Abstract
Dried fruits have gained more and more space in the food market. Osmotic dehydration (OD) can be applied as a pre-treatment to convective drying, aiming to produce foods with different characteristics. Therefore, the present study evaluated the OD process of banana slices using coconut sugar and sucrose, as well as its influence on convective drying (CD) and the physicochemical parameters of the product. Osmotic solutions at 40 and 60% were prepared, and OD was conducted at 30 and 50 °C. OD and CD kinetic parameters were analyzed. The dried product was characterized by moisture, water activity, shrinkage, texture, color, bioactive and volatile compounds. The higher concentration (60%) and higher temperature (50 °C) resulted in higher values of water loss, solid gain, and weight reduction during OD for both sugars. CD time varied between 225 and 345 min. OD as pre-treatment reduced drying time by up to 65%. The dried banana had low moisture content and low water activity. The shrinkage was up to 73.44%, associated with the higher concentration treatment and higher temperature during OD. OD reduced product hardness after CD. In general, using coconut sugar resulted in greater changes in color parameters and higher levels of bioactive compounds in dried bananas. Volatile compounds highly related to banana flavor were present after drying. Therefore, the use of coconut sugar as an alternative did not compromise the efficiency of the process and resulted in a product with some distinct characteristics compared to those obtained using sucrose.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The banana is one of the most consumed fruits in the world. Its production occurs in most tropical countries, including Brazil (Kowalski et al., 2013; Mercali et al., 2010). In recent years, there has been a significant increase in the number of exports of this fruit, reaching 21 million tons in 2019. The leading exporters are countries in Central America, South America, and the Philippines, while the main importers are the European Union, the United States, China, the Russian Federation, and Japan (FAO, 2023).
Due to the volume produced, banana losses after harvest can be quite significant, as they are highly perishable, and the fruits cannot go through freezing processes. In this way, drying techniques become helpful in maintaining quality and reducing losses (Mercali et al., 2010). Furthermore, the dry product has advantages such as smaller volume, ease of distribution, year-round availability, and low perishability (Braga et al., 2009). Although there are several ways to dry food, drying with heated air is the most traditional method (Rodriguez et al., 2019).
Due to the long exposure period of the material to hot air, drying is an essential step in producing dried bananas. In this way, pre-treatment studies for drying have been developed to minimize physical–chemical and nutritional changes in food and reduce process costs (Bai et al., 2023). Among the available pre-treatments, OD can be highlighted; its main characteristics are the simplicity of the process and low operating cost (Taşova et al., 2022).
OD comprises a complex and dynamic mass exchange process between food and hypertonic solution (Mercali et al., 2010). One of the advantages of OD over traditional drying methods is that it allows the moisture content of the product to be reduced at low temperatures or even at room temperature (Chiu et al., 2017). OD as a pre-treatment for convective drying has been shown to reduce energy consumption during drying and minimize color changes and loss of flavor and functional compounds (An et al., 2013; Pravitha et al., 2022).
Several studies have evaluated the OD of fruits and vegetables using mainly solutes such as sucrose (An et al., 2013; Ayetigbo et al., 2019; Chiu et al., 2017; da Silva et al., 2014; Delgado et al., 2017; Mercali et al., 2010), sodium chloride (de Jesus Junqueira et al., 2017; Grzelak-Błaszczyk et al., 2020), polyols (Macedo et al., 2022a; Mendonça et al., 2017), and isomaltulose (Giannakourou et al., 2020; Macedo, Corrêa, Petri Júnior, et al., 2022a, b, c; Macedo, Corrêa, Vimercati, et al., 2022a, b, c). However, although sucrose is the most-used solute in OD, it is a highly caloric compound and does not contain nutritional compounds. Therefore, there is a need to find alternative solutions that are more nutritious and contain fewer calories in order to serve consumers who are more concerned about their health (Pravitha et al., 2022; Saraiva et al., 2020).
Coconut sugar can be considered healthier when compared to sucrose, given its levels of antioxidant compounds, minerals, vitamins, and lower glycemic index (Pravitha et al., 2022). Among the minerals and vitamins found in coconut sugar, iron, potassium, zinc, magnesium, and vitamins B1, B2, B3, and B6 deserve mention (Hebbar et al., 2015). This natural sweetener is obtained by cutting and harvesting the sap that flows from the coconut tree (Cocos nucifera L.) (Saraiva et al., 2020).
Considering that each osmotic agent can interfere with the OD and convective drying processes differently, it is clear that evaluating these changes is essential to improve these processes. However, despite all its documented qualities, the use of coconut sugar has yet to face further examinations. Thus, this study aimed to evaluate how sucrose and coconut sugar use in different concentrations (40 and 60%) and temperatures (30 and 50 °C) affects mass exchange during OD, convective drying curves, and the physicochemical characteristics of dried banana slices.
Materials and Methods
Materials
Bananas (Prata cultivar) and refined sugar were purchased from local stores. Coconut sugar was purchased from Cerealista Castelo®.
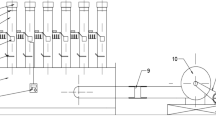
Bananas were selected for their physical integrity and degree of maturation (predominantly yellow peel and green tips, 20.72 ± 1.65°Brix). For the experiments, the banana peels were manually removed, and the fruit was cut into cylinders (5 mm thickness and 28 mm diameter) with the help of a food slicer and a stainless steel mold. The experimental steps are shown in Fig. 1.
Osmotic Dehydration (OD)
Osmotic solutions were prepared by dissolving sucrose or coconut sugar in distilled water at concentrations of 40 and 60% (w/w) (Table 1).
The osmotic solutions were added to cylindrical polystyrene containers concurrently with banana slices (1:20 w/v), which were then packaged in an incubator to maintain the process temperature (30 or 50 °C) (Table 1). The proportion of fruit/solution used aimed to keep the solution concentration unchanged during the experiments (da Silva et al., 2014; Luchese et al., 2015).
Samples were removed from the solutions at predetermined times (0.5, 1, 2, 3, 4, and 5 h) to calculate the dehydration kinetics. After removal, the slices were immersed in ice-cold water for 10 s, followed by draining the surface with absorbent paper and weighing on an analytical balance (de Jesus Junqueira et al., 2017; Macedo et al., 2021a, b).
Mass Transfer Parameters
The values of water loss (WL), solid gain (SG), and weight reduction (WR) during OD were calculated according to Eqs. (1), (2), and (3), respectively (Macedo et al., 2023a, b).
where W represents the weight of the sample, in kg; M represents the moisture of the sample in kg water/kg sample; and “0” and “t” indicate the initial and t times, respectively.
Peleg's model (Peleg, 1988) was adjusted to the values of WL, SG, and WR, according to Eq. 4, 5, and 6 (Macedo et al., 2023a, b).
where \({k}_{1}\) and \({k}_{2}\) are the Peleg model constants for WL, SG, and WR, and t is the time in minutes.
