Abstract
Purpose
To develop a population pharmacokinetic (PPK) model for methotrexate (MTX) dosage for all ages, assess the association between concentration and clearance, and determine covariates affecting MTX disposition.
Methods
We compared MTX PK profiles among neonates, children, and adults by performing a systematic literature search for published population MTX models and conducted a Monte Carlo-based meta-analysis. Subsequently, we evaluated study quality and covariates significantly affecting dosage regimens and compared LDMTX and HDMTX PK profiles.
Results
Of the total 40 studies included, 34 were HDMTX, and six were LDMTX studies. For HDMTX, three studies involving neonates reported estimated apparent clearances (median, range) of 0.53 (0.27–0.77) L/kg/h; for 14 studies involving children, 0.23 (0.07–0.23) L/kg/h; and for 13 involving adults, 0.11 (0.03–0.22) L/kg/h. Neonates had a higher volume of distribution than children and adults. For LDMTX studies, apparent clearance was 0.085 (0.05–1.68) L/kg/h, and volume of distribution was 0.25 (0.018–0.47) L/kg, lower than those of HDMTX studies, with large between-subject variability. Bodyweight significantly influenced apparent clearance and volume of distribution, whereas renal function mainly influenced clearance. Mutations in certain genes reduced MTX clearance by 8–35.3%, whereas those in others increased it by 15–48%. Body surface area (BSA) significantly influenced apparent clearance with a median reduction of 51% when BSA increased in pediatric patients.
Conclusions
Methotrexate dosage regimens were primarily based on body surface area and renal function. Further studies are needed to evaluate MTX pharmacokinetics and pharmacodynamics in both children (especially infants) and adults.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Methotrexate (MTX), a folate antimetabolite, can be administered over a broad range of doses via different routes for its antitumor and anti-inflammatory effects [1]. An MTX dosage < 50 mg/m2 is defined as low-dose MTX (LDMTX) and is mainly used for the treatment of rheumatoid arthritis (RA), breast cancer, and prevention of acute graft-versus-host disease (aGVHD) [2]. Dosages of MTX > 500 mg/m2 are defined as high-dose MTX (HDMTX) and are used effectively to treat infant, pediatric, or adult patients with acute lymphoblastic leukemia (ALL), osteosarcoma (OS), or lymphoma malignancies [3].
Methotrexate can be administered orally, subcutaneously, intramuscularly, or intravenously. Oral MTX absorption is highly variable, ranging from 23 to 95% in the dose range used to treat RA, with a dose-dependent response [4]; its absorption declines at higher doses, especially at > 40 mg/m2 for pediatric patients and > 80 mg/m2 for adult patients [5]. However, MTX absorption is complete after intramuscular injection. The time-to-peak for oral administration is 0.7–4 h for children and 0.75–6 h for adults; for intramuscular administration, it is 30–60 min for both children and adults. MTX penetrates slowly into third-space fluids (e.g., pleural effusions and ascites) and exits more slowly from these compartments than from plasma.
Major side effects of LDMTX include nausea, stomatitis, abnormal liver chemistry, and rash [6]. HDMTX is associated with toxicity, including acute kidney injury (AKI), oral mucositis, hepatotoxicity, and myelosuppression [7]. The activity and toxic effects of MTX are closely related to the dosage regime and the duration of exposure above the threshold concentration. There is also a higher probability of disease relapse among patients with rapid clearance [8]. These effects are not readily distinguishable between HD and LD indications. As the MTX dose affects both its PK and pharmacodynamics (PD), various pharmacogenetic associations may also have an impact. HDMTX in chemotherapy and LDMTX in immunosuppression therapy have different mechanisms of action. For LDMTX, the PK may be nonlinear and exhibit large heterogeneity, indicating that accurate LDMTX dosing is complicated [9]. To ensure the safety and effectiveness of MTX, therapeutic drug monitoring (TDM) can be employed to measure the MTX plasma concentration [10]. However, most TDM has been focused on HDMTX, which is guided by population means reflected in nomograms, and is not based on the individual characteristics of patients [11].
The population pharmacokinetic model (PPK) is used to analyze sparse TDM data for highly diverse populations, estimate intra- and inter-individual variability, and identify the impact of covariates. Bayesian estimation using PPK parameters has been effectively applied for dosage adjustment to avoid under or overestimating MTX concentrations [12, 13]. Although many PPK studies have been conducted to quantitatively describe the pharmacokinetic characteristics of MTX, these studies have mainly focused on identifying covariates with potentially significant effects on MTX PK.
The overarching aim of our analysis was to perform a Monte Carlo simulation of concentration-time profiles to assess the statistical and structural models within each MTX PPK model [14]. A robust PPK model with Monte Carlo simulation of various dosing regimens and patient populations can generate the expected range of MTX concentrations throughout the therapy and assist in predicting the therapeutic effect and risk of MTX toxicity in patients. Further, we aimed to compare the clearance (CL) of the included MTX models via formal meta-analysis, analyze the covariates significantly affecting MTX PK, and identify challenges that remain to be explored.
Methods
Information sources and search strategy
A systematic literature search for data published on PPK models for MTX until December 31, 2022, was conducted using the following electronic databases: PubMed, Embase, and MEDLINE. The language was limited to English. The PPK studies on MTX were searched using the following terms: “methotrexate,” “population pharmacokinetic,” “pharmacokinetic modeling,” “nonlinear mixed effect model,” “nonmem or P-metrics,” “WINNONMIX,” “ADAPT,” “P-PHARM,” “nlmixed,” “NLME,” “USC*PACK,” “MONOLIX,” or “Bayes,” and “adult,” “adolescent,” “children,” or “infant.” We also checked the references of related studies. The literature search was performed by two authors, and a senior researcher was consulted to resolve discrepancies.
All relevant articles selected from the databases and reference lists were screened to evaluate their eligibility for inclusion, according to the following inclusion criteria: (1) patients receiving MTX; (2) MTX dosage, administration route, and sampling time provided; (3) use of the PPK method to analyze data; and (4) availability of essential PK parameters. The following studies were excluded: (1) the article was a review or external evaluation article; (2) the PK parameters were incomplete; (3) the articles were duplicated; (4) the full text could not be obtained; and (5) non-English articles.
Data extraction
We extracted the following information from the included articles: (1) basic demographics, including dosage regimens (e.g., MTX dosage, administration route), age, weight, diagnosis, study design (e.g., study type, number of patients and sample points, and sampling time), and (2) information on PPK-related parameters that contained the PPK formula, model evaluation methods, structural models, between-subject variability (BSV), and residual unexplained variability (RUV).
Study comparison
The concentration–time profiles of the virtual patients were generated based on the final PPK models. For HDMTX-based articles, we performed 2000 simulations in three age groups (infants, children, and adults) with three disease types (OS, ALL, or malignant lymphoma); for LDMTX used to treat RA or breast cancer or prevent aGVHD, we simulated 2000 virtual adult male patients each. They received intravenous or oral MTX at different dosage regimes, and we simulated the MTX concentration at the end of the administration and elimination phase. Patients were assumed to have received monotherapy if they reached a steady state for oral administration, with MTX plasma measured in μmol.
Standard HDMTX doses for ALL, lymphoma, and OS were determined according to the following relevant guidelines:
-
ALL: 500 mg/m2 over 0.5 h, followed by 4500 mg/m2 over 23.5 h, as per the ALL BFM95 [15].
-
Malignant lymphoma: 3000 mg/m2 for 6 h infusion, according to the guidelines of lymphoma [16].
-
OS: 12,000 mg/m2 for 6 h infusion [8].
Standard LDMTX doses for breast cancer, aGVHD, and RA were administered according to the following guidelines:
-
Breast cancer: 40 mg/m2 intravenously for 6–12 cycles [17].
