Abstract
The incidence of gallstone disease is two to three times higher in women than in men, and female sex hormones, particularly estrogens, have been implicated as contributory factors. Cholesterol nucleation is the initial step in gallstone pathogenesis and proceeds from cholesterol-rich phospholipid vesicles. The aim of this study was to investigate if there is a difference in cholesterol nucleation rates in male and female bile and whether estrogen influences nucleation rates by interacting with cholesterol-rich regions known as “lipid rafts” that exist within the cholesterol-phospholipid vesicles of the bile. Cholesterol nucleation from native prairie dog bile and the interaction of estrogens with lipid rafts in model bile solutions were investigated using Förster resonance energy transfer (FRET). Female native bile samples showed a greater reduction in energy transfer than did male native bile, indicating that cholesterol nucleation occurred more readily in female bile than in male bile. Model bile experiments demonstrated that the addition of estrogen has a significant effect, either cholesterol nucleation or raft disruption, but only in samples containing cholesterol-rich rafts. These results suggest that estrogen interacts with cholesterol-rich rafts in vesicles within bile to promote cholesterol nucleation and predispose females to gallstone formation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gallstone disease is one of the most common and costly digestive diseases, affecting 10–15% of the U.S. population and accounting for 600,000 cholecystectomies per year, with an annual cost of more than six billion dollars (Friedman et al. 1966; NIH Consensus Development Panel on Gallstones and Laparoscopic Cholecystectomy 1993). The incidence of cholesterol gallstones is two to three times higher in women than in men (Diehl 1991). Female sex hormones have been implicated as contributory factors to the promotion of gallstones. Increased parity is also considered as an independent risk factor in gallstone disease, especially among young women (Bernstein et al. 1973). In addition, estrogen treatment in men with prostate cancer has been found to increase the incidence of gallstones compared to men treated with other methods (Henriksson et al. 1989), further suggesting a contributory role of estrogens in gallstones. It is believed that female steroids promote gallstones by decreasing gallbladder motility and increasing biliary cholesterol saturation (Everson et al. 1991; White et al. 1976).
Hepatic secretion of cholesterol-saturated bile is a necessary (Admirand and Small 1968), but not sufficient, prerequisite for gallstone formation since cholesterol-supersaturated bile is common in the healthy population (Holzbach et al. 1973). This suggests that additional defects such as alterations in nucleation time (Holan et al. 1979; Sedaghat and Grundy 1980) and gallbladder absorption (Lee 1978; Conter et al. 1986; Giurgiu et al. 1997) may be required to complete the process. It has become apparent that a “nucleation defect” exists in patients with cholesterol gallstones, although the exact nature of this abnormality has yet to be defined. While some investigators suggest that antinucleating factors are absent in the bile of gallstone-containing patients (Holzbach et al. 1984), others suggest that pronucleating factors are the cause of this nucleation defect (Burnstein et al. 1983; Levy et al. 1984; Groen et al. 1990).
Formation of gallstones occurs via a multistep process. Cholesterol is secreted as unilamellar cholesterol and phospholipid vesicles into the bile, where bile salts micellize the vesicles (Donovan 1999). Depending on the bile salt:cholesterol ratio, equilibrium consists of either micelles alone or both vesicles and micelles. The vesicles remaining at equilibrium (if there are any) are enriched in cholesterol because phospholipids are more easily micellized than cholesterol (Strasberg and Harvey 1990; Somjen and Gilat 1985; Ulloa et al. 1987). Aggregation of these small unilamellar vesicles into larger, multilamellar vesicles (MLVs) has been observed to directly precede cholesterol nucleation (Halpern et al. 1986; Peled et al. 1989). It has been well established that phospholipid and cholesterol vesicles are heterogeneous, containing small, cholesterol-rich “rafts” within a cholesterol-depleted continuous phase (Brown and London 1997; Simons and Ikonen 1997). Therefore, it is likely that the cholesterol-rich vesicles that remain in the bile after micellization contain rafts.
Estrogens have profound effects on bile composition. They are excreted into bile, after metabolism in the liver, in female prairie dogs (Longwell and McKee 1942). Within the bile, estrogens alter bile composition in humans, by increasing the fraction of cholesterol and changing the bile salt composition (Heuman et al. 1980), and estrogen treatment (either oral or transdermal) has been shown to decrease nucleation time (Uhler et al. 1998). The mechanism by which estrogen mediates these effects has not yet been determined, however. It has been shown that estrogen affects membrane fluidity by intercalating into the membrane (Tsuda et al. 2001; Tsuda and Nishio 2004; Liang et al. 2001; Whiting et al. 2000), and it is possible that this effect occurs through the disruption of rafts in the vesicle. Lipid rafts, therefore, represent a possible nucleation site and estrogen, a possible nucleation factor.
