Abstract
This work evaluated the production of dehydrated mangoes (Mangifera indica L.) and the effectiveness of ultrasonic-assisted osmotic dehydration on the drying kinetics of mangoes. Cube shaped mango samples were pretreated using ultrasound-assisted osmotic dehydration (UAOD) and dried in a circulating drying oven. An experimental design was created to evaluate the effect of pretreatment time and osmotic solution concentration on the water loss and sugar gain in the osmotic dehydration and on the drying time. The ultrasonic pretreatment was carried out in a bath ultrasound operating at 25 kHz and outputting 55 W/m3 of power. Osmotic solution ranging from 0 to 500 kg sucrose/m3 was applied in the treatments, and air drying was carried out at 60 °C. A mathematical model was developed for the osmotic pretreatment, and Fick’s law was used to model the air-drying process. The mass transfer coefficients were estimated for the ultrasonic-assisted osmotic dehydration, and the apparent water diffusivity was estimated for the air-drying process. The mass transfer coefficient ranged from 0.017 to 0.109 m2/s and the resistance to mass transfer at the surface ranged from 0.26 × 10−6 to 1.22 × 10−6 m2/s on the UAOD, while the apparent water diffusivity during air drying ranged from 5.94 × 10−9 to 8.41 × 10−9 m2/s. Mangoes presented a different behavior when compared to other fruits. The ultrasonic pretreatment was effective only when associated with an osmotic solution at 500 kg sucrose/m3.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The application of ultrasound technology has been studied in drying processes since the early 2000’s. Ultrasound technology has been used directly in air-drying or as a pretreatment. Early studies addressed the suitability of the ultrasound pretreatment before drying processes (Cárcel et al. 2007; Fernandes and Rodrigues 2012; Corrêa et al. 2017). Recent studies evolved into studying the effect of the pretreatment in quality aspects of dehydrated fruits and vegetables (Fernandes et al. 2016; Nascimento et al. 2016; Sangeeta 2016; Amami et al. 2017; Delgado et al. 2017; Soquetta et al. 2018).
The ultrasonic pretreatment reduces the process time of the more expensive air-drying process when applied to fruits and vegetables that have porous tissue structure, thin and fragile cell wall or a tissue structure containing hard phenolic-filled cells (Fernandes et al. 2008a; Rodríguez et al. 2014; Dehghannya et al. 2015). The suitability of the ultrasonic pretreatment in the processing of roots, such as carrots, cassava, and other is questionable.
The use of ultrasound-assisted osmotic dehydration (UAOD) enhances the changes caused by the ultrasonic pretreatment. The higher osmotic potential between the sample and the osmotic solution boosts the mass transfer amid the solid and the solution. It makes the technology suitable for application is less porous fruits, such as papayas and genipap (Rodrigues et al. 2009b; Fernandes and Rodrigues 2012). It increases the deformation of the tissue structure due to the ultrasonic waves and osmotic pressure generating microscopic channels. These channels diminish the diffusion boundary layer and enhance the convective mass transfer in the sample (Nowacka et al. 2014; La Fuente and Tadini 2017).
The application of the ultrasound technology is still troublesome in dense and less porous fruit and vegetables, such as mango and avocado. Although some studies addressed these fruits, the studies are incomplete (Santos et al. 2015; Méndez-Calderón et al. 2018). A complete understanding of the phenomena is still required, such as in the mathematical modeling of the process by applying a theoretical model rather than statistical models as commonly has been done.
In this study, we investigate the application of ultrasound-assisted osmotic dehydration as a pretreatment to air-drying of mangoes. The effect of processing time and osmotic solution concentration on soluble solids gain, water loss, and water diffusivity was evaluated and modeled. Its effect in the drying kinetics throughout convective air-drying was evaluated and modeled. The process (ultrasound-assisted osmotic dehydration and air-drying) was optimized using the mathematical model developed for the process.
