Abstract
The effects of air drying temperature, slice thickness and pre-treatment application on the drying kinetics of carrot slices during convective drying in the range 50–70 °C were investigated. Results indicated that drying time, rehydration ratio and colour characteristics of carrot slices were more affected by drying air temperature, followed by pre-treatment applications. Five thin-layer drying models were applied to describe the drying kinetics. Midilli et al. model was the best model to characterize the drying kinetics of carrot slices. The moisture effective diffusivity calculated from the second Fick’s law of diffusion ranged from 3.46 × 10−10 to 1.02 × 10−9 m2/s. The values of activation energy determined from the slope of the Arrhenius plot, ln(D eff ) versus 1/(T + 273.15), were 35.53, 43.42, and 37.75 kJ/mol for blanch, potas and control samples, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Carrot is root vegetables that are cultivated worldwide. The annual world production of carrot in 2012 was 36.9 million tons. China is the leading grower of carrot in the world. Russian Federation, United States of America, Uzbekistan, Poland, Turkey, Morocco, United Kingdom and Japan are the other major producing countries. Carrot production in Turkey was approximately 0.71 million tons in 2012 [1]. Carrot is the most commonly used vegetable for human. It is excellent source of β-carotene, vitamin A and potassium, and contains cholesterol-lowering pectin, vitamin C, vitamin B6, thiamine, folic acid and magnesium [2, 3]. Fresh carrots contain about 434 mg a-carotene/g solid, 1153 mg b-carotene/g solid, 0.11–0.50 mg a-tocopherol/100 g solid and 770 mg vitamin C/g solid [4]. Like other vegetables, carrot is highly seasonal and abundantly available at particular times of the year. For extending the availability of this product, several preservation processes can be used such as freezing and drying. Drying is one of the most important, because it is not only significantly extends vegetable shelf life but also diversifies the offer of foods for consumers [3]. Usually dried carrots are used in soups, pastries, sauces, spices, and other types of read-made meals [5].
Drying is a preservation method whose main purpose is to prolong the self life of products by reducing the water activity to a level low enough for the growth of microorganisms, enzymatic reactions, and other deteriorative reactions to be inhibited [6, 7]. It is a complicated process involving simultaneous, coupled mass and heat transfer, particularly under transient conditions [8]. From an engineering point of view, it is important to develop a better understanding of the controlling parameters of this complex process. Mathematical models of the drying processes are used for designing new or improving existing drying systems or even for the control of the drying process. Many mathematical models have proposed to describe the drying process, of them thin-layer drying models have been widely in use. These models can be categorized as theoretical, semi-theoretical, and empirical [9]. The drying characteristics and kinetics of various agricultural products have been studied by many investigators [3, 5, 10–12].
Pretreatments of some agricultural foods prior to drying have been reported to help reduce some of undesired changes. The main purpose of pretreatment is generally to inactivate enzymes such as polyphenoloxidase, peroxidase and phenolase and to inhibit some undesirable chemical reactions, which cause many adverse changes of a product [13]. Potassium and sodium hydroxide, potassium carbonate, potassium metabisulphate, methyl and ethyl ester emulsions, citric and ascorbic acids are some of the most common and commercially used pretreatments [14, 15]. Blanching of vegetables is a common pretreatment used prior to thermal processing, freezing and drying. The primary purpose of blanching is to inactivate naturally occurring enzymes in addition to removal of gases from surfaces and intercellular spaces to prevent oxidation, discoloration, and off-flavour development as well as reducing the initial number of microorganisms [16]. Generally, fruits and vegetables are blanched by heating with steam or hot water.
Many studies done to process carrot by air drying [2, 4, 5, 17], microwave drying [18] and infrared drying [19]. Several researches have been done to the effect of some process parameters such as temperature, sample thickness and air velocity [14, 20]. However, the influence of pre-treatment solutions on drying characteristics and quality parameters of carrot has not been studied in detail. The objectives of this research was to: (1) determine the effect of drying air temperature and pre-treatment on drying time of carrot slices, (2) fit the drying curves with five thin-layer drying models and investigate the goodness of fit, (3) calculate effective moisture diffusivity and activation energy of the carrot slices, (4) analysis the quality parameters such as rehydration and colour.