In this work, we attempted to determine if there is a difference in cholesterol nucleation between males and females and, if so, if estrogen is a contributory factor responsible for these differences. Specifically, we were interested in determining whether estrogen interacts with lipid rafts during the nucleation process, thereby predisposing females to gallstone pathogenesis in the prairie dog, which has emerged as an important animal model for the study of human cholesterol gallstone disease (Holzbach 1984).
Materials and Methods
Materials
Cholesterol, β-estradiol (98% pure), dehydroergosterol (DHE), sodium chloride (NaCl), calcium chloride (CaCl2), sodium azide (NaN3), bovine serum albumin (BSA), β-estradiol-6-(O-carboxymethyl)oxime:BSA and 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid (HEPES) were purchased from Sigma (St. Louis, MO). 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC) and 1-myristoyl-2-[12-[(5-dimethylamino-1-naphthalenesulfonyl)amino]dodecanoyl]-sn-glycero-3-phosphocholine (DAN-PC) were purchased from Avanti Polar Lipids (Alabaster, AL). All chemicals were used without further purification.
Animal Care and Gallbladder Bile Harvesting
Adult black-tailed male and female prairie dogs (Cynomys ludovicianus), trapped in the wild and obtained from Flyers Speciality Pets (Lubbock, TX), were caged individually in a 23°C thermoregulated room. In order to examine whether females are predisposed to gallstones, the animals were fed a control nonlithogenic diet consisting of normal laboratory chow (Purina Laboratory Chow; Ralston-Purina, St. Louis, MO), sufficient to maintain body weight. After a 16-h fast with water ad libitum, the animals were intramuscularly anesthetized with ketamine (100 mg/kg body weight) and xylazine (1.5 mg/kg), cholecystectomy was performed via a midline laparotomy, gallbladders were harvested and bile was collected. Use of animals followed approval by the Institutional Animal Care and Use Committee (IACUC) of Drexel University College of Medicine.
Native Bile Compositional Analysis
Gallbladder bile samples were aliquoted fresh and stored at –20°C until analyzed using methods that have been long established in our laboratory (Abedin et al. 1989) for levels of cholesterol (Roschlau et al. 1974), phospholipids (Dryer et al. 1957) and total bile acids (Iwata and Yamasaki 1964). Carey’s critical tables were used to calculate the cholesterol saturation index (CSI) on the basis of the total lipid concentration (Carey and Small 1978). Total calcium was determined by a previously published method (Anderegg et al. 1951; Connerty and Briggs 1966). Total and conjugated bilirubin were measured by a modification of the methods of Michaelsson (1961) and Nosslin (1960). Total protein was measured by the method of Lowry et al. (1951).
Förster Resonance Energy Transfer
We have developed Förster resonance energy transfer (FRET) assays to quantify true nucleation times in solutions of model bile (Wrenn et al. 1999, 2001) as well as to measure raft size (Brown et al. 2007a). In this work, DHE was used as a FRET donor and DAN-PC was used as a FRET acceptor. DHE is a fluorescent analogue of cholesterol which acts similarly to cholesterol, while DAN-PC is a fluorescently labeled phospholipid, which has been observed to act similarly to an unsaturated phospholipid (Brown et al. 2007a). The probes partition differentially between the raft and nonraft phases, with DHE partitioning preferentially into the raft phase along with cholesterol and DAN-PC being almost completely excluded from the raft phase (Brown et al. 2007a). This differential partitioning allows for domain size measurement. In addition, this pair can be used to detect cholesterol nucleation directly (Wrenn et al. 1999, 2001) as DHE nucleates and crystallizes with cholesterol.
FRET can be measured in several ways; for this work, two methods were used. To study cholesterol nucleation, the ratio of donor and acceptor peaks (R) was measured as follows:
where F D is the intensity of the donor at its maximal wavelength (373 nm for DHE) and F A is the intensity of the acceptor at its maximal wavelength (515 nm for DAN-PC). During cholesterol nucleation/crystallization, DHE leaves the vesicle along with cholesterol, while DAN-PC remains in the vesicle. This increased distance between the probes causes FRET to decrease, which is observed by an increase in donor peak and a decrease in acceptor peak, leading to an increased ratio (Wrenn et al. 2001).
Relative raft size is measured through the use of FRET efficiency (%E). This measure is calculated from the intensity of the donor at its maximal emission wavelength (373 nm for DHE) in the presence and absence of acceptor:
where F DA and F D are the donor emission intensities in the presence and absence of acceptor, respectively (Lakowicz 1999). Because of the differential partitioning of DAN-PC and DHE between raft and nonraft phases, this experimental efficiency can be related to domain size (Brown et al. 2007b); larger domains correspond to lower efficiency values and smaller domains correspond to higher efficiency values.
