Abstract
Purpose
The study aims to analyse overall as well as subgroup-specific outpatient paediatric macrolide use in five European countries, including time trends of macrolide prescription rates, and to provide potential targets for future interventions aiming to promote judicious macrolide use.
Methods
Macrolide prescription rates per 1000 person years to paediatric outpatients (≤18 years) were calculated using healthcare databases from Denmark, Germany, Italy, The Netherlands and the UK. Poisson regression analysis was used to estimate the influence of increasing calendar year on total macrolide and subgroup-specific prescription rates based on monthly data, adjusted for seasonal variations. Time periods for which data were available varied between 4 (Italy 2007–10, Germany 2005–8) and 10 years (UK 2000–9).
Results
Paediatric macrolide use in 2008 varied between 199 (Italy) and 47 (Netherlands) prescriptions per 1000 person years. Prescription rates of short-acting macrolides declined significantly in all countries but the UK. The use of intermediate-acting macrolides significantly rose with increasing calendar year in Denmark (rate ratio (RR) = 1.12) and the UK (RR = 1.06), but decreased in Germany (RR = 0.84) and The Netherlands (RR = 0.97). Prescription rates of long-acting agents increased in Denmark (RR = 1.05), The Netherlands (RR = 1.05) and the UK (RR = 1.11) (all trends p < 0.05). The greatest seasonal variations of macrolide use between summer and winter months were observed in Italy and Germany.
Conclusions
The observed trend toward increased prescribing of intermediate- and/or long-acting agents might further increase resistance pressure on bacterial pathogens due to their prolonged plasma half-life and broader antibacterial activity. Marked seasonality of prescription rates in the high-utilising countries, Italy and Germany, suggests frequent prescription of macrolides to treat respiratory infections which may be of viral origin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Macrolides are among the most frequently prescribed antibiotics in the treatment of childhood and adolescent infections in primary care [1]. Several studies suggest that there is a strong association between high macrolide use and the emergence of macrolide-resistant strains of common pathogens [2–5]. Moreover, dual beta-lactam/macrolide resistance is frequent, particularly among paediatric serotypes, and a high use of macrolides has been considered as a major factor in the increase in dual beta-lactam/macrolide resistance [6].
Overall, the outpatient utilisation of macrolides increased throughout Europe in recent years, mostly due to increased use of intermediate- (e.g. clarithromycin) and long-acting agents (e.g. azithromycin) [7]. This coincided with a general shift toward increased outpatient prescription of broad-spectrum antibiotics in many European countries, [8] and the burden of broad-spectrum prescribing might be particularly pronounced in paediatric primary care [9].
In contrast to short-acting agents such as erythromycin, intermediate- and long-acting macrolides feature a more favourable dosing profile, less frequent side effects and a broader antibacterial spectrum [7]. Nevertheless, the longer plasma half-life and broader antimicrobial activity of these agents might even enhance resistance selection in bacterial pathogens [10] and possibly result in new phenotypes of resistant bacteria [11].
The European Surveillance of Antimicrobial Consumption Project (ESAC) was established in 2001 with the aim to gather reliable and comparable information on the utilisation of antibiotics throughout Europe [8]. From this project, data about outpatient macrolide use across Europe is available, albeit without distinguishing between adults and children [7]. So far, comprehensive data on macrolide use among children and adolescents from European countries are lacking. Detailed knowledge of prescribing patterns is mandatory to identify targets for future interventions designed to promote rational macrolide use. In this regard, the current study aimed to analyse patterns of paediatric macrolide use in Denmark, Germany, Italy, The Netherlands and the UK, based on a standardised protocol for data extraction and analysis for each country developed within the EU-funded collaborative ARITMO project (www.aritmo-project.org). The specific objectives were (1) to describe overall and subgroup-specific (short-, intermediate- and long-acting agents) prescription rates of macrolides in children and adolescents of different age groups per country and (2) to estimate country-specific time trends in total and subgroup-specific paediatric macrolide prescription rates.
