Abstract
Introduction
Spinal cord infarction is a rare clinical diagnosis characterized by a sudden onset of paralysis, bowel and bladder dysfunction, and loss of pain and temperature perception, with preservation of proprioception and vibration sense. Magnetic resonance imaging (MRI) usually demonstrates intramedullary hyperintensity on T2-weighted MR images with cord enlargement. However, in approximately 45% of patients, MR shows no abnormality. Diffusion-weighted MR imaging (DWI) has been widely used for the evaluation of a variety of brain disorders, especially for acute stroke. Preliminary data suggest that DWI has the potential to be useful in the early detection of spinal infarction.
Methods
We performed DWI, using navigated, interleaved, multishot echo planar imaging (IEPI), in a series of six patients with a clinical suspicion of acute spinal cord ischemia.
Results
In all patients, high signal was observed on isotropic DWI images with low ADC values (0.23 and 0.86×10−3 cm2/s), indicative of restricted diffusion.
Conclusion
We analyzed the imaging findings from conventional MR sequences and diffusion-weighted MR sequences in six patients with spinal cord infarction, compared the findings with those in published series, and discuss the value of DWI in spinal cord ischemia based on current experience. Although the number of patients with described DWI findings totals only 23, the results of previously published studies and those of our study suggest that DWI has the potential to be a useful and feasible technique for the detection of spinal infarction.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Spinal cord infarction is an uncommon condition, usually presenting clinically with a sudden onset of paralysis, sensory loss, and urinary and bowel dysfunction [1–5]. On MRI, high signal on T2-weighted (T2-W) images of the spinal cord with cord enlargement are usually present [6]. However, in one clinical study, only 45% of the patients with acute spinal cord ischemia had signal intensity changes on T2-W MR images [1]. Diffusion-weighted MR imaging (DWI) has been widely used for the evaluation of a variety of diseases that affect the brain, especially for acute stroke [7–15].
While there are numerous reports about the high sensitivity and usefulness of DWI in the brain, the experience with DWI in the spinal cord is much more limited [16–30]. The main reason for this is the much greater technical difficulty associated with obtaining DWI in the spinal canal. Pulse sequences specifically designed for the spine and spinal cord are not widely available and require much more optimization.
We analyzed imaging findings from conventional MR sequences and diffusion-weighted MR sequences in six patients with spinal cord infarction, compared the findings with those in published series, and discuss the value of DWI in spinal cord ischemia based on current experience. We attempted to prove that: (a) DWI is a sufficiently robust technique for use in suspected cases of spinal cord infarction; and (b) the results of our small series would confirm changes in ADC values in spinal cord ischemia.
Methods
Subjects
Six patients (one male, five females; age range 14–67 years; mean age 52 years) with clinical suspicion of spinal cord infarction were examined with conventional MRI and DWI. In three patients (patients 1, 4 and 6), no obvious cause for spinal ischemia was found.
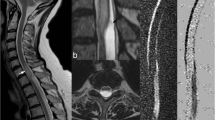
Patient 3 was a 14-year-old previously healthy boy. He suddenly felt a burning, belt-like back pain during soccer training and had progressive walking difficulty. On admission 4 days later, he had a spastic paraparesis, a dissociated anesthesia for pain and temperature below the T2 level, and bladder dysfunction. Patient 5 was a 49-year-old female with a confirmed dural AV fistula at the level of T11, which had been successfully embolized in April 2004, at which time, the patient was asymptomatic and was discharged. She was admitted to hospital 4 months later because of severe pain and weakness in both lower extremities. On initial MR examination performed 1 day later (24 h), high signal intensity was observed in the spinal cord that extended into three vertebral segments in the region of the conus medullaris with spinal cord enlargement (Fig. 1). DWI was not performed. Identical signal alterations were present on all previous MR scans, and spinal infarction was suspected. Follow-up MR examination 8 days later showed a T2-W abnormality and high signal on DWI in the conus region. Marked enhancement was also present on post-contrast T1-W images. Spinal cord infarction in the subacute stage, associated with changed hemodynamics after embolization of the dural AV fistula, was diagnosed. A third follow-up MR scan 1 month later showed residual hyperintensity without enhancement or DWI abnormalities. Patient 2 was diagnosed with degenerative disease with spinal cord compression and consequent venous congestion (Fig. 2).