-
Hematopoietic stem cell transplant (HSCT):15 mg/m2 on day 1 and 10 mg/m2 on days 3, 6, and 11 after HSCT for aGVHD [18].
-
RA: 7.5 mg oral dose once weekly [19].
The simulation profiles were plotted according to the established PPK models, as follows: neonates (3 kg, postmenstrual age [PMA] 36 weeks), serum creatinine (SCr) set to 0.5 mg/dL; children (10 years, 30 kg), SCr set to 0.5 mg/dL, according to a multicenter study of children [20]; and adults (40 years, 70 kg), SCr set to 1 mg/dL, according to the mean estimated glomerular filtration rate of 120 mL/min for patients between 20 and 60 years [21].
We analyzed the effects of the potential covariates retained in the included PPK studies on MTX CL; the results are represented using a forest map. Continuous covariates (e.g., weight, age, creatinine clearance [CrCL], and body surface area [BSA]) were standardized to the same range for comparison. The weight ranges were 16–40 kg for children and 40–100 kg for adults. The CrCL range was 20–200 mL/min/1.73 m2. For some continuous covariates that were retained in only one study (e.g., hematocrit), the minimum and maximum values were obtained from the study. For binary covariates, such as sex, 0 and 1 were used. We tested the range of covariates in each study and the calculated minimum and maximum apparent clearance (CL) values; CL was normalized to the median covariate values in each study. We defined effects on CL greater than 80–125% to have a significant clinical correlation [22].
We conducted simulations using NONMEM (version 7.4; ICON Development Solutions, Ellicott City, MD, USA). R (version 3.5.1; http://www.r-project.org/) was used to generate concentration–time profiles and forest plots.
Results
Study identification
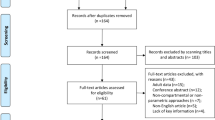
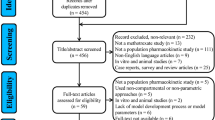
We preliminarily screened 1085 articles identified using the search strategy from PubMed (n = 263), Embase (n = 104), and Medline (n = 718). Twelve additional articles were identified using the reference lists of the selected articles. After removing the duplicates, 154 studies were screened, and 63 full-text articles were retrieved for a more detailed evaluation of eligibility. After literature retrieval and screening (Fig. 1 and Online Resource S1), we finally included 40 articles containing 34 studies on HDMTX and six studies on LDMTX for evaluation.
Study characteristics
The HDMTX studies all applied an infusion time of 1–24 h to treat ALL, OS, and lymphoma; four of these were for infants [23,24,25,26], 14 for adults [27,28,29,30,31,32,33,34,35,36,37,38,39,40], 11 for children [41,42,43,44,45,46,47,48,49,50,51]; the remaining five addressed both children and adults [52,53,54,55,56]. The LDMTX studies were all conducted using adults [57,58,59,60,61,62]. Of the HDMTX PPK studies, seven addressed only ALL [24, 41, 44, 47,48,49,50]; eight, only primary central nervous system lymphoma (PSCNL) [23, 25, 26, 34, 36, 39, 40, 53]; four, only OS [42, 46, 52, 54]; and one, only non-Hodgkin lymphoma (NHL) [35]. The remaining studies addressed two or more diagnoses. For LDMTX, three studies used oral MTX for patients with RA [57, 59, 62], two used intravenous MTX for breast cancer [58, 60], and one used intravenous MTX for preventing aGVHD [61]. Nine articles were prospective studies [24, 29, 31, 46, 48, 52, 53, 59, 61], and the rest were retrospective studies.
Only three articles were multicenter studies [25, 46, 53]; the remainder were single-center studies. Ten studies were conducted in China [26, 29, 34, 35, 40, 45, 47, 50, 51, 54], nine in the USA [23,24,25, 33, 36, 49, 55,56,57], five in France [27, 30, 31, 37, 46], four in Japan [28, 32, 39, 59], two in Spain [41, 42], two in the UK [52, 58], and one each in Slovenia [43], Switzerland [53], Germany [38], India [60], Korea [61], México [48], and Egypt [44].
Thirteen of the studies, all using HDMTX, had > 100 subjects [25, 26, 37, 38, 46, 47, 49,50,51, 53,54,55,56], and one [53] was a PK/PD study. The number of patients in each study ranged from 14 and 772, with MTX observations per individual ranging from 1 to 10. The daily dose of intravenous MTX ranged between 15 and 18,000 mg/m2 and the oral dose between 7.5 and 15 mg weekly. Four articles did not mention the MTX bioassay method [33, 36, 44, 45]. Fluorescence polarization immunoassay (FPIA), enzyme multiplied immunoassay technique (EMIT), aldo–keto reductase (AKR), chemiluminescent immunoassay (CMIA), high-performance liquid chromatography(HPLC) with an ultraviolet detector, a special mode of HPLC called ultra-high performance liquid chromatography with tandem mass spectrometry (UPLC–MS/MS), and liquid chromatography with tandem mass spectrometry (LC–MS/MS) were employed as the MTX bioassay methods. The lowest limit for quantitative assays ranged between 0.238 and 5000 nM. The modeling strategies and final PPK parameters of each study are summarized in Tables 1 and 2.
In these studies, a two-compartment model with first-order elimination (FOCE) was used in most of the included studies; four used a three-compartment model [31, 38, 49, 50], and only one used a compartment model [26, 62].
Comparison of studies
For HDMTX, three studies were conducted in neonates, with an estimated CL (median, range) of 0.53 (0.27–0.77) L/kg/h; 14 articles addressed children with estimated CL (median, range) of 0.23 (0.07–0.23) L/kg/h; and 13 addressed adults with estimated CL (median, range) of 0.11 (0.03–0.22) L/kg/h. The median CL was higher in neonates than in children and adults. The volume of distribution (Vd) was determined as 1.51 (0.91–1.82) L/kg for neonates, 0.51 (0.19–1.22) L/kg for children, and 0.37 (0.03–1.13) L/kg for adults. Vd was also higher for neonates than for children and adults. For the six studies that applied LDMTX in adults, the estimated CL (median, range) was 0.085 (0.05–1.68) L/kg/h, Vd was 0.25 (0.018–0.47) L/kg, and the BSV was large. The median CL values were lower for LDMTX than for HDMTX studies. The concentration–time profiles of these six studies are presented in Fig. 2.
Concentration–time profiles of LDMTX for HSCT patients (a), breast cancer patients (b), and RA patients (c), respectively. The solid line represents the median of the simulated concentration–time profile, and the light shadows represent the 10th–90th percentiles of the simulated concentration–time profiles
In all the included studies, BSV was described mainly using exponential models. For CL and Vd, the BSV median (range) was as follows: CL 22.9% (0.3–81.73%) [n = 39], Vd 25.9% (0.03–116%) [n = 30]. The RUV, commonly described using proportional models, ranged from 2 to 67.7%. In one study, the median RUV in patients receiving LDMTX was considerably lower than that in patients receiving HDMTX (18.6% vs. 33.94%, respectively).
Comparison of different dosing regimens for HDMTX
HDMTX over-exposure was defined as a serum MTX concentration > 1.0 μM at 48 h (MTX C48h) and > 0.1 μM at 72 h (MTX C72h) and was associated with increased MTX-related toxicity [39].
OS patients
Recent studies have reported that a serum MTX concentration of 700 μM after a 6 h (MTX C6h) infusion can achieve good efficacy in treating OS, while a higher incidence of MTX-induced side effects was associated with MTX C6h > 1000 μM [63]. Three studies, by Johansson et al. [52], Zhang et al. [54], and Kawakatsu et al. [55], included both children and adults, and their concentration–time profiles differed substantially. However, two studies, by Johansson et al. [52] and Watanabe et al. [32], revealed that the target Cmax values were not achieved in either adults or children.