Methods
Data were retrieved from one general-practice database (The Health Improvement Network (THIN), UK), one outpatient pharmacy dispensing database (PHARMO, The Netherlands) and three claim databases (Aarhus University Hospital Database, Denmark; the German Pharmacoepidemiological Research Database (GePaRD), Germany; Emilia Romagna regional database, Italy).
These electronic healthcare databases cover a total source population of approximately 23 million persons. The Aarhus University Hospital Database covers the entire population of the Northern and Central region of Jutland (∼1.8 million Danish inhabitants), which accounts for about 30 % of the total Danish population. The Emilia Romagna regional database contains information on all healthcare services reimbursable by the Italian National Health Service for the about 4.5 million inhabitants of the Emilia Romagna region in Northern Italy. GePaRD collects insurance claims from four statutory health insurance (SHI) providers in Germany of more than 17 million insurants from all German federal states. Three of the four SHIs contributing data to GePaRD approved the study, resulting in a source population of about 8 million insurants. PHARMO gathers healthcare data from 3.2 million inhabitants of 65 municipal areas in The Netherlands. THIN includes information from primary care medical records from over 500 general practices of about 5.9 million active registered patients from the UK. Overall population coverage varied between countries ((estimated values) Denmark 30 %; Germany 10 %; Italy 8 %; Netherlands 19 %; UK 9 %).
All five databases comprise medical information of a defined population and are in compliance with European Union guidelines on the usage of medical data for research. The study was approved by regulatory agencies or by scientific advisory boards of the databases, whenever applicable. A detailed description of database characteristics, regarding validity and representativeness of databases, approvals for use of data, and methods used to locally extract and aggregate data can be found elsewhere [1].
The study was conducted in an open cohort design. The study population in each country comprised all children and adolescents up to the age of 18 years who contributed data to one of the databases during the time period for which data was available. Data availability varied between databases. Database coverage was longest for the UK (2000–2009), followed by The Netherlands (2001–2009), Denmark (2001–2008), Italy (2007–2010) and Germany (2005–2008). Cohort entry was defined as the beginning of the time period covered by the respective database or—if later—the first date a person entered into the database. Cohort exit was defined as exit of the person from the respective database, 18th birthday, death, first interruption of follow-up in the database or the end of the time period covered by the database, whichever came first.
Outpatient prescriptions of macrolides were divided into three subgroups according to their mean plasma elimination half-life [7] (agents and ATC-codes in brackets): short-acting macrolides (erythromycin (J01FA01); spiramycin (J01FA02); miocamycin (J01FA11); rokitamycin (J01FA12)), intermediate-acting macrolides (roxithromycin (J01FA06); josamycin (J01FA07); clarithromycin (J01FA09); telithromycin (J01FA15)) and long-acting macrolides (azithromycin (J01FA10)). Prescription rates, i.e. the number of prescriptions of macrolide subgroups per 1000 person years in the year 2008, were estimated for different age groups (≤4, 5–9, 10–14 and 15–18 years) per country. The choice of these age bands was based on previous studies of antibiotic utilisation in the paediatric setting, which used similar or identical age group classifications [1, 12, 13]. The year 2008 was chosen, since it was the most recent year covered by all five databases.
For each country, a Poisson regression analysis was performed to investigate the influence of increasing calendar year and season on monthly macrolide prescription rates. Analyses were based on monthly macrolide prescription rates per country. We assumed that there was a log-linear relationship between the year and the prescription rate. A visual examination of the Pearson residuals did not indicate a violation of this assumption. Season was included as a dummy-coded variable with four categories (spring March to May, summer June to August (reference period), autumn September to November, winter December to February). Rate ratio estimates for calendar year and the (dummy-) coded season variable as well as corresponding likelihood ratio test p values were calculated. The analyses were repeated for each macrolide subgroup. Since over-dispersion was expected (and present in all regression analyses), all regression models included a dispersion parameter. Statistical analyses were conducted with SAS 9.3.