Patient 5, a 49-year-old female with known dural AV fistula in the thoracolumbar region, presented with severe pain and weakness in both legs. She was treated with embolization 4 months before the onset of symptoms. a Sagittal T2-W MR (TR/TE 3000/140) images revealed a high signal intensity lesion in the conus medullaris. Note the low signal intensity on the dorsal surface of the cord, representing pathological vessels. b On axial T2-W MR image, high signal intensity was observed in the ventral parts of the cord. c High signal was observed at the corresponding level on sagittal DWI, performed using navigated, interleaved, multishot echo planar imaging (IEPI) (5 mm slice thickness, b max 700 s/mm2). d Low ADC value (0.69×10−3 mm2/s) measured on the ADC map confirmed the hypothesis of spinal cord infarction due to hemodynamic disturbance and increased venous pressure
Patient 2, a 60-year-old female with sudden onset of severe low back pain. On conventional MR images, degenerative changes with multiple disc bulging were noted. a On axial DWI (b max 700 s/mm2), a marked high signal abnormality was observed in the spinal cord at the level of C7/T1 vertebral bodies. b Measurements of ADC values on the ADC map at the corresponding level revealed decreased diffusion with an ADC of 0.25×10−3 mm2/s, indicating spinal cord ischemia. c Hyperintensity was also present on sagittal diffusion-weighted (b max 700 s/mm2) images
Imaging
MRI studies were performed on a clinical Philips 1.5-T (Gyroscan ACS-NT 1.5 T, Philips, Best, The Netherlands) magnet using a spine array coil. Sagittal T1-W TSE MR images (TR/TE 400/10 ms), T2-W MR images (TR/TE 3000/140 ms), STIR images (TR/TE/TI 2500/70/170 ms), and axial T2-W TSE MR images (TR/TE 2500/120 ms) were acquired in all patients. DWI was performed using navigated, interleaved, multishot echo planar imaging (IEPI) with the following imaging parameters: FOV 280 mm; slice thickness 5 mm; AP phase-encode direction; EPI factor 13; partial Fourier acquisition; number of b factors 2; max b factor 700 s/mm2; finger pulse triggering; NSA 4; and total scan time, 4:38 min. DWI was performed in the sagittal plane in all patients, and, in three patients, axial DWI images were also obtained. Diffusion encoding gradients were played out sequentially along all three principal axes. Isotropic, diffusion-weighted images (trace DWI) were generated by computing the geometric mean value from the principal axis DWI scans on a per-pixel basis. Apparent diffusion coefficient (ADC) maps were generated from the DWI on a pixel-by-pixel basis with software supplied by the manufacturer. Regions of interest (ROI) were defined in areas with high signal intensity on trace DWI and copied to the ADC map. In addition, the same ROI was placed in the portion of the spinal cord without signal abnormality.
Results
Table 1 summarizes the MRI findings, findings with DWI, and ADC values measured in the lesion and normal-appearing spinal cord (NASC) on MR examinations of our six patients. Spinal cord infarction was located in the cervical spinal cord in one patient (patient 2), in the thoracic cord in two patients (patients 1 and 3), and in the region of the conus medullaris in three patients (patients 4, 5 and 6). On T2-W MR images, high signal was detected in the spinal cord in all patients. Cord enlargement was present in four patients. In all six patients, hyperintense signal abnormalities were present on DWI, corresponding to areas of abnormal signal intensity seen on the T2-W MR images. ADC values ranged from 0.23 to 0.86×10−3 cm2/s. Table 1 summarizes the imaging findings in our six patients with spinal cord infarction, and Table 2 summarizes the imaging findings reported in the literature in 23 patients with spinal cord infarction.