2 Materials and methods
2.1 Materials
Fresh carrot (Daucus carota L.) were purchased from a local market in Istanbul, and kept in a refrigerator at 4 °C prior to use. The samples were removed from refrigeration and left to equilibrate at the room temperature before experimentations. The carrots were washed with tap water peeled and sliced manually into disks of 2.5 ± 0.1 cm diameter and 0.4, 0.7 and 1.0 cm (±0.1) thickness (d) using a food slicer. Then, the samples were pre-treated with solution of potassium carbonate solution or blanched with hot water for inactivation of enzymes. The pre-treatments investigated in this study are shown in Table 1. The initial moisture content of the fresh carrot was determined using the AOAC method [21]. The initial moisture content of the fresh samples was measured in triplicate and was determined as 8.33 ± 0.05 kg water/kg dry matter (d.b.).
2.2 Drying procedure
The drying of carrot slices was investigated in experimental drying cabinet (APV & PASILAC Limited of Carlisle, Cumbria, UK). The cabinet dryer was described previously by Doymaz [14]. It basically consists of a centrifugal fan to supply the air-flow, an electric heater, an air filter and an electronic proportional controller. The air temperature is controlled by means of a proportional controller. The air velocity above the product is measured with an anemometer (AM-4201, Lutron, Taipei, Taiwan) with a sensitivity of ±0.1 m/s. The air passed through heating unit and heated to be desired temperature and channelled to the drying tunnel. The air temperature in the dryer is regulated to ±1 °C using a temperature controller. The samples were dried in a perforated tray (radius: 29 cm, and height: 7 cm). Weight loss of samples was recorded by using a digital balance (model BB3000, Mettler-Toledo AG, Grefensee, Switzerland), which has 0–3000 g measurement range with reading accuracy of ±0.1 g.
The dryer was started about 30 min before drying experiments to achieve steady-state conditions before each drying run. Then, the samples weighing about 50 ± 0.5 g was spread on the tray. The drying experiments were conducted at 50, 60 and 70 °C air temperatures and constant air velocity of 2 ± 0.1 m/s. Air flowed perpendicular to drying surfaces of the samples. Weight loss of samples was recorded at regular intervals of 15 min during drying. Drying was stopped when the moisture content of samples were approximately 0.11 ± 0.05 kg water/kg dry matter (d.b.) [22]. Then, the dried samples were packed into polyethylene bags, which were then heat-sealed and stored in incubators at ambient temperature. The experiments were replicated three times and the average of the moisture ratio at each value was used for drawing the drying curves.
2.3 Mathematical modelling of the drying curves
The data derived from drying of carrot were fitted with five drying models (Table 2) typically used for the modeling of drying curves. The moisture ratio (MR) of the samples is defined according to Eq. (1):
where M t , M 0 and M e are moisture content at any time, initial moisture content, and equilibrium moisture content (kg water/kg dry matter), respectively, and t is drying time (min). The moisture ratio (MR) was simplified to M t /M 0 instead of (M t − M e )/(M 0 − M e ) by some investigators [28, 29] because of the values of M e small compared with M t or M 0 for long drying time.
The drying rate (DR) of carrot slices was calculated using Eq. (2):
where M t+Δt is moisture content at t + Δt (kg water/kg dm), t is time (min).
2.4 Data analysis
Data were analyzed using Statistica 8.0.550 (StatSoft Inc., Tulsa, OK, USA) software package. The parameters of models were estimated using a non-linear regression procedure based on the Levenberg–Marquardt algorithm. The goodness of fit was deduced using the parameters, i.e. coefficient of determination (R 2), reduced Chi square (χ 2), and root mean square error (RMSE). These parameters can be described in equations from as (3) and (5):
where MR exp,i and MR pre,i are experimental and predicted dimensionless moisture ratios, respectively; N is number of observations; z is number of constants. Higher R 2 and lower χ 2, and RMSE values indicate good fitness of the established model [29, 30].
2.5 Determination of effective moisture diffusivity
The most widely studied theoretical model in thin-layer drying of various foods is given by solution of Fick’s second law. The Fick’s law in three-dimensional rectangular coordinates can be expressed as:
The solution of diffusion equation (Eq. 6) for slab geometry is solved by Crank [31] and supposed uniform initial moisture distribution, negligible external resistance, constant diffusivity and negligible shrinkage:
where D eff is the effective moisture diffusivity (m2/s), L is the half-thickness of the slab in samples (m), and n is positive integer. As time increased, just the first term can be used to estimate the drying rate (n = 0), and then Eq. (7) converges into:
The effective moisture diffusivity (D eff ) values were calculated by plotting experimental drying data in terms of ln (MR) were plotted versus drying time. ln(MR) versus drying time results in a straight line with negative slope and k is related to D eff by Eq. (9).