Results
The average annual study population, i.e. the average annual number of children covered by the included databases, comprised 336,576 children from Denmark, 799,194 children from Italy, 1,340,163 children from Germany, 601,280 children from The Netherlands and 768,631 children from the UK.
In 2008, total macrolide use varied between 46.8 (Netherlands) and 198.6 (Italy) prescriptions per 1000 person years (Table 1). In Denmark, macrolide prescription rates were most frequent in the age group 15–18 years while they were highest among very young children (0–4 years) in the four other countries (Table 1).
Intermediate-acting agents were most frequently prescribed in Italy and Germany, whereas preferential use of short-acting agents was observed in the UK and Denmark. In contrast, relative use of short-acting agents in Italy was negligible. Only in The Netherlands, where overall macrolide use was very low, were long-acting macrolides the most prescribed subgroup (Table 1).
The ranking of macrolide subgroups by their magnitude of use was similar in all age groups in Italy, The Netherlands and the UK. In Germany, intermediate-acting agents were most frequently prescribed to patients above 9 years of age, whereas in younger children, short-acting agents were preferred. In Denmark, long-acting macrolides were most frequently prescribed to adolescents aged 15–18 years, but short-acting macrolides were the subgroup with the highest prescription rates in the three remaining age groups (Table 1).
In the year 2008, erythromycin was the only short-acting agent prescribed to children and adolescents from Denmark, Germany, The Netherlands and the UK, whereas rokitamycin (57.7 %) was most commonly prescribed in Italy, followed by erythromycin (25.8 %), spiramycin (9.9 %) and miocamycin (6.6 %, data not shown). Use of intermediate-acting agents in The Netherlands and the UK was limited to clarithromycin, and in Italy, it covered 96.4 % of this subgroup. In Denmark and Germany, roxithromycin was frequently used, as well, and accounted for 56.8 and 37.8 % of prescribing of intermediate-acting macrolides, respectively.
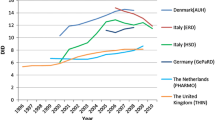
A statistically significant change in total macrolide prescription rates with increasing calendar year was only observed for Germany (RR = 0.88 p < 0.05, Table 2), indicating an overall decrease of about 32 % from 2005 to 2008. Prescription rates of short-acting agents showed a significant decline over time in all countries, except the UK. The use of intermediate-acting macrolides rose significantly with increasing calendar year in Denmark (RR = 1.12, p < 0.05) and the UK (RR = 1.06, p < 0.05), but decreased in Germany (RR = 0.84, p < 0.05) and The Netherlands (RR = 0.97, p < 0.05, Table 2). Prescription rates of long-acting agents significantly increased with calendar year in Denmark (RR = 1.05, p < 0.05), The Netherlands (RR = 1.05, p < 0.05) and the UK (RR = 1.11, p < 0.05, Table 2). In all countries, macrolide prescription rates were highest during the winter and lowest during the summer months (Fig. 1). The rate ratio of summer-to-winter macrolide prescriptions per year was highest in Germany (RR = 2.44, p < 0.05) and lowest in Denmark (RR = 1.49, p < 0.05, Table 2).
Discussion
The results of this population-based, multi-database study provide a comprehensive examination of paediatric outpatient use of macrolides, overall and by subgroups, over time in five European countries.
Our data illustrates striking variations of overall paediatric macrolide use across countries. In 2008, paediatric macrolide prescription rates in Italy were more than four times higher than those in The Netherlands, the country with the lowest use. The ranking of countries by magnitude of macrolide use was consistent with their respective total antibiotic use as previously observed [1]. Accordingly, variations of paediatric macrolide use between the studied countries are mostly explained by different levels of overall paediatric antibiotic use. The observed considerable differences of macrolide prescription rates as much as antibiotic use in general likely reflect differences in the appropriateness of prescribing patterns rather than strong variations in the burden of paediatric bacterial infections between countries.
