Abstract
Purpose
The aim of the present study was to investigate the effect of age on venlafaxine and escitalopram serum concentrations in various cytochrome P450 (CYP) 2D6 and CYP2C19 genotype subgroups.
Methods
Serum concentration measurements from CYP-genotyped patients treated with venlafaxine (n = 255) or escitalopram (n = 541) were collected retrospectively from a therapeutic drug monitoring database. Patients were divided into three CYP2D6 (venlafaxine) or CYP2C19 (escitalopram) phenotype subgroups according to inherited genotype, i.e., poor metabolizers (PMs), heterozygous extensive metabolizers (HEMs), and extensive metabolizers (EMs), and subsequently distributed into three age groups, i.e., <40 (control), 40–65, and >65 years. The effect of age on dose-adjusted serum concentrations (i.e., nmol/L/mg/day) of venlafaxine and escitalopram in each of the phenotype subgroups was evaluated by separate multivariate mixed model analyses.
Results
In CYP2D6 PMs, the mean dose-adjusted serum concentration of venlafaxine was 8-fold higher in patients >65 years compared with those <40 years (p < 0.001). In comparison, the respective age-related differences in mean dose-adjusted serum concentrations of venlafaxine were much less pronounced in CYP2D6 HEMs and EMs (<2-fold differences between age groups). A similar genotype-related effect of age was not observed for escitalopram (<1.5-fold age differences in all CYP2C19 subgroups).
Conclusion
This study suggests that the effect of age on serum concentration of venlafaxine is dependent on CYP genotype, in contrast to escitalopram. Thus, to prevent potential side effects, it might be particularly relevant to consider CYP2D6 genotyping prior to initiation of venlafaxine treatment in older patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Late-life depression is a prevalent condition, especially among elderly patients with chronic diseases [1]. In 2011, the proportion of Norwegian individuals aged 70 years or more that used antidepressant drugs was 12 % compared to 6 % of the whole population [2, 3]. Venlafaxine, a combined serotonin and noradrenaline reuptake inhibitor, and escitalopram, a selective serotonin reuptake inhibitor, are commonly used antidepressants, also among elderly patients [2]. About 50 % of antidepressant-using Norwegians aged 65 years or older are treated with either venlafaxine or escitalopram [2].
Many antidepressants display a substantial interpatient pharmacokinetic variability, and the first reports showing the pronounced individual variability in dose-adjusted serum concentrations of tricyclic antidepressants (TCAs) were published more than 40 years ago [4, 5]. Later, similar interindividual variability (>10-fold) in dose-adjusted serum concentrations has been reported for newer antidepressants such as venlafaxine and escitalopram [6–8].
Genetic polymorphisms in drug-metabolizing enzymes and age are two important sources to pharmacokinetic variability, which might lead to poor response or adverse reactions in patients treated with recommended doses. Both venlafaxine and escitalopram are primarily metabolized by genetically polymorphic cytochrome P450 (CYP) enzymes, i.e., CYP2D6 and CYP2C19, respectively [9–12]. For CYP2D6 and CYP2C19, individuals are divided into different phenotype subgroups according to genotype, i.e., extensive metabolizers (EMs, noncarriers of variant alleles), heterozygous extensive metabolizers (HEMs, carriers of one defective variant allele), poor metabolizers (PMs, homozygous carriers of defective variant alleles), and ultrarapid metabolizers (UMs, carriers of variant alleles encoding increased metabolism).
In CYP2D6 PMs treated with venlafaxine, serum concentrations and adverse effect frequency have been reported to be 7-fold and 5-fold higher, respectively, compared to EMs [13, 14]. For escitalopram, severalfold differences in dose-adjusted serum concentrations have been reported between CYP2C19 PMs and EMs [8, 11, 12, 15–17]. Although clinical evidence is lacking, these extensive pharmacokinetic differences are likely to impact the therapeutic response and/or adverse effect risk of escitalopram as well.