The value of k1 can be related to the initial mass transfer rate (t = 0), calculated as shown in Eqs. 7, 8 and 9 (Mendonça et al., 2017).
In the equilibrium condition (t → ∞), the parameters were calculated according to Eqs. 10, 11, and 12 (Mendonça et al., 2017).
Drying Kinetics and Mathematical Model Adjustment
Fresh bananas (FB) and the samples subjected to OD treatments were dried in a cabin dryer (Polidryer, PD-25, Viçosa, Brazil) at 60 °C and an air speed of 1.5 m/s. For this, the samples (~ 60 g) were placed in perforated stainless steel trays, which were inserted into the dryer and had their masses measured every 15 min until the end of the drying process. Drying was stopped when the samples reached a moisture content of 17.24 ± 1.08%wb, aiming to achieve water activity values below 0.6 (Macedo et al., 2020; Macedo et al., 2021b).
The moisture ratio (MR) was calculated according to Eq. 13.
where xt is the water content on a dry basis at any instant of time t, xe is the water content in the equilibrium condition, and x0 is the initial water content.
The moisture content at the equilibrium condition was determined by drying the samples for 1440 min under the same experimental conditions used to obtain the dried samples (Macedo et al., 2023b).
The Page model (Eq. 14) was fitted to MR data using non-linear regression (Macedo et al., 2020).
where t is the drying time (minutes), k and n are model parameters.
Characterization of Samples
FB, dried banana (DB), and the samples subjected to OD treatments were evaluated in terms of moisture content, water activity, shrinkage, texture, color, bioactive compounds, and volatile compounds.
Moisture and Water Activity (aw)
The moisture content of the samples after convective drying was determined gravimetrically, according to (AOAC, 2010).
The aw of the samples and osmotic solutions were determined at 25 °C by an electronic hygrometer (Aqualab, series 3TE, Washington, USA).
Shrinkage
Shrinkage was determined by calculating the volume of the samples (Macedo, Corrêa, Vimercati, et al., 2022a, b, c). A digital pachymeter (Stainless Hardened) was used to measure the dimensions of the bananas, which were used to calculate the cylinder volume. The degree of shrinkage was calculated according to Eq. 15.
where V and V0 represent the volumes (m3) of dried banana and fresh slices, respectively.
Texture
The hardness of fresh and dry samples was determined through compression testing using a texture analyzer device (Brookfield Engineering Labs, CT3). The test tip was knife-edge (TA7), 10000 g load cell and the test speed/return speed was 2 mm/s.
Color
The color of the samples was measured using a bench colorimeter (Konica Minolta, model CR-400, Osaka, Japan). The parameters L*, a*, b*, chroma (C*), and tone angle (°h) were obtained. The total color difference value (∆E) was calculated using the fresh sample as standard (subindex 0), according to Eq. 16 (Rai et al., 2021).
Bioactive compounds
Extract Preparation
The extracts used to analyze total phenolic compounds and antioxidants were made as described by (Rufino et al., 2010). Briefly, compounds from fresh and dried bananas were extracted from 40 mL of methanolic solution (50% v/v) and 40 mL of acetone solution (70% v/v). After adding the methanolic solution, the samples remained at rest for 1 h in darkness, followed by centrifugation for 15 min. The acetone solution was added to the residue from the first extraction, and the mixture remained at rest for another 1 h, followed by centrifugation for 15 min. Then, the two extracts were mixed, and the final extract volume was made up to 100 mL with distilled water.
Total Phenolic Content (TPC)
The TPC content was determined by the Fast Blue BB method, according to the methodology described by (Medina, 2011). 0.3 mL of Fast Blue BB reagent (0.1%), 0.3 mL of NaOH (5%), and 3 mL of extract were used. The reaction lasted 90 min, and the absorbance was read at 420 nm on a spectrophotometer (Global analyzer, GTA-96, China). The blank consisted of adding water in place of the sample. A standard curve of gallic acid diluted in water (0 to 500 ppm) was made, and the final result was expressed as mg of gallic acid equivalent per g sample (mg GAE/g) on a dry basis.
Antioxidant Capacity (AC)
The antioxidant capacity of the samples was determined by the ABTS (2,20-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid) and FRAP (2,4,6-tris(2-pyridyl)-s-triazine) assays, according to methodologies described by Rufino et al. (2010).
ABTS
Solutions of 7 mM ABTS and 145 mM potassium persulfate were prepared to carry out the ABTS assay. To carry out the ABTS assay, solutions of 7 mM ABTS and 145 mM potassium persulfate were prepared. The ABTS radical was prepared by mixing 5 mL of ABTS stock solution with 88 µL of potassium persulfate, which remained at rest and in darkness for 16 h. Subsequently, the ABTS radical was diluted in ethanol to an absorbance of 0.70 at 734 nm, and 30 µL of the sample was mixed with 3 mL of the radical for the reaction for 6 min. The absorbance was read in a spectrophotometer (Global analyzer, GTA-96, China) at 734 nm. Ethyl alcohol was used as a blank to calibrate the equipment. A standard curve with known concentrations of Trolox (100 to 2000 µL) was constructed and the results were expressed µmol trolox/g fruit on a dry basis.
FRAP
For the FRAP assay, 90 µL of the extract, 270 µL of water, and 2.7 mL of the FRAP reagent (TPTZ, FeCl3, and acetate buffer) were used. The test tubes were kept at 37 °C in a water bath for 30 min. Subsequently, the absorbance of the sample was read at 595 nm in a spectrophotometer (Global analyzer, GTA-96, China). FRAP reagent was used as a blank. A standard curve for ferrous sulfate (500 to 2000 µM) was prepared, and the results were expressed as µmol of ferrous sulfate/g fruit on a dry basis.
Volatile Compounds
The volatile compounds from fresh and dried bananas were isolated by headspace solid phase micro-extraction (HS-SPME) and analyzed by gas chromatography, according to the methodology proposed by de Jesus Filho et al. (2018), with minor modifications.
To extract the volatile components, 2 g of sample was mixed with NaCl (5%) and the vials were kept in a water bath (60 °C) for 30 min. A divinylbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS) fiber was manually inserted into the headspace of the sample container. The volatile compounds adsorbed on the fiber were identified by gas chromatography coupled to a mass spectrometer (QP-PLUS-2010, SHIMADZU). The capillary column was fused silica (30 m long and 0.25 mm internal diameter) with Rtx-5MS stationary phase. The column temperature started at 60 °C and increased at a rate of 3 °C per minute until it reached a maximum of 240 °C. The injector and detector temperatures were 220 and 300 °C, respectively. Helium was used as a carrier gas.
The volatile components were identified by comparing the samples' mass spectra with the equipment database (Wiley7) and the linear retention index (LRI), calculated after the injection of a standard of linear alkanes.
