Abstract
Purpose
The purpose of this study was to investigate the interactions of itraconazole (ITCZ) with orally administered calcineurin inhibitors (CNIs) in Japanese allogeneic hematopoietic stem cell transplant (HSCT) recipients.
Methods
Sixteen HSCT patients (8 patients each receiving tacrolimus or cyclosporine) were enrolled. An ITCZ oral solution was administered from day 30 after the initiation of ITCZ administration as a loading dose. Before the co-administration of ITCZ and CNI and 1 week daily thereafter, whole blood ITCZ and CNI (tacrolimus or cyclosporine) concentrations were measured in samples taken just before (C0h) and 2 h (C2h) after CNI administration.
Results
The median dose-adjusted C0h values of tacrolimus and cyclosporine on day 7 after the start of ITCZ co-administration were 5.6- and 2.7-fold higher, respectively, than the corresponding values obtained before the initiation of ITCZ treatment. On day 7 after ITCZ treatment, the mean single dosages of tacrolimus and cyclosporine were reduced to 33.7 and 66.5 % of the dosages before ITCZ co-administration, respectively, to adjust the CNI target concentration. Although ITCZ co-administration did not alter the dose-adjusted C0h values of tacrolimus in a patient with a CYP3A5*1/*1 allele, it did change this value of tacrolimus in patients with CYP3A5*3 alleles. However, in patients receiving cyclosporine, no such tendency was observed.
Conclusion
The magnitude of the interaction between orally administered tacrolimus and ITCZ was significantly greater than that between cyclosporine and ITCZ. Prospective analysis of the CYP3A5 polymorphism may be important to ensure safe and reliable immunosuppressive therapy with tacrolimus in patients treated with ITCZ.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The clinical management of orally or intravenously administered calcineurin inhibitors (CNIs), such as tacrolimus or cyclosporine, which are widely used immunosuppressive agents in hematopoietic stem cell transplantation (HSCT) recipients, is complicated by their narrow therapeutic range and their inter- and intra-individual variable pharmacokinetics [1]. Given that CNIs are substrates of cytochrome P450 (CYP) 3A4/5, which is expressed in the small intestine and hepatocytes, the variability in their pharmacokinetics, in particular, their bioavailability, has been attributed to individual differences in CYP3A expression [2–4].
The antifungal agent itraconazole (ITCZ) is routinely used as prophylaxis against Aspergillus infection and often administered to HSCT recipients receiving CNIs. In several multicenter, prospective clinical trials, the elimination of Aspergillus infection in allogeneic stem cell transplant recipients was achieved using ITCZ prophylaxis. A significant difference was observed in the incidence of Aspergillus infection between prophylaxis with ITCZ and that with fluconazole, particularly from day 30 after HSCT [5, 6]. However, in both in vitro and in vivo studies, ITCZ potently inhibited the activity of CYP3A4 [7–13]. Therefore, the blood concentration of CNIs may increase on their co-administration with ITCZ. In contrast, ITCZ is not a substrate of CYP3A5 [10, 14]. Although ITCZ may inhibit CYP3A5 in vitro [15], whether it inhibits CYP3A5 in vivo is unknown.
Polymorphic expression in the liver and small intestine of polymorphic forms of CYP3A5 is strongly correlated with the 6986A > G single nucleotide polymorphism (SNP) within intron 3 of CYP3A5, which is designated CYP3A5*3 [16]. Recently, we reported that the maintenance oral dosage of tacrolimus was 40-fold lower than the dose before co-administration with ITCZ in allogeneic HSCT recipients with CYP3A5*3/*3 alleles [17]. To our knowledge, thus far, no study has systematically evaluated the interactions between orally administered CNIs and ITCZ or determined whether the magnitude of interaction is different among patients harboring genetic polymorphisms in CYP3A5. Therefore, the aim of the study reported here was to investigate the interactions between orally administered ITCZ and tacrolimus or cyclosporine in Japanese allogeneic HSCT recipients.
Material and methods
Patients and protocols
A single-institute, prospective pilot study was conducted to evaluate both the efficacy and safety of an oral solution of ITCZ and its interactions with tacrolimus or cyclosporine in allogeneic HSCT recipients.
The patient eligibility criteria for the study were as follows: (1) HSCT recipient treated using identical immunosuppressive regimens that included tacrolimus (Prograf®) and cyclosporine (Neoral®); (2) age of >16 years; (3) no hypersensitivity to ITCZ; (4) no liver dysfunction [aspartate aminotransferase (AST) or alanine aminotransferase (ALT) level of <3-fold the upper normal range and total bilirubin level of <2.0 mg/dL); (5) no renal dysfunction (serum creatinine level of <2.0 mg/dL or creatinine clearance of >30 mL/min); (6) nonsmoker; (7) no consumption of drugs or food affecting CYP3A and P-glycoprotein levels.