For patients with OS, MTX concentrations were higher in adults than in pediatric patients. In six studies of OS in children, MTX C6h (median, range) was 702 μM (475–1199 μM), with large BSV. Three studies of patients with OS defined MTX C6h < 700 μM as subtherapeutic, and one defined MTX C6h > 1000 μM as supratherapeutic. Four studies simulated adults with MTX C6h (median, range) of 877 μM (566–1006 μM). MTX C72h (median, range) was 0.17 μM (0.06–0.28 μM) in children and 0.21 μM (0.055–0.267 μM) in adults (Fig. 3).
ALL patients
For ALL patients receiving 24 h infusion, target MTX concentrations were defined based on previous literature, which suggested a serum MTX concentration after 24 h infusion (MTX C24h) < 16 μM as subtherapeutic with risk of relapse and MTX C24h > 100 μM as supratherapeutic and associated with increased toxicity [64].
Nader et al. [33] studied ALL in Middle Eastern populations, for CL in this article was higher than that estimated in other PPK studies of non-Middle Eastern populations (Fig. 4). Our simulation results revealed that dosing regimens of 5 g/m2 provided sufficient MTX exposure based on the 24-h MTX target of 16 μM. However, eight of the ALL simulation studies [44,45,46,47,48,49,50,51] examined supratherapeutic MTX concentrations (> 100 μM) at 24 h. Our results revealed that only one study was conducted in infants with supratherapeutic MTX C24h for the simulated treatment period, suggesting the need for further research in this special population. The simulated MTX concentrations at 24, 48, and 72 h were higher in children than in adults, indicating that children are more likely to experience MTX toxicity.
Lymphoma malignancy
There are no studies regarding the optimal effective target MTX concentration in patients with lymphoma malignancy (Fig. 5). For PSCNL patients, Joerger et al. [53] found that MTX C24h of 4.0–5.0 μM could achieve a target area under the concentration–time curve (AUC) of 1000–1100 μM at an MTX dose ≥ 3000 mg/m2 administered via 4- or 6-h infusions. Three studies were for adults with PCNSL; the MTX C24h in Pai et al. [36] was supratherapeutic, whereas that in the others was subtherapeutic (Fig. 6).
Comparison of studies for covariates
All of the included PPK studies were used to identify potential covariates to describe the BSV of MTX PK. Three studies did not report covariates [24, 32, 60]. The most frequently identified covariates were CrCL, SCr, BSA, and weight. CrCL significantly influenced MTX PK. Fifteen studies retained CrCL as a significant predictor of MTX CL [25, 26, 28, 30, 31, 35, 39, 40, 45, 53,54,55, 57, 58, 61]. For adults, most of these applied the Cockcroft–Gault formula, while Yang et al. [35] used the Chronic Kidney Disease Epidemiology Collaboration 2009 SCr formula; Johansson et al. [52] used the Rhodin formula [65]; and Batey et al. [58] used an estimate of CrCL obtained from the EDTA PPK model [66]. For pediatric patients, most of the studies applied the Schwartz formula, while Panetta et al. [25] used the St. Jude equation, and Johansson et al. [52] used a linear extrapolation based on the oldest pediatric group predicted by Ceriotti et al. [67]. For patients with renal impairment (CrCL 20 mL/min), the median CL (range) was 52.5% (40–85%), lower than for those with median renal function.
Eight studies of HDMTX that retained SCr as significant revealed that HDMTX CL decreased as SCr increased [27, 29, 34, 36, 38, 49, 50, 52]. Taylor et al. [49] reported a nonlinear relationship between SCr and MTX CL that varied with time. Some studies were unable to detect a nonlinear relationship between SCr and CL, possibly owing to the narrow distribution of SCr or small sample size. Three studies revealed an effect of SCr on CL exceeding 40%. Based on these studies, for patients with renal failure (SCr > 3 mg/dL), the median estimated CL was 58% lower than in patients with median SCr (Fig. 7).
Covariate effect on the clearance of MTX. The horizontal bars represent the covariate effect on clearance in each study. The typical value of clearance in each study was considered to be 1. The effect of each covariate for clearance is displayed by the ratio of clearance in the range each covariate to the typical clearance value. The shade area ranges from 80 to 125%
HDMTX dosage is calculated based on the patient’s BSA. Eight studies retained BSA as a covariate; in all cases, it was positively correlated with CL [25, 34, 38, 44, 45, 47, 48, 53]. Five studies of pediatric patients revealed that BSA significantly influenced CL, with a median decline of CL 51% (range 37–77.8%). Therefore, it is necessary to consider the influence of BSA influence on CL in pediatric patients.
Seven studies retained gene polymorphism as a covariate and identified several genes that contribute to the vast variability in MTX PK. These included MTHFR 677C > T (rs1801133) [43], a folate pathway gene associated with MTX; MTX-related transporter genes including ABCC2 (rs717620) [30], ABCB1 (rs1045642) [61], ABCG2 (rs13120400) [46], ABCC3 (rs4148416) [51], the SLCO1B1 521 T > C (rs4149056) and SLCO1B1 388A > G (rs2306283) variants which encodes OATP1B1 [56], SLC19A1 (rs17004785) [51], and OATP1B1 (rs2306283) [62]. Our results revealed that a mutation in MTHFR reduced enzyme activity, thus reducing MTX CL by ca. 26.2%. Mutations in ABCG2, SLCO1B1 388A > G, and OATP1B1 reduced MTX CL. Patients carrying the OATP1B1 388A > G mutation exhibited a 35.3% reduction in CL, which has clinical significance. Expression of ABCC2, ABCC3, and ABCB1 increased CL by 43%, 48%, and 30%, respectively, demonstrating that all of these genes have clinically significant effects.
Seven articles reported the effects of bodyweight on CL; of these, six addressed HDMTX in children and young adults [26, 41,42,43, 52, 56, 57], and one addressed LDMTX in adults [57]. These studies all revealed that MTX CL increased with bodyweight. Aumente et al. [41] showed that, for children, age is closely related to weight. Our results revealed that, for the same weight (40 kg), children younger than 10 years had 24% higher CL than those older than 10 years. This is consistent with the findings of Donelli et al. [66], who reported that in children older than 10 years, the drug reached higher plasma concentrations and was cleared at a lower rate.
Seven studies retained age as a covariate, and six studies of adults found that age was negatively associated with CL [23, 27, 36,37,38, 40, 42]. One study of children and one of infants revealed that age was positively correlated with CL [23].
Four studies revealed that co-medication influenced MTX CL, with inhibition of transporters involved in MTX uptake as the potential mechanism. HDMTX combined with both benzimidazoles and β-lactams [31] reduced MTX CL by 11%, whereas co-administration of penicillin reduced it by 38.7% [61]. Dexamethasone [25, 26] and vancomycin [25] were associated with a small increase in MTX clearance (ca. 20%).
Three studies retained sex as a significant covariate, finding that CL was higher for males than females. Two of these included patients with OS. One LDMTX study found that MTX dosage increased the clinical significance of MTX CL.
Other covariates retained as significant for CL included hematocrit (HCT) [33], urine volume to hydration (UV/HV) [39], vertebral body height (VBH) [36], difference in the urinary coproporphyrin I (UCP I): UCP I + III ratio between hospital discharge and methotrexate infusion (DP3) [33], and albumin (ALB) [36, 40].