Labeling of Native Gallbladder Bile
Films of DHE and/or DAN-PC were prepared using the film deposition technique (Szoka and Papahadjopoulos 1980). DHE (0.3 mg) and DAN-PC (0.45 mg) dissolved in chloroform were added to scintillation vials. The chloroform was evaporated under a stream of nitrogen, and any residual chloroform was removed by exposing the samples to a vacuum overnight. It was necessary to dilute the bile samples in order to obtain a sufficient sample volume, as well as to eliminate the inner filter effect during fluorescence spectroscopy. Gallbladder bile samples were diluted six times with buffer containing 0.15 m NaCl, 5 mm CaCl2, 5 mm HEPES and 3 mm NaN3 at a pH of 7.4. Diluted bile (2 ml) was added to the DHE/DAN-PC films on day 0. The samples were vortex-mixed and incubated with the fluorescent probes for 1 h before use.
Model Bile Preparation
Model bile solutions were prepared using the rapid solvent exchange (RSE) technique (Buboltz and Feigenson 1999). Stock solutions of DMPC, cholesterol, DHE, DAN-PC and estradiol dissolved in chloroform were added to 20 ml flat-bottomed vials in the required amounts. Heated (60°C) aqueous buffer (3 ml) was added. The solution was then vortex-mixed while exposed to a vacuum (4.92 inches mercury absolute pressure) for 1 min and then diluted with buffer to a final lipid concentration of 1 mm. The buffer (pH of 7.4) contained 0.15 m NaCl, 5 mm CaCl2, 5 mm HEPES and 3 mm NaN3.
Three sets of model vesicles were created in this work to investigate three regions of the phase diagram for the DMPC-Chol model system at 30°C. The first set contained 5% sterol, making it completely liquid-disordered (ld). The second set contained 20% sterol so that it resided within the two-phase region (the region of interest in the study of rafts). The third set contained 50% sterol and was completely liquid-ordered (lo) (Almeida et al. 1992; Tampe et al. 1991). Each of these samples also contained 5% DHE, DAN-PC at an acceptor-to-lipid ratio (ALR) of 0.0–12.0 and 0–10% estrogen.
Fluorescence Measurements
Fluorescence measurements were obtained using a steady-state fluorescence spectrometer (Photon Technology, Ontario, Canada; model Q-5/W-601) with a circulating water bath to maintain the sample temperature. The temperature was read on a cuvette thermometer (Fisher, Philadelphia, PA; model 15-078 J). All native bile experiments were performed at room temperature, and all model bile experiments were performed at 30°C.
Results
Native Bile Compositional Analysis
Lipids
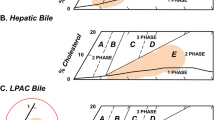
The amounts of cholesterol, total bile acids, phospholipids and total lipids in each native bile sample are shown in Table 1 along with the CSI. In five of the six pairs of samples, the female sample contained more cholesterol than the male did. A majority of the female samples likewise contained a larger amount of total bile acids, phospholipids and total lipids than the male samples. In addition, the CSI was higher in four of the six female samples than in the male samples. Overall, the female bile samples contained significantly more cholesterol, total bile acids, phospholipids and total lipids and had a greater CSI than the male samples. These bile compositions, on a mol% basis, were overlaid on the equilibrium phase diagram for bile (Wang and Carey 1996) in Fig. 1. Each of these bile samples resides within the one-phase micellar region, with little difference between male and female samples overall.
Native bile compositions for male (filled circles) and female (open circles) samples overlaid on the equilibrium phase diagram for bile (redrawn from Carey and Small 1978). Region 1 represents a one-phase micellar region. Region 2 contains micelles and cholesterol crystals. Region 3 contains micelles, vesicles and cholesterol crystals. Region 4 contains micelles and vesicles
Nucleating Factors
Table 2 contains an analysis of some proposed nucleating factors in each of the native bile samples. A majority of female samples contained more Ca2+, total proteins and unconjugated bilirubin than the male samples, while a majority of male samples contained more total bilirubin and conjugated bilirubin than the female samples. In addition, more of the male samples contained a higher conjugated:unconjugated bilirubin ratio than the female ones. Overall, the female samples contained more Ca2+, total proteins, total bilirubin and unconjugated bilirubin and the male samples contained more conjugated bilirubin and had a higher conjugated:unconjugated bilirubin ratio.