Materials and methods
Preparation of samples
Mangoes (Mangifera indica L. var Tommy Atkins) bought from a local market (Fortaleza, Brazil) were cut into cubes (0.010 m × 0.010 m × 0.010 m) using a cutting device. Its moisture content was attained by heating in a drying oven (Tecnal model TE-394, Brazil) at 105 °C for 48 h following the method recommended by AOAC (AOAC 1997).
Ultrasound-assisted osmotic dehydration
Mango cubes (0.035 ± 0.003 kg) were immersed in an aqueous solution of sucrose and subjected to ultrasonic waves for 5, 10, 20, 30 and 40 min in an ultrasonic bath (Unique model USC25, volume = 0.0027 m3, frequency = 25 kHz). The effective ultrasonic power density was 55 kW/m3.
Sucrose solutions of 120, 250 and 500 kg/m3 were prepared. An experiment was also carried out with samples immersed in distilled water. The experiments were done in Beakers of 250 mL. The water to sample weight ratio was set to 4:1, because this was the ratio, found as optimal in previous works (Fernandes et al. 2006; Garcia-Noguera et al. 2014). The dilution of the osmotic solution was imperceptible under this condition.
The experiments were done at 23 °C (ambient temperature). The maximum temperature rise was less than 2 °C, which is not negligible for the process. All experiments were done in triplicate.
The mango cubes were drained, and the excess of osmotic solution blotted with absorbent paper at the end of the osmotic dehydration. The weight of the samples and their moisture content were measured and used to determine the solid gain (SG) and water loss (WL) during the osmotic dehydration (Eqs. 1, 2).
Where, SG is the soluble solids gain (%), Xi is the initial fruit moisture on wet basis (kg water/kg), Xf is the final fruit moisture on wet basis (kg water/kg), wi is the initial mass of the cubes (kg), wf is the final mass of the cubes (kg), and WL is the water loss (%)
The mass balance considered the water loss of the fruit to the osmotic solution and the gain of soluble solids by the mangoes (Eqs. 3, 4). The mathematical model developed for osmotic dehydration of mango is an extension of the mathematical model developed in previous works of our group (Oliveira et al. 2006; Fernandes et al. 2008b). The original model only considered the mass transfer between the fruit and the osmotic solution, but it was not able to represent the process when the samples had a high soluble solid gradient between the surface and the center of the fruit sample. Dense fruits, like mangoes, will impose a higher resistance to water and soluble solids transfer because the surface will saturate with the solute. Thus, a second term representing this surface resistance to mass transfer caused by the impregnation of soluble solids was added to the model.
Where, AFR is the total surface area of the fruit (m2), C is the concentration (kg/m3), Km is the mass transfer coefficient (1/m2 s), M is the mass (kg), R Ws is the surface resistance to water mass transfer (kg water/kg solids m2 s), R Ss is the surface resistance to soluble solids mass transfer (1/m2 s), VFR is the total volume of the fruit (m3), the subscript FR refers to the fruit and OS to the osmotic solution, and the superscript W refer to water and S to soluble solids.
The mathematical model considered the shrinkage of the samples. This assumption improves the physical representation of the process and increases the certainty of the mass transfer coefficients. The model assumed that the shrinkage was proportional to the change in the mass of water in the samples (Eq. 5).
Where α is the shrinkage coefficient (m3/kg).
For the osmotic solution, the mass balance considered the gain of water coming from the sample and the loss of sucrose to the fruit (Eqs. 6 and 7).
Convective air-drying
The mangoes were transferred to a circulating air-drying oven (Tecnal model TE-394, Brazil) after the pretreatment. The temperature was set at 60 °C, and air velocity was 0.5 m/s. The air relative humidity, determined by psychrometry, was 46%. The mango cubes were weighed every 30 min until constant weight.
The mathematical model for the process assumed diffusion-controlled mass transfer following Fick’s 2nd law of diffusion (Crank 1975). The falling-rate period was the only period considered for modeling because the constant-rate period was not observed after analyzing the data. The diffusion equation was solved assuming the samples to be symmetrical, cubical in shape, and with uniform moisture and temperature. The model assumed that mass transfer was controlled by diffusion mechanisms.