Seven articles retained bodyweight as a covariate of MTX CL [29, 33, 41,42,43,44, 48]. Bodyweight was positively correlated with Vd; therefore, bodyweight affected both MTX distribution and elimination. One study fixed Vd at 32.8 L/kg [26] because it could not be accurately estimated owing to the lack of distribution of blood collection points. The inter-individual variability in Vd was partially explained by the fact that this parameter was weight-dependent, supporting the need for dose adjustment following significant weight gain or loss. Other covariates of Vd included age [34, 41] BSA [25, 45], alanine aminotransferase (ALT) [55], and co-medication with dexamethasone and vancomycin [50].
Several age-dependent factors can influence the distribution of drugs in the body, most notably of drugs with high plasma protein levels [68]. Age positively influenced Vd in the study of Mei et al. [34], with a median age of 57.16 years. In the study by Kawakatsu et al. [55] (median age 15 years), age was negatively correlated with Vd. The reason for this inconsistency may be that bodyweight increases with age in patients ≤ 50 years, decreasing after 50 years. The distribution of MTX and accumulation of MTX-polyglutamate in erythrocytes increases with age [69]. Two studies of pediatric patients by Hui et al. [45] and Panetta et al. [25] found that Vd was significantly related to BSA because of the rapid increase in body size as children grow. Hui et al. [45] found that height influenced Vd in patients with OS. Panetta et al. [25] found that dexamethasone and vancomycin use influenced Vd, probably as an indirect result of the patient’s clinical care and conditions, such as increased fluid administration and increased MTX Vd.
Kawakatsu et al. [55] found that ALT significantly affected Vd and Vp, suggesting that ALT is an indicator of liver dysfunction and that increased ALT could lead to decreased albumin production. Changes in liver function may affect MTX binding and affinity in plasma and tissue. Gallais et al. [37] and Schulte et al. [56] found that Vp was directly correlated with weight, implying that the higher the weight, the longer the MTX half-life. Simon et al. [30] found that ABCC2 24C > T was involved in MTX elimination and distribution, although the mechanism underlying the association between ABCC2 and MTX PK remains unclear. For the HDMTX peripheral clearance rate (QP), age influenced Qp in patients with ALL, and weight influenced Qp in those with OS. Yang et al. [35] and Zhang et al. [54] found that BSA was positively correlated with Qp, and Hui et al. [45] found that age influenced Qp in patients with ALL.
Discussion
Our study reveals that, after administering MTX at a fixed dose for the same diagnosis, MTX CL exhibited substantial inter- and intra-patient variability, varying tenfold in patients with normal renal function, and MTX plasma concentration varied substantially between individuals. The selected HDMTX studies, of infants, children, and adults, reported different concentration–time profiles. MTX CL varied substantially between the LDMTX and HDMTX studies. Since MTX is contraindicated during pregnancy, none of the studies included this special population.
Based on our findings, HDMTX should be applied in conjunction with TDM to optimize initial dosing regimens to reach target concentrations and achieve elimination-phase concentrations. This will guide rescue strategies to prevent MTX-induced toxicity. In contrast, it is unnecessary to perform TDM routinely for LDMTX, except in cases of MTX-related toxicity and suspected ineffective MTX treatment.
Although some PPK studies have been conducted for adult and pediatric patients, most of these were single-center studies that were limited by not fully investigating the factors that may affect MTX PK. Our study is one of the most comprehensive evaluations of MTX PK to date, representing a wide range of ages and disease types. Our findings can potentially help in optimizing initial MTX dosing regimens for patients with different physical characteristics.
Our results revealed that HDMTX CL was higher in infants than in children and adults, because renal excretion is its primary route of elimination, accounting for ca. 70–90% of MTX clearance [8]. The reduction in HDMTX CL with age is due to the maturation of renal tubule function, glomerular filtration rate, and renal blood flow [24], and to the increase in CL in the first year of life as the metabolic pathways mature. CL gradually declines after the first year of life, reaching the adult level in adolescence [70]. However, for infants, the concentration profiles illustrate that BSA influences MTX pharmacokinetics.; infants had significantly lower MTX elimination than children and adults, with a greater possibility of metabolic delays. For infants aged < 1 year, further PK data is required to describe MTX disposition in this vulnerable population. Future studies are therefore needed to elucidate the relationship between MTX efficacy and toxicity in infants, and the use of BSA-dependent dosing for infants should be re-evaluated. Children exhibit higher renal function than adults, potentially explaining the difference in CL between children and adults [70]. Our Monte Carlo simulations revealed considerable variation in blood MTX levels within individuals. Therefore, to ensure that all patients achieve similar plasma MTX, we propose individualizing MTX dosing via TDM, which involves determining the individual MTK PK.
We found that adults had lower Vd than children, potentially because body water content declines with age. Moreover, substantial amounts of MTX can be distributed into third-space fluids [8], potentially affecting its disposition in infants, who are known to undergo significant changes in total body water content, from ca. 75% during the neonatal period to 55% in adulthood [25]. Furthermore, MTX is 50% protein-bound, and glomerular filtration is limited to unbound drugs; as infants’ physiological parameters potentially alter the plasma protein-binding of drugs, they exhibit lower total plasma protein than adults [70]. Therefore, prior knowledge of age- and weight-related changes would be useful for designing pediatric clinical trials and defining the initial MTX dose to be administered in the pediatric population.
The aim of reviewing these PPK studies was to identify potential covariates describing the BSV of MTX PKs. Two of the selected studies included ethnicity as a covariate, finding no significant associations [25, 56]. However, CL and Vd were reportedly higher in Middle Eastern populations than in other populations [33], indicating that ethnicity may influence the MTX PK; this requires further study.
Renal function parameters (CrCL and SCr) significantly influence MTX CL. One of the selected trials (of adults receiving HDMTX for ALL) recommended that the HDMTX dose should be based on SCr on the day of treatment [71] and suggested reducing the dose to 50% of the usual dose if SCr is greater than 2.0 mg/dL. Our results showed that, for adults, when SCr was increased from 1 to 3 mg/dL, the CL of MTX for Faltaos et al. [27] and Mei et al. [34] decreased by 33% and 36%, respectively. The optimal CrCL-based MTX dose adjustment is unclear because there is no consensus on the optimal dose-reduction scheme and institutional practices vary. Our results reveal that MTX CL was 24% lower in patients with renal insufficiency (eGFR < 60 mL/min/1.73 m2) and 47.5% lower in those with renal impairment (eGFR < 20 mL/min/1.73 m2) than in those with normal renal function (eGFR > 90 mL/min/1.73 m2). Therefore, for patients with renal impairment, the MTX dosing regimen must be adjusted before infusion. A pooled HDMTX PPK model is needed to analyze the relationship between age and renal function to achieve individualized MTX dosing. Monitoring of SCr alone is inadequate for this purpose because there are large inter-individual variations in MTX clearance. If the patient develops HDMTX-related nephrotoxicity during treatment, SCr should be serially monitored, and MTX administration should be paused until SCr has returned to baseline.
Until now, dose adjustment for hepatic impairment has not been provided in the MTX drug insert. Hepatic elimination is estimated to account for only 5–10% of MTX elimination, and no studies have considered the effects of hepatic function on CL (such as by measuring serum bilirubin or transaminase). The liver plays a key role in the metabolic conversion of MTX to 7-hydroxy-MTX via aldehyde oxidases [3]. Two studies conducted on children found that HDMTX co-administration with dexamethasone increased HDMTX CL by 19%, potentially because dexamethasone may induce aldehyde oxidase. The SLCO1B1 gene encoding the basolateral hepatocellular transporters (OATPs) is almost exclusively expressed in the liver. Schulte et al. [56] found that genetic variation in SLCO1B1 was associated with reduced MTX clearance, consistent with the findings of a systematic review by Taylor et al. [49], which revealed that SLCO1B1 was the only gene that influenced MTX PK. These results indicate that for patients co-administrated dexamethasone and MTX and possessing the SLCO1B1 gene mutation, hepatic function may be impaired; such patients, and especially children, may require MTX dosage adjustment.
