, resulting in a clearer image and therefore theoretically allowing better detection of smaller lesions and complex anatomy [19]. The superior spatial resolution with 3 T images allows greater confidence in the extent of tumor resection and determination of margins of the FLAIR signal abnormality on the iMRI and postoperative exams [20]. One potential disadvantage of 3 T is greater susceptibility for artifacts (such as in areas of blood products) although this does not seem to be a problem in determining the peripheral margins of LGG in most cases [21].
Therefore, the primary aim of the presented study was to evaluate 3 T intraoperative MRI FLAIR in the estimation of residual LGG tumor volume compared to early and late postoperative 3 T MRI.
Methods
Patient population and study design
The study is an IRB-approved retrospective cohort review evaluating patients who were treated for LGG at The Ohio State University Wexner Medical Center in the three-year time period 2015–2018. The initial cohort consisted of 79 patients that had a pathology-proven diagnosis of LGG and underwent surgical resection. Exclusion criteria included patients that underwent adjuvant therapy prior to a follow up MRI at 3 months and any patients without any of three standard MRI time points: an intraoperative 3 T MRI, a postoperative 3 T MRI within 24 h of their procedure, and a follow up 3 T MRI at 3 months post-surgical resection. The 24 h time point was based on previous literature that denoted increases in perilesional FLAIR parenchymal abnormalities secondary to retraction, hemoglobin and granulation tissue [22, 23]. The final cohort consisted of 32 patients. Patient demographics, surgical reports, pathological evaluation, and intraoperative and postoperative imaging records were reviewed.
Imaging evaluation
During surgery, iMRI scans were performed utilizing a 3 T Espree Navigation System (Discovery™ MR750w GEM—70 cm, Software DV25.1, GE Healthcare, Chicago, USA). The parameters of a standard FLAIR sequence used in this study: TR 9000 ms and TE 114 ms, flip angle 150° 4 mm slice thickness, number of averages 1, echo train length 22, number of phase encoding steps 326, acquisition matrix size 384 × 348. All iMRI sequences were 2D sequences. iMRI sequences included FLAIR. Early postoperative MRI was performed within 24 h following surgery, and late postoperative MRI was obtained 3–4 months after surgery. Postoperative MRIs were performed with 3 T MRI Symphony system (Siemens AG, Erlangen, Germany). Post-operative and late postoperative MR images were obtained with the same type of scanner with the same protocol.
Volumetric tumoral analysis
Tumor volumes were measured using TeraRecon iNtuition software (Version 4.4.13.P2; Foster City, CA). The process involved auto-segmentation with additional manual contouring in the axial, sagittal, and coronal planes as well as 3-D image overlay for verification of segmentation accuracy. Auto-segmentation was performed using the TeraRecon iNtuition software which approximated the margins of a targeted signal abnormality. Then the radiologists manually contoured each lesion freehand to fine tune the margins for accuracy. The fine tuning was performed on all three planes (axial, sagittal, and coronal) to ensure a high degree of accuracy. This was performed for each case. Following this step, 3-D overlay of a shaded rendering of the expected volume was placed over the lesion to ensure accuracy of the final volume. If discrepancies were noted, the process was repeated until no visible errors or discrepancies between the measure volume and visualized lesion were noted. Circumscribed LGG was defined as a compact lesion without visible spread along white matter fibers. The threshold was 1.5 cm3 for FLAIR sequences and 1 cm3 for T2 sequences, demarcation of residual disease signal abnormality was achieved on FLAIR sequences compared to T2 sequences, and therefore a higher threshold for minimal residual disease on FLAIR sequences (1.5 cm3) was utilized with higher confidence when measuring signal abnormality.
Statistical analysis
Statistics were calculated using SPSS v25 (IBM Corp. Released 2017. IBM SPSS Statistics for Macintosh, Version 25.0. Armonk, NY: IBM Corp.). Normality was tested via a Shapiro–Wilk test. Patient characteristics were compared using analysis of variance (ANOVA), Kruskal–Wallis and Chi-square tests. A two-sided p-value less than 0.05 was considered to be statistically significant.