Native Bile Nucleation
An established FRET assay, which relates FRET between DHE, a fluorescent cholesterol analogue, and DAN-PC, a fluorescently labeled phospholipid, to cholesterol nucleation (Brown et al. 2007b), was used to detect cholesterol nucleation in native prairie dog bile. Figure 2 shows representative emission scans for one fluorescently labeled bile set (set 2). The emission intensities are normalized by the DAN-PC peak intensity (measured at 515 nm). On day 0, for both male and female samples, the DHE peak (at 373 nm) was much lower than the DAN-PC peak (515 nm). This corresponded to a low R value. By day 14, the DHE peak in both samples had increased relative to the DAN-PC peak, resulting in an increased value of R, a trend which continued through day 42. In this sample set, the DHE peak increased relative to the DAN-PC peak to a greater extent in the female sample than in the male sample. This increase in DHE peak relative to the DAN-PC peak, resulting in an increase in R, indicates a decrease in energy transfer due to an increased distance between DHE and DAN-PC. DHE is believed to partition similarly to cholesterol, and this decrease in energy transfer is interpreted as the initiation of cholesterol nucleation.
Figure 3 shows the FRET ratio (R) for all six pairs of female and male prairie dog bile samples (fluorescently labeled with DHE and DAN-PC) measured over a 2-month period. In the first three sets of samples, readings were obtained on days 0, 14, 28 and 56; in the second three sets of samples, readings were obtained every 7 days in order to better determine the kinetic behavior. Both male and female samples displayed a fairly steep initial upturn in R, followed by a plateau, as seen in the representative emission scans (Fig. 2). In five of the six pairs, the females displayed a higher ratio than the males. It appears that both male and female samples began to nucleate almost immediately (as observed by the initial sharp increase in R) and that the cholesterol in the female samples more often crystallized to a greater extent than it did in the male samples.
FRET ratio for six pairs of male (filled diamond) and female (open square) prairie dog bile samples, fluorescently labeled with DHE and DAN-PC. a Pair 1, b pair 2, c pair 3, d pair 4, e pair 5 and f pair 6. It can be seen that in five of the six pairs the FRET ratio is higher in the female sample than the male sample
Figure 4a shows the overall summary of the first three sets of male and female bile, and Fig. 4b shows the overall summary of the second two sets of male and female bile. In both cases, the female ratio is higher than the male, indicating increased extent of cholesterol nucleation in the females.
Model Bile Nucleation
FRET in DHE- and DAN-PC-labeled DMPC-Chol vesicles containing estrogen is shown in Fig. 5. In samples containing 5% cholesterol (Fig. 5a), which are completely ld, the FRET profile increases slightly with addition of up to 10% estrogen. This increase in FRET indicates a decreased distance between donor and acceptor, suggesting an increase in order upon addition of estrogen. Likewise, samples containing 50% cholesterol (Fig. 5c), which are completely lo, show a FRET profile that increases significantly with addition of estrogen, indicating an estrogen-induced increase in order. However, samples within the two-phase (raft-containing) region, containing 20% cholesterol (Fig. 5b), display a markedly different FRET behavior upon addition of estrogen. These samples exhibit a FRET profile that is seen to decrease with addition of estrogen, representing an increase in donor–acceptor distance due to one of two possibilities: (1) an increase in raft radius or (2) initiation of cholesterol nucleation.
Discussion
FRET studies in native bile indicate that cholesterol may nucleate faster in female bile than in male bile. In most cases, the female sample showed a greater extent of nucleation compared to the male sample, and on average, the female bile samples showed a greater extent of nucleation than the male samples.
Comparison of the nucleation results (Fig. 2) with the biliary lipid analysis (Table 1) shows a striking correlation between extent of nucleation and cholesterol content in the bile. Those samples containing more cholesterol were observed to nucleate to a greater extent than those with less cholesterol. However, when the biliary lipid, on a mol% basis (Fig. 1), is compared to the nucleation results (Fig. 2), the correlation is less apparent. On a percentage basis, all of the samples reside within the one-phase micellar region of the bile phase diagram and are therefore not expected to form crystals without some external driving force.
Several factors which have been previously shown to promote cholesterol nucleation and gallstone formation were quantified in the native bile samples. Certain proteins, such as mucin, immunoglobulins, phospholipase C and fibronectin, have been proposed as pronucleating factors (Harvey et al. 1991; Pattinson and Willis 1991; Chijiiwa et al. 1991; Upadhya et al. 1993; Smith 1987). Likewise, the biliary Ca2+ concentration has been shown to have pronucleating effects (Gleeson et al. 1992; Strichartz et al. 1988), and the concentration of bilirubin has been identified as a potential pronucleating agent (Nakai et al. 2001; Alponat et al. 1997). However, in the native bile samples reported here, the extent of nucleation does not noticeably correlate with these nucleating factors; a correlation between nucleation and sex is much clearer in these native bile samples. On average, the female native bile samples nucleated to a greater extent than the male samples did. This observation raises the possibility that estrogen may act as a pronucleating factor.