Age is also a significant determinant of venlafaxine and escitalopram pharmacokinetic variability. Reis et al. have previously reported 1.4- and 1.9-fold higher median serum concentrations of venlafaxine and escitalopram, respectively, in patients ≥65 years compared with younger patients [7]. In a recent study, we confirmed these findings [18]. Potentially, the impact of age on serum concentrations of venlafaxine and escitalopram might differ according to the patients’ inherent metabolic phenotype. The aim of the present study was therefore to investigate the effect of age on venlafaxine and escitalopram serum concentrations in various CYP2D6 and CYP2C19 genotype subgroups.
Materials and methods
Patients and samples
Serum concentrations of venlafaxine or escitalopram from CYP-genotyped patients were collected retrospectively from a routine therapeutic drug monitoring (TDM) database at Center for Psychopharmacology, Diakonhjemmet Hospital, Oslo, Norway, during the period August 2007 to November 2011. For some patients, CYP genotyping had been performed prior to initiation of antidepressant treatment, whereas for other’s, genotyping was done retrospectively. It also varied whether genotyping had been performed in association to use of the study drugs or not.
Information about the determined serum concentrations, the respective patients’ CYP2D6 and CYP2C19 genotypes, age, gender, drug dosage, and sampling time (time interval between last drug intake and sample withdrawal) were recorded from the TDM files. When multiple serum concentration analyses of venlafaxine/escitalopram had been performed for the same patient, all measurements were considered for inclusion. Serum concentration measurements were included in the study if (i) serum samples had been withdrawn 10–26 h after the last dose intake, (ii) requisition forms provided information about drug dosage, (iii) dose adjustments were made 3 days or more prior to sampling for venlafaxine and 6 days or more prior to sampling for escitalopram, or (iv) measured serum concentrations were above the lower limit of quantification.
Patients carrying multiplied, functional CYP2D6 alleles were not included in the study. Further, if some of the patients contributed with serum concentration measurements in two different age groups, as a consequence of the time span of the retrospective data collection, measurements in the youngest age group were excluded. In cases where the requisition forms provided information about possible noncompliance, or concomitant use of drugs inhibiting or inducing the respective CYP enzymes involved in venlafaxine (CYP2D6 and CYP3A4) and escitalopram metabolism (CYP2C19 and CYP3A4), measurements were also excluded from the study. As a reference to agents described as enzyme inhibitors/inducers, we used the “P450 Drug Interaction Table” published at the website http://medicine.iupui.edu/clinpharm/ddis/clinical-table/[19].
The study was approved by the Regional Committee for Medical and Health Research Ethics and the Hospital Investigational Review Board.
Drug analyses
Serum concentrations of venlafaxine, O-desmethylvenlafaxine, N-desmethylvenlafaxine, escitalopram, and N-desmethylescitalopram, were determined by the same analytical assay developed for routine TDM analyses at Center for Psychopharmacology, Diakonhjemmet Hospital, a laboratory certified by Norwegian Accreditation in accordance with the ISO 15189 standard. The assay had been validated according to international guidelines for bioanalytical methods and had been subjected to external quality control four times a year (for details on the chromatographic assay, see Supplementary document).
CYP genotyping
Two different routine methods for CYP genotyping had been used during the time span of the retrospective data collection. These have previously been described in detail [20, 21]. The methods had some minor differences in detectable mutations but commonly involved determination of the following variant alleles encoding defective enzyme function: CYP2C19*2, *3, and *4; CYP2D6*3, *4, *5, and *6 (the included variants of the present investigation). Cross-validation of the two methods showed 100 % concordance. Absence of variant alleles was interpreted as presence of the functional wild-type allele (*1).
Data analyses
Samples were divided into the following three phenotype subgroups according to genotype: PMs (homozygous carriers of noncoding variant alleles), HEMs (heterozygous carriers of noncoding variant alleles), and EMs (absence of noncoding variant alleles). To enable inclusion of multiple serum measurements from the same individuals, multivariate mixed model analyses were performed to evaluate the effect of age on dose-adjusted serum concentrations (i.e., concentration to dose (C/D) ratios; nmol/L/mg/day) of venlafaxine and escitalopram in each of the above-mentioned phenotype subgroups. Gender and sampling time were included in the mixed model analyses as potential covariates, but only statistically significant covariates (p < 0.05) were included in the final models. Additionally, potential differences in sampling time and drug dosage between the test groups and controls were examined by mixed model analysis.