Statistical Analysis
The experiment was conducted in a completely randomized design with five repetitions for osmotic processes and three repetitions for convective drying. The physical–chemical analyses were carried out in triplicate, and the data were evaluated by analysis of variance and Tukey test for comparing means at a significance level of 5%.
Results and Discussion
Kinetic of OD Evaluation
In Fig. 2 and 3, variations in WL (a), SG (b) and WR (c) can be seen as a function of OD time using coconut sugar and sucrose at different concentrations and temperatures.
WL, SG, and WR increased over time, as also observed in other OD kinetics studies (Mercali et al., 2011; Tortoe et al., 2007; X. Wang et al., 2022). There was a noticeable increase in responses in the first few minutes compared to the rest of the process due to the greater osmotic pressure gradient between both systems. The osmotic pressure gradient is the driving force for mass exchange during OD. As the systems exchange mass, the gradient decreases, reducing the intensity of the exchanges until equilibrium is reached (Assis et al., 2016).
For both sugars, a similar behavior was observed for the analyzed variables, in which WL, SG, and WR increased as the immersion time in the osmotic solution increased. Furthermore, it was found that the highest WL, SG, and WR values were associated with the highest osmotic solution concentration and temperature values. This result is in line with that reported by other authors. Chiu et al. (2017) also observed an increase in WL with increasing sucrose concentration and temperature during OD. The same behavior was reported by Song et al. (2020), who found an increase in SG and WL values as the OD time advanced and the temperature and concentration of the osmotic solution were raised. According to An et al. (2013), raising the temperature reduces the viscosity of the hypertonic solution and increases the speed of movement of water molecules across cell membranes, elevating WL. Another possible effect is swelling of the cell membrane with increasing temperature. When this occurs, an increase in the membrane's permeability to the passage of water and solid compounds is expected (Rai et al., 2021).
The values found for WL and SG are close to those reported by Shukla et al. (2018), who report WL values ranging from 38.66 to 48.57% and SG ranging between 5.87 and 6.68% for osmo-dehydrated banana slices in sucrose solution in concentrations of 40, 50 and 60% and temperatures of 40, 50 and 60 °C, for 4 h. On the other hand, the WL and SG values reported in this study were lower than the 47% WL and 15.39% SG found by Rai et al. (2021). However, the experimental conditions used for the latter differed from those of the present work, using a 65% sucrose solution, 50 °C temperature, and 6 h of dehydration process.
In general, WL curves tend to have higher values when compared to SG curves due to the lower molecular weight and greater diffusivity of water concerning solids (Ayetigbo et al., 2019). It is observed that the SG value tends to rise with increasing immersion time. Previous studies have demonstrated that the sample incorporates more solute in more concentrated solutions and at higher temperatures (An et al., 2013; Ayetigbo et al., 2019). This fact is related to the greater diffusion rate when greater concentration gradients are used (Song et al., 2020).
Table 2 presents the values of the parameters obtained after adjusting the Peleg model, which presented high coefficient of determination (R2) values and low standard error of estimate (SEE) values for all treatments. Additionally, the values predicted by the Peleg model were close to the experimental values (Fig. 4), given that the data dispersion was close to a 45° slope.
At constant temperature, the k1 values obtained for WL, SG, and WR decreased with increasing concentration of osmotic solutions (Table 2), indicating increased mass transfer rates when using more concentrated solutions.
The k2 values can be used to estimate the equilibrium conditions during OD. Thus, lower values of this parameter indicate higher equilibrium content (Ganjloo et al., 2012). As seen in Table 2, the k2 values for WL and WR reduced with increasing concentration of the osmotic solution, obtaining similar values for both sugars. Similarly, there was a reduction in the value of k2 for WR as the temperature was increased from 30 to 50 °C. However, for WL, the increase in temperature only resulted in a lower value of the k2 parameter when 60% solutions were used.
The WL, SG, and WR values obtained at the end of 300 min of OD are presented in Fig. 5. For the variables WL (Fig. 5a) and WR (Fig. 5c), a significant interaction (p < 0.05) was verified between the three factors under study (type of osmotic agent, concentration, and temperature). However, for SG (Fig. 5b), only the concentration of the osmotic solution was significant (p < 0.05); it was verified that when raising the concentration of the osmotic solution from 40 to 60%, the SG value also increased. For WL and WR, it was found that the increase in temperature and concentration resulted in higher values for these variables. Regarding the type of sugar, only the SC60%30 °C and CS60%30 °C treatments differed concerning WL and WR, with coconut sugar presenting the lowest value compared to sucrose. In general, coconut sugar and sucrose showed very similar behavior for WL, SG, and WR. These results are in line with those reported by Macedo et al., (2023a, b), who evaluated the use of coconut sugar and sucrose for osmotic dehydration of strawberries and observed that the two osmotic agents did not differ in the values from WL, SG, and WR.
Values of water loss (a), solid gain (b), and weight reduction (c) obtained after 300 min of OD using coconut sugar and sucrose. Values are expressed as the mean ± standard deviation. Values with the same uppercase letter do not differ (p > 0.05) concerning the type of osmotic agent. Values with the same lowercase letter do not differ (p > 0.05) concerning the concentration of the osmotic solution. Values with the same amount of asterisk do not differ (p > 0.05) concerning the OD temperature
Drying Kinetics
In the second stage of this study, samples of fresh bananas pre-treated by OD were subjected to convective drying. The drying kinetics at 60 °C for fresh banana and osmotic treatments can be seen in Fig. 6. It was observed that moisture decreases over time for all treatments. In the initial minutes of the drying process, moisture removal from the osmo-dehydrated samples occurred slightly faster than the fresh banana. This effect was more evident for samples that were dehydrated with coconut sugar (Fig. 6a). Pravitha et al. (2022) found that OD increased the effective diffusivity values in osmo-dehydrated coconut samples with different sugars, presenting a shorter drying time than untreated coconut.
The behavior of moisture ratio data over drying time was very similar for both osmotic agents, culminating in very similar drying times. This phenomenon can be explained by the similarity in composition between these two osmotic agents. According to Pravitha et al. (2022), 86.86% of sucrose is found in coconut sugar.
Comparing the curves obtained (Fig. 6), it was verified that the drying time was different between the treatments. The longest drying time was obtained for fresh banana samples, while bananas dehydrated at a higher sugar concentration (60%) and higher OD temperature (50 °C) had a shorter drying time. A longer drying time for fresh bananas may be related to this sample's higher initial moisture content (71.06%) compared to the moisture values of osmo-dehydrated samples, ranging from 50 to 64%. which ranged from 50 to 64%. For all treatments, drying times were between 225 and 345 min. The dried product obtained is shown in Fig. 7.