Where a is the width of the cube (m), D is the apparent diffusivity coefficient (m2/s), and W is the moisture content (kg water/kg), Wcrit is the critical moisture content (kg water/kg), and Weq is the equilibrium moisture content (kg water/kg).
The prediction of the apparent diffusion coefficient was based on the minimization of the error sum of squares. The model was applied in the optimization of the total processing time demanded to dry mangoes to a moisture content of 0.09 ± 0.01 kg water/kg (removal of 90% of the initial moisture content of the mangoes).
The optimization applied the Levenberg–Marquardt method to search for the minimal total processing time (pre-treatment + air-drying) as an objective function (Silva et al. 2009). The algorithm was coded in the Python programming language.
Results and discussion
The fresh mangoes presented initial moisture of 0.872 ± 0.003 kg water/kg and a soluble solids content of 0.128 ± 0.3 kg soluble solids/kg.
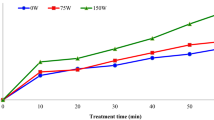
Mangoes exposed to the ultrasound in distilled water lost soluble solids. The mangoes samples lost between 13.2 and 25.9% of soluble solids to the liquid medium (Fig. 1a), producing mangoes with low sugar content.
The mango cubes gained from 9.7% to 64.9% of soluble solids depending on the concentration of the osmotic solution and processing time in the UAOD. The soluble solids gain increased both with processing time and the osmotic solution concentration, as expected for this kind of mass transfer process (Fernandes and Rodrigues 2007; Garcia-Noguera et al. 2010; Kek et al. 2013; Thippanna and Tiwari 2017). The mass transfer of soluble solids to the mango cubes was rapid in the first 10 min of ultrasound processing and less intense after that.
Water loss followed the same trend (Fig. 1b). The mango cubes lost between 0.4 and 20.6% of water depending on the concentration of the osmotic solution and processing time. Water loss increased with processing time and the osmotic solution concentration. The mass transfer of water from the fruit to the osmotic solution was intense in the first 5 min and lost intensity in the next 15 min. The mass change was not significant between 30 and 40 min of processing, denoting the end of the mass transfer process. Data show that the ultrasound-assisted osmotic dehydration is not useful in accomplishing dehydration after 20 min of processing.
It is interesting to notice that soluble solids gain, and water loss was observed even in the process done with an isotonic solution (120 kg/m3 of sucrose). Theoretically, the process should not result in the exchange of solids or water amid the mangoes and the solution because the concentration gradient was zero. However, the fruit is composed of glucose and fructose, rather than sucrose (as in the osmotic solution). The concentration gradients of each of these sugars are not zero. Thus mass transfer occurs with glucose and fructose leaving the fruit and sucrose entering the fruit. The net gain of sugars happens because the concentration gradient of sucrose is higher than the concentration gradient of fructose and glucose and because sucrose adheres to the surface of the fruit cubes.
Fruits like apple, pineapple, and strawberry have a porous tissue structure with thin cell walls. For these fruits, water and soluble solids mass transfer from the fruit to the solution and vice versa suffers little resistance at the border of the fruit samples. Mass transfer modeling for these fruits is simple. The model needs to account for mass transfer and mass transfer resistance imposed by soluble solids saturation at the border of the fruit can be neglected (Rodrigues and Fernandes 2007; Fernandes et al. 2008b). Modeling the osmotic process for mangoes is more complicated due to its tissue structure. Mangoes are dense and much less porous (porosity = 0.04 to 0.05) than most fruits (pineapple porosity = 0.16 to 0.25; apple porosity = 0.18 to 0.22; strawberry porosity = 0.47) (Ozcan and Haciseferogullan 2007; Yan et al. 2008; Singh et al. 2015). This tissue structure imposes a higher resistance to mass transfer. Soluble solids tend to saturate at the border of the fruit creating an extra resistance to mass transfer, as observed previously in melons (Rodrigues and Fernandes 2007). In this case, the mass transfer resistance at the border of the fruit cannot be neglected. Table 1 presents the coefficients for mass transfer of water and soluble solids calculated for the osmotic concentrations applied in this study, which ranged from 0.017 to 0.059 m−2/s for water mass transfer and from 0.014 to 0.109 m−2/s for soluble solids mass transfer.