Results
A total of 32 patients treated for WHO grade II LGGs were assessed. The most common histologic subtype of LGG was diffuse astrocytoma (n = 21, 65.6%), followed by oligodendroglioma (n = 11, 34.4%). A median age of 36.5 (range 22–75) years was noted. The majority of patients were male (56%, n = 18). The majority, 28(87.5%) patients were positive for IDH1R132H mutation, 1(3.1%) patient had IDH wildtype and 3(9.4%) patients the IDH status was not performed. The mean initial tumor volume was 30.7 ± 4.6 cm3.
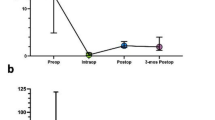
All patients included in the analysis underwent iMRI. Tumor volumes from the iMRI are reflective of last iMRI if multiple iMRI were performed. The perilesional FLAIR parenchymal abnormality volumeof iMRI FLAIR was 2.17 ± 0.45 cm3. Gross total resection was obtained in 84.4% (27/32, volume < 3 cm3) and near total resection in 15.6% (5/32, volume < 8 cm3, > 90% of initial volume [24]). All early postoperative MRIs were obtained within 24 h of resection. No patients required additional surgery based on findings of early MRI. The perilesional FLAIR parenchymal abnormality volumeof early postoperative MRI FLAIR was 5.47 ± 1.07 cm3. A significant difference of FLAIR abnormalities was found comparing intraoperative and early postoperative MRI perilesional FLAIR parenchymal abnormality volume (p = 0.0002), in which the iMRI perilesional FLAIR parenchymal abnormality volume was significant smaller than the early post-operative perilesional FLAIR parenchymal abnormality volume. The late postoperative MRI was at approximately 3-months (median 92.0 ± 5.0 days) from the time of resection. No patients were recommended for additional surgery based on late MRI findings. The perilesional FLAIR parenchymal abnormality volume of late postoperative MRI FLAIR was 3.22 ± 0.64 cm3. A significant difference of FLAIR abnormalities was found in early postoperative and late postoperative MRI perilesional FLAIR parenchymal abnormality volume (p = 0.0001), in which the early post-operative MRI tumor perilesional FLAIR parenchymal abnormality volume was larger than the 3 month follow up perilesional FLAIR parenchymal abnormality volume. There was no significant difference between intraoperative and late postoperative perilesional FLAIR parenchymal abnormality volume (Fig. 1). Additionally Fig. 2 shows the trend of the pre-operative to immediate post-operative MRI. An illustrative case is shown in Fig. 3 in which the early post-operative FLAIR images over-estimate any possible residual disease as compared to iMRI FLAIR images.
Discussion
For patients with newly diagnosed LGG, EOR has become a prognostic factor for progression free survival and overall survival [25]. Remnants of tumor suggested on postoperative imaging may lead to recommendations for repeat surgery for completion of resection or influence recommendations regarding adjuvant therapy [26]. The management of LGG is often based on the volume of tumor seen post-operatively on FLAIR MRI sequences [27, 28], as the FLAIR sequences are able to delineate tumor boundaries more precisely than T2-weighted sequences, especially when tumors are near cerebrospinal fluids spaces [29]. However, residual blood and degradation products influence relaxation time, which limits optimal determination of FLAIR inversion time. FLAIR signal alterations attributable to surgical trauma, changes in hemoglobin and early stages of fibrosis may lead to overestimation of residual LGG on post-operative MRI depending on the time point after surgery [22, 23, 30,31,32,33,34]. Blood and degradation products may also result in artifact obscuring tumor remnants on early postoperative imaging [35]. Thus, it has been postulated that the routine use of either a final iMRI and/or very early MRI (completed within 24 h) after resection may minimize artifact and better define any remaining tumor.