Discussion
Acute spinal cord ischemia is a severe neurological condition with a poor prognosis, and is associated with neuronal death, functional neurological loss, and paraplegia in up to 33% of patients [1]. Atherosclerosis, aortic dissection (3–5% risk), and aortic surgery (1–10% risk) are the most common causes of acute spinal cord ischemia [1]. Other rare causes of spinal cord infarction include: cardiac embolism, decompression sickness, coagulopathy, spinal arteriovenous malformations, systemic hypotension, epidural anesthesia, trauma, and vasculitis. Vascular risk factors are usually present in the majority of patients with spinal cord ischemia; however, fewer than 50% of patients have a definite cause [2]. In 50% of patients, the final etiology remains unclear [3]. The most common location is the central territory of the anterior spinal artery [2]. In one recently published study, 21 of 24 patients with spinal cord ischemia had an infarct located in the central territory of the anterior spinal artery [2].
The short-term outcome in patients with spinal cord infarction depends primarily on the severity of the initial neurological deficit [2, 4]. Of 28 patients in one published series, 15 had a good outcome, and a poor outcome was seen in 13 [2].
On MRI, intramedullary high signal on T2-W images and cord enlargement are usually present. Sagittal T2-W images showed “pencil-like” hyperintensity in 16 of 16 patients in one study, whereas cord enlargement was present in only 9 of 16 patients in another study [5]. The appearance of “snake-eyes” with bilateral hyperintensities on axial T2-W MR images, primarily suggests involvement of the anterior part of the gray matter, which is known to have the highest vulnerability to ischemia [6]. Contrast enhancement may be present in the subacute stage, as well as hemorrhagic transformation, usually recognized as a high signal intensity abnormality in the peripheral parts of the infarction area on T1-W MR images [5]. However, in one clinical study, only 45% of the patients with acute spinal cord ischemia had signal intensity changes on T2-W MR images [1]. In another study, MR did not show any abnormality in two patients, 2 and 3 h, respectively, after the onset of neurological symptoms [5].
The value of DWI in the detection and characterization of cerebral processes has been documented in numerous published reports [7–14]. The extreme sensitivity of DWI in visualizing the hyperacute stroke, compared to the apparent disadvantages of computed tomography (CT) and conventional MR sequences, made DWI the method of choice for the early detection of cerebral infarction in several studies [9, 10, 15]. DWI combined with perfusion MR imaging (PI) is a powerful and important investigational tool in the acute phase of ischemic stroke, as it may differentiate reversible from irreversible brain tissue damage [15].
The results of published reports on DWI in patients with cerebral vascular disorders have subsequently suggested that DWI might also improve early detection of spinal cord infarction. The initial report describing the use of DWI in spinal cord infarction was published in 2000 [22]. Gass et al. reported hyperintensity on T2-W MR images in the central portion of the lumbar cord [22]. DWI was performed 30 h after symptom onset and showed high signal in the spinal cord with a reduction in the ADC value (0.49×10−3 cm2/s), confirming a spinal infarct. Soon after the first report of a patient in 2001, Stepper and Lovblad reported a patient with spinal cord infarction, seen on echo-planar DWI, who had undergone grafting of the descending thoracoabdominal aorta [23]. An area of hyperintensity, corresponding to a decrease in diffusion, was observed on DWI as early as 12 h after the onset of symptoms, with an ADC decrease of 75% compared to the normal spinal cord. Likewise, Weidauer et al. described two patients with anterior spinal artery syndrome, in whom a clear diagnostic benefit was observed for DWI over conventional sequences [24]. In one of the patients, 4 h after the onset of symptoms, conventional MR sequences showed no abnormality, whereas with DWI, high signal intensities were noted in the cord that were consistent with spinal infarction [24].
Reports of larger series (three to five patients), in which DWI findings in spinal cord infarction are described, have followed the initial case reports [27–30]. The findings with DWI, combined with clinical information, enabled the authors of these reports to confidently make the diagnosis of spinal cord infarction. High signal intensities on DWI and low ADC values, compared with the normal cord, were present in these patients as well.