The concentration gradient of water and soluble solids between the mango samples and the osmotic solution increased with the increase of the concentration of the solution. This condition would raise the water and soluble solids mass transfer coefficients in most fruits, but mangoes showed a different behavior. The mass transfer tends to increase with the increase in the concentration gradient, but the fast saturation of the surface of the samples with sugars results in a net decrease in the water and soluble solids mass transfer coefficient at high concentrations of the osmotic solution.
Convective air-drying of mangoes presented only the falling-rate period. Figure 2 presents the drying kinetics of mangoes and UAOD pretreated mangoes. The behavior and pattern of the drying kinetics were like the behavior observed for other fruits like apples, pineapples, sapota and strawberry (Fernandes and Rodrigues 2008; Garcia-Noguera et al. 2014; Rodríguez et al. 2017).
The effect of the ultrasound was also noticed during air-drying, changing the apparent water diffusivity in mangoes. The apparent water diffusivity decreased when ultrasound and UAOD were applied. Table 2 presents the diffusivities obtained as a function of the pre-treatment condition. The decrease in the water diffusivity ranged from 9.3 to 29.4%. The decrease was higher for more concentrated osmotic solutions.
The reduction in the water diffusivity is not standard. For most fruits, the diffusivity increases when ultrasound-assisted osmotic dehydration is applied (Garcia-Noguera et al. 2010; Fernandes and Rodrigues 2012; Rodríguez et al. 2014). For example, the water diffusivity in papayas increased by 28.8% when it was subjected to 20 min of ultrasonic treatment (Rodrigues et al. 2009b). Pineapples presented an effective water diffusivity 64.3% higher when 30 min of ultrasound as applied (Fernandes et al. 2008b), and melons increased by 39.3% in the water diffusivity after ultrasound application for 20 min (Rodrigues and Fernandes 2007).
The ultrasonic pretreatment acts through cavitation and the sponge effect. Both induce the formation of microscopic channels in the fruit as has been demonstrated in several fruits (Prinzivalli et al. 2006; Fernandes et al. 2008a; Rodrigues et al. 2009a). The sponge effect induces contraction and dilatation motion in the fruit, expelling and incorporating water and soluble solids thought the boundary amid the fruit and the solution. The long-term result of cavitation and sponge effect in the fruit is the creation of microscopic channels and breakdown of the tissue structure, which facilitates the mass transfer of water and soluble solids amid the fruit and the solution and increases the water diffusivity in the fruit.
Mangoes are dense and have more fibers than most fruits. This kind of tissue structure differs from the other fruits and is less susceptible to the effects induced by ultrasound application. The water diffusivity presented a significant drop in the first 10 min of pretreatment, which might have been caused by saturation of soluble solids on the boundary between the fruit and the osmotic solution creating an even denser structure that raised the mass transfer resistance and reduced the mobility of water during air-drying.
The microscopic channels were formed around 20 and 30 min of ultrasound application, as happened with most fruits that were previously studied (Fernandes et al. 2008a, 2009; Rodrigues et al. 2009a). This fact can be noticed by the slight increase in the water diffusivity that occurred from 20 to 30 min of pretreatment, caused by the increase in water mobility allowed by the microscopic channels.
The diffusivity in mangoes increased again when the fruit was pretreated for more than 30 min most probably because of the saturation of the microscopic channels with soluble solids from the osmotic solution. Mangoes immersed in water during the ultrasonic pretreatment did not present this behavior, and the water diffusivity did not decrease when pretreated for more than 20 min.