Age above 65 years was classified as “elderly” in line with the definition by the World Health Organization [22]. Prior to data analyses, the patients were divided into three age subgroups, i.e., <40 (control), 40–65, and >65 years (test groups). Differences in C/D ratios of the parent drugs, metabolites, active moiety (venlafaxine only), and metabolic ratios in the test groups were compared with controls. In addition, we compared the proportion of PMs among those with absolute serum concentrations above the recommended drug concentration range in the different age groups. For the active moiety of venlafaxine (i.e., venlafaxine + O-desmethylvenlafaxine), the upper range was defined as 1,500 nmol/L, while the upper range of escitalopram was defined as 250 nmol/L [23]. Fisher’s exact tests were applied to compare the proportions of PMs achieving supratherapeutic serum concentrations in the subgroup >65 years to the subgroups <40 and 40–65 years, respectively. Further, we examined the simple linear correlations between age (as a continuous variable) and the venlafaxine/O-desmethylvenlafaxine metabolic ratio and the C/D ratio of venlafaxine active moiety in the three CYP2D6 phenotype subgroups.
Prior to the mixed model analyses, C/D ratios were logarithmically transformed. After analyses, group estimates were transformed back to original scale and presented as geometric mean values with 95 % confidence intervals. Statistical significance was considered as p < 0.05. SPSS Software version 20.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analyses, while GraphPad Prism version 4 (GraphPad Software Inc., La Jolla, CA, USA) was used for graphical presentations.
Results
Altogether, 462 serum concentration measurements from 255 patients were included for venlafaxine, and 953 serum concentration measurements from 541 patients were included for escitalopram (Supplementary table). Overall, approximately 16 % of the patients were older than 65 years. The respective dose ranges of venlafaxine and escitalopram were 18.75–900 and 2.5–120 mg/day, regardless of age and inherited CYP phenotype.
The main reason for excluding serum concentration measurements from the study was concomitant treatment with an enzyme inhibitor or inducer. For venlafaxine, this reason for exclusion comprised concomitant treatment with the CYP2D6 inhibitors bupropion (n = 17) or paroxetine (n = 1) and the CYP3A4 inducer carbamazepine (n = 1). For escitalopram, samples were excluded due to coadministration of the CYP2C19 inhibitors omeprazole (n = 3), esomeprazole (n = 5), lanzoprazole (n = 7), or ethinyl estradiol (n = 5) and the CYP3A4 inducers phenytoin (n = 3) or carbamazepine (n = 8).
Venlafaxine and effect of age in CYP2D6 subgroups
In CYP2D6 PMs, the mean C/D ratio of venlafaxine was about 8-fold and 2.5-fold higher in patients >65 and 40–65 years, respectively, compared with those <40 years (p ≤ 0.001, Table 1). In comparison, the respective age-related differences in mean C/D ratios of venlafaxine were much less pronounced and not significantly different between CYP2D6 HEMs and EMs (Table 1). A similar genotype-dependent effect of age was observed for the sum of venlafaxine and O-desmethylvenlafaxine, where mean C/D ratios within the PM subgroup were approximately 5-fold higher in patients >65 than <40 years (p < 0.001, Table 1). In comparison, the differences in mean C/D ratios of venlafaxine + O-desmethylvenlafaxine were only 1.5-fold between older and younger CYP2D6 HEMs and EMs (p < 0.01, Table 1).
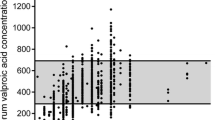
Among venlafaxine-treated PMs >65 years, all three subjects (100 %) had measured absolute serum concentrations above the upper recommended concentration range of venlafaxine + O-desmethylvenlafaxine (Fig. 1a). In comparison, 2 of 8 PMs <40 years (25 %) and 6 of 15 PMs aged 40–65 years (40 %) had measured absolute serum concentrations above the upper recommended concentration range of the pharmacological active fraction (p > 0.05 compared to PMs >65 years).