Among the selected studies, only a study addressing HCT [33] revealed that it had a clinically significant impact on CL. Our simulation results revealed that MTX C72h was highest in adults with ALL. Because MTX can be distributed into red blood cells, erythrocytes could be considered storage compartments for MTX, with several pharmacological implications [72]. Hemoglobin is highly correlated with HCT levels. Similar to MTX, 7-OH-MTX is also polyglutamylated in cells, and the retention of these polyglutamated forms in erythrocytes may contribute to MTX toxicity. Differences in HCT levels between patients, or changes in HTC within the same patient during treatment, may necessitate dose adjustments. The effects of HCT and hemoglobin on MTX CL require further study [73, 74].
Of the selected studies, two studies of patients with OS found that MTX dosage influenced CL. Hui et al. [45] found that the ratio of the MTX dose to BSA was positively associated with MTX CL. Fukuhara et al. [28] showed that MTX dosage was the most influential categorical covariate for MTX CL; MTX dose ≥ 10 g resulted in a difference in non-renal clearance of MTX. First, patients receiving higher doses of MTX are likely to frequently require extra hydration and urine alkalinization, which may increase CL. Second, HDMTX increases CL via a compensatory clearance mechanism caused by saturation of the process involving active tubular secretion [75], indicating that MTX is secreted, and not reabsorbed, by the renal tubules. However, tubular secretion is saturated at higher plasma concentrations, playing only a minor role in MTX elimination during and immediately after HDMTX infusion [76]. Further exploration of the potential nonlinearity of HDMTX PK is required. An LDMTX study of patients with RA found that the CL was lower after single-dose administration than multiple doses (single-dose, 0.39 L/kg; multiple doses, 1 L/kg).
Our results confirm that HDMTX PK parameters are influenced by the type of disease, MTX dosage, and the duration of infusion [55]. It demonstrates that the treatment response at the end of infusion and HDMTX toxicity in the terminal elimination phase vary significantly with MTX serum concentration, thus emphasizing the need to develop adaptive dosing methods [77].
Our study had some limitations. The primary limitation is that our study is based on sparse data. Furthermore, only articles published in English were included, and we excluded articles that were missing PK parameters. In most of the selected studies, the data were collected retrospectively from routine TDM.
Conclusion
MTX PK differed among infants, children, and adults with different diagnoses. Infants showed higher CL and Vd than adults and children following HDMTX administration at the same dosage per kilogram bodyweight. These findings reveal that MTX dose individualization should depend on both renal function and BSA. Further PPK studies are required to characterize the MTX PK in infants. Prospective MTX PK/PD studies should be conducted to clarify how the MTX exposure–response relationship varies between patients.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Guichard N, Guillarme D, Bonnabry P, Fleury-Souverain S (2017) Antineoplastic drugs and their analysis: a state of the art review. Analyst 142:2273–2321. https://doi.org/10.1039/c7an00367f
Cronstein BN (1996) Molecular therapeutics. methotrexate and its mechanism of action. Arthritis Rheum 39:1951–1960. https://doi.org/10.1002/art.1780391203
Taylor ZL, Vang J, Lopez-Lopez E, Oosterom N, Mikkelsen T, Ramsey LB (2021) Systematic review of pharmacogenetic factors that influence high-dose methotrexate pharmacokinetics in pediatric malignancies. Cancers (Basel) 13:2837. https://doi.org/10.3390/cancers13112837
Methotrexate (Jiangsu Hengrui Pharmaceutical Co., LTD): drug information
Weinblatt ME, Trentham DE, Fraser PA, Holdsworth DE, Falchuk KR, Weissman BN, Coblyn JS (1988) Long-term prospective trial of low-dose methotrexate in rheumatoid arthritis. Arthritis Rheum 31:167–175. https://doi.org/10.1002/art.1780310203
Morgan C, Lunt M, Brightwell H, Bradburn P, Fallow W, Lay M, Silman A, Bruce IN (2003) Contribution of patient related differences to multidrug resistance in rheumatoid arthritis. Ann Rheum Dis 62:15–19. https://doi.org/10.1136/ard.62.1.15
American Society of Clinical Oncology, Kris MG, Hesketh PJ, Somerfield MR, Feyer P, Clark-Snow R, Koeller JM, Morrow GR, Chinnery LW, Chesney MJ, Gralla RJ, Grunberg SM (2006) American Society of Clinical Oncology guideline for antiemetics in oncology: update 2006. J Clin Oncol 24:2932–2947. https://doi.org/10.1200/JCO.2006.06.9591
Ramsey LB, Balis FM, MM, Schmiegelow K, Pauley JL, Bleyer A, Widemann BC, Askenazi D, Bergeron S, Shirali A, Schwartz S, Vinks AA, Heldrup J, (2018) Consensus guideline for use of glucarpidase in patients with high-dose methotrexate induced acute kidney injury and delayed methotrexate clearance. Oncologist 23:52–61. https://doi.org/10.1634/theoncologist.2017-0243
Shen DD, Azarnoff DL (1978) Clinical pharmacokinetics of methotrexate. Clin Pharmacokinet 3:1–13. https://doi.org/10.2165/00003088-197803010-00001
Paci A, Veal G, Bardin C, Levêque D, Widmer N, Beijnen J, Astier A, Chatelut E (2014) Review of therapeutic drug monitoring of anticancer drugs part 1 – cytotoxics. Eur J Cancer 50:2010–2019. https://doi.org/10.1016/j.ejca.2014.04.014
Holmboe L, Andersen AM, Mørkrid L, Slørdal L, Hall KS (2012) High dose methotrexate chemotherapy: pharmacokinetics, folate and toxicity in osteosarcoma patients. Br J Clin Pharmacol 73:106–114. https://doi.org/10.1111/j.1365-2125.2011.04054.x
Sabot C, Debord J, Roullet B, Marquet P, Merle L, Lachatre G (1995) Comparison of 2- and 3-compartment models for the Bayesian estimation of methotrexate pharmacokinetics. Int J Clin Pharmacol Ther 33:164–169
Sheiner LB, Rosenberg B, Melmon KL (1972) Modelling of individual pharmacokinetics for computer-aided drug dosage. Comput Biomed Res 5:411–459. https://doi.org/10.1016/0010-4809(72)90051-1
Lu X, Xu G, Chen L, Fan J, Li M, Zhu L (2020) Assessment of micafungin loading dosage regimens against Candida spp. in ICU patients by Monte Carlo simulations. Eur J Clin Pharmacol 76:695–702. https://doi.org/10.1007/s00228-020-02840-0
Möricke A, Reiter A, Zimmermann M, Gadner H, Stanulla M, Dördelmann M, Löning L, Beier R, Ludwig WD, Ratei R, Harbott J, Boos J, Mann G, Niggli F, Feldges A, Henze G, Welte K, Beck JD, Klingebiel T, Niemeyer C, Zintl F, Bode U, Urban C, Wehinger H, Niethammer D, Riehm H, Schrappe M, German-Austrian-Swiss ALL-BFM Study Group (2008) Risk-adjusted therapy of acute lymphoblastic leukemia can decrease treatment burden and improve survival: treatment results of 2169 unselected pediatric and adolescent patients enrolled in the trial ALL-BFM 95. Blood 111:4477–4489. https://doi.org/10.1182/blood-2007-09-112920