The Page model adjusted adequately to the experimental data, exhibiting high R2 values (above 0.99) and low SEE values for all treatments (Table 3). The predicted versus experimental moisture ratio data were close, as shown in Fig. 8, demonstrating a dispersion close to a 45° slope. Thus, we can infer that this model can accurately estimate the relationship between the moisture ratio and drying time of osmo-dehydrated banana samples with both osmotic agents. The parameters k and n of the Page model were similar between the samples. k is associated with the diffusion rate and was slightly higher for samples that went through OD. On the other hand, the parameter n indicates the type of diffusion, with an n value lower than 1 being observed for all treatments, which may indicate a sub-diffusion phenomenon during drying (Simpson et al., 2017).
Physicochemical Analysis
Figures 9, 10 and 11 present the effects of OD and convective drying processes on the physicochemical properties of dried banana.
Moisture and Water Activity (aw)
The moisture content of foods is an essential indicator of their stability during prolonged storage (Pravitha et al., 2021). The highest moisture value was reported for the fresh sample (FB) sample (Fig. 9a), equal to 71.06 ± 1.55%. After drying, moisture values were between 15.08 ± 0.23% and 18.87 ± 0.06% (Fig. 9a). It was observed that some samples that underwent the OD treatment had a moisture content significantly higher than the dried banana (DB) treatment (without osmotic treatment), namely SC40%50 °C, SC60%30 °C, SC60%50 °C, CS40%30 °C and CS60%50 °C.
As occurred with the moisture content, the aw values also decreased in the samples after drying. As expected, the highest aw value was found for the FB, equal to 0.965 ± 0.004 (Fig. 9b). On the other hand, the DB and CS40%30 °C samples did not differ statistically and presented the lowest values for this parameter. It was found that, for all treatments, the aw value was lower than 0.60, ensuring good stability and microbiological safety of the dry product. The aw value of 0.60 is taken as a reference, as below this value, the growth of microorganisms is highly unfavorable, guaranteeing a longer shelf life for the product. The aw of food products is an important quality indicator, as it is related to the level of susceptibility of food to the growth of microorganisms and chemical reactions. Therefore, higher aw values result in shorter shelf-life products (Kroehnke et al., 2021).
Shrinkage
Shrinkage is an important physical property of solid foods that undergo drying processes. Sample shrinkage occurs due to moisture loss, generating an imbalance of pressure inside the product, causing tensions that lead to shrinkage or even rupture of the sample (Nahimana et al., 2011).
Sample shrinkage ranged from 59.26 ± 0.60 to 73.44 ± 0.72% (Fig. 9c). The shrinkage values found for bananas were similar to those reported in other studies that used OD for strawberries (42.6% to 72.2%) (Macedo et al., 2023a, b) and bananas (54.9 to 67.5%) (Tabtiang et al., 2012). Thuwapanichayanan et al. (2011) found that during drying, changes in banana diameter were minimal, around 10%, while changes in thickness were much more pronounced, reaching values of up to 70%.
The treatments using the highest concentrations of coconut sugar and sucrose for both temperatures did not differ from each other and resulted in samples with higher shrinkage values. However, osmotic treatments at lower concentrations and temperatures (CS40%30 and SC40%30) demonstrated shrinkage equal to that of the sample not subjected to OD.
It was also observed that the treatments with the greatest shrinkage presented the highest WL and WR values in the OD stage. According to Nahimana et al. (2011), water loss and solids gain during OD are closely related to the level of sample shrinkage. Furthermore, a likely explanation for the more significant shrinkage in samples subjected to OD at higher sugar concentrations is the fact that there is a greater probability of interaction between the hydroxyl group in the solution and the hydroxyl group in the banana via hydrogen bonds, causing distortions in the sample (Tabtiang et al., 2012).
Texture
The texture of the banana slices was compared concerning the hardness parameter, as shown in Fig. 9d. It was found that the drying process increased in hardness concerning the FB. The DB treatment resulted in samples with greater hardness (88.87 ± 7.20 N) when compared to treatments that used previous osmotic treatment. The treatments using higher concentrations of sucrose (SC60%30 °C and SC60%50 °C) and those using a high concentration of coconut sugar and higher temperature (SC60%50 °C) showed lower hardness than the others. Rai et al. (2021) also reported lower hardness values for banana slices after OD than fresh bananas. These authors suggest that changes in cellular structure, with loss of turgidity, may explain this phenomenon. Furthermore, this effect can be enhanced at higher OD temperatures.
Color
The evaluation of the color of products intended for human consumption is highly relevant due to its close relationship with the level of food acceptance by the consumer (Pravitha et al., 2022). The values obtained for the parameters L*, C*, °h, and ∆E are shown in Fig. 10.
The parameter L* (lightness) is related to the degree of lightness of the sample. According to the results presented in Fig. 10a, the L* value was lower for all samples in which coconut sugar was used in OD, especially for treatments with the highest concentrations of this sugar (AC60%50 °C and AC60%30 °C), indicating a tendency towards a darker color in these samples. The color given to these treatments is predominantly related to the characteristics of the coconut sugar used, which has a brownish color. This same behavior was reported in previous studies. Macedo et al., (2023a, b) and Pravitha et al. (2022), when using coconut sugar for osmotic dehydration of strawberries and coconuts, respectively, also found a reduction in the L* values of the samples. On the other hand, higher L* values were obtained for samples that did not undergo OD or that underwent OD using sucrose. The DB treatment did not differ statistically from the FB concerning the sample's lightness level.
The C* (chroma) parameter indicates how intense the color of a given sample is. Pathare et al. (2013). According to Fig. 10b, no significant differences were observed between the C* values for the FB, DB, and osmotic processes using sucrose treatments, these values being higher than the C* of the samples that underwent OD with coconut sugar. The higher C* values of these samples demonstrate greater color intensity. Macedo et al., (2023a, b) also observed a lower C* value for osmo-dehydrated strawberries with coconut sugar compared to sucrose.
For the parameter °h (tone angle) it was observed that the lowest values were obtained for osmotic treatments using coconut sugar. For the FB sample, the highest °h value was obtained (Fig. 10c), equal to 85.25 ± 1.43. °h values closer to 90° indicate the yellower color of the sample (Pathare et al., 2013). Therefore, osmo-dehydrated samples with coconut sugar showed a greater distance from the yellow hue characteristic of fresh bananas.
The ∆E values can be seen in Fig. 10d. Treatments using coconut sugar resulted in samples with a higher ∆E value, indicating a greater color difference concerning the fresh sample. On the other hand, the DB and sucrose-treated samples had less color difference than the FB. These results align with those found by Pravitha et al. (2022), who also found a higher ∆E between osmotically dehydrated coconut using brown sugar and coconut sugar concerning fresh coconut. According to Adekunte et al. (2010), E values can be classified into ranges so that ∆E > 3 indicates that the samples have a very distinct color; 1.5 < ∆E < 3 the samples are distinct, and ∆ E < 1.5 samples have only a little color difference. Therefore, according to this classification, only the DB sample (∆E = 2.34 ± 0.12) presents a different color from the FB. In contrast, the other treatments present a very different color compared to FB.