The ADC measured in previously reported patients with spinal cord infarction ranges from 0.23 to 0.9×10−3 mm2/s. The largest series of patients with spinal cord infarction and DWI findings included six patients [28]. In that study, ADC values ranged from 0.23×10−3 to 0.47×10−3 mm2/s [28]. In the present series, ADC values ranged from 0.23 to 0.86×10−3 mm2/s, which is in accordance with previously published data.
The shortest time reported in the literature between the onset of clinical symptoms and abnormalities shown on DWI is 3 h after ictus. It is known from brain studies that DWI is capable of showing abnormalities 30 min after the onset of symptoms [10]. Larger studies, with MR examinations performed soon after the onset of symptoms, are necessary to establish the time threshold for detection of signal abnormalities on DWI in patients with spinal cord ischemia.
In a majority of the previously reported patients, signal abnormalities were demonstrated on T2-W images and on DWI. However, conventional MR sequences failed to show an abnormality in four reported patients at 3, 4, 10, and 46 h after the onset of spinal ischemia [24, 26, 30]. In those patients, DWI showed a clear benefit for patient management. In our small series, all patients had abnormalities on T2-W images as well as on DWI. Considering the fact that the outcome in spinal cord infarction depends on the initial neurological deficit, the possibility of early detection of the abnormality, with subsequent therapeutic consequences, could possibly result in fewer neurological deficits [2, 4].
In some of the published series, follow-up MR examinations (including DWI) were performed. In a patient reported by Gass et al., follow-up MR scans 11 days after the initial MR showed increased ADC, which was interpreted as pseudonormalization [22]. In one of three patients, reported by Fujikawa et al., an increase in ADC was observed on follow-up scans, again indicating pseudonormalization. In two other patients, the ADC values remained low, possibly because of venous contributions to spinal cord infarctions, with delayed clearance of necrotic cells by macrophages due to the individual variations in anastomoses [27, 31]. In three patients with spinal cord infarction, reported by Küker et al. in 2004, a strong diffusion abnormality and abnormalities on T2-W images were demonstrated in all patients [29]. However, diffusion abnormalities showed a rapid decline, suggesting early signal normalization. In one of the patients, the diffusion abnormality disappeared, and ADC returned to normal after 7 days, whereas in the other patient, normalization of ADC was combined with residual hyperintensity on DWI [29]. In the brain, pseudonormalization of the ADC is usually seen in the late subacute phase of an ischemic stroke [32, 33]. In that phase, on T2-W scans, these lesions appear hyperintense, and lesions cannot be distinguished from normal tissue based on imaging or ADC measurements.
In another study, signal abnormalities were detected on DWI with decreased ADC values 26 h after the onset of symptoms in a patient with anterior spinal artery stroke of the cervical spinal cord, which showed a significant decrease in ADC (0.77×10−3 mm2/s) [25]. Follow-up MR imaging 28 days later showed cord atrophy and residual hyperintensity on T2-W MR images, consistent with a chronic stage of infarction. Although an increase in the ADC was noted, compared with the acute stage (1.026 10−3 mm2/s), DWI still demonstrated persistent hyperintensity. In 14 of 28 reported patients, follow-up MR examination with DWI was performed in a time frame ranging from 2 to 28 days. Persistent hyperintensity on DWI was reported in 9 of 14 patients when MR images were obtained between 2 and 9 days. Disappearance of signal abnormalities on DWI was reported after 1 week in only one patient [29]. In one of our patients who was examined 9 days after the onset of symptoms, a diffusion abnormality was still present, with a low ADC value of 0.24×10−3 mm2/s. According to the published reports, diffusion abnormalities can be found after a few hours in patients with spinal cord ischemia, but they may not persist for longer than 1 week, which would be shorter than in brain ischemia. However, further study is required, in a much larger patient cohort, to establish the time-course of spinal cord ischemia.