The statistical test of means shows that both water diffusivity and air-drying time were affected by the ultrasonic pretreatment period and the concentration of the solution (Fig. 3). The pretreatment time had a smaller influence on the process, denoted by the values of water diffusivity within the deviation of this variable. The only exception was at the pretreatment time of 20 min, which presented the highest increment in the water diffusivity, caused most probably by the creation of microscopic channels in the structure of the mango tissue samples (Fig. 3a). The concentration of the solution had a higher influence on the water diffusivity and in the air-drying. Although the increase in the solution concentration decreased the apparent water diffusivity, the loss of water during the process resulted in lower initial moisture content with a consequent reduction in the air-drying period, regardless of the lower water diffusivity (Fig. 3b, d).
Table 3 presents processing time (pretreatment + air drying) required to eliminate 90% of the initial moisture content of the mangoes. The simulations and experimental data show that mangoes without pretreatment require 168 min to dry, removing 90% of its initial moisture. The uncertainty of the simulations is ± 3 min given the reproducibility tests that were carried out.
The air-drying time did not reduce when the pretreatments were carried out with distilled water and with the solutions of 120 kg/m3, and 250 kg/m3. The UAOD reduced the water content in the mango samples, but the increase in the apparent water diffusivity induced an increase in drying time and consequently in a rise in the total processing time. Under such condition, considering lower technical viability and the higher costs involved with both processes, the ultrasound pretreatment was not satisfactory.
The total processing time obtained when the ultrasound pretreatment was carried out with a 500 kg/m3 osmotic solution was statistically similar (within the uncertainty of the analysis and simulations) to the air-drying time of the untreated mango. The use of the ultrasonic pretreatment can be attractive, under such condition, because it reduces the more expensive air-drying process as has been previously calculated for other fruits (Fernandes et al. 2008b).
Previous studies reported that the optimum processing time is attained when the osmotic dehydration is applied while the water loss rate is higher than the rate that would be attained by air-drying (Garcia-Noguera et al. 2010). For ultrasound, its application should be made while the rise in water diffusivity it causes results in a continuous decrease of total processing time. From an economic perspective, the optimal process for mangoes should apply UAOD for 20 min in a 500 kg/m3 osmotic solution, followed by 147 min of air-drying.
Conclusion
The application of ultrasound as a pre-treatment immersing the fruits in distilled water enabled the production of dried mangoes with low sugar content. The use of osmotic solutions with a concentration lower than 500 kg/m3 is not technically viable to decrease air-drying time.
Mangoes pretreated with UAOD reduced the apparent water diffusivity during the air-drying, due to the saturation of the surface of the mango samples with soluble sugars, which increased the mass transfer resistance amid the fruit and the solution. Despite the lower water diffusivity, the reduction in the initial moisture content after the osmotic process culminated in reduced drying time and shorter total process time when a 500 kg/m3 osmotic solution was applied.