Reiter A, Tiemann M, Ludwig WD, Wacker HH, Yakisan E, Schrappe M, Henzler D, Sykora KW, Brandt A, Odenwald E (1994) NHL-BFM 90 therapy study in treatment of malignant non-Hodgkin’s lymphomas in children and adolescents. Part 1: classification and allocation to strategic therapy groups. BIF study group Klin Pädiatr 206:222–233. https://doi.org/10.1055/s-2008-1046608
Bonadonna G, Valagussa P, Moliterni A, Zambetti M, Brambilla C (1995) Adjuvant cyclophosphamide, methotrexate, and fluorouracil in node-positive breast cancer: the results of 20 years of follow-up. N Engl J Med 332:901–906. https://doi.org/10.1056/NEJM199504063321401
Chao NJ, Schmidt GM, Niland JC, Amylon MD, Dagis AC, Long GD, Nademanee AP, Negrin RS, O’Donnell MR, Parker PM, Smith EP, Snyder DS, Stein AS, Wong RM, Blume KG, Forman SJ (1993) Cyclosporine, methotrexate, and prednisone compared with cyclosporine and prednisone for prophylaxis of acute graft-versus-host disease. N Engl J Med 329:1225–1230. https://doi.org/10.1056/NEJM199310213291703
Tugwell P, Bennett K, Gent M (1987) Methotrexate in rheumatoid arthritis. Indications, contraindications, efficacy, and safety. Ann Intern Med 107:358–366. https://doi.org/10.7326/0003-4819-107-2-358
Uemura O, Honda M, Matsuyama T, Ishikura K, Hataya H, Yata N, Nagai T, Ikezumi Y, Fujita N, Ito S, Iijima K, Kitagawa T (2011) Age, gender, and body length effects on reference serum creatinine levels determined by an enzymatic method in Japanese children: a multicenter study. Clin Exp Nephrol 15:694–699. https://doi.org/10.1007/s10157-011-0452-y
Cockcroft DW, Gault MH (1976) Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41. https://doi.org/10.1159/000180580
Burns DR, Elswick RK (2001) Equivalence testing with dental clinical trials. J Dent Res 80:1513–1517. https://doi.org/10.1177/00220345010800060701
Wright KD, Stewart CF (2015) Response to: comment on ‘Delayed methotrexate excretion in infants and young children with primary central nervous system tumors and postoperative fluid collections.’ Cancer Chemother Pharmacol 75:877–878. https://doi.org/10.1007/s00280-015-2699-6
Beechinor RJ, Thompson PA, Hwang MF, Vargo RC, Bomgaars LR, Gerhart JG, Dreyer ZE, Gonzalez D (2019) The population pharmacokinetics of high-dose methotrexate in infants with acute lymphoblastic leukemia highlight the need for bedside individualized dose adjustment: a report from the Children’s Oncology Group. Clin Pharmacokinet 58:899–910. https://doi.org/10.1007/s40262-018-00734-0
Panetta JC, Roberts JK, Huang J, Lin T, Daryani VM, Harstead KE, Patel YT, Onar-Thomas A, Campagne O, Ward DA, Broniscer A, Robinson G, Gajjar A, Stewart CF (2020) Pharmacokinetic basis for dosing high-dose methotrexate in infants and young children with malignant brain tumours. Br J Clin Pharmacol 86:362–371. https://doi.org/10.1111/bcp.14160
Shi ZY, Liu YO, Gu HY, Xu XQ, Yan C, Yang XY, Yan D (2020) Population pharmacokinetics of high-dose methotrexate in Chinese pediatric patients with medulloblastoma. Biopharm Drug Dispos 41:101–110. https://doi.org/10.1002/bdd.2221
Faltaos DW, Hulot JS, Urien S, Morel V, Kaloshi G, Fernandez C, Xuan K, Leblond V, Lechat P (2006) Population pharmacokinetic study of methotrexate in patients with lymphoid malignancy. Cancer Chemother Pharmacol 58:626–633. https://doi.org/10.1007/s00280-006-0202-0
Fukuhara K, Ikawa K, Morikawa N, Kumagai K (2008) Population pharmacokinetics of high-dose methotrexate in Japanese adult patients with malignancies: a concurrent analysis of the serum and urine concentration data. J Clin Pharm Ther 33:677–684. https://doi.org/10.1111/j.1365-2710.2008.00966.x
Min Y, Qiang F, Peng L, Zhu Z (2009) High dose methotrexate population pharmacokinetics and Bayesian estimation in patients with lymphoid malignancy. Biopharm Drug Dispos 30:437–447. https://doi.org/10.1002/bdd.678
Simon N, Marsot A, Villard E, Choquet S, Hoang-Xuan K, Zahr N, Lechat P, Leblond V, Hulot JS (2013) Impact of ABCC2 polymorphisms on high-dose methotrexate pharmacokinetics in patients with lymphoid malignancy. Pharmacogenom J13:507–513. https://doi.org/10.1038/tpj.2012.37
Benz-de Bretagne I, Zahr N, Le Gouge A, Hulot JS, Houillier C, Hoang-Xuan K, Gyan E, Lissandre S, Choquet S, Le Guellec C (2014) Urinary coproporphyrin I/(I + III) ratio as a surrogate for MRP2 or other transporter activities involved in methotrexate clearance. Br J Clin Pharmacol 78:329–342. https://doi.org/10.1111/bcp.12326
Watanabe M, Fukuoka N, Takeuchi T, Yamaguchi K, Motoki T, Tanaka H, Kosaka S, Houchi H (2014) Developing population pharmacokinetic parameters for high-dose methotrexate therapy: implication of correlations among developed parameters for individual parameter estimation using the Bayesian least-squares method. Biol Pharm Bull 37:916–921. https://doi.org/10.1248/bpb.b13-00672
Nader A, Zahran N, Alshammaa A, Altaweel H, Kassem N, Wilby KJ (2017) Population pharmacokinetics of intravenous methotrexate in patients with hematological malignancies: utilization of routine clinical monitoring parameters. Eur J Drug Metab Pharmacokinet 42:221–228. https://doi.org/10.1007/s13318-016-0338-1
Mei S, Li X, Jiang X, Yu K, Lin S, Zhao Z (2018) Population pharmacokinetics of high-dose methotrexate in patients with primary central nervous system lymphoma. J Pharm Sci 107:1454–1460. https://doi.org/10.1016/j.xphs.2018.01.004
Yang L, Wu H, de Winter BCM, Sheng CC, Qiu HQ, Cheng Y, Chen J, Zhao QL, Huang J, Jiao Z, Xie RX (2020) Pharmacokinetics and pharmacogenetics of high-dose methotrexate in Chinese adult patients with non-Hodgkin lymphoma: a population analysis. Cancer Chemother Pharmacol 85:881–897. https://doi.org/10.1007/s00280-020-04058-4
Pai MP, Debacker KC, Derstine B, Sullivan J, Su GL, Wang SC (2020) Comparison of body size, morphomics, and kidney function as covariates of high-dose methotrexate clearance in obese adults with primary central nervous system lymphoma. Pharmacotherapy 40:308–319. https://doi.org/10.1002/phar.2379
Gallais F, Oberic L, Faguer S, Tavitian S, Lafont T, Marsili S, Brice A, Chatelut E, Puisset F (2021) Body surface area dosing of high-dose methotrexate should be reconsidered, particularly in overweight, adult patients. Ther Drug Monit 43:408–415. https://doi.org/10.1097/FTD.0000000000000813