While overall paediatric macrolide use remained stable throughout the country-specific observational periods in all countries but Germany, changes of prescription rates of macrolide subgroups over time were common. Our analysis demonstrated that prescription rates of short-acting macrolides declined significantly in all countries, except the UK. Concurrently, use of intermediate- and/or long-acting agents rose in Denmark, The Netherlands, and the UK and showed no relevant change in Italy. This is in line with findings by Adriaennssens et al. who observed a common trend toward increased prescribing of intermediate- and long-acting macrolides relative to short-acting agents in the general European population [7]. In the current study, annual increases in prescription rates of intermediate- and long-acting macrolides were most pronounced among paediatric outpatients in Denmark and the UK, amounting to overall increases of 121 % in 8 and 156 % in 10 years, respectively. The shift of prescribing preferences from short-acting toward intermediate- and long-acting macrolides can likely be explained by the superiority of the most commonly prescribed agents clarithromycin and azithromycin in terms of dosing profile, side effects and spectrum of antimicrobial activity in contrast to erythromycin [7]. Nevertheless, the observed trend might further accelerate the development of antibiotic resistance, due to increased plasma half-life and broader antimicrobial activity of intermediate- and long-acting macrolides [7, 10].
Only in Germany, significant reductions of overall macrolide use were observed over time, which coincided with a general trend toward lower prescribing of systemic antibiotics to the paediatric population in Germany from 2005 to 2008 [1]. However, with 16 % overall reductions of crude annual paediatric antibiotic prescription rates were less pronounced than those observed for macrolides (34 %). Since Adriaenssens et al. found no relevant decrease in the utilisation of macrolides (measured as defined daily doses per 1000 inhabitants per day) in the German population of all ages from 2005 to 2008 [7], the observed strong decrease of macrolide prescription rates seems to be limited to the paediatric population.
Despite these overall reductions, macrolide use among German children and adolescents in 2008 remained the second highest after Italy and exceeded the use in The Netherlands by a factor of 2.4. Taken in conjunction with the strong seasonal variations of macrolide prescription rates in Italy and Germany, the high total use in these countries suggests common high prescribing of macrolides to potentially treat winter respiratory infections of mostly viral origin in these two countries [14].
Irrespective of common trends in subgroup-specific prescription rates, distributions of macrolide subgroups varied markedly across countries in 2008, both overall and by age groups. Only in the UK, short-acting macrolides were more often prescribed in all age groups than intermediate- and long-acting macrolides combined. This pattern of prescribing is probably driven by financial incentives of the National Health Service to reward cost-conscious prescribing by general practitioners [7]. By contrast, prescription rates of short-acting macrolides in Italy decreased strongly from 2007 to 2010 and use of this subgroup in 2008 was negligible across all age groups. In 2008, overall prescription rates of each of the two other subgroups alone in Italy were higher than the total paediatric macrolide use in Denmark, The Netherlands and the UK, respectively. This suggests frequent prescribing of these agents as first-line treatment which is not recommended for common childhood infections by international practice guidelines [1, 15–17]. Moreover, total paediatric use of intermediate- and long-acting macrolides in Italy might have been even higher than suggested by our data from the northern Italian region Emilia Romagna, due to considerably higher prescribing of these agents in southern regions [18]. The observed patterns of prescribing to paediatric outpatients in Italy may facilitate development of substantial resistance to bacterial pathogens. So far, the frequency of macrolide resistance among invasive pneumococci isolates from Italy is already the highest in Europe, according to European surveillance data [6].
In general, due to strong selective pressure of macrolides on and already high resistance rates among common pathogens across Europe [6, 19], their use in the treatment of childhood infections should be limited. Potential benefits due to favourable dosing profiles and shorter courses of therapy in contrast to penicillins, which offer a similar antibacterial spectrum, are outweighed by the risk of further increasing selective pressure on bacteria, in many cases. In addition, restricting the broad utilisation of macrolides would contain the risk of bacteriologic failure of macrolides in β-lactam allergic patients.