In order to eliminate sample-to-sample compositional variation and to clearly determine the role of estradiol on cholesterol nucleation in bile, we created several model bile samples of defined composition. We found that the effect of estradiol in model bile systems is highly dependent on lipid composition and, specifically, on the presence or absence of lipid rafts. In vesicles with lipid rafts, addition of estradiol caused a strong dose-dependent increase in raft size or cholesterol nucleation. However, in vesicles without lipid rafts, addition of estradiol had an opposite effect, possibly increasing the membrane order slightly. Previous studies have found that lipid composition is extremely important in cholesterol nucleation. It has been established that supplementation with phospholipids can increase nucleation time and that the type of phospholipid used in the supplementation plays a major role in the nucleation time (Halpern et al. 1993; Mendez-Sanchez et al. 2001). Phospholipid supplementation likewise shifts the location of biliary cholesterol from vesicles to micelles and thereby decreases the cholesterol-to-phospholipid ratio of the vesicles (Halpern et al. 1993). In addition, the hydrophobicity of bile has been shown to impact nucleation times, with less hydrophobic model biles displaying faster cholesterol crystal growth rates (Ochi et al. 1996).
In this work we found an additional effect of lipid composition on gallstone pathogenesis in response to estradiol presence. We have shown that estrogen has a significant effect on vesicles containing lipid rafts, while having a limited effect on vesicles without lipid rafts. The exact mechanism of this effect has not been established completely; however, the finding has a significant impact on interpretations of the effect of estradiol on vesicles. Currently, a large disparity in reported results regarding the effect of estradiol on membrane fluidity exists, with some groups reporting an increase in order (Tsuda et al. 2001; Whiting et al. 2000), others reporting a decrease in order (Clarke et al. 1990; Liang et al. 2001; Schwarz et al. 1996) and others reporting no effect (Roy et al. 1990). While the purpose of this work was not specifically to investigate the effect of estradiol on membrane fluidity, we did ultimately find that the effect of estradiol on vesicles varies considerably, depending on the membrane composition and cholesterol content and, specifically, on the presence or absence of lipid rafts. This finding could explain, in part, this disparity in findings regarding the effect of estradiol on membrane fluidity.
The increase in FRET that was observed upon addition of estradiol in the non-raft-containing vesicles suggests that estradiol increases the order of the vesicle, thereby decreasing the distance (and increasing FRET) between donor and acceptor molecules. In vesicles containing 5% cholesterol, estradiol may interdigitate within the vesicles, creating local order, similar, but perhaps reduced in strength, to the ordering behavior of cholesterol. In the vesicles containing 50% cholesterol, estradiol may act in concert with cholesterol to again increase vesicle order, resulting in the increase in FRET that was observed upon estradiol addition.
Most interesting, however, is the effect of estradiol on raft-containing vesicles. In these vesicles, we observed a decrease in FRET, suggesting one of two possibilities: raft growth or cholesterol nucleation. It has been suggested that sterols may accumulate preferentially in regions of the vesicle containing high cholesterol content (Whiting et al. 2000). Accumulation of estradiol in the cholesterol-rich raft region would increase the raft size. Furthermore, estradiol could increase the local sterol composition within the raft to a composition that is sufficient to initiate cholesterol nucleation. An additional possibility is that within the raft, which is already highly ordered, estradiol may increase order enough to force the cholesterol to nucleate. Regardless of the exact mechanism of action, it is apparent that estradiol has a profound effect on raft-containing vesicles. Although the model bile studies were necessarily performed at nonphysiological concentrations, one can clearly see that even small amounts of estradiol have significant effects on lipid phase behavior, specifically on lipid rafts.
Rafts have been found to play a role in a wide variety of membrane processes (Simons and Ikonen 1997; Golden et al. 1999; de Gassart et al. 2003; Dykstra et al. 2003; Cherukuri et al. 2001; Fiedler et al. 1993), although their possible role in cholesterol nucleation has not yet been reported. We found that estrogen has a greater effect on vesicles containing rafts and propose that these rafts might present a nucleation point in the formation of gallstones. A schematic of the proposed mechanism of estrogen effect on gallstone pathogenesis is shown in Fig. 6.
Proposed mechanism of estrogen effects on lipid rafts. Cholesterol is secreted into bile as phospholipid-cholesterol vesicles, which are micellized by the bile salts present in bile to form phospholipid-cholesterol-bile salt micelles and unstable cholesterol-rich vesicles when bile is saturated with cholesterol. These unstable vesicles aggregate and fuse to form large vesicles, enriched in cholesterol. If these large vesicles contain rafts, it is proposed that estrogen interacts with the cholesterol-rich rafts, causing cholesterol nucleation and gallstone formation
While the exact means by which estrogen acts on lipid rafts has not been elucidated, we have shown clearly that female bile nucleates to a greater extent on average than male bile and that variation in bile composition alone cannot fully explain this difference. Additionally, we found that estrogen disrupts lipid rafts in some manner, presenting a potential means by which estrogen influences cholesterol crystallization from bile that may partly explain the gender differences in gallstone pathogenesis.