2.6 Computation of activation energy
Drying characteristics of moist material are mainly dependent on bonding potential of moisture. The value of activation energy is an index indicating the bonding potential of moisture in moist material. Activation energy is defined as the necessary energy while removing 1 mol moisture form the material with given moisture content and constant composition [32]. The dependence of the effective moisture diffusivity on the different drying temperature was predicted using an Arrhenius type equation:
here D 0 is the pre-exponential factor (m2/s), E a is the activation energy (kJ/mol), R is the universal gas constant [kJ/(mol K)], and T is temperature (°C).
2.7 Rehydration experiments
Rehydration experiments were determined by soaking known weight (2.5 g) samples in 300 mL of water at 25 and 65 °C. At the end of the rehydration period, that is, 300 min, the samples were then removed, blotted with tissue paper to eliminate excess water on the surface, and weighed with an electronic digital balance (Precisa, model XB220A, Precisa Instruments AG, Dietikon, Switzerland) having a sensitivity of 0.001 g. The rehydration ratio (RR) was calculated according to Eq. (11):
where W 1 is the weight of dried matter (kg) and W 2 is the weight of material (kg).
2.8 Colour analysis
The colour values of the fresh and dried samples were evaluated using a hand-held tristimulus colorimeter (Chroma Meter-CR-400 from Konica Minolta, Osaka, Japan). The colour brightness coordinate L measures the whiteness value of a colour and ranges from black at 0 to white at 100. The chromaticity coordinate a measures red when positive and green when negative, and the chromaticity coordinate b measures yellow when positive and blue when negative. Five replicate measurements were performed and results were averaged.
3 Results and discussion
3.1 Drying curves
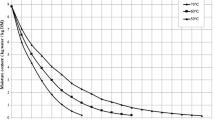
Figure 1 represents the variations of moisture content with drying time for drying of carrot slices at 50, 60 and 70 °C. The results showed that the increase in drying air temperature resulted in a decrease in the drying time. For example, the drying time of control code samples reduced from 315 to 165 min when the air temperature was increased from 50 to 70 °C. Similar trend was observed for pre-treated samples at same temperatures. These results agreed with the findings of Özgen [33] for Cornelian cherry, Vega-Gálvez et al. [7] for Cape gooseberry, and Xiao et al. [4] for carrot cubes.
3.2 Effect of pre-treatment on drying time
According to the results in Fig. 1, pre-treatment is very important parameter that affects the drying time. The samples dipped in hot water (blanch code) prior to drying were found to have a shorter drying time compared to potas and control samples. The drying time required reaching final water content (0.11 kg water/kg dry matter) for potas and control samples were 300 and 315 min at 50 °C, respectively. Corresponding value for the blanched samples was 240 min at the same temperature. The difference in drying times was close to 20 and 23.8 %, respectively. These results show that blanched with hot water contributed to increase the permeability of the cell membranes of carrot slices, leading to an increase in water diffusivity. Similar trends were observed at drying temperatures of 60, and 70 °C. The effect of pre-treatment on drying time was also reported in previous studies for different biological products [11, 26, 34].
3.3 Slice thickness
Figure 2 shows the drying curves of control slices at 50 °C for different slice thicknesses. It is clearly evident from these curves that the drying rate was higher at thin slices, and the total drying time reduced substantially with the decrease in slice thickness. The drying times of control samples were 315, 405 and 510 min, respectively, in relation to the slice thickness of 0.4, 0.7 and 1.0 cm. The drying time of 0.4 cm slice thickness samples was shortened by 28.5 and 61.9 % compared with the drying process realized at 0.7 and 1.0 cm slice thickness samples, respectively. Thinly sliced products dried faster due to the reduced distance the moisture travels and increased surface area exposed for a given volume of the product. The similar observations were found by Ertekin and Yaldiz [35] for hot-air drying of eggplant slices and Wu et al. [12] for infrared and hot-air drying of carrot slices.
3.4 Drying rate
The drying rates defined as the quantity of water removed per unit time versus drying time are shown in Fig. 3. It is apparent that drying rate decreased continuously with improving drying time. The drying rate reached its maximum values at higher drying air temperatures. The moisture removal inside the carrot slices were higher at high drying air temperatures, because the migration of moisture to the surface and the evaporation rate from surface to air slows down with decreasing the moisture in the product, the drying rate clearly decrease (Fig. 3). Constant drying rate period was not observed in the drying curves and the whole drying process take place in the falling drying rate period. This shows that diffusion was domain physical mechanism governing moisture movement in drying process. These results are in agreement with the observations of earlier researchers on drying various vegetables [5, 12].