After HSCT, continuous infusion of 0.03 mg/kg/day tacrolimus or 2.0–3.0 mg/kg/day cyclosporine (from day −1 before transplantation) was administered to prevent acute graft-versus-host disease (aGVHD). On days 20–30 after transplantation, patients were switched from continuous CNI infusion to 0.06 mg/kg/day orally administered tacrolimus or 6–12 mg/kg/day orally administrated cyclosporine in equal doses every 12 h at 0800 and 2000 hours. After the initiation of 200 mg ITCZ twice daily (1000 and 2200) for the first 2 days as a loading dose, a 200 mg/day ITCZ oral solution was administered at 0600 hours on days 30–100 after transplantation. The target trough concentrations of tacrolimus and cyclosporine were 5–15 ng/mL and 150–300 ng/mL, respectively.
Treatment was terminated if participants met any one of the following criteria: (1) fungal infection; (2) no resolution of fever (temperature >38 °C for 4–7 days) by a broad-spectrum antibacterial agent during neutropenia; (3) drug-related toxicity; (4) AST or ALT level of >5-fold the upper normal range; (5) total bilirubin level of >3 mg/dL; (6) serum creatinine level of >2.0 mg/dL or creatinine clearance of <30 mL/min; (7) aggravation of a hematological disease or a complication; (8) grade 3/4 aGVHD.
A total of 16 Japanese HSCT patients (12 men, 4 women) who received grafts between October 2009 and August 2011 were enrolled in the study. The study protocol was approved by the Ethics Committee of Akita University Graduate School of Medicine, and all patients gave written informed consent.
Sample collection and analytical methods
Before the co-administration of ITCZ and daily for 1 week thereafter, we collected venous blood samples just before (C0h) and 2 h (C2h) after tacrolimus or cyclosporine administration (0800 hours) to determine the blood concentrations of tacrolimus and cyclosporine and the plasma concentrations of ITCZ and hydroxyitraconazole. Tacrolimus and cyclosporine blood concentrations were determined using chemiluminescence magnetic microparticle immunoassay (CMIA) on the Architect-i1000® system (Abbott Laboratories, Abbott Park, IL) according to the manufacturer’s instructions. The limit of quantitation of tacrolimus and cyclosporine for the Architect-i1000® instrument is stated to be 0.5 ng/mL [18] and 20 ng/mL [19], respectively, and their coefficients of variation for this assay were <10 % in the concentration range 2–8 ng/mL [18] and <15 % in the concentration range 87.5–916 ng/mL [19], respectively. Plasma concentrations of ITCZ and hydroxyitraconazole were monitored using high-performance liquid chromatography (HPLC) [20]. The limit of quantitation of this HPLC system for ITCZ and hydroxyitraconazole is stated to be 0.5 ng/mL [20], and the coefficient of variation for both substances in this assay is <13 % in the concentration range 5–2,500 ng/mL [20].
Genotyping
DNA was extracted from a peripheral blood sample using the QIAamp Blood kit (Qiagen, Hilden, Germany) and stored at –80 °C until analysis. For genotyping, the CYP3A5*3 allele was detected using a PCR–restriction fragment length polymorphism (RFLP) method [21]. The results obtained from PCR-RFLP were confirmed using a fully automated SNP detection system (prototype i-densy™; ARKRAY, Kyoto, Japan).
Statistical analyses
The Kolmogorov–Smirnov test was used to assess distribution. The doses of tacrolimus or cyclosporine were expressed as the mean ± standard deviation (SD). The clinical characteristics of patients with HSCT, dose-adjusted blood concentrations, and changes in these parameters were expressed as medians (quartiles 1–3). The chi-square test was used to examine differences in categorical data, except when expected number of cells was <5, in which case the Fisher’s exact test was used. Statistically significant differences between two populations were assessed using the Student’s t test if the population data conformed to a normal distribution; otherwise, the Mann–Whitney U test was applied. The Wilcoxon paired signed rank test was used to determine the difference in continuous values within each patient. Spearman’s rank correlation coefficient test was used to assess the correlation between the dose-adjusted blood concentration of tacrolimus or cyclosporine and the plasma concentration of ITCZ. These results were expressed as a determination coefficient (r 2). A P value of <0.05 was considered to be statistically significant. Statistical analyses were performed using SPSS ver. 20.0 software for Windows (SPSS IBM Japan, Tokyo, Japan).
Results
Patient characteristics are listed in Table 1. The genotype frequencies of the CYP3A5 polymorphisms were almost equal in the tacrolimus and cyclosporine groups.
No patients were removed from this study because of the previously mentioned criteria, which included breakthrough fungal infections, severe aGVHD [22], or severe adverse events (Table 2).