References
Abedin MZ, Strichartz SD, Festekdjian S, Roslyn JJ (1989) Increased biliary calcium in cholesterol and pigment gallstone disease: the role of altered bile acid composition. Lipids 24:572–578
Admirand WH, Small DM (1968) The physicochemical basis of cholesterol gallstone formation in man. J Clin Invest 47:1043–1052
Almeida PFF, Vaz WLC, Thompson TE (1992) Lateral diffusion in the liquid phases of dimyristoylphosphatidylcholine/cholesterol lipid bilayers: a free volume analysis. Biochemistry 31:6739–6747
Alponat A, Kum CK, Rajnakova A, Koh BC, Goh PMY (1997) Predictive factors for synchronous common bile duct stones in patients with cholelithiasis. Surg Endosc 11:928–932
Anderegg C, Flaschka H, Sallman R, Schwarzenback GM (1951) Metallindikatoren IV. Ein auf erdkaliionen ansprechendes phtalien und seine analytische Verwendung. Helv Chim Acta 37:113–120
Bernstein RA, Werner LH, Rimm AA (1973) Relationship of gallbladder disease to parity, obesity, and age. Health Serv Res 88:925–936
Brown AC, Towles KB, Wrenn SP (2007a) Measuring raft size as a function of membrane composition in PC-based systems: part II. Ternary systems. Langmuir 23:11188–11196
Brown AC, Towles KB, Wrenn SP (2007b) Measuring raft size as a function of membrane composition in PC-based systems: part I. Binary systems. Langmuir 23:11180–11187
Brown DA, London E (1997) Structure of detergent-resistant membrane domains: does phase separation occur in biological membranes? Biochem Biophys Res Commun 240:1–7
Buboltz JT, Feigenson GW (1999) A novel strategy for the preparation of liposomes: rapid solvent exchange. Biochim Biophys Acta 1417:232–245
Burnstein MJ, Ilson RG, Petrunka CN, Taylor RD, Strasberg SM (1983) Evidence for a potent nucleating factor in the gallbladder bile of patients with cholesterol gallstones. Gastroenterology 85:801–807
Carey MC, Small DM (1978) The physical chemistry of cholesterol solubility in bile. Relationship to gallstone formation and dissolution in man. J Clin Invest 61:998–1026
Cherukuri A, Dykstra M, Pierce S (2001) Floating the raft hypothesis: lipid rafts play a role in immune cell activation. Immunity 14:657–660
Chijiiwa Z, Koga A, Yamasaki T, Shimada K, Noshiro H, Nakayama F (1991) Fibronectin: a possible factor promoting cholesterol monohydrate crystallization in bile. Biochim Biophys Acta 1086:44–48
Clarke R, van den Berg HW, Murphy RF (1990) Reduction of the membrane fluidity of human breast cancer cells by tamoxifen and 17beta-estradiol. J Natl Cancer Inst 82:1702–1705
Connerty HV, Briggs AR (1966) Determination of serum calcium by means of orthocresolphthalein complexone. Am J Clin Pathol 45:290–296
Conter RL, Roslyn JJ, Porter-Fink V, DenBesten L (1986) Gallbladder absorption increases during early cholesterol gallstone formation. Am J Surg 91:184–191
de Gassart A, Geminard C, Revrier B, Raposo G, Vidal M (2003) Lipid raft–associated protein sorting in exosomes. Blood 102:4336–4344
Diehl A (1991) Epidemiology and natural history of gallstone disease. Gastroenterol Clin North Am 20:1–19
Donovan JM (1999) Physical and metabolic factors in gallstone pathogenesis. Gastroenterol Clin North Am 28:75–97
Dryer RL, Tammes AR, Routh JI (1957) The determination of phosphorus and phosphatase with n-phenyl-p-phenylenediamine. J Biol Chem 225:177–184
Dykstra M, Cherukuri A, Sohn HW, Tzeng S, Pierce SK (2003) Location is everything: lipid rafts and immune cell signaling. Annu Rev Immunol 21:457–481
Everson GT, McKinley C, Kern F Jr (1991) Mechanisms of gallstone formation in women. J Clin Invest 87:237–246
Fiedler K, Kobayashi T, Kurzchalia TV, Simons K (1993) Glycosphingolipid-enriched, detergent-insoluble complexes in protein sorting in epithelial cells. Biochemistry 32:6365–6373
Friedman GD, Kannel WB, Dawber TR (1966) The epidemiology of gallbladder disease: observations in the Framingham Study. J Chronic Dis 19:273–292
Giurgiu DIN, Saunders-Kirkwood KD, Roslyn JJ, Abedin MZ (1997) Sequential changes in biliary lipids and gallbladder ion transport during gallstone formation. Ann Surg 225:382–390