Absolute serum concentrations of the sum of a venlafaxine and O-desmethylvenlafaxine and b escitalopram, in patients with various inherent CYP phenotypes subgrouped according to age, i.e., <40 (squares), 40–65 (circles), and >65 years (triangles). Note that multiple samples are present for some patients, see Supplementary table. Dotted lines indicate the upper recommended concentration levels [23]. PM poor metabolizer, HEM heterozygous extensive metabolizer, EM extensive metabolizer
In the multivariate mixed model analyses, gender was found to significantly influence the C/D ratio of N-desmethylvenlafaxine in CYP2D6 HEMs (2.1-fold higher in women vs men, p = 0.005, data not shown). Further, in CYP2D6 HEMs, gender was found to significantly influence the metabolic ratio venlafaxine/N-desmethylvenlafaxine (33 % lower in women vs men, p = 0.043, data not shown). Gender was also found to significantly influence the metabolic ratio venlafaxine/O-desmethylvenlafaxine in CYP2D6 EMs (28 % lower in women vs men, p = 0.045, data not shown).
When plotting the individual venlafaxine/O-desmethylvenlafaxine metabolic ratios and C/D ratios of venlafaxine active moieties versus age as a continuous variable, the strongest positive correlations were found within the subgroup of CYP2D6 PMs (Supplementary figure).
Escitalopram and effect of age in CYP2C19 subgroups
For CYP2C19 PMs, a 1.4-fold higher mean C/D ratio of escitalopram was observed in patients >65 years compared with those <40 years, but the difference was not significant (p = 0.1, Table 2). In the other CYP2C19 phenotype subgroups, similar degrees of differences in mean C/D ratios of escitalopram were found to be significant between patients aged >65 versus <40 years (p < 0.02, Table 2).
Among PMs >65 years, none had measured absolute serum concentrations above the upper recommended concentration range of escitalopram (Fig. 1b). In comparison, 2 of 8 PMs <40 years (25 %) and 4 of 16 PMs aged 40–65 years (25 %) had measured absolute serum concentrations above the upper recommended concentration range of escitalopram (p > 0.5 compared to PMs >65 years).
In the multivariate mixed model analyses, gender was found to significantly influence the C/D ratio of escitalopram in CYP2C19 PMs (1.8-fold higher in women vs men, p < 0.001, data not shown). Further, gender was found to significantly influence the metabolic ratio escitalopram/desmethylescitalopram in CYP2C19 PMs (1.8-fold higher in women vs men, p = 0.003, data not shown). Gender was also found to significantly influence the metabolic ratio in CYP2C19 EMs (15 % lower in women vs men, p = 0.017, data not shown).
Discussion
The results of this study indicate that inherent CYP2D6 phenotype is important for the age-related differences in venlafaxine serum concentrations. While only modest age differences in dose-adjusted serum concentrations of venlafaxine and its active moiety were observed within EMs and HEMs, more than 5-fold higher levels were observed in old versus young CYP2D6 PMs. These findings suggest that it might be particularly relevant to genotype elderly patients before initiation of venlafaxine treatment in order to individualize dosing and prevent adverse drug reactions.
A 5-fold increase in occurrence of side effects of venlafaxine has previously been reported in CYP2D6 PMs [14]. Gastrointestinal side effects like nausea, vomiting, and diarrhea, and cardiovascular side effects were reported to be more frequent in CYP2D6 PMs than EMs [14, 24]. The potential cardiac toxicity of venlafaxine is dose-related [25], and higher rates of side effects, such as hypertension, tachycardia, and prolonged QTc interval, have been reported in both CYP2D6 PMs and elderly patients [24, 26], probably due to high serum concentrations at therapeutic doses in these patient subgroups. Although our study included a limited number of elderly CYP2D6 PMs, an important observation was that all three PMs above 65 years had serum concentration measurements of the sum of venlafaxine and O-desmethylvenlafaxine (pharmacologically active concentration) above the upper recommended therapeutic limit, defined as 1,500 nmol/L [23]. Actually, two of three elderly PMs also had absolute serum concentrations above the toxic limit, defined as 3,600 nmol/L [27]. Our study therefore suggests that being an older CYP2D6 PM implies a particular risk of overexposure at standard dosing of venlafaxine.