Bioactive Compounds
The product's content of phenolic compounds and antioxidants was lower after drying when compared to FB (Fig. 11). The content of phenolic compounds found for FB is close to that found in the literature for different banana varieties, as reported by Rózek et al. (2010), Morais et al. (2015), Al Amri and Hossain (2018) found values equal to 1.04 mg/g, 2.64 mg/g, and 3.86 mg/g, respectively. Factors affecting phenolic compounds' content in bananas include the extraction technique, the extracting agent, and the variety of fruit (Al Amri and Hossain, 2018; Sulaiman et al., 2011).
As found in this work, the reduction of banana phenolic compounds after drying was also evidenced by Guiné et al. (2015), who observed a 20% loss of phenolic compounds after drying at 50 °C. Furthermore, OD processes can reduce the content of water-soluble bioactive compounds due to the leaching of these components into the osmotic solution (de Jesus Junqueira et al., 2018).
According to Fig. 11a, only the treatments using coconut sugar at the highest concentration (CS60%30 °C and CS60%50 °C) resulted in a content of phenolic compounds that did not differ from the treatment that did not undergo OD (DB). In some cases, sugar impregnation during OD may have a protective effect on phenolic compounds so that, after convective drying, these compounds remain preserved (Rózek et al., 2010). Furthermore, drying temperature may have a beneficial effect by releasing phenolic compounds from the vacuole structure due to tissue collapse, increasing extractable phenolics (Sulaiman et al., 2011). On the other hand, other authors have observed a reduction in the values of bioactive compounds in samples submitted to OD. de Jesus Junqueira et al. (2018) observed lower values of phenolic compounds in beetroot, carrots, and eggplant. Macedo et al., (2023a, b) reported a reduction in the values of several bioactive compounds in strawberries, including anthocyanins, ascorbic acid, phenolic compounds, and antioxidants.
It was observed differences between the two methods used for antioxidant activity. According to the results of the ABTS method, only the fresh banana differed from the others, having greater antioxidant activity (Fig. 11c). On the other hand, according to the FRAP method (Fig. 11b), the AC60%30 °C treatment was the closest to the value found for FB, having presented the highest antioxidant activity among all osmotic treatments. In contrast, the SC40%50 treatment °C resulted in lower antioxidant content. These results are in line with those found by Pravitha et al. (2022), who reported greater antioxidant activity in coconut samples that underwent OD using coconut sugar and brown sugar than in samples that underwent OD with sucrose. The greater antioxidant activity of osmo-dehydrated samples with coconut sugar is related to the antioxidant properties of this osmotic agent. Karseno et al., (2018) demonstrated that the Maillard reaction products generated during the production of this sugar confer antioxidant activity. They also further demonstrated that there is a linear relationship between the increase in the product's color intensity and the antioxidant activity found.
Volatile Compounds
The majority volatile component found for fresh, dried and dried osmo-dehydrated bananas was isoamyl butyrate (Table 4). Previous studies have reported this compound as the component in the most significant quantity of fresh bananas (de Jesus Filho et al., 2018; Thuwapanichayanan et al., 2011). Furthermore, the other compounds reported in Table 4 are in agreement with the results found by other authors (Boudhrioua et al., 2003; Saha et al., 2018; J. Wang et al., 2007). It was observed that some compounds found in dried bananas were also present in fresh bananas, demonstrating that the OD and drying processes resulted in a product with aroma characteristics similar to fresh food.
According to Saha et al. (2018), rapid surface drying can have a protective effect on the banana volatile compounds trapped inside the slices. These authors observed that this effect is more pronounced at higher drying temperatures, so important compounds for banana aroma, such as isoamyl acetate and isobutyl, are better preserved. However, changes occur primarily due to the effects of heat, oxygen, and chemical interactions with other compounds. The loss of volatile compounds is among the main quality degradations caused by drying (Karam et al., 2016).
According to Thuwapanichayanan et al. (2011), isoamyl butyrate, isoamyl isovalerate, and isoamyl acetate are the main compounds responsible for the banana aroma. Brat et al. (2004) demonstrated that the aromatic profile of this fruit can be greatly influenced by factors such as altitude, cultivar, and degree of ripeness. Wang et al. (2007) dried banana and found that acetic acid, butanoic acid, and isovalerate esters formed the dominant volatile compound category in the dried product. Some compounds reported by these authors include hexyl isovalerate, isoamyl butyrate, 3-methylbutanoic acid 3-methylbutyl ester, butanoic acid 1-methylhexyl ester, and 3-methylbutyl acetate.
Compounds such as acetic acid, and decanoic acid found in treatments carried out with coconut sugar (CS60%50 °C and CS40%50 °C, respectively) can be attributed to the presence of these compounds in this sugar (Saraiva et al., 2023). Kabir and Lorjaroenphon (2014) found forty volatile compounds in coconut sugar, with acetic acid being predominant.
It has already been demonstrated that the type of solute and the time of osmotic treatment can influence the aromatic profile of fruits, promoting the loss of some aromatic compounds to the solution but being able to favor the formation of others via fermentative processes (Rizzolo et al., 2007).
Conclusions
Coconut sugar demonstrated very similar behavior to sucrose regarding WL, SG, and WR parameters during OD. Furthermore, comparing both sugars, few differences were observed regarding the dried product's drying curves and physical–chemical properties. However, using coconut sugar demonstrated the potential to increase antioxidant activity and greater color change in the samples. Furthermore, some volatile compounds characteristic of coconut sugar were detected in the dried product, which may contribute to a sensorial experience different from that of the conventional product.
The results expand the range of possible uses for coconut sugar and generate opportunities to develop a product with greater added value. However, a sensory evaluation can be helpful in indicating whether consumers will accept dehydrated bananas with coconut sugar well.
Data Availability
No datasets were generated or analysed during the current study.
References
Adekunte, A. O., Tiwari, B. K., Cullen, P. J., Scannell, A. G. M., & O’Donnell, C. P. (2010). Effect of sonication on colour, ascorbic acid and yeast inactivation in tomato juice. Food Chemistry, 122(3), 500–507. https://doi.org/10.1016/j.foodchem.2010.01.026
Al Amri, F. S., & Hossain, M. A. (2018). Comparison of total phenols, flavonoids and antioxidant potential of local and imported ripe bananas. Egyptian Journal of Basic and Applied Sciences, 5(4), 245–251. https://doi.org/10.1016/j.ejbas.2018.09.002
An, K., Ding, S., Tao, H., Zhao, D., Wang, X., Wang, Z., & Hu, X. (2013). Response surface optimisation of osmotic dehydration of C hinese ginger (Z ingiber officinale R oscoe) slices. International journal of food science & technology, 48(1), 28–34. https://doi.org/10.1111/j.1365-2621.2012.03153.x
AOAC. (2010). Official methods of analysis (18th ed.). AOAC Internacional Association of Official Analytical Chemists.