The current study is a retrospective assessment of patients undergoing surgery for LGG in which no subsequent resection was performed after iMRI due to surgeon and radiology determining GTR and/or additional resection was felt to be unsafe due to high risk of neurologic morbidity, and in which the FLAIR volume abnormalities for intraoperative, early postoperative and 3-month follow-up MRI were assessed using 3 T MRI. We found significantly smaller FLAIR volumes on intraoperative MRI compared to early postoperative MRI, whereas no significant difference was seen comparing intraoperative MRI to 3-month follow up MRI. This indicates that early postoperative MRI may underestimate extent of resection compared to intraoperative and late MRI and should have less influence over immediate surgical and medical decisions compared to iMRI. These results are complementary to prior work that demonstrated that residual tumor volumes were 49% smaller on late postoperative compared with early postoperative FLAIR imaging [35], confirming that early postoperative MRI overestimates the residual volume and that later postoperative MRI defines this volume more precisely.
Previous work by Pala et al. [14] and Masuda et al. [15] has shown that tumor volume and EOR are much better assessed with iMRI compared to early postoperative FLAIR. The Pala et al. [14] study defined the early postoperative MRI as MRIs obtained within 48 h of the resection, compared with the current study which confines the early postoperative MRI window to 24 h. This longer window may account for the larger difference in the FLAIR volumes that were seen in that study. Additionally the Pala study included all LGG regardless of extent of resection, accounting for the larger residual volumes which cumulatively may account for the larger variation in iMRI and early postoperative MRI. A major limitation of the Masuda et al. [15] study was the mix of glioma types that were analyzed and that only 8 LGG were included. Another major limitation of both prior studies was the use of different magnet strengths at different time points. In the present study, eliminating this variable by using the same magnet strength at all time points in the present study helped strengthen confidence in the comparisons of MRIs at different time points.
A recent prospective study evaluated differences in radiation planning volumes for high-grade gliomas when patients were scanned on 3 T MR versus 1.5 T MR. The authors found that radiation planning volumes based on 3 T MRI tended to be smaller compared to volumes based on 1.5 T MRI, and appeared more accurate to level of disease [36]. Additionally in a different study, tumor contrast-to-noise ratio (CNR) was significantly higher for FLAIR at 1.5 T when 3 T on the same patients with WHO II and III grade gliomas [17]. Overall, a higher strength magnet may better delineate residual FLAIR signal and thus more accurately identify residual tumor volumes at different time points. With improved early radiographic assessment, clinicians may be better informed when counseling patients regarding the need for additional surgery or adjuvant therapy.
The differences in volume comparing early to late postoperative MRI were significant and were not accounted for by ischemia-dependent tumor shrinkage. Rather, dissipation of edema likely accounts for a significant component of the reduction in FLAIR volume over this 3 month period. Intraoperative MRI FLAIR volumes showed no significant difference compared to late postoperative MRI. However there is the potential for tumor to grow between the time of iMRI and late postop MR images obtained 3 months later. Therefore, the decision to defer adjuvant therapy in high risk patients while awaiting late post-operative MRI should involve active discussion with neuro-oncology providers and staff based on patient history, symptoms and initial pathology/molecular characterization.
There are limitations with this study including its small sample size and potential for differences in MRI quality based on specific MRI machine variables such as age, manufacturer and MRI technologist. Another limitation is the reliance on a single MRI sequence for each data point. FLAIR hyperintensity can be caused by LGG, edema, gliosis, blood products and ischemia among other causes. It is possible that each MRI time point is identifying different levels of each of these entities. For example, the iMRI may identify edema and blood products, whereas the late MRI, though similar in FLAIR hyperintensity, may be identifying gliosis and aging ischemic changes [37]. Ongoing studies are needed to better clarify MRI variables key for analysis and optimization of patient outcomes.
Conclusion
iMRI helps maximize extent of resection in patients with LGG. The 3 T iMRI FLAIR sequence appears to be superior to early postoperative 3 T MRI FLAIR sequences with regard to assessing residual tumor in patients undergoing surgical resection of LGG. Future work may help better define the role, if any, of routine immediate postoperative MRI.
Data availability
Presented data is available upon request.