Arshad U, Taubert M, Seeger-Nukpezah T, Ullah S, Spindeldreier KC, Jaehde U, Hallek M, Fuhr U, Vehreschild JJ, Jakob C (2021) Evaluation of body-surface-area adjusted dosing of high-dose methotrexate by population pharmacokinetics in a large cohort of cancer patients. BMC Cancer 21:719. https://doi.org/10.1186/s12885-021-08443-x
Isono T, Hira D, Morikochi A, Fukami T, Ueshima S, Nozaki K, Terada T, Morita SY (2021) Urine volume to hydration volume ratio is associated with pharmacokinetics of high-dose methotrexate in patients with primary central nervous system lymphoma. Pharmacol Res Perspect 9:e00883. https://doi.org/10.1002/prp2.883
Mao J, Li Q, Li P, Qin W, Chen B, Zhong M (2022) Evaluation and application of population pharmacokinetic models for identifying delayed methotrexate elimination in patients with primary central nervous system lymphoma. Front Pharmacol 13:817673. https://doi.org/10.3389/fphar.2022.817673
Aumente D, Buelga DS, Lukas JC, Gomez P, Torres A, García MJ (2006) Population pharmacokinetics of high-dose methotrexate in children with acute lymphoblastic leukaemia. Clin Pharmacokinet 45:1227–1238. https://doi.org/10.2165/00003088-200645120-00007
Colom H, Farré R, Soy D, Peraire C, Cendros JM, Pardo N, Torrent M, Domenech J, Mangues MA (2009) Population pharmacokinetics of high-dose methotrexate after intravenous administration in pediatric patients with osteosarcoma. Ther Drug Monit 31:76–85. https://doi.org/10.1097/FTD.0b013e3181945624
Faganel Kotnik B, Grabnar I, Bohanec Grabar P, Dolžan V, Jazbec J (2011) Association of genetic polymorphism in the folate metabolic pathway with methotrexate pharmacokinetics and toxicity in childhood acute lymphoblastic leukaemia and malignant lymphoma. Eur J Clin Pharmacol 67:993–1006. https://doi.org/10.1007/s00228-011-1046-z
Desoky ESE, Ghazal MH, Singh RP, Abdelhamid ON, Derendorf H (2013) Population pharmacokinetics of methotrexate in Egyptian children with lymphoblastic leukemia. Pharmacol Pharm 4:139–145. https://doi.org/10.4236/pp.2013.42020
Hui KH, Chu HM, Fong PS, Cheng WTF, Lam TN (2019) Population pharmacokinetic study and individual dose adjustments of high-dose methotrexate in Chinese pediatric patients with acute lymphoblastic leukemia or osteosarcoma. J Clin Pharmacol 59:566–577. https://doi.org/10.1002/jcph.1349
Lui G, Treluyer JM, Fresneau B, Piperno-Neumann S, Gaspar N, Corradini N, Gentet JC, Marec Berard PM, Laurence V, Schneider P, Entz-Werle N, Pacquement H, Millot F, Taque S, Freycon C, Lervat C, Le Deley MC, Mahier Ait Oukhatar C, Brugieres L, Le Teuff G, Bouazza N, Sarcoma Group of UNICANCER (2018) A pharmacokinetic and pharmacogenetic analysis of osteosarcoma patients treated with high-dose methotrexate: data from the OS2006/Sarcoma-09 trial. J Clin Pharmacol 58:1541–1549. https://doi.org/10.1002/jcph.1252
Zang YN, Wang SZ, Qin Y, Zhang JR, Zhao LB, Wang XL (2019) Population pharmacokinetic study of delayed methotrexate excretion in children with acute lymphoblastic leukemia. Int J Clin Pharmacol Ther 57:402–407. https://doi.org/10.5414/CP203423
Medellin-Garibay SE, Hernández-Villa N, Correa-González LC, Morales-Barragán MN, Valero-Rivera KP, Reséndiz-Galván JE, Ortiz-Zamudio JJ, Milán-Segovia RDC, Romano-Moreno S (2020) Population pharmacokinetics of methotrexate in Mexican pediatric patients with acute lymphoblastic leukemia. Cancer Chemother Pharmacol 85:21–31. https://doi.org/10.1007/s00280-019-03977-1
Taylor ZL, Mizuno T, Punt NC, Baskaran B, Navarro Sainz A, Shuman W, Felicelli N, Vinks AA, Heldrup J, Ramsey LB (2020) MTXPK.org: a clinical decision support tool evaluating high-dose methotrexate pharmacokinetics to inform post-infusion care and use of glucarpidase. MTXPK.org. Clin Pharmacol Ther 108:635–643. https://doi.org/10.1002/cpt.1957
Gao X, Qian XW, Zhu XH, Yu Y, Miao H, Meng JH, Jiang JY, Wang HS, Zhai XW (2021) Population pharmacokinetics of high-dose methotrexate in Chinese pediatric patients with acute lymphoblastic leukemia. Front Pharmacol 12:701452. https://doi.org/10.3389/fphar.2021.701452
Zhan M, Sun Y, Zhou F, Wang H, Chen Z, Yan L, Li X (2022) Population pharmacokinetics of methotrexate in paediatric patients with acute lymphoblastic leukaemia and malignant lymphoma. Xenobiotica 52:265–273. https://doi.org/10.1080/00498254.2022.2069060
Johansson ÅM, Hill N, Perisoglou M, Whelan J, Karlsson MO, Standing JF (2011) A population pharmacokinetic/pharmacodynamic model of methotrexate and mucositis scores in osteosarcoma. Ther Drug Monit 33:711–718. https://doi.org/10.1097/FTD.0b013e31823615e1
Joerger M, Ferreri AJ, Krähenbühl S, Schellens JH, Cerny T, Zucca E, Huitema ADR (2012) Dosing algorithm to target a predefined AUC in patients with primary central nervous system lymphoma receiving high dose methotrexate. Br J Clin Pharmacol 73:240–247. https://doi.org/10.1111/j.1365-2125.2011.04084.x
Zhang W, Zhang Q, Tian X, Zhao H, Lu W, Zhen J, Niu X (2015) Population pharmacokinetics of high-dose methotrexate after intravenous administration in Chinese osteosarcoma patients from a single institution. Chin Med J (Engl) 128:111–118. https://doi.org/10.4103/0366-6999.147829
Kawakatsu S, Nikanjam M, Lin M, Le S, Saunders I, Kuo DJ, Capparelli EV (2019) Population pharmacokinetic analysis of high-dose methotrexate in pediatric and adult oncology patients. Cancer Chemother Pharmacol 84:1339–1348. https://doi.org/10.1007/s00280-019-03966-4
Schulte RR, Choi L, Utreja N, Van Driest SL, Stein CM, Ho RH (2021) Effect of SLCO1B1 polymorphisms on high-dose methotrexate clearance in children and young adults with leukemia and lymphoblastic lymphoma. Clin Transl Sci 14:343–353. https://doi.org/10.1111/cts.12879
Godfrey C, Sweeney K, Miller K, Hamilton R, Kremer J (1998) The population pharmacokinetics of long-term methotrexate in rheumatoid arthritis. Br J Clin Pharmacol 46:369–376. https://doi.org/10.1046/j.1365-2125.1998.t01-1-00790.x
Batey MA, Wright JG, Azzabi A, Newell DR, Lind MJ, Calvert AH, Boddy AV (2002) Population pharmacokinetics of adjuvant cyclophosphamide, methotrexate and 5-fluorouracil (CMF). Eur J Cancer 38:1081–1089. https://doi.org/10.1016/s0959-8049(02)00024-2
Yukawa E, Mori S, Ueda K, Nakada Y (2007) Population pharmacokinetic investigation of low-dose methotrexate in rheumatoid arthritics Japanese patients. J Clin Pharm Ther 32:573–578. https://doi.org/10.1111/j.1365-2710.2007.00859.x
Nagulu M, Kiran VU, Nalini Y, Reddy YN, Krishna DR (2010) Population pharmacokinetics of methotrexate in Indian cancer patients. Asian Pac J Cancer Prev 11:403–407
Kim IW, Yun HY, Choi B, Han N, Park SY, Lee ES, Oh JM (2012) ABCB1 C3435T genetic polymorphism on population pharmacokinetics of methotrexate after hematopoietic stem cell transplantation in Korean patients: a prospective analysis. Clin Ther 34:1816–1826. https://doi.org/10.1016/j.clinthera.2012.06.022