Assis, F. R., Morais, R. M. S. C., & Morais, A. M. M. B. (2016). Mass transfer in osmotic dehydration of food products: comparison between mathematical models. Food Engineering Reviews, 8(2), 116–133. https://doi.org/10.1007/s12393-015-9123-1
Ayetigbo, O., Latif, S., Abass, A., & Müller, J. (2019). Osmotic dehydration kinetics of biofortified yellow-flesh cassava in contrast to white-flesh cassava (Manihot esculenta). Journal of Food Science and Technology, 56(9), 4251–4265. https://doi.org/10.1007/s13197-019-03895-3
Bai, J.-W., Zhang, L., Aheto, J. H., Cai, J.-R., Wang, Y.-C., Sun, L., & Tian, X.-Y. (2023). Effects of different pretreatment methods on drying kinetics, three-dimensional deformation, quality characteristics and microstructure of dried apple slices. Innovative Food Science & Emerging Technologies, 83(301), 1–11. https://doi.org/10.1016/j.ifset.2022.103216
Boudhrioua, N., Giampaoli, P., & Bonazzi, C. (2003). Changes in aromatic components of banana during ripening and air-drying. LWT-Food Science and Technology, 36(6), 633–642. https://doi.org/10.1016/S0023-6438(03)00083-5
Braga, A. M. P., Pedroso, M. P., Augusto, F., & Silva, M. A. (2009). volatiles identification in pineapple submitted to drying in an ethanolic atmosphere. Drying Technology, 27(2), 248–257. https://doi.org/10.1080/07373930802606097
Brat, P., Yahia, A., Chillet, M., Bugaud, C., Bakry, F., Reynes, M., & Brillouet, J.-M. (2004). Influence of cultivar, growth altitude and maturity stage on banana volatile compound composition. Fruits, 59(2), 75–82. https://doi.org/10.1051/fruits:2004007
Chiu, M. T., Tham, H. J., & Lee, J. S. (2017). Optimization of osmotic dehydration of Terung Asam (Solanum lasiocarpum Dunal). Journal of Food Science and Technology, 54(10), 3327–3337. https://doi.org/10.1007/s13197-017-2785-3
da Silva, W. P., e Silva, C. M., Lins, M. A., & Gomes, J. P. (2014). Osmotic dehydration of pineapple (Ananas comosus) pieces in cubical shape described by diffusion models. LWT-Food Science and Technology, 55(1), 1–8. https://doi.org/10.1016/j.lwt.2013.08.016
de Jesus Filho, M., do Carmo, L. B., Della Lucia, S. M., Saraiva, S. H., Costa, A. V., Osório, V. M., & Teixeira, L. J. Q. (2018). Banana liqueur: Optimization of the alcohol and sugar contents, sensory profile and analysis of volatile compounds. LWT, 97, 31–38. https://doi.org/10.1016/j.lwt.2018.06.044
de Jesus Junqueira, J. R., Corrêa, J. L. G., de Mendonça, K. S., Resende, N. S., & de Barros Vilas Boas, E. V. (2017). Influence of sodium replacement and vacuum pulse on the osmotic dehydration of eggplant slices. Innovative Food Science and Emerging Technologies, 41, 10–18. https://doi.org/10.1016/j.ifset.2017.01.006
de Jesus Junqueira, J. R., Corrêa, J. L. G., de Mendonça, K. S., de Mello Júnior, R. E., & de Souza, A. U. (2018). Pulsed vacuum osmotic dehydration of beetroot, carrot and eggplant slices: effect of vacuum pressure on the quality parameters. Food and Bioprocess Technology, 11, 1863–1875. https://doi.org/10.1007/s11947-018-2147-9
Delgado, T., Pereira, J. A., Ramalhosa, E., & Casal, S. (2017). Osmotic dehydration effects on major and minor components of chestnut (Castanea sativa Mill.) slices. Journal of Food Science and Technology, 54(9), 2694–2703. https://doi.org/10.1007/s13197-017-2706-5
FAO - Food and Agriculture Organization of the United Nations (2023). Markets and trade. https://www.fao.org/markets-and-trade/commodities/bananas/en/
Ganjloo, A., Rahman, R. A., Bakar, J., Osman, A., & Bimakr, M. (2012). Kinetics modeling of mass transfer using Peleg’s equation during osmotic dehydration of seedless guava (Psidium guajava L.): Effect of process parameters. Food and Bioprocess Technology, 5(6), 2151–2159. https://doi.org/10.1007/s11947-011-0546-2
Giannakourou, M. C., Lazou, A. E., & Dermesonlouoglou, E. K. (2020). Optimization of osmotic dehydration of tomatoes in solutions of non-conventional sweeteners by response surface methodology and desirability approach. Foods, 9(10), 1393. https://doi.org/10.3390/foods9101393
Grzelak-Błaszczyk, K., Czarnecki, A., & Klewicki, R. (2020). The effect of osmotic dehydration on the polyphenols content in onion. Acta Scientiarum Polonorum Technologia Alimentaria, 19(1), 37–45. https://doi.org/10.17306/J.AFS.2020.0766
Guiné, R. P. F., Barroca, M. J., Gonçalves, F. J., Alves, M., Oliveira, S., & Mendes, M. (2015). Artificial neural network modelling of the antioxidant activity and phenolic compounds of bananas submitted to different drying treatments. Food Chemistry, 168, 454–459. https://doi.org/10.1016/j.foodchem.2014.07.094
Hebbar, K. B., Arivalagan, M., Manikantan, M. R., Mathew, A. C., Thamban, C., Thomas, G. V., & Chowdappa, P. (2015). Coconut inflorescence sap and its value addition as sugar - collection techniques, yield, properties and market perspective. Current Science, 109(8), 1411–1417. https://doi.org/10.18520/v109/i8/1411-1417
Kabir, M. A., & Lorjaroenphon, Y. (2014, February). Identification of aroma compounds in coconut sugar. In Proceedings of the 52nd Kasetsart University Annual Conference, Bangkok, Thailand (pp. 4-7).