3.5 Evaluation of drying models
Non-linear regression analysis was done according to five drying models presented in Table 2. The best mathematical model fitting experimental data was chosen based on maximized R 2 and minimized χ 2 and RMSE. The results of statistical analysis undertaken on the models for air drying are summarized in Table 3. For the drying of carrot slices, the Midilli et al. model was found to be the best descriptive model. From Table 3, it was determined that for three drying air temperatures, the R2 value was highest and χ 2 and RMSE values were lowest for the Midilli et al. model. The values of R 2, χ 2, and RMSE for the Midilli et al. model vary between 0.9972 and 0.9998, 0.000010 and 0.000438, and 0.013090 and 0.041931, respectively. Figure 4 compare the experimental data with the predicted ones using different models for carrot slices at 50, 60 and 70 °C. The predictions using the models showed MR values banded along a straight line, which proved the suitability of these models in describing the drying characteristics of carrot slices.
3.6 Effective moisture diffusivity
The determined values of the effective moisture diffusivity (D eff ) were found to range between 3.46 × 10−10 and 1.02 × 10−9 m2/s. As expected that the effective moisture diffusivity increased greatly with increasing drying temperature. The values of D eff obtained from this study are within the general ranged from 10−12 to 10−8 m2/s for biological materials [36]. D eff values in this study are close to the values of 2.65 × 10−10–1.052 × 10−9 m2/s reported for the hot-air drying of carrot cubes at 40–60 °C [4], 1.35 × 10−9–3.45 × 10−9 m2/s for the hot-air drying of carrot slices at 50–70 °C [37], 1.43 × 10−9–5.50 × 10−9 m2/s for the hot-air drying of carrot slices at 60–80 °C [12], 2.38 × 10−9–10.30 × 10−9 m2/s for the infrared drying of carrot slices at 60–80 °C [12]. The differences between the results could be due to the composition structure, shape and initial moisture content of material, as well as the drying temperature, pretreatments, and drying equipment.
3.7 Activation energy
The activation energy using Eq. (10) was calculated by plotting ln(D eff ) versus the reciprocal of the temperature (1/(T + 273.15)), and presented in Fig. 6. Thus, the values of activation energy found to be 35.53, 43.42 and 37.75 kJ/mol for blanch, potas and control samples, respectively. The values obtained in this study are in the range of 12.7–110 kJ/mol for various foods reported by Zogzas et al. [36]. The similar value to those proposed in the literature for drying of carrots: 35.74–59.06 kJ/mol for infrared drying of carrot slices [12], 23.63–33.24 kJ/mol hot-air drying of carrot slices [12], and 20.17 kJ/mol for hot-air drying of carrot slices [4].
3.8 Rehydration ratio
Rehydration characteristics have been considered as a measure of the induced damage in the material during drying. The ability of food products to reconstitute depends primarily on the internal structure of the dried pieces and the extent to which the water-holding components (e.g., proteins and starch) have been damaged during drying [7]. The values of rehydration ratio of carrot slices at different rehydration temperatures, calculated from Eq. (11) are shown in Fig. 7. In the same figure, rehydration ratio was affected by the drying temperatures, since absorbed water decreased with temperature. The lowest rehydration ratio values were obtained with the samples dried at 50 °C, this could be explained due to cellular structure damage resulting in modifications of osmotic properties of the cell as well as lower diffusion of water through the surface during rehydration [38]. The rehydration ratio increased when rehydration temperature increased from 25 to 65 °C. From Fig. 7, rehydration ratio at 65 °C was more rapid than at 25 °C. Rehydration at high temperatures improves due to the effect of temperature on cell wall and tissue [39]. As seen in Fig. 7, the rehydration ratio of potas samples resulted in the highest rehydration, compared to blanched and control samples.
3.9 Colour values
Colour is one of the most important quality parameters of dried products and a decisive factor for the consumer acceptance of the product. The colour values (L, a and b) of the fresh samples was measured as 60.04, 20.41, and 26.97, respectively. The colour parameters of dried carrots are given in Fig. 8. Figure 8 shows that L values of blanch, potas and control samples decreased from 58.28 to 55.13, 56.13 to 54.13, and 55.34 to 53.41 at drying air temperatures ranging from 50 to 70 °C, respectively. This result can be related to oxidative reactions that occur during drying process which is potentiated by drying air temperature. However, the blanched samples displayed higher L (lightness) values compared to those other samples.