Wang Z, Zhang N, Chen C, Chen S, Xu J, Zhou Y, Zhao X, Cui Y (2019) Influence of the OATP polymorphism on the population pharmacokinetics of methotrexate in Chinese patients. Curr Drug Metab 20:592–600. https://doi.org/10.2174/1389200220666190701094756
Crews KR, Liu T, Rodriguez-Galindo C, Tan M, Meyer WH, Panetta JC, Link MP, Daw NC (2004) High-dose methotrexate pharmacokinetics and outcome of children and young adults with osteosarcoma. Cancer 100:1724–1733. https://doi.org/10.1002/cncr.20152
Evans WE, Relling MV, Rodman JH, Crom WR, Boyett JM, Pui CH (1998) Conventional compared with individualized chemotherapy for childhood acute lymphoblastic leukemia. N Engl J Med 338:499–505. https://doi.org/10.1056/NEJM199802193380803. (PubMed: 9468466)
Rhodin MM, Anderson BJ, Peters AM, Coulthard MG, Wilkins B, Cole M, Chatelut E, Grubb A, Veal GJ, Keir MJ, Holford NHG (2009) Human renal function maturation: a quantitative description using weight and postmenstrual age. Pediatr Nephrol 24:67–76. https://doi.org/10.1007/s00467-008-0997-5
Donelli MG, Zucchetti M, Robatto A, Perlangeli V, D’Incalci M, Masera G, Rossi MR (1995) Pharmacokinetics of HD-MTX in infants, children, and adolescents with non-B acute lymphoblastic leukemia. Med Pediatr Oncol 24:154–159. https://doi.org/10.1002/mpo.2950240303
Ceriotti F, Boyd JC, Klein G, Henny J, Queraltó J, Kairisto V, Panteghini M, Committee IFCC, on Reference Intervals and Decision Limits (C-RIDL), (2008) Reference intervals for serum creatinine concentrations: assessment of available data for global application. Clin Chem 54:559–566. https://doi.org/10.1373/clinchem.2007.099648
den Boer E, de Rotte MC, Pluijm SM, Heil SG, Hazes JM, de Jonge R (2014) Determinants of erythrocyte methotrexate polyglutamate levels in rheumatoid arthritis. J Rheumatol 41:2167–2178. https://doi.org/10.3899/jrheum.131290
Stamp LK, O’Donnell JL, Chapman PT, Zhang M, Frampton C, James J, Barclay ML (2009) Determinants of red blood cell methotrexate polyglutamate concentrations in rheumatoid arthritis patients receiving long-term methotrexate treatment. Arthritis Rheum 60:2248–2256. https://doi.org/10.1002/art.24653
Foissac F, Bouazza N, Valade E, De Sousa MM, Fauchet F, Benaboud S, Hirt D, Tréluyer JM, Urien S (2015) Prediction of drug clearance in children. J Clin Pharmacol 55:739–747. https://doi.org/10.1002/jcph.488
Kantarjian HM, O’Brien S, Smith TL, Cortes J, Giles FJ, Beran M, Pierce S, Huh Y, Andreeff M, Koller C, Ha CS, Keating MJ, Murphy S, Freireich EJ (2000) Results of treatment with hyper-CVAD, a dose-intensive regimen, in adult acute lymphocytic leukemia. J Clin Oncol 18:547–561. https://doi.org/10.1200/JCO.2000.18.3.547
Fabre G, Fabre I, Matherly LH, Cano JP, Goldman ID (1984) Synthesis and properties of 7-hydroxymethotrexate polyglutamyl derivatives in Ehrlich ascites tumor cells in vitro. J Biol Chem 259:5066–5072. https://doi.org/10.1016/S0021-9258(17)42956-5
Dupuis C, Mercier C, Yang C, Monjanel-Mouterde S, Ciccolini J, Fanciullino R, Pourroy B, Deville JL, Duffaud F, Bagarry-Liegey D, Durand A, Iliadis A, Favre R (2008) High-dose methotrexate in adults with osteosarcoma: a population pharmacokinetics study and validation of a new limited sampling strategy. Anticancer Drugs 19:267–273. https://doi.org/10.1097/cad.0b013e3282f21376
Relling MV, Fairclough D, Ayers D, Crom WR, Rodman JH, Pui CH, Evans WE (1994) Patient characteristics associated with high-risk methotrexate concentrations and toxicity. J Clin Oncol 12:1667–1672. https://doi.org/10.1200/JCO.1994.12.8.1667
Hendel J, Nyfors A (1984) Nonlinear renal elimination kinetics of methotrexate due to saturation of renal tubular reabsorption. Eur J Clin Pharmacol 26:121–124. https://doi.org/10.1007/BF00546719
Mikkelsen TS, Thorn CF, Yang JJ, Ulrich CM, French D, Zaza G, Dunnenberger HM, Marsh S, McLeod HL, Giacomini K, Becker ML, Gaedigk R, Leeder JS, Kager L, Relling MV, Evans W, Klein TE, Altman RB (2011) PharmGKB summary: methotrexate pathway. Pharmacogenet Genomics 21:679–686. https://doi.org/10.1097/FPC.0b013e328343dd93
Borsi JD, Moe PJ (1987) A comparative study on the pharmacokinetics of methotrexate in a dose range of 0.5 to 33.6 g/m2 in children with acute lymphoblastic leukemia. Cancer 60:5–13
Acknowledgements
We extend our heartfelt gratitude to the following individuals and institutions for their invaluable contributions: Dr. Daniel Gonzalez from the UNC Eshelman School of Pharmacy, The University of North Carolina at Chapel Hill, USA; Dr. Tai Ning Lam from the Lo Kwee-Seong Integrated Biomedical Sciences Building, The Chinese University of Hong Kong, Hong Kong; Dr. Tetsuichiro Isono and Dr. Shin-ya Morita from the Department of Pharmacy, Shiga University of Medical Science Hospital, Otsu, Shiga, Japan; Dr. Zhigang Zhao from the Department of Pharmaceutics, School of Pharmaceutical Sciences, Peking University, Beijing, China; and Dr. Joseph F. Standing from the Department of Pharmacy, Great Ormond Street Hospital for Children, United Kingdom for providing the NONMEM control stream from their previously published population pharmacokinetic models. We would also like to thank Editage (www.editage.cn) for English language editing.
Funding
This study was supported by the Shanghai Changhai Hospital Youth Start-up Fund Project, “Study on the dose–response relationship mechanism of Wuzhi capsule increasing tacrolimus concentration based on population pharmacokinetic model” (grant number: 2020QNB11), and the Special youth cultivation project for basic medical research of Changhai Hospital, “Establishment of cyclosporine PK/PD model in patients with RIF based on trophoblastic organs and its pharmacodynamic mechanism” (grant number: 2021JCQN19).
Author information
Authors and Affiliations
Contributions
YYY, ZW, and ZJ designed the review and planned the work that led to the manuscript. ZL and CW performed the literature search and data analysis. YYY, ZW, and ZJ drafted and revised the manuscript. All authors approved the final version of this manuscript.
Corresponding authors
Ethics declarations
Ethics approval
No ethical approval is required/exemption granted.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, Y., Liu, Z., Chen, J. et al. Factors influencing methotrexate pharmacokinetics highlight the need for individualized dose adjustment: a systematic review. Eur J Clin Pharmacol 80, 11–37 (2024). https://doi.org/10.1007/s00228-023-03579-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-023-03579-0