In a majority of the studies, single-shot echo planar imaging (EPI) was used for DWI of the spinal cord, with maximum b factors between 600 and 1000 s/mm2, and a slice thickness of 5 mm [22–25, 29, 30]. In one study, a single-shot fast spin echo (SSFSE) technique was used [26, 27]. In our study, multi-shot IEPI was used with a maximum b factor of 700 s/mm2. Multi-shot, IEPI has been proven to be less sensitive to geometric and susceptibility distortions than single-shot EPI, yet it is faster than other methods [19]. IEPI provides better spatial resolution and less blurring than single-shot EPI. IEPI was rated superior to fast spin-echo FSE-DWI in one study [19]. Periodically Rotated Overlapping ParallEL Lines with Enhanced Reconstruction (PROPELLER/BLADE) DWI has been introduced recently, but it often allows only axial images and no sagittal images [34]. Even at higher fields, this technique has difficulties with regard to the signal-to-noise ratio. Susceptibility distortions around the spinal cord can lead to “pile-up” artifacts (hyperintensities) that can mimic cord infarction. Based on experience with DWI on our MR system, and also on previous studies of DWI of the spinal cord performed by one of the authors, we used AP phase correction in our patients [19]. All published DWI studies were performed on 1.5-T clinical scanners with sufficient image quality suitable for clinical use.
Although the largest number of patients with described DWI findings is small (six patients), these preliminary data suggest that DWI has the potential to be a useful and feasible technique for the early detection of spinal cord infarction. Early diagnosis of acute spinal cord ischemia contributes to improved patient management and allows earlier application of pharmacological agents that may reduce neuronal apoptosis, and thus, subsequent neurological deficits.
References
Nadeltchev K, Loher TJ, Stepper F, et al (2004) Long-term outcome of acute spinal cord ischemia syndrome. Stroke 35:560–565
Masson C, Pruvo JP, Meder JF, et al (2004) Spinal cord infarction: clinical and magnetic resonance imaging findings and short term outcome. J Neurol Neurosurg Psychiatry 75:1431–1435
Yuh W, Marsh EE, Wang AK, et al (1992) MR imaging of spinal cord and vertebral body infarction. AJNR Am J Neuroradiol 13:145–154
Iseli E, Cavigelli A, Dietz V, Curt A (1999) Prognosis and recovery in ischemic and traumatic spinal cord injury: clinical and electrophysiological evaluation. J Neurol Neurosurg Psychiatry 67:567–571
Weidauer S, Nichtweiss M, Lanfermann H, Zanella FE (2002) Spinal cord infarction: MR imaging and clinical features in 16 cases. Neuroradiology 44:851–857
Hundsberger T, Thömke F, Hopf HC, Fitzek C (1998) Symmetrical infarction of the cervical spinal cord due to spontaneous bilateral vertebral artery dissection. Stroke 29:1742
Le Bihan D (1991) Molecular diffusion nuclear magnetic resonance imaging. Magn Reson Q 7:1–30
Bammer R (2003) Basic principles of diffusion-weighted imaging. Eur J Radiol 45:169–184
Lansberg MG, Mangin JF, Poupon C, et al (2000) Comparison of diffusion-weighted MRI and CT in acute stroke. Neurology 54:1557–1561
Burdette J, Ricci PE, Petitti N, Elster AD (1998) Cerebral infarction: time course of signal intensity changes on diffusion-weighted MR images. AJR Am J Roentgenol 171:791–795
Christensen P, Gideon P, Thomsen C, Stubgaard M, Henriksen O, Larsson HBW (1993) Increased water self-diffusion in chronic plaques and in apparently normal white matter in patients with multiple sclerosis. Acta Neurol Scand 87:195–197
Nakahara M, Ericsson K, Bellander BM (2001) Diffusion-weighted MR and apparent diffusion coefficient in the evaluation of severe brain injury. Acta Radiol 42:365–369