References
Amami E, Khezami W, Mezrigui S et al (2017) Effect of ultrasound-assisted osmotic dehydration pretreatment on the convective drying of strawberry. Ultrason Sonochem 36:286–300. https://doi.org/10.1016/j.ultsonch.2016.12.007
AOAC (1997) Official methods of analysis. Association of Official Analytical Chemists, Arlington
Cárcel JA, Benedito J, Rosselló C, Mulet A (2007) Influence of ultrasound intensity on mass transfer in apple immersed in a sucrose solution. J Food Eng 78:472–479
Corrêa JLG, Rasia MC, Mulet A, Cárcel JA (2017) Influence of ultrasound application on both the osmotic pretreatment and subsequent convective drying of pineapple (Ananas comosus). Innov Food Sci Emerg Technol 41:284–291. https://doi.org/10.1016/j.ifset.2017.04.002
Crank J (1975) The mathematics of diffusion, 2nd edn. Oxfort University Press, Glasgow
Dehghannya J, Gorbani R, Ghanbarzadeh B (2015) Effect of ultrasound-assisted osmotic dehydration pretreatment on drying kinetics and effective moisture diffusivity of mirabelle plum. J Food Process Preserv 39:2710–2717
Delgado T, Pereira JA, Ramalhosa E, Casal S (2017) Osmotic dehydration effects on major and minor components of chestnut (Castanea sativa Mill.) slices. J Food Sci Technol 54:2694–2703
Fernandes FAN, Rodrigues S (2007) Ultrasound as pre-treatment for drying of fruits: dehydration of banana. J Food Eng 82:261–267. https://doi.org/10.1016/j.jfoodeng.2007.02.032
Fernandes FAN, Rodrigues S (2008) Application of ultrasound and ultrasound-assisted osmotic dehydration in drying of fruits. Dry Technol 26:1509–1516. https://doi.org/10.1080/07373930802412256
Fernandes FA, Rodrigues S (2012) Ultrasound as pre-treatment for drying of genipap (Genipa americana L.). Int J Food Eng 8:36. https://doi.org/10.1515/1556-3758.2480
Fernandes FAN, Rodrigues S, Gaspareto OCP, Oliveira EL (2006) Optimization of osmotic dehydration of papaya followed by air-drying. Food Res Int 39:492–498. https://doi.org/10.1016/j.foodres.2005.10.004
Fernandes FAN, Gallão MI, Rodrigues S (2008a) Effect of osmotic dehydration and ultrasound pre-treatment on cell structure: melon dehydration. LWT Food Sci Technol 41:604–610
Fernandes FAN, Linhares FE, Rodrigues S (2008b) Ultrasound as pre-treatment for drying of pineapple. Ultrason Sonochem 15:1049–1054. https://doi.org/10.1016/j.ultsonch.2008.03.009
Fernandes FAN, Gallão MI, Rodrigues S (2009) Effect of osmosis and ultrasound on pineapple cell tissue structure during dehydration. J Food Eng 90:186–190. https://doi.org/10.1016/j.jfoodeng.2008.06.021
Fernandes FAN, Rodrigues S, García-Pérez JV, Cárcel JA (2016) Effects of ultrasound-assisted air-drying on vitamins and carotenoids of cherry tomatoes. Dry Technol 34:986–996. https://doi.org/10.1080/07373937.2015.1090445
Garcia-Noguera J, Oliveira FIP, Gallão MI et al (2010) Ultrasound-assisted osmotic dehydration of strawberries: effect of pretreatment time and ultrasonic frequency. Dry Technol 28:294–303
Garcia-Noguera J, Oliveira FIP, Weller CL et al (2014) Effect of ultrasonic and osmotic dehydration pre-treatments on the colour of freeze dried strawberries. J Food Sci Technol 51:2222–2227. https://doi.org/10.1007/s13197-012-0724-x
Kek SP, Chin NL, Yusof YA (2013) Direct and indirect power ultrasound assisted pre-osmotic treatments in convective drying of guava slices. Food Bioprod Process 91:495–506. https://doi.org/10.1016/j.fbp.2013.05.003
La Fuente CIA, Tadini CC (2017) Unripe banana flour produced by air-drying assisted with ultrasound—description of the mechanisms involved to enhance the mass transfer in two approaches. Int J Food Eng 13:1–13. https://doi.org/10.1515/ijfe-2017-0178
Méndez-Calderón EK, Ocampo-Castaño JC, Orrego CE (2018) Optimization of convective drying assisted by ultrasound for mango tommy (Mangifera indica L.). J Food Process Eng 41:12634. https://doi.org/10.1111/jfpe.12634