The steady-state median (quartiles 1–3) plasma concentrations of ITCZ in the tacrolimus and cyclosporine groups were 1,005 (768–1,403) and 1,357 (682–2,122) ng/mL, respectively, and those of hydroxyitraconazole were 1,023 (872–1,674) and 1,367 (1,056–1,904) ng/mL, respectively. The median (quartiles 1–3) hydroxyitraconazole/ITCZ concentration ratios in the tacrolimus and cyclosporine groups were 1.23 (0.81–1.67) and 1.20 (1.08–1.38), respectively. ITCZ co-administration did not result in any serious renal or hepatic dysfunction, and no laboratory abnormalities were observed (Table 3).
The ratio of dose-adjusted C0h and C2h values with or without ITCZ is plotted against the number of days after co-administration with ITCZ for the tacrolimus and cyclosporine groups and shown in Figs. 1 and 2, respectively. ITCZ treatment significantly increased the dose-adjusted C0h of tacrolimus and cyclosporine from day 3 after the start of ITCZ co-administration (Fig. 1). The median (quartiles 1–3) dose-adjusted C0h of tacrolimus and cyclosporine on day 7 increased by 5.6- (3.1–7.5) or 2.7- (2.1–3.6) fold, respectively, compared with the values before the initiation of ITCZ. A significant inter-group difference was observed in the dose-adjusted C0h after ITCZ treatment (P = 0.0357), indicating that the drug interaction with ITCZ was greater than that for tacrolimus.
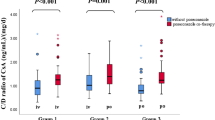
The ratio of dose-adjusted blood trough concentration (C 0h ) with or without itraconazole (ITCZ) is plotted as a function of the number of days after the co-administration of ITCZ with tacrolimus (open column) and cyclosporine (closed column) in hematopoietic stem cell transplant recipients. This graphical analysis was carried out using the SPSS (SPSS, Chicago, IL) box and whiskers plot. The box spans data between the two quartiles (interquartile range, IQR), with the median represented as a bold horizontal line. The ends of the whiskers (vertical lines) represent the smallest and largest values that are not outliers. Outliers (circles) are values between 1.5 and 3 IQRs from the end of a box. Values of >3 IQRs from the end of a box are defined as extreme (asterisk). †P < 0.05 indicates that a significant difference was observed before and after ITCZ treatment
The ratio of dose-adjusted blood concentration (C 2h ) with or without ITCZ is plotted as a function of the number of days after the co-administration of ITCZ for tacrolimus (open column) and cyclosporine (closed column) in hematopoietic stem cell transplant recipients. Graphical analysis was carried out using the SPSS box and whiskers plot. The box spans data between the two quartiles (IQRs), with the median represented as a bold horizontal line. The ends of the whiskers (vertical lines) represent the smallest and largest values, respectively, that are not outliers. Outliers (circles) are values between 1.5 and 3 IQRs from the end of a box. †P < 0.05 indicates that a significant difference was observed before and after ITCZ treatment
Moreover, ITCZ treatment significantly increased the dose-adjusted C2h of tacrolimus from day 4 after the start of ITCZ co-administration, whereas no significant difference was observed in the dose-adjusted C2h of cyclosporine up to 7 days after the start of ITCZ co-administration (Fig. 2). The median (quartiles 1–3) dose-adjusted C2h of tacrolimus and cyclosporine on day 7 after the start of ITCZ co-administration increased by 6.0- (2.9–7.7) or 2.1-fold (1.5–3.5), respectively, compared with the median before the initiation of ITCZ therapy. Similar to the C0h results, the tacrolimus and cyclosporine groups were significantly different in terms of the dose-adjusted C2h after the start of ITCZ co-administration (P = 0.0389).
The reduction in the single dosage of tacrolimus and cyclosporine after the start of ITCZ co-administration is shown in Fig. 3. The mean single dosage of tacrolimus and cyclosporine was decreased to 33.7 % and 66.5 %, respectively, of that before ITCZ co-administration. The reduction rate and the time for the single dosage of tacrolimus were significantly greater and faster, respectively, than that for cyclosporine.
The correlation between the C0h of tacrolimus or cyclosporine and the plasma concentrations of ITCZ from days 3 to 7 is shown in Fig. 4. The correlation between the C0h of cyclosporine and plasma ITCZ concentrations was high (r 2 = 0.4141, P < 0.0002); however, no correlation was found between tacrolimus and ITCZ.
The changes in the dose-adjusted C0h of tacrolimus and cyclosporine before and on day 7 after the start of ITCZ co-administration in individual patients and their CYP3A5 genotypes are shown in Fig. 5. The dose-adjusted C0h of tacrolimus in the single patient with the CYP3A5*1/*1 genotype did not change following ITCZ co-administration; however, the concentration of tacrolimus in the other patients with the CYP3A5*1/*3 and *3/*3 genotypes did significantly increase after the start of ITCZ co-administration. In contrast, this CYP3A5 genotype-associated tendency was not observed as a clear trend in patients receiving cyclosporine.