The values of a and b are shown in Fig. 8. It showed that an increase in relative a values and decrease in b values during drying under different drying temperatures. The initial and final a values of blanch, potas, and control samples varied from 20.41 to 28.66, 28.50 and 29.95 as the drying air temperature increased from 50 to 70 °C. In similar, the initial and final b values of blanch, potas, and control samples varied from 26.97 to 22.79, 22.82 and 23.17 as the drying air temperature increased from 50 to 70 °C. The loss of b value indicates that the yellowness of samples decreased due to application of drying air temperatures, and it may be due to degradation of carotenoid pigments, nonenzymatic Maillard browning and formation of brown pigments [17, 40].
4 Conclusions
Drying characteristics of carrot slices were investigated in a cabinet dryer at various temperatures of 50, 60 and 70 °C and constant air velocity of 2 m/s. The air temperature and pre-treatment had more significant effects on drying time and quality characteristics such as rehydration and colour. The drying process of carrot occurred in the falling drying rate period. In order to explain the drying kinetics of carrot slices, five models in the literature were applied and fitted to the experimental data. According to the results of regression analysis, the experimental data were well predicted by the Midilli et al. model. The effective moisture diffusivity was found in the range of 3.46 × 10−10–1.02 × 10−9 m2/s as the drying temperature increased from 50 to 70 °C. The values of activation energy were determined to be 35.53, 43.42, and 37.75 kJ/mol for blanch, potas and control samples, respectively.
References
FAO 2014. FaoStat: Agriculture data. http://faostat.fao.org/site/567/DesktopDefault.aspx?PageID=567#ancor. Updated 04 August 2014
Vázquez-Vila MJ, Chenlo-Romero F, Moreira-Martínez R, Pacios-Penelas B (2009) Dehydration kinetics of carrots (Daucus carota L.) in osmotic and air convective drying processes. Span J Agric Res 7:869–875
Guiné RPF, Sério SIA, Correia PMR, Barroca MJ (2014) Effect of pre-treatment on some physical–chemical properties of dried carrots. J Hyg Eng Des 6:187–191
Xiao HW, Gao ZJ, Lin H, Yang WX (2010) Air Impingement drying characteristics and quality of carrot cubes. J Food Process Eng 33:899–918
Zielinska M, Markowski M (2010) Air drying characteristics and moisture diffusivity of carrots. Chem Eng Process 49:212–218
Rodríguez Ó, Llabrés PJ, Simal S, Femenia A, Rosselló C (2015) Intensification of predrying treatments by means of ultrasonic assistance: effects on water mobility, PPO activity, microstructure, and drying kinetics of apple. Food Bioprocess Technol 8:503–515
Vega-Gálvez A, Zura-Bravo L, Lemus-Mondaca R, Martinez-Monzó J, Quispe-Fuentes I, Puente L, Di Scala K (2015) Influence of drying temperature on dietary fibre, rehydration properties, texture and microstructure of Cape gooseberry (Physalis peruviana L.). J Food Sci Technol 52:2304–2311
Diamante LM, Ihns R, Savage GP, Vanhanen L (2010) A new mathematical model for thin layer drying fruits. Int J Food Sci Technol 45:1956–1962
McMinn WAM (2006) Thin-layer modeling of the convective, microwave, microwave-convective and microwave-vacuum drying of lactose powder. J Food Eng 72:113–123
Madhiyanon T, Phila A, Soponronnarit A (2009) Models of fluidized bed drying for thin-layer chopped coconut. Appl Therm Eng 29:2849–2854
Falade KO, Solademi OJ (2010) Modeling of air drying of fresh and blanched sweet potato slices. Int J Food Sci Technol 45:278–288
Wu B, Ma H, Qu W, Wang B, Zhang X, Wang P, Wang J, Atungulu GG, Pan Z (2014) Catalytic infrared and hot air dehydration of carrot slices. J Food Process Eng 37:111–121
Hiranvarachat B, Devahastin S, Chiewchan N (2011) Effects of acid pretreatments on some physicochemical properties of carrot undergoing hot air drying. Food Bioprod Process 89:116–127
Doymaz I (2004) Effect of pre-treatments using potassium metabisulphide and alkaline ethyl oleate on the drying kinetics of apricots. Biosyst Eng 89:281–287