References
Smith JS, Chang EF, Lamborn KR et al (2008) Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J Clin Oncol 26(8):1338–1345
van den Bent MJ (2014) Practice changing mature results of RTOG study 9802: another positive PCV trial makes adjuvant chemotherapy part of standard of care in low-grade glioma. Neuro Oncol 16(12):1570–1574
Solomons MR, Rees JH (2020) Seizure outcomes and survival in adult low-grade glioma over 11 years: living longer and better. Neurooncol Pract 7(5):578–579
Alexander BM, Cloughesy TF (2017) Adult glioblastoma. J Clin Oncol 35(21):2402–2409
Ius T, Isola M, Budai R et al (2012) Low-grade glioma surgery in eloquent areas: volumetric analysis of extent of resection and its impact on overall survival. A single-institution experience in 190 patients: clinical article. J Neurosurg 117(6):1039–1052
Jakola AS, Skjulsvik AJ, Myrmel KS et al (2017) Surgical resection versus watchful waiting in low-grade gliomas. Ann Oncol 28(8):1942–1948
Hervey-Jumper SL, Berger MS (2014) Role of surgical resection in low- and high-grade gliomas. Curr Treat Options Neurol 16(4):284
de Leeuw CN, Vogelbaum MA (2019) Supratotal resection in glioma: a systematic review. Neuro Oncol 21(2):179–188
Kavouridis VK, Boaro A, Dorr J, et al (2019) Contemporary assessment of extent of resection in molecularly defined categories of diffuse low-grade glioma: a volumetric analysis. J Neurosurg 1–11
Scherer M, Ahmeti H, Roder C et al (2020) Surgery for diffuse WHO grade II gliomas: volumetric analysis of a multicenter retrospective cohort from the german study group for intraoperative magnetic resonance imaging. Neurosurgery 86(1):E64–E74
Senft C, Bink A, Franz K, Vatter H, Gasser T, Seifert V (2011) Intraoperative MRI guidance and extent of resection in glioma surgery: a randomised, controlled trial. Lancet Oncol 12(11):997–1003
Kubben PL, ter Meulen KJ, Schijns OE, ter Laak-Poort MP, van Overbeeke JJ, van Santbrink H (2011) Intraoperative MRI-guided resection of glioblastoma multiforme: a systematic review. Lancet Oncol 12(11):1062–1070
Li P, Qian R, Niu C, Fu X (2017) Impact of intraoperative MRI-guided resection on resection and survival in patient with gliomas: a meta-analysis. Curr Med Res Opin 33(4):621–630
Pala A, Brand C, Kapapa T et al (2016) The value of intraoperative and early postoperative magnetic resonance imaging in low-grade glioma surgery: a retrospective study. World Neurosurg 93:191–197
Masuda Y, Akutsu H, Ishikawa E et al (2018) Evaluation of the extent of resection and detection of ischemic lesions with intraoperative MRI in glioma surgery: is intraoperative MRI superior to early postoperative MRI? J Neurosurg 131(1):209–216
Zwanenburg JJ, Hendrikse J, Visser F, Takahara T, Luijten PR (2010) Fluid attenuated inversion recovery (FLAIR) MRI at 7.0 Tesla: comparison with 1.5 and 3.0 Tesla. Eur Radiol 20(4):915–922
Tselikas L, Souillard-Scemama R, Naggara O et al (2015) Imaging of gliomas at 15 and 3 Tesla - A comparative study. Neuro Oncol 17(6):895–900
Regnery S, Knowles BR, Paech D et al (2019) High-resolution FLAIR MRI at 7 Tesla for treatment planning in glioblastoma patients. Radiother Oncol 130:180–184
Kounelakis MG, Dimou IN, Zervakis ME et al (2011) Strengths and weaknesses of 1.5T and 3T MRS data in brain glioma classification. IEEE Trans Inf Technol Biomed 15(4):647–654
Truwit CL, Hall WA (2006) Intraoperative magnetic resonance imaging-guided neurosurgery at 3-T. Neurosurgery 58(4 Suppl 2):ONS-338–345; discussion ONS-345–336.