The development and wide implementation of tailored interventions to promote rational use of antibacterial substances is of mayor public health relevance. The current study allows healthcare practitioners and policy makers to audit country-specific patterns of paediatric macrolide use in the outpatient setting with regard to total level of prescribing, the distribution of macrolide subgroups and corresponding time trends to help plan future strategies. Our findings highlight potential target areas for future activities to improve judicious prescribing of macrolides to children and adolescents in the studied countries. Promising effectiveness in increasing the appropriateness of antibiotic use has been shown for multifaceted interventions, which combine strategies such as education of patients and the general public with intervention components directly addressing providers’ prescribing behaviour [20–22]. Public campaigns alone might be particularly effective in reducing unnecessary prescribing in high-utilising countries [23, 24]. Programmes aiming at modifying parents’ care seeking behaviour show favourable effects when educational interventions are delivered prior to the child’s illness and when the focus is on specific symptoms rather than generic massages [25]. However, generalizability of findings from interventional studies to different cultural settings might be limited due to varying attitudes, knowledge and cultural beliefs of patients/parents and healthcare providers. Hence, programme implementation in different settings has to be accompanied by rigorous evaluation.
Strengths and limitations
Our study had a large sample size and provides a detailed population-based analysis of outpatient paediatric macrolide use across five European countries. Data extraction and analysis for each database in these countries were based on a standardised protocol, ensuring high inter-country comparability of study findings. However, some limitations exist. Due to variations of upward winter peaks over time within a given country and between countries in a given year, comparability of seasonal differences between countries based on uniform grouping of months into seasonal categories might be limited. Downward summer troughs almost exclusively appeared in July or August in all countries, whereas upward winter peaks could be observed between December and March. However, the ranking of countries by magnitude of seasonal variation based on the rate ratio of summer-to-winter macrolide prescription rates from Poisson regression remained stable, when the onset of the four seasons was moved ahead by 1 month. Finally, due to a high heterogeneity of drug use among Italian regions, [18] our findings may not reflect paediatric antibiotic prescribing in Italy as a whole.
Conclusions
Outpatient paediatric macrolide use showed substantial variation in terms of total use and use of macrolide subgroups between European countries. A common trend toward increased prescribing of intermediate- and/or long-acting macrolides relative to short-acting agents was observed in most of the studied countries and might unnecessarily accelerate the development of antibiotic resistance. The magnitude of seasonality in prescription rates in the high-utilising countries, Germany and Italy, may indicate common inappropriate prescribing of macrolides to treat viral respiratory infections. Further efforts are needed to promote and sustain judicious prescribing in all countries. In particular, extensive use of intermediate- and long-acting agents in the (Italian) outpatient setting appears unjustified.
Competing interests
JH, FI, AO, FK, EP, AP, SPU and GT declare that they have no competing interest. IB is an employee of the PHARMO Institute. This independent research institute performs financially supported studies for government and related healthcare authorities and several pharmaceutical companies. MM has received funding from Pfizer, AstraZeneca and the International Serious Adverse Events Consortium for studies of drug safety. MCS is coordinating a research group that occasionally conducts research for pharmaceutical companies. These companies include Pfizer, GlaxoSmithKline and Boehringer Ingelheim. EG has been a consultant to Bayer-Schering, Nycomed, GlaxoSmithKline, Schwabe, Teva and Novartis. EG is running a department that occasionally performs studies for pharmaceutical industries. DE and TS work at this department. The companies include Bayer, Celgene, GlaxoSmithKline, Mundipharma, Novartis, Sanofi-Aventis, Sanofi Pasteur MDS and STADA.
Funding
The current study is part of the EU-funded ARITMO study which aims to assess the utilisation and arrhythmogenic potential of antiinfectives, antihistamines and antipsychotics. ARITMO is a Research and Development project funded by the Health Area of the European Commission under the VII Framework Programme (FP7/2007–2013) under grant agreement no. 241679-the ARITMO project.