Baomeng Z, Xuesen W, Guodong W (2014) Effect of pre-treatments on drying characteristics of Chinese jujube (Zizyphus jujuba Miller). Int J Agric Biol Eng 7:94–102
Yucel U, Alpas H, Bayindirli A (2010) Evaluation of high pressure pretreatment for enhancing the drying rates of carrot, apple, and green bean. J Food Eng 98:266–272
Demiray E, Tulek Y (2014) Color degradation kinetics of carrot (Daucus carota L.) slices during hot air drying. J Food Process Preserva. doi:10.1111/jfpp.12290
Cui ZW, Xu SY, Sun DW (2004) Microwave-vacuum drying kinetics of carrot slices. J Food Eng 65:157–164
Kocabiyik H, Tezer D (2009) Drying of carrot slices using infrared radiation. Int J Food Sci Technol 44:953–959
Kaya A, Aydin O, Demirtas C (2009) Experimental and theoretical analysis of drying carrots. Desalination 237:285–295
AOAC (1990) Official method of analysis. Association of Official Analytical Chemists, Arlington
Nahimana H, Mujumdar AS, Zhang M (2011) Drying and radial shrinkage characteristics and changes in color and shape of carrot tissues (Daucus carota L.) during drying. Afr J Biotechnol 10(68):15327–15345
Chimplee S, Klinkesorn U (2015) Thin-layer drying model of rambutan (Nephelium lappaceum L.) kernel and its application in fat extraction process. Int J Food Eng 11:243–253
Pirone BN, De Michelis A, Salvatori DM (2014) Pretreatments effect in drying behaviour and colour of mature and immature ‘Napolitana’ sweet cherries. Food Bioprocess Technol 7:1640–1655
Midilli A, Kucuk H, Yapar Z (2002) A new model for single layer drying. Dry Technol 20:1503–1513
Bi J, Yang A, Liu X, Wu X, Chen Q, Wang Q, Lv J, Wang X (2015) Effects of pretreatments on explosion puffing drying kinetics of apple chips. LWT Food Sci Technol 60:1136–1142
Corzo O, Bracho N, Pereira A, Vásquez A (2008) Weibull distribution for modelling air drying of coroba slices. LWT Food Sci Technol 41:2023–2028
Dissa AO, Bathiebo DJ, Desmorieux H, Coulibaly O, Koulidiati J (2011) Experimental characterisation and modelling of thin layer direct solar drying of Amelia and Brooks mangoes. Energy 36:2517–2527
Baslar M, Karasu S, Kilicli M, Us AA, Sagdic O (2014) Degradation kinetics of bioactive compounds and antioxidant activity of pomegranate arils during the drying process. Int J Food Eng 10:839–848
Falade KO, Ogunwolu OS (2014) Modeling of drying patterns of fresh and osmotically pretreated cooking banana and plantain slices. J Food Process Preserva 38:373–388
Crank J (1975) The mathematics of diffusion. Clarendon Press Inc, Oxford
Fang S, Wang Z, Hu X (2009) Hot air drying of whole fruit Chinese jujube (Zizyphus jujuba Miller): thin-layer mathematical modeling. Int J Food Sci Technol 44:1818–1824
Ozgen F (2014) Experimental investigation of drying characteristics of Cornelian cherry fruits (Cornus mas L.). Heat Mass Transfer 51:343–352
Tunde-Akintunde TY (2011) Mathematical modeling of sun and solar drying of chilli pepper. Renew Energy 36:2139–2145
Ertekin C, Yaldiz O (2004) Drying of eggplant and selection of a suitable thin layer drying model. J Food Eng 63:349–359
Zogzas NP, Maroulis ZB, Marinos-Kouris D (1996) Moisture diffusivity data compilation in foodstuffs. Dry Technol 14:2225–2253
Liu Y, Wu J, Miao S, Chong C, Sun Y (2014) Effect of a modified atmosphere on drying and quality characteristics of carrots. Food Bioprocess Technol 7:2549–2559
Kaymak-Ertekin F (2002) Drying and rehydrating kinetics of green and red peppers. J Food Sci 67:168–175
Singh B, Panesar PS, Nanda V (2006) Utilization of carrot pomace for the preparation of a value added product. World J Dairy Food Sci 1(1):22–27
Koca N, Burdurlu HS, Karadeniz F (2007) Kinetics of colour changes in dehydrated carrots. J Food Eng 78:449–455
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Doymaz, İ. Drying kinetics, rehydration and colour characteristics of convective hot-air drying of carrot slices. Heat Mass Transfer 53, 25–35 (2017). https://doi.org/10.1007/s00231-016-1791-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00231-016-1791-8