Lavdas E, Tsougos I, Kogia S et al (2014) T2 FLAIR artifacts at 3-T brain magnetic resonance imaging. Clin Imaging 38(2):85–90
Leao DJ, Craig PG, Godoy LF, Leite CC, Policeni B (2020) Response assessment in neuro-oncology criteria for gliomas: practical approach using conventional and advanced techniques. AJNR Am J Neuroradiol 41(1):10–20
Champ CE, Siglin J, Mishra MV et al (2012) Evaluating changes in radiation treatment volumes from post-operative to same-day planning MRI in High-grade gliomas. Radiat Oncol 7:220
Pouratian N, Asthagiri A, Jagannathan J, Shaffrey ME, Schiff D (2007) Surgery insight: the role of surgery in the management of low-grade gliomas. Nat Clin Pract Neurol 3(11):628–639
McGirt MJ, Chaichana KL, Attenello FJ et al (2008) Extent of surgical resection is independently associated with survival in patients with hemispheric infiltrating low-grade gliomas. Neurosurgery 63(4):700–707
Coburger J, Merkel A, Scherer M et al (2016) Low-grade glioma surgery in intraoperative magnetic resonance imaging: results of a multicenter retrospective assessment of the german study group for intraoperative magnetic resonance imaging. Neurosurgery 78(6):775–786
Essig M, Metzner R, Bonsanto M et al (2001) Postoperative fluid-attenuated inversion recovery MR imaging of cerebral gliomas: initial results. Eur Radiol 11(10):2004–2010
Fouke SJ, Benzinger T, Gibson D, Ryken TC, Kalkanis SN, Olson JJ (2015) The role of imaging in the management of adults with diffuse low grade glioma: a systematic review and evidence-based clinical practice guideline. J Neurooncol 125(3):457–479
Essig M, Hawighorst H, Schoenberg SO et al (1998) Fast fluid-attenuated inversion-recovery (FLAIR) MRI in the assessment of intraaxial brain tumors. J Magn Reson Imaging 8(4):789–798
Scherer M, Jungk C, Gotz M, et al (2018) Early postoperative delineation of residual tumor after low-grade glioma resection by probabilistic quantification of diffusion-weighted imaging. J Neurosurg 1–9
Sarbu N, Oleaga L, Valduvieco I, Pujol T, Berenguer J (2016) Increased signal intensity in FLAIR sequences in the resection cavity can predict progression and progression-free survival in gliomas. Neurocirugia 27(6):269–276
Zhang J, Song Y, He M et al (2018) Diagnostic performance of increased signal intensity within the resection cavity on fluid-attenuated inversion recovery sequences for detection of progression in patients with glioma. World Neurosurg 110:434–441
Winterstein M, Munter MW, Burkholder I, Essig M, Kauczor HU, Weber MA (2010) Partially resected gliomas: diagnostic performance of fluid-attenuated inversion recovery MR imaging for detection of progression. Radiology 254(3):907–916
Bette S, Gempt J, Huber T et al (2017) FLAIR signal increase of the fluid within the resection cavity after glioma surgery: generally valid as early recurrence marker? J Neurosurg 127(2):417–425
Belhawi SM, Hoefnagels FW, Baaijen JC et al (2011) Early postoperative MRI overestimates residual tumour after resection of gliomas with no or minimal enhancement. Eur Radiol 21(7):1526–1534
Guarnaschelli JN, Vagal AS, McKenzie JT et al (2014) Target definition for malignant gliomas: no difference in radiation treatment volumes between 1.5T and 3T magnetic resonance imaging. Pract Radiat Oncol 4(5):e195–e201
Lasocki A, Gaillard F (2019) Non-contrast-enhancing tumor: a new frontier in glioblastoma research. AJNR Am J Neuroradiol 40(5):758–765
Author information
Authors and Affiliations
Contributions
KH, MM, MD, PG, WS, BE made substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data; or the creation of new software used in the work; drafted the work or revised it critically for important intellectual content; approved the version to be published; and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Research involving human participants and/or animals
This study was a retrospective study of patients and no identifiable information was utilized.
Informed consent
Informed consent was not needed as this was retrospective study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Huntoon, K., Makary, M.S., Damante, M. et al. Intraoperative 3 T MRI is more correlative to residual disease extent than early postoperative MRI. J Neurooncol 154, 345–351 (2021). https://doi.org/10.1007/s11060-021-03833-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-021-03833-4