Karam, M. C., Petit, J., Zimmer, D., Baudelaire Djantou, E., & Scher, J. (2016). Effects of drying and grinding in production of fruit and vegetable powders: A review. Journal of Food Engineering, 188, 32–49. https://doi.org/10.1016/j.jfoodeng.2016.05.001
Karseno, E., Yanto, T., Setyowati, R., & Haryanti, P. (2018). Effect of pH and temperature on browning intensity of coconut sugar and its antioxidant activity. Food Research, 2(1), 32–38. https://doi.org/10.26656/fr.2017.2(1).175
Kowalski, S. J., Łechtańska, J. M., & Szadzińska, J. (2013). Quality Aspects of Fruit and Vegetables Dried Convectively with Osmotic Pretreatment. Chemical and Process Engineering, 34(1), 51–62. https://doi.org/10.2478/cpe-2013-0005
Kroehnke, J., Szadzińska, J., Radziejewska-Kubzdela, E., Biegańska-Marecik, R., Musielak, G., & Mierzwa, D. (2021). Osmotic dehydration and convective drying of kiwifruit (Actinidia deliciosa)–The influence of ultrasound on process kinetics and product quality. Ultrasonics Sonochemistry, 71, 105377. https://doi.org/10.1016/j.ultsonch.2020.105377
Luchese, C. L., Gurak, P. D., & Marczak, L. D. F. (2015). Osmotic dehydration of physalis (Physalis peruviana L.): Evaluation ofwater loss and sucrose incorporation and the quantification ofcarotenoids. LWT, 63(2), 1128–1136. https://doi.org/10.1016/j.lwt.2015.04.060
Macedo, L. L., Vimercati, W. C., Araújo, C., Saraiva, S. H., & Teixeira, L. J. Q. (2020). Effect of drying air temperature on drying kinetics and physicochemical characteristics of dried banana. Journal of Food Process Engineering, 43(April), 1–10. https://doi.org/10.1111/jfpe.13451
Macedo, L. L., Corrêa, J. L. G., Araújo, C. D. S., Vimercati, W. C., & Pio, L. A. S. (2021a). Process optimization and ethanol use for obtaining white and red dragon fruit powder by foam mat drying. Journal of Food Science, 86(2), 426–433. https://doi.org/10.1111/1750-3841.15585
Macedo, L. L., Corrêa, J. L. G., da Silva Araújo, C., Vimercati, W. C., & Júnior, I. P. (2021b). Convective drying with ethanol pre-treatment of strawberry enriched with isomaltulose. Food and Bioprocess Technology, 14(11), 2046–2061. https://doi.org/10.1007/s11947-021-02710-2
Macedo, L. L., Corrêa, J. L. G., da Silva Araújo, C., & Vimercati, W. C. (2022a). Effect of osmotic agent and vacuum application on mass exchange and qualitative parameters of osmotically dehydrated strawberries. Journal of Food Processing and Preservation, 46(7), e16621. https://doi.org/10.1111/jfpp.16621
Macedo, L. L., Corrêa, J. L. G., Júnior, I. P., da Silva Araújo, C., & Vimercati, W. C. (2022b). Intermittent microwave drying and heated air drying of fresh and isomaltulose (Palatinose) impregnated strawberry. Lwt, 155, 112918. https://doi.org/10.1016/j.lwt.2021.112918
Macedo, L. L., Corrêa, J. L. G., Vimercati, W. C., & Araújo, C. (2022c). The impact of using vacuum and isomaltulose as an osmotic agent on mass exchange during osmotic dehydration and their effects on qualitative parameters of strawberries. Journal of Food Process Engineering, 45(March), 1–13. https://doi.org/10.1111/jfpe.14057
Macedo, L. L., Corrêa, J. L. G., Araújo, C. D. S., Oliveira, D. D. S., & Teixeira, L. J. Q. (2023a). Use of coconut sugar as an alternative agent in osmotic dehydration of strawberries. Journal of Food Science, 88(9), 3786–3806. https://doi.org/10.1111/1750-3841.16715
Macedo, L. L., Corrêa, J. L. G., da Silva Araújo, C., & Cardoso, W. S. (2023b). Use of ethanol to improve convective drying and quality preservation of fresh and sucrose and coconut sugar-impregnated strawberries. Food and Bioprocess Technology, 16(10), 2257–2271. https://doi.org/10.1007/s11947-023-03066-5
Medina, M. B. (2011). Determination of the total phenolics in juices and superfruits by a novel chemical method. Journal of Functional Foods, 3(2), 79–87. https://doi.org/10.1016/j.jff.2011.02.007
Mendonça, K., Correa, J., Junqueira, J., Angelis-Pereira, M., & Cirillo, M. (2017). Mass transfer kinetics of the osmotic dehydration of Yacon slices with polyols. Journal of Food Processing and Preservation, 41(1), e12983. https://doi.org/10.1111/jfpp.12983
Mercali, G. D., Tessaro, I. C., Noreña, C. P. Z., & Marczak, L. D. F. (2010). Mass transfer kinetics during osmotic dehydration of bananas (Musa sapientum, shum.). International Journal of Food Science and Technology, 45(11), 2281–2289. https://doi.org/10.1111/j.1365-2621.2010.02418.x
Mercali, G. D., Ferreira Marczak, L. D., Tessaro, I. C., & Zapata Noreña, C. P. (2011). Evaluation of water, sucrose and NaCl effective diffusivities during osmotic dehydration of banana (Musa sapientum, shum.). LWT - Food Science and Technology, 44(1), 82–91. https://doi.org/10.1016/j.lwt.2010.06.011
Morais, D. R., Rotta, E. M., Sargi, S. C., Schmidt, E. M., Bonafe, E. G., Eberlin, M. N., Sawaya, A. C. H. F., & Visentainer, J. V. (2015). Antioxidant activity, phenolics and UPLC-ESI(-)-MS of extracts from different tropical fruits parts and processed peels. Food Research International, 77, 392–399. https://doi.org/10.1016/j.foodres.2015.08.036
Nahimana, H., Zhang, M., Mujumdar, A. S., & Ding, Z. (2011). Mass Transfer Modeling and Shrinkage Consideration during Osmotic Dehydration of Fruits and Vegetables. Food Reviews International, 27(4), 331–356. https://doi.org/10.1080/87559129.2010.518298
Pathare, P. B., Opara, U. L., & Al-Said, F. A. J. (2013). Colour Measurement and Analysis in Fresh and Processed Foods: A Review. Food and Bioprocess Technology, 6(1), 36–60. https://doi.org/10.1007/s11947-012-0867-9
Peleg, M. (1988). An Empirical Model for the Description of Moisture Sorption Curves. Journal of Food Science, 53(4), 1216–1217. https://doi.org/10.1111/j.1365-2621.1988.tb13565.x
Pravitha, M., Manikantan, M. R., Ajesh Kumar, V., Beegum, S., & Pandiselvam, R. (2021). Optimization of process parameters for the production of jaggery infused osmo-dehydrated coconut chips. Lwt, 146, 111441. https://doi.org/10.1016/j.lwt.2021.111441
Pravitha, M., Manikantan, M. R., Kumar, V. A., Beegum, P. S., & Pandiselvam, R. (2022). Comparison of drying behavior and product quality of coconut chips treated with different osmotic agents. Lwt, 162, 113432. https://doi.org/10.1016/j.lwt.2022.113432
Rai, R., Rani, P., & Tripathy, P. P. (2021). Osmo-air drying of banana slices: Multivariate analysis, process optimization and product quality characterization. Journal of Food Science and Technology. https://doi.org/10.1007/s13197-021-05261-8