Whereas inherent CYP2D6 phenotype seemed to be of critical importance for the age-related difference in venlafaxine exposure, no difference in the age effect on serum concentrations of escitalopram with respect to inherent CYP2C19 phenotype was observed in the study. One possible explanation for the variable impact of inherent CYP phenotype on the age-related differences in serum concentrations of venlafaxine and escitalopram might be the difference in hepatic extraction ratio between the two drugs. Venlafaxine has a high hepatic extraction ratio, while the hepatic extraction ratio of escitalopram is low [28]. Hepatic blood flow decreases by 20–50 % with age [29], and this change might reduce the elimination of high-clearance drugs [30]. However, the elimination of low-clearance drugs, where the metabolic rate is not limited by hepatic blood flow, will not be affected by change in blood flow to the same extent [30].
Another possible explanation for the discrepancy in findings between venlafaxine and escitalopram might be differences in the impact of age on secondary enzymes/elimination pathways. The primary enzymes involved in venlafaxine and escitalopram metabolism are CYP2D6 and CYP2C19, respectively [9–12], but other CYP enzymes are also of importance. CYP3A4 is involved in the metabolism of both drugs, but this enzyme is probably quantitatively more important in metabolism of venlafaxine than escitalopram, as interpreted from interaction studies with the potent CYP3A4 inhibitor ketoconazole [31, 32]. Although the literature is somewhat inconsistent, reduced CYP3A4 activity has been reported with increasing age [33, 34]. Thus, reduced CYP3A4 activity by age might have reinforced the effect of age on venlafaxine serum concentrations in CYP2D6 PMs.
Gender has also been associated to altered CYP activities, but the literature is conflicting with regard to potential differences in CYP3A4 phenotypes between females and males [34–37]. In our study, the observed lower venlafaxine to N-desmethylvenlafaxine ratio in females versus males might indicate higher CYP3A4 activity in females, since formation of N-desmethylvenlafaxine is mediated by CYP3A4 [10]. The formation of the desmethylated escitalopram metabolite is mediated by multiple enzymes, including CYP3A4 [11, 12]. It is therefore difficult to interpret if the observed higher escitalopram metabolic ratio in females versus males was due to gender differences in CYP3A4 or other enzymes.
In the main (multivariate) analysis of this study, the effect of age in various phenotype subgroups was investigated as a categorical variable, where age above 65 years was classified as elderly in line with the World Health Organization’s definition [22]. The most substantial effect of age on venlafaxine variables was found in the subgroup of inherent CYP2D6 PMs. This finding, which was based on a limited number of observations, was supported by the fact that the effect of age, as a continuous variable, on venlafaxine variables was most prominent in CYP2D6 PMs.
In addition to the limited inclusion of older PM subjects in the present investigation, the naturalistic study setting has some methodological weaknesses. These include uncertain or unconfirmed information regarding dosage, steady-state conditions, patient adherence, and concurrent use of potentially interacting drugs or herbal agents. In addition, we had no information available on whether the physicians were aware of the patients’ CYP genotype or not before dosing of venlafaxine or escitalopram. All of these uncertainties have the potential to affect the results of the study. However, the relatively high sample number included probably outweighs the disadvantages. Another strength of the study was the application of multivariate mixed model analyses, which enabled inclusion of multiple serum concentration measurements from the same individuals. In this type of multivariate analysis, the data is weighted according to the number of measurements per person. This means that the serum concentration estimate in the subgroup of older venlafaxine-treated PMs was strengthened by the fact that the three patients were represented by five serum concentration measurements.