Nascimento EMGC, Mulet A, Ascheri JL et al (2016) Effects of high-intensity ultrasound on drying kinetics and antioxidant properties of passion fruit peel. J Food Eng 170:108–118
Nowacka M, Tylewicz U, Laghi L et al (2014) Effect of ultrasound treatment on the water state in kiwifruit during osmotic dehydration. Food Chem 144:18–25. https://doi.org/10.1016/j.foodchem.2013.05.129
Oliveira IM, Fernandes FAN, Rodrigues S et al (2006) Modeling and optimization of osmotic dehydration of banana followed by air drying. J Food Process Eng 29:400–413. https://doi.org/10.1111/j.1745-4530.2006.00067.x
Ozcan MM, Haciseferogullan H (2007) The strawberry (Arbutus unedo L.) fruits: chemical composition, physical properties and mineral contents. J Food Eng 78:1022–1028
Prinzivalli C, Brambilla A, Maffi D et al (2006) Effect of osmosis time on structure, texture and pectic composition of strawberry tissue. Eur Food Res Technol 224:119–127. https://doi.org/10.1007/s00217-006-0298-9
Rodrigues S, Fernandes FAN (2007) Dehydration of melons in a ternary system followed by air-drying. J Food Eng 80:678–687. https://doi.org/10.1016/j.jfoodeng.2006.07.004
Rodrigues S, Gomes MCF, Gallão MI, Fernandes FAN (2009a) Effect of ultrasound-assisted osmotic dehydration on cell structure of sapotas. J Sci Food Agric 89:665–670. https://doi.org/10.1002/jsfa.3498
Rodrigues S, Oliveira FIP, Gallão MI, Fernandes FAN (2009b) Effect of immersion time in osmosis and ultrasound on papaya cell structure during dehydration. Dry Technol 27:220–225. https://doi.org/10.1080/07373930802605883
Rodríguez O, Santacatalina JV, Simal S et al (2014) Influence of power ultrasound application on drying kinetics of apple and its antioxidant and microstructural properties. J Food Eng 129:21–29
Rodríguez Ó, Gomes W, Rodrigues S, Fernandes FAN (2017) Effect of acoustically assisted treatments on vitamins, antioxidant activity, organic acids and drying kinetics of pineapple. Ultrason Sonochem 35:92–102. https://doi.org/10.1016/j.ultsonch.2016.09.006
Sangeeta BSH (2016) Instant vegetable from osmo-air drying of jimikand (A. campanulatus) in NaCl solution: nutritional, functional, micro-structural and other quality aspects. J Food Sci Technol 53:3512–3521
Santos JG, Fernandes FAN, de Siqueira Oliveira L, de Miranda MRA (2015) Influence of ultrasound on fresh-cut mango quality through evaluation of enzymatic and oxidative metabolism. Food Bioprocess Technol 8:1532–1542. https://doi.org/10.1007/s11947-015-1518-8
Silva CK, Silva ZE, Mariani VC (2009) Determination of the diffusion coefficient of dry mushrooms using the inverse method. J Food Eng 95:1–10
Singh F, Katiyar VK, Singh BP (2015) Mathematical modeling to study influence of porosity on apple and potato during dehydration. J Food Sci Technol 52:5442–5455
Soquetta MB, Schmaltz S, Righes FW et al (2018) Effects of pretreatment ultrasound bath and ultrasonic probe, in osmotic dehydration, in the kinetics of oven drying and the physicochemical properties of beet snacks. J Food Process Preserv 42:e13393. https://doi.org/10.1111/jfpp.13393
Thippanna KS, Tiwari RB (2017) Quality changes in osmotically dehydrated banana var. “Robusta” and “Ney Poovan” as affected by syrup concentration and immersion time. J Food Sci Technol 52:399–406
Yan Z, Sousa-Gallagher MJ, Oliveira FAR (2008) Shrinkage and porosity of banana, pineapple and mango slices during air-drying. J Food Eng 84:430–440
Acknowledgements
The authors acknowledge the financial support of the Brazilian funding agency CNPq and the INCT Frutos Tropicais.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fernandes, F.A.N., Braga, T.R., Silva, E.O. et al. Use of ultrasound for dehydration of mangoes (Mangifera indica L.): kinetic modeling of ultrasound-assisted osmotic dehydration and convective air-drying. J Food Sci Technol 56, 1793–1800 (2019). https://doi.org/10.1007/s13197-019-03622-y
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-019-03622-y