Sundgren PC, Reinstrup P, Romner B, Holtas S, Maly P (2002) Value of conventional, and diffusion- and perfusion weighted MRI in the management of patients with unclear cerebral pathology, admitted to the intensive care unit. Neuroradiology 44:674–680
Stadnik TW, Chaskis C, Michotte A, et al (2001) Diffusion-weighted MR-Imaging of intracerebral masses: comparison with conventional MR-imaging and histologic findings. AJNR Am J Neuroradiol 22:969–976
Thurnher M, Castillo M (2005) Imaging in acute stroke. Eur Radiol 15:408–415
Barker GJ (2001) Diffusion-weighted imaging of the spinal cord and optic nerve. J Neurol Sci 186 [Suppl 1]:S45–S49
Schwartz ED, Chin CL, Takahashi M, Hwang SN, Hackney DB (2002) Diffusion-weighted imaging of the spinal cord. Neuroimaging Clin N Am 12:125–146
Holder CA, Muthupillai R, Mukundan S, Eastwood J, Hudgins PA (2000) Diffusion-weighted MR imaging of the normal human spinal cord in vivo. AJNR Am J Neuroradiol 21:1799–1806
Bammer R, Augustin M, Prokesch R, et al (2002) Diffusion-weighted imaging of the spinal cord: interleaved echo-planar imaging is superior to fast spin-echo. J Magn Reson Med 15:364–373
Bammer R, Fazekas F (2003) Diffusion imaging of the human spinal cord and the vertebral column. Top Magn Reson Imaging 14:461–476
Nagayoshi K, Kimura S, Ochi M, et al (2000) Diffusion-weighted echo planar imaging of the normal human cervical spinal cord. J Comput Assist Tomogr 24:482–485
Gass A, Back T, Behrens S, Maras A (2000) MRI of spinal cord infarction. Neurology 54:2195
Stepper F, Lovblad KO (2001) Anterior spinal artery stroke demonstrated by echo-planar DWI. Eur Radiol 11:2607–2610
Weidauer S, Dettmann E, Krakow K, Lanfermann H (2002) Darstellung von zwei Fällen und Literaturübersicht. Nervenarzt 73:999–1003
Sagiuchi T, Iida H, Tachibana S, Kusumi M, Kan S, Fujii K (2003) Case report: diffusion-weighted MRI in anterior spinal artery stroke of the cervical spinal cord. J Comput Assist Tomogr 27:410–414
Fujikawa A, Tsuchiya K, Takeuchi S, Hachiya J (2004) Diffusion-weighted MR imaging in acute spinal cord ischemia. Eur Radiol 14:2076–2078
Fujikawa A, Tsuchiya K, Koppera P, Aoki C, Hachiya J (2003) Spinal cord infarction demonstrated on diffusion-weighted MR imaging with a single-shot fast spin-echo sequence. J Comput Assist Tomogr 27:415–419
Zhang J, Huan Y, Qian Y, Sun L, Ge Y (2005) Multishot diffusion-weighted imaging features in spinal cord infarction. J Spinal Disord Tech 18:277–282
Küker W, Weller M, Klose U, Krapf H, Dichgans J, Nägele T (2004) Diffusion-weighted MRI of spinal cord infarction—high resolution imaging and time course of diffusion abnormality. J Neurol 251:818–824
Loher TJ, Bassetti CL, Lövblad KO, et al (2003) Diffusion-weighted MRI in acute spinal cord ischemia. Neuroradiology 45:557–561
Zhang Z, Nonaka H, Nagayama T, et al (2001) Circulatory disturbance of rat spinal cord induced by occluding of the dorsal spinal vein. Acta Neuropathol 102:335–338
Ahlhelm F, Schneider G, Backens M, Reith W, Hagen T (2002) Time course of the apparent coefficient after cerebral infarction. Eur Radiol 12:2322–2329
Copen WA, Schwamm LH, Gonzalez RG, et al (2001) Ischemic stroke: effects of etiology and patient age on the time course of the core apparent diffusion coefficient. Radiology 221(1):27–34
Pipe JG, Farthing VG, Forbes KP (2002) Multishot diffusion-weighted FSE using PROPELLER MRI. Magn Reson Med 47:42–52
Conflict of interest statement
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thurnher, M.M., Bammer, R. Diffusion-weighted MR imaging (DWI) in spinal cord ischemia. Neuroradiology 48, 795–801 (2006). https://doi.org/10.1007/s00234-006-0130-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-006-0130-z