Conclusion
The results of this study suggest that the effect of age on venlafaxine serum concentration is dependent on inherent CYP2D6 phenotype, while this is not the case for escitalopram with respect to inherent CYP2C19 phenotype. In older patients starting venlafaxine treatment, CYP2D6 genotyping might be particularly relevant to individualize dosing and prevent adverse drug reactions.
References
Mulsant BH, Ganguli M (1999) Epidemiology and diagnosis of depression in late life. J Clin Psychiatry 60(suppl 20):9–15
Norwegian Institute of Public Health, Norwegian Prescription Database. http://www.reseptregisteret.no. Accessed 11 September 2013
Statistics Norway. Population at population censuses in 2001 and 2011, by age. http://www.ssb.no/a/english/kortnavn/fobhoved_en/tab-2012-06-21-02-en.html. Accessed 11 September 2013
Hammer W, Sjöqvist F (1967) Plasma levels of monomethylated tricyclic antidepressants during treatment with imipramine-like compounds. Life Sci 6:1895–1903
Alexanderson B, Evans DAP, Sjöqvist F (1969) Steady-state plasma levels of nortriptyline in twins: influence of genetic factors and drug therapy. Br Med J 4:764–768
Reis M, Lundmark J, Björk H et al (2002) Therapeutic drug monitoring of racemic venlafaxine and its main metabolites in an everyday clinical setting. Ther Drug Monit 24:545–553
Reis M, Aamo T, Spigset O et al (2009) Serum concentrations of antidepressant drugs in a naturalistic setting: compilation based on a large therapeutic drug monitoring database. Ther Drug Monit 31:42–56
Rudberg I, Mohebi B, Hermann M et al (2008) Impact of the ultrarapid CYP2C19*17 allele on serum concentration of escitalopram in psychiatric patients. Clin Pharm Ther 83:322–327
Fogelman SM, Schmider J, Venkatakrishnan K et al (1999) O- and N-demethylation of venlafaxine in vitro by human liver microsomes and by microsomes from cDNA-transfected cells: effect of metabolic inhibitors and SSRI antidepressants. Neuropsychopharmacol 20:480–490
Otton SV, Ball SE, Cheung SW et al (1996) Venlafaxine oxidation in vitro is catalysed by CYP2D6. Br J Clin Pharmacol 41:149–156
Sindrup SH, Brosen K, Hansen MG et al (1993) Pharmacokinetics of citalopram in relation to the sparteine and the mephenytoin oxidation polymorphisms. Ther Drug Monit 15:11–17
Yu BN, Chen GL, He N et al (2003) Pharmacokinetics of citalopram in relation to genetic polymorphism of CYP2C19. Drug Metab Dispos 31:1255–1259
Hermann M, Hendset M, Fosaas K et al (2008) Serum concentrations of venlafaxine and its metabolites O-desmethylvenlafaxine and N-desmethylvenlafaxine in heterozygous carriers of the CYP2D6*3, *4 or *5 allele. Eur J Clin Pharmacol 64:483–487
Shams ME, Arneth B, Hiemke C et al (2006) CYP2D6 polymorphism and clinical effect of the antidepressant venlafaxine. J Clin Pharm Ther 31:493–502
Herrlin K, Yasui-Furukori N, Tybring G et al (2003) Metabolism of citalopram enantiomers in CYP2C19/CYP2D6 phenotyped panels of healthy Swedes. Br J Clin Pharmacol 56:415–421
Jin Y, Pollock BG, Frank E et al (2010) Effect of age, weight and CYP2C19 genotype on escitalopram exposure. J Clin Pharmacol 50:62–72
Tsai MH, Lin KM, Hsiao MC et al (2010) Genetic polymorphisms of cytochrome P450 influence metabolism of escitalopram and treatment response. Pharmacogenomics 11:537–546
Waade RB, Molden E, Refsum H et al (2012) Serum concentrations of antidepressants in the elderly. Ther Drug Monit 34:25–30
Flockhart DA (2007) Drug interactions: cytochrome P450 drug interaction table. Indiana University School of Medicine. http://medicine.iupui.edu/clinpharm/ddis/clinical-table/Accessed 10 March 2014