Gleeson D, Hood KA, Murphy GM, Dowling RH (1992) Calcium and carbonate ion concentrations in gallbladder and hepatic bile. Gastroenterology 102:1707–1716
Golden GA, Mason RP, Tulenko TN, Zubenko GS, Rubin RT (1999) Rapid and opposite effects of cortisol and estradiol on human erythrocyte Na + , K + -atpase activity: relationship to steroid intercalation into the cell membrane. Life Sci 65:1247–1255
Groen AK, Noordam C, Drapers JA, Egbers P, Jansen PL, Tytgat GN (1990) Isolation of a potent cholesterol nucleation-promoting activity from human gallbladder bile: role in the pathogenesis of gallstone disease. Hepatology 11:525–533
Halpern Z, Dudley MA, Kibe A, Lynn MP, Breuer AC, Holzbach RT (1986) Rapid vesicle formation and aggregation in abnormal human biles. A time-lapse video-enhanced contrast microscopy study. Gastroenterology 80:875–885
Halpern Z, Moshkowitz M, Laufer H, Peled Y, Gilat T (1993) Effect of phospholipids and their molecular species on cholesterol solubility and nucleation in human and model biles. Gut 34:110–115
Harvey PR, Upadhya GA, Strasberg SM (1991) Immunoglobulins and nucleating proteins in the gallbladder bile of patients with cholesterol gallstones. J Biol Chem 266:13966–14003
Henriksson P, Einarsson K, Eriksson A, Kelter U, Angelin B (1989) Estrogen-induced gallstone formation in males. J Clin Invest 84:811–816
Heuman R, Larsson-Cohn U, Hammar M, Tiselius HG (1980) Effects of postmenopausal ethinylestradiol treatment on gallbladder bile. Maturitas 2:69–72
Holan KR, Holzbach RT, Hermann RE, Cooperman AM, Claffey WJ (1979) Nucleation time: a key factor in the pathogenesis of cholesterol gallstone disease. Gastroenterology 77:611–617
Holzbach RT (1984) Animal models of cholesterol gallstone disease. Hepatology 4:191S–198S
Holzbach RT, Marsh M, Olszewski M, Holan K (1973) Cholesterol solubility in bile. Evidence that supersaturated bile is frequent in healthy man. J Clin Invest 52:611–617
Holzbach RT, Kibe A, Thiel E, Howell JH, Marsh M, Hermann RE (1984) Biliary proteins. Unique inhibitor of cholesterol crystal nucleation in human gallbladder bile. J Clin Invest 73:35–45
Iwata T, Yamasaki K (1964) Enzymatic determination and thin-layer chromatography of bile acids in blood. J Biochem 56:424–431
Lakowicz JR (1999) Principles of fluorescence spectroscopy, 2nd edn. Plenum, New York
Lee SP (1978) Enhanced fluid transport across gallbladder mucosa in experimental cholelithiasis. Am J Physiol 234:E575–E578
Levy PF, Smith BF, Lamont JT (1984) Human gallbladder mucin accelerates nucleation of cholesterol in artificial bile. Gastroenterology 84:270–275
Liang Y, Belford S, Tang F, Prokai L, Simpkins JW, Hughes JA (2001) Membrane fluidity effects of estratrienes. Brain Res Bull 54:661–668
Longwell BB, McKee FS (1942) The excretion of estrogens in the bile and urine after the administration of estrone. J Biol Chem 142:757–764
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Mendez-Sanchez M, Gonzalez V, Aguaryo P, Sanches JM, Tanimoto MA, Elizondo J, Uribe M (2001) Fish oil (n-3) polyunsaturated fatty acids beneficially affect biliary cholesterol nucleation time in obese women losing weight. J Nutr 9:2300–2303
Michaelsson M (1961) Bilirubinate determination in serum and urine. Scand J Clin Lab Invest 13:40–50
Nakai K, Tazuma S, Ochi H, Chayama K (2001) Does bilirubin play a role in the pathogenesis of both cholesterol and pigment gallstone formation? Direct and indirect influences of bilirubin on bile lithogenicity. Biochim Biophys Acta Mol Cell Biol Lipids 1534:78–84
NIH Consensus Development Panel on Gallstones, Laparoscopic Cholecystectomy (1993) Gallstones and Laparoscopic Cholecystectomy. JAMA 269:1018–1024
Nosslin B (1960) The direct diazo reaction of bile pigments in serum. Scand J Clin Lab Invest 12:1–17
Ochi H, Tazuma S, Kajiyama G (1996) Lecithin hydrophobicity modulates the process of cholesterol crystal nucleation and growth in supersaturated model bile systems. Biochem J 318:139–144
Pattinson NR, Willis KE (1991) Effect of phospholipase C on cholesterol solubilization in model bile. A conanavalin A–binding nucleation-promoting factor from human gallbladder bile. Gastroenterology 101:1339–1344