Rizzolo, A., Gerli, F., Prinzivalli, C., Buratti, S., & Torreggiani, D. (2007). Headspace volatile compounds during osmotic dehydration of strawberries ( cv Camarosa ): Influence of osmotic solution composition and processing time. LWT - Food Science and Technology, 40(3), 529–535. https://doi.org/10.1016/j.lwt.2006.02.002
Rodriguez, A., Sancho, A. M., Barrio, Y., Rosito, P., & Gozzi, M. S. (2019). Combined drying of Nopal pads (Opuntia ficus-indica): Optimization of osmotic dehydration as a pretreatment before hot air drying. Journal of Food Processing and Preservation, 43(11), 1–9. https://doi.org/10.1111/jfpp.14183
Rózek, A., García-Pérez, J. V., López, F., Güell, C., & Ferrando, M. (2010). Infusion of grape phenolics into fruits and vegetables by osmotic treatment: Phenolic stability during air drying. Journal of Food Engineering, 99(2), 142–150. https://doi.org/10.1016/j.jfoodeng.2010.02.011
Rufino, M. D. S. M., Alves, R. E., de Brito, E. S., Pérez-Jiménez, J., Saura-Calixto, F., & Mancini-Filho, J. (2010). Bioactive compounds and antioxidant capacities of 18 non-traditional tropical fruits from Brazil. Food chemistry, 121(4), 996–1002. https://doi.org/10.1016/j.foodchem.2010.01.037
Saha, B., Bucknall, M. P., Arcot, J., & Driscoll, R. (2018). Profile changes in banana flavour volatiles during low temperature drying. Food Research International, 106, 992–998. https://doi.org/10.1016/j.foodres.2018.01.047
Saraiva, A., Carrascosa, C., Raheem, D., Ramos, F., & Raposo, A. (2020). Natural Sweeteners: The Relevance of Food Naturalness for Consumers, Food Security Aspects, Sustainability and Health Impacts. International Journal of Environmental Research and Public Health, 17(17), 6285. https://doi.org/10.3390/ijerph17176285
Saraiva, A., Carrascosa, C., Ramos, F., Raheem, D., Lopes, M., & Raposo, A. (2023). Coconut Sugar: Chemical Analysis and Nutritional Profile; Health Impacts; Safety and Quality Control; Food Industry Applications. International Journal of Environmental Research and Public Health, 20(4), 3671. https://doi.org/10.3390/ijerph20043671
Shukla, R. N., Khan, M. A., & Srivastava, A. K. (2018). Mass transfer kinetics during osmotic dehydration of banana in different osmotic agent. International Journal of Agricultural Engineering, 11(1), 108–122. https://doi.org/10.15740/HAS/IJAE/11.1/108-122
Simpson, R., Ramírez, C., Nuñez, H., Jaques, A., & Almonacid, S. (2017). Understanding the success of Page’s model and related empirical equations in fitting experimental data of diffusion phenomena in food matrices. Trends in Food Science and Technology, 62, 194–201. https://doi.org/10.1016/j.tifs.2017.01.003
Song, C., Ma, X., Li, Z., Wu, T., Raghavan, G. V., & Chen, H. (2020). Mass transfer during osmotic dehydration and its effect on anthocyanin retention of microwave vacuum-dried blackberries. Journal of the Science of Food and Agriculture, 100(1), 102–109. https://doi.org/10.1002/jsfa.9999
Sulaiman, S. F., Yusoff, N. A. M., Eldeen, I. M., Seow, E. M., Sajak, A. A. B., & Supriatno, & Ooi, K. L. (2011). Correlation between total phenolic and mineral contents with antioxidant activity of eight Malaysian bananas (Musa sp.). Journal of Food Composition and Analysis, 24(1), 1–10. https://doi.org/10.1016/j.jfca.2010.04.005
Tabtiang, S., Prachayawarakon, S., & Soponronnarit, S. (2012). Effects of osmotic treatment and superheated steam puffing temperature on drying characteristics and texture properties of banana slices. Drying Technology, 30(1), 20–28. https://doi.org/10.1080/07373937.2011.613554
Taşova, M., Polatcı, H., & Gökdoğan, O. (2022). Effect of osmotic dehydration pre-treatments on physicochemical and energy parameters of Kosia (Nashi) pear slices dried in a convective oven. Journal of Food Processing and Preservation, 46(11), 1–12. https://doi.org/10.1111/jfpp.16945
Thuwapanichayanan, R., Prachayawarakorn, S., Kunwisawa, J., & Soponronnarit, S. (2011). Determination of effective moisture diffusivity and assessment of quality attributes of banana slices during drying. LWT - Food Science and Technology, 44(6), 1502–1510. https://doi.org/10.1016/j.lwt.2011.01.003
Tortoe, C., Orchard, J., & Beezer, A. (2007). Osmotic dehydration kinetics of apple, banana and potato. International Journal of Food Science and Technology, 42(3), 312–318. https://doi.org/10.1111/j.1365-2621.2006.01225.x
Wang, J., Li, Y., Chen, R., Bao, J., & Yang, G. (2007). Comparison of volatiles of banana powder dehydrated by vacuum belt drying, freeze-drying and air-drying. Food Chemistry, 104(4), 1516–1521. https://doi.org/10.1016/j.foodchem.2007.02.029
Wang, X., Kahraman, O., & Feng, H. (2022). Impact of Osmotic Dehydration With/Without Vacuum Pretreatment on Apple Slices Fortified With Hypertonic Fruit Juices. Food and Bioprocess Technology, 15(7), 1588–1602. https://doi.org/10.1007/s11947-022-02834-z
Acknowledgements
To the Federal University of Espírito Santo (UFES) and the Espírito Santo Research and Innovation Support Foundation (FAPES) for the Espírito Santo researcher scholarship granted to Sérgio Henriques Saraiva and financial support (704/2022).
Funding
This work was carried out with support from the Espírito Santo Research and Innovation Support Foundation (FAPES) – 704/2022.
Author information
Authors and Affiliations
Contributions
Araújo: Conceptualization, Methodology, Vali- dation, Formal analysis, Investigation, Data Curation, Writing—Original Draft, Visualization. Macedo: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Data Curation, Writing—Review & Editing, Visualization. Vimercati: Formal analysis, Data Curation, Writing—Review & Editing. Osório: Formal analysis, Data Curation, Writing—Review & Editing. Saraiva: Conceptualization, Methodology, Data Curation, Writing—Review & Editing, Supervi- sion, Project administration, Funding acquisition.
Corresponding author
Ethics declarations
Competing Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Araújo, C.d.S., Macedo, L.L., Vimercati, W.C. et al. Quality Attributes of Dried Osmo-Dehydrated Banana Slices Using Coconut Sugar and Sucrose as Osmotic Agents. Food Bioprocess Technol (2024). https://doi.org/10.1007/s11947-024-03558-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11947-024-03558-y