This research was supported by the National Institute for Health Research (NIHR) Biomedical Research Centre at Guy’s and St. Thomas’ NHS Foundation Trust and King’s College London. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. The funding sources had no involvement in study design; in the collection, analysis and interpretation of data; in the writing of the report and in the decision to submit the article for publication.
Ethical approval
This study did not involve human participants; prior ethical approval was not required.
Contributions of Authors
JH conceived and designed the study, conducted data analysis, drafted the article and had final approval. DE conducted the regression analyses, helped with interpretation of results, revised the article for content and gave final approval. TS helped acquire the data and interpret the results and revise the article for content and gave final approval. FI helped design the study, helped with data acquisition and interpretation, and made revisions to article draughts, and gave final approval for publication. AO helped acquire the data and interpret the results and revise the article for content, and gave final approval. IB was involved with data acquisition, helped revise the article and gave final approval for publication. FK helped with data analysis and interpretation of results and revised the article for content, and gave final approval. MM was involved with data acquisition, helped revise the article and gave final approval for publication. EP helped acquire the data and interpret the results, made revisions to article draughts and gave final approval. AP was involved with data acquisition, helped revise the article and gave final approval for publication. SPU helped acquire the data and interpret the results, and made revisions to article draughts, and gave final approval. MCS is the primary investigator of the ARITMO project. She was involved with data acquisition, helped revise the article and gave final approval for publication. GT was involved with data acquisition and interpretation, helped revise the article and gave final approval for publication. EG helped design the study, and acquire and interpret the data, made revisions to the article draught and gave final approval for publication.
References
Holstiege J, Schink T, Molokhia M, Mazzaglia G, Innocenti F, Oteri A, et al. (2014) Systemic antibiotic prescribing to paediatric outpatients in 5 European countries: a population-based cohort study. BMC Pediatr 14:174
Granizo JJ, Aguilar L, Casal J, Garcia-Rey C, Dal-Re R, Baquero F (2000) Streptococcus pneumoniae resistance to erythromycin and penicillin in relation to macrolide and beta-lactam consumption in Spain (1979–1997). J Antimicrob Chemother 46:767–773
Granizo JJ, Aguilar L, Casal J, Dal-Re R, Baquero F (2000) Streptococcus pyogenes resistance to erythromycin in relation to macrolide consumption in Spain (1986–1997). J Antimicrob Chemother 46:959–964
Bergman M, Huikko S, Huovinen P, Paakkari P, Seppälä H (2006) Macrolide and azithromycin use are linked to increased macrolide resistance in Streptococcus pneumonia. Antimicrob Agents Chemother 50:3646–3650
García-Rey C, Aguilar L, Baquero F, Casal J, Dal-Ré R (2002) Importance of local variations in antibiotic consumption and geographical differences of erythromycin and penicillin resistance in Streptococcus pneumonia. J Clin Microbiol 40:159–164
European Centre for Disease Prevention and Control (2014) Antimicrobial resistance surveillance in Europe 2013. Annual report of the European antimicrobial resistance surveillance network (EARS-Net). Stockholm: ECDC.
Adriaenssens N, Coenen S, Versporten A, Muller A, Minalu G, Faes C et al. (2011) European Surveillance of Antimicrobial Consumption (ESAC): outpatient macrolide, lincosamide and streptogramin (MLS) use in Europe (1997–2009). J Antimicrob Chemother 66 Suppl 6:vi37-vi45.