Rudberg I, Hendset M, Uthus LH et al (2006) Heterozygous mutation in CYP2C19 significantly increases the concentration/dose ratio of racemic citalopram and escitalopram (S-citalopram). Ther Drug Monit 28:102–105
Schaeffeler E, Schwab M, Eichelbaum M et al (2003) CYP2D6 genotyping strategy based on gene copy number determination by TaqMan real-time PCR. Hum Mutat 22:476–485
World Health Organization. Definition of an older or elderly person. http://www.who.int/healthinfo/survey/ageingdefnolder/en/Accessed 20 March 2014
Hiemke C, Baumann P, Bergemann N et al (2011) AGNP consensus guidelines for therapeutic drug monitoring in psychiatry: update 2011. Pharmacopsychiatry 44:195–235
Lessard É, Yessine MA, Hamelin BA et al (1999) Influence of CYP2D6 activity on the disposition and cardiovascular toxicity of the antidepressant agent venlafaxine in humans. Pharmacogenetics 9:435–443
Howell C, Wilson AD, Waring WS (2007) Cardiovascular toxicity due to venlafaxine poisoning in adults: a review of 235 consecutive cases. Br J Clin Pharmacol 64:192–197
Johnson EM, Whyte E, Mulsant BH et al (2006) Cardiovascular changes associated with venlafaxine in the treatment of late-life depression. Am J Geriatr Psychiat 14:796–802
TIAFT reference blood level list of therapeutic and toxic substances. September 2004. http://www.gtfch.org/cms/images/stories/Updated_TIAFT_list_202005.pdf. Accessed 25 September 2013
Adis data information BV (2006) Dose adjustment of drugs with high hepatic extraction are required in patients with severe liver disease. Drugs Ther Perspect 22:23–26, 1172-0360/06/0005-0023
Klotz U (2009) Pharmacokinetics and drug metabolism in the elderly. Drug Metab Rev 41:67–76
Cusack BJ (2004) Pharmacokinetics in older persons. Am J Geriatr Pharmacother 2:274–302
Gutierrez M, Abramowitz W (2001) Lack of effect of a single dose of ketoconazole on the pharmacokinetics of citalopram. Pharmacotherapy 21:163–168
Lindh JD, Annas A, Meurling L et al (2003) Effect of ketoconazole on venlafaxine plasma concentrations in extensive and poor metabolisers of debrisoquine. Eur J Clin Pharmacol 59:401–406
Kinirons MT, O'Mahony MS (2004) Drug metabolism and ageing. Br J Clin Pharmacol 57:540–544
Bebia Z, Buch SC, Wilson JW et al (2004) Bioequivalence revisited: influence of age and sex on CYP enzymes. Clin Pharmacol Ther 76:618–627
Hunt CM, Westerkam WR, Stave GM (1992) Effect of age and gender on the activity of human hepatic CYP3A. Biochem Pharmacol 44:275–283
Harris RZ, Benet LZ, Schwartz JB (1995) Gender effects in pharmacokinetics and pharmacodynamics. Drugs 50:222–239
McCune JS, Lindley C, Decker JL et al (2001) Lack of gender differences and large intrasubject variability in cytochrome P450 activity measured by phenotyping with dextromethorphan. J Clin Pharmacol 41:723–731
Contribution of authors
All authors provided substantial contributions to the conception and design of the study and were responsible for interpretation of data contained within this manuscript. HLM was the primary contributor of the data collection. RBW was responsible for data processing and statistical analyses and also prepared the initial draft of the manuscript. All authors revised the manuscript critically for important intellectual content and read and approved the final version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Waade, R.B., Hermann, M., Moe, H.L. et al. Impact of age on serum concentrations of venlafaxine and escitalopram in different CYP2D6 and CYP2C19 genotype subgroups. Eur J Clin Pharmacol 70, 933–940 (2014). https://doi.org/10.1007/s00228-014-1696-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-014-1696-8