Peled Y, Halpern Z, Eitan B, Goldman G, Konokoff F, Gilat T (1989) Biliary micellar cholesterol nucleates via the vesicular pathway. Biochim Biophys Acta 1003:246–249
Roschlau P, Bernt E, Gruber W (1974) Enzymatic determination of total cholesterol in serum. Z Klin Chem Klin Biohem 12:403–407
Roy EJ, Buyer DR, Licari VA (1990) Estradiol in the striatum: effects on behavior and dopamine receptors but no evidence for membrane steroid receptors. Brain Res Bull 25:221–227
Schwartz Z, Gates PA, Nasatzky E, Sylvia VL, Mendez J, Dean DD, Boyan BD (1996) Effect of 17β-estradiol on chondrocyte membrane fluidity and phospholipid metabolism is membrane-specific, sex-specific, and cell maturation-dependent. Biochim Biophys Acta Biomembr 1282:1–10
Sedaghat A, Grundy SM (1980) Cholesterol crystals and the formation of cholesterol gallstones. N Engl J Med 302:1274–1277
Simons K, Ikonen E (1997) Functional rafts in cell membranes. Nature 387:569–572
Smith BF (1987) Human gallbladder mucin binds biliary lipids and promotes cholesterol crystal nucleation in model bile. J Lipid Res 28:1088–1097
Somjen GJ, Gilat T (1985) Contribution of vesicular and micellar carriers to cholesterol transport in human bile. J Lipid Res 26:699–704
Strasberg SM, Harvey PR (1990) Biliary cholesterol transport and precipitation. Introduction and overview of conference. Hepatology 12:1S–5S
Strichartz SD, Abedin MZ, Abdou MS, Roslyn JJ (1988) Increased biliary calcium in cholesterol gallstone formation. Am J Surg 155:131–137
Szoka F, Papahadjopoulos D (1980) Comparative properties and methods of preparation of lipid vesicles (liposomes). Annu Rev Biophys Bioeng 9:467–508
Tampe R, von Lukas A, Galla HJ (1991) Glycophorin-induced cholesterol-phospholipid domains in dimyristoylphosphatidylcholine bilayer vesicles. Biochemistry 30:4909–4916
Tsuda K, Kinoshita Y, Kimura K, Nishio I, Masuyama Y (2001) Electron paramagnetic resonance investigation of modulatory effect of 17β-estradiol on membrane fluidity of erythrocytes in postmenopausal women. Arterioscler Thromb Vasc Biol 21:1306–1312
Tsuda K, Nishio I (2004) Role of estrogen in the regulation of membrane microviscosity. Circ Res 94:17–21
Uhler ML, Marks JW, Voigt BJ, Judd HL (1998) Comparison of the impact of transdermal versus oral estrogens on biliary markers of gallstone formation in postmenopausal women. J Clin Endocrinol Metab 83:410–414
Ulloa N, Garrido J, Nervi F (1987) Ultracentrifugal isolation of vesicular carriers of biliary cholesterol in native human and rat bile. Hepatology 7:235–244
Upadhya GA, Harvey PR, Strasberg SM (1993) Effect of human biliary immunoglobulins on the nucleation of cholesterol. J Biol Chem 268:5193–5200
Wang DQ, Carey MC (1996) Characterization of crystallization pathways during cholesterol precipitation from human gallbladder biles: identical pathways to corresponding model biles with three predominating sequences. J Lipid Res 37:2539–2549
White CM, Howat JM, Schofield PF (1976) Lithogenic bile: a study in women taking oral contraceptives. Br J Surg 63:664
Whiting KP, Restall CJ, Brain PF (2000) Steroid hormone-induced effects on membrane fluidity and their potential roles in non-genomic mechanisms. Life Sci 67:743–757
Wrenn SP, Kaler EW, Lee SP (1999) A fluorescence energy transfer study of lecithin-cholesterol vesicles in the presence of phospholipase C. J Lipid Res 40:1483–1494
Wrenn SP, Gudheti M, Veleva AN, Kaler EW, Lee SP (2001) Characterization of model bile using fluorescence energy transfer from dehydroergosterol to dansylated lecithin. J Lipid Res 42:923–934
Acknowledgements
This work was supported by National Institutes of Health grant DK 070865 (to Mohammad Z. Abedin and Steven P. Wrenn).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brown, A.C., Wrenn, S.P., Suresh, N. et al. Gender Differences in Cholesterol Nucleation in Native Bile: Estrogen Is a Potential Contributory Factor. J Membrane Biol 232, 35–45 (2009). https://doi.org/10.1007/s00232-009-9214-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00232-009-9214-0