Adriaenssens N, Coenen S, Versporten A, Muller A, Minalu G, Faes C, et al. (2011) European Surveillance of Antimicrobial Consumption (ESAC): outpatient antibiotic use in Europe (1997–2009). J Antimicrob Chemother 66(Suppl 6):vi3–v12
Fossum GH, Lindbaek M, Gjelstad S, Dalen I, Kvaerner KJ (2013) Are children carrying the burden of broad-spectrum antibiotics in general practice? Prescription pattern for paediatric outpatients with respiratory tract infections in Norway. BMJ Open 3:1–8
Malhotra-Kumar S, Lammens C, Coenen S, Van HK, Goossens H (2007) Effect of azithromycin and clarithromycin therapy on pharyngeal carriage of macrolide-resistant streptococci in healthy volunteers: a randomised, double-blind, placebo-controlled study. Lancet 369:482–490
Leclercq R, Courvalin P (2002) Resistance to macrolides and related antibiotics in Streptococcus pneumoniae. Antimicrob Agents Chemother 46:2727–2734
Schneider-Lindner V, Quach C, Hanley JA, Suissa S (2011) Secular trends of antibacterial prescribing in UK paediatric primary care. J Antimicrob Chemother 66:424–433
Högberg L, Oke T, Geli P, Lundborg CS, Cars O, Ekdahl K. (2005) Reduction in outpatient antibiotic sales for pre-school children: interrupted time series analysis of weekly antibiotic sales data in Sweden 1992–2002. J Antimicrob Chemother 56: 208–215
Giannattasio A, Lo Vecchio A, Napolitano C, Di FL, Guarino A (2014) A prospective study on ambulatory care provided by primary care pediatricians during influenza season. Ital J Pediatr 40:38
American Academy of Pediatrics Subcommittee on Management of Acute Otitis Media (2004) Diagnosis and management of acute otitis media. Pediatrics 113:1451–1465
Chiappini E, Regoli M, Bonsignori F, Sollai S, Parretti A, Galli L, et al. (2011) Analysis of different recommendations from international guidelines for the management of acute pharyngitis in adults and children. Clin Ther 33:48–58
NICE Short Clinical Guidelines Technical Team (2008) Respiratory tract infections—antibiotic prescribing. Prescribing of antibiotics for self-limiting respiratory tract infections in adults and children in primary care. London: National Institute for Health and Clinical Excellence
Piovani D, Clavenna A, Cartabia M, Bonati M (2012) The regional profile of antibiotic prescriptions in Italian outpatient children. Eur J Clin Pharmacol 68:997–1005
Richter SS, Heilmann KP, Dohrn CL, Beekmann SE, Riahi F, Garcia-de-Lomas J, Ferech M, Goossens H, Doern GV (2008) Increasing telithromycin resistance among Streptococcus pyogenes in Europe. J Antimicrob Chemother 61(3):603–611
Ranji SR, Steinman MA, Shojania KG, Gonzales R (2008) Interventions to reduce unnecessary antibiotic prescribing: a systematic review and quantitative analysis. Med Care 46:847–862
Arnold SR, Straus SE. (2005) Interventions to improve antibiotic prescribing practices in ambulatory care. Cochrane Database Syst Rev 4:CD003539.
Fürst J, Cizman M, Mrak J, Kos D, Campbell S, Coenen S, et al. (2014) The influence of a sustained multifaceted approach to improve antibiotic prescribing in Slovenia during the past decade: findings and implications. Expert Rev Anti-Infect Ther 13(2):279–289
Huttner B, Goossens H, Verheij T, Harbarth S (2010) Characteristics and outcomes of public campaigns aimed at improving the use of antibiotics in outpatients in high-income countries. Lancet Infect Dis 10(1):17–31
Sabuncu E, David J, Bernède-Bauduin C, Pépin S, Leroy M, Boëlle PY et al. (2009) Significant reduction of antibiotic use in the community after a nationwide campaign in France, 2002–2007. PLoS Med (6):e1000084.
Andrews T, Thompson M, Buckley DI, Heneghan C, Deyo R, Redmond N, Lucas PJ, Blair PS, Hay AD. (2012) Interventions to influence consulting and antibiotic use for acute respiratory tract infections in children: a systematic review and meta-analysis. PLoS One 7:e30334
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Holstiege, J., Enders, D., Schink, T. et al. Trends in paediatric macrolide use in five European countries—a population-based study. Eur J Clin Pharmacol 71, 991–999 (2015). https://doi.org/10.1007/s00228-015-1870-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-015-1870-7