Discussion
The present study is the first report, to our knowledge, of a systematic evaluation of the interactions between orally administered ITCZ and CNIs and an investigation of possible variations in the magnitude of drug interactions among patients with different CYP3A5 genetic polymorphisms.
In an in vitro study using human liver microsomes from subjects harboring the CYP3A5*3/*3 genotype, Yamazaki et al. reported that the inhibition constant (Ki) of ITCZ for CYP3A5*3/*3 is approximately half that of the CYP3A5*1/*3 genotype [15]. The authors of this study expected that the increased area under the concentration–time curve (AUC) of CYP3A5 substrate resulting from ITCZ co-administration in patients with the CYP3A5*1/*1 allele would be the lowest of the three CYP3A5 genotype groups [15]. In our study, the dose-adjusted C0h of tacrolimus in the one patient with the CYP3A5*1/*1 genotype was not changed by ITCZ co-administration; however, the concentration of tacrolimus in the other patients with the CYP3A5*1/*3 and *3/*3 alleles significantly increased after the start of ITCZ co-administration.
Taken together, these findings suggest that in homozygous expressors of CYP3A5*1/*1, the interaction is minimal, presumably because the CYP3A5 pathway is fully capable of compensating for the lack of drug metabolism of the inhibited CYP3A4 pathway. In heterozygous expressors of CYP3A5*1/*3, the interaction manifests more than that of the homozygous (*1/*1) phenotype because there are fewer active CYP3A5 molecules to compensate for the effects of the inhibited CYP3A4 pathway. Our present findings support the results reported by Yamazaki et al. [15]. In contrast, it appears that the cyclosporine and ITCZ interaction is not as influenced by or is sensitive to CYP3A5 polymorphisms. The authors of several studies also report that CYP3A5 polymorphisms significantly influenced treatment with tacrolimus, but not with cyclosporine [23, 24]. Leather et al. reported that intravenously administered ITCZ increased the mean serum concentration of tacrolimus and cyclosporine by 83 and 80 %, respectively, in allogeneic HSCT recipients [25]. This result shows that the interactions between ITCZ and intravenously administered CNIs involve hepatic CYP3A and that the magnitude of the drug interactions between intravenous tacrolimus or cyclosporine and ITCZ are very similar [25]. However, in our study, the magnitude of the interaction between orally administered tacrolimus and ITCZ was significantly greater than that between orally administered cyclosporine and ITCZ. Therefore, intestinal CYP3A5 might play an important role in the metabolism of tacrolimus [26]. If tacrolimus is selected as an immunosuppressive agent for allogeneic HSCT patients, the prospective analysis of CYP3A5 polymorphism may be important to ensure safe and reliable immunosuppressive therapy with tacrolimus.
The ITCZ metabolites hydroxyitraconazole, keto-itraconazole, and N-desalkyl-itraconazole also inhibit CYP3A4 in vitro, and they may contribute to the overall inhibition of CYP3A4 in vivo if they circulate at sufficiently high concentrations [10, 27]. Although only hydroxyitraconazole was measured in our study, this metabolite might influence the metabolism of tacrolimus via CYP3A4. Moreover, tacrolimus inhibits CYP3A4 and CYP3A5, whereas cyclosporine only inhibits CYP3A4 [28]. Because the inhibition of CYP by tacrolimus is much stronger than that of cyclosporine, the co-administration of CNIs and ITCZ in allogeneic HSCT patients may be easier to manage using cyclosporine rather than tacrolimus.
CNIs are also a substrate of P-glycoprotein, and ITCZ is a known potent inhibitor of P-glycoprotein activity, both in vitro and in vivo [29, 30]. P-glycoprotein is a membrane efflux transporter that is normally expressed in human tissues, such as the small intestine, the biliary canalicular front of hepatocytes, and renal proximal tubules [31]. Orally administered ITCZ may also increase the blood concentrations of CNIs by inhibiting P-glycoprotein activity in the small intestine or on the biliary canalicular front of hepatocytes.
No laboratory abnormalities were observed in the recipients of ITCZ co-administration. Although allogeneic HSCT patients received a loading dose of ITCZ intravenously for 2 days followed by oral treatment with 200 mg of ITCZ, the dose-adjusted C0h values of tacrolimus and cyclosporine were significantly increased from day 3 and then stabilized on day 5 after the start of ITCZ co-administration. Thus, the interactions between the CNIs and ITCZ were observed 3 days after replacement of the oral solution. Because sufficient time may be required for the appearance of drug interactions, the CNI C0h should be monitored daily for at least 5 days after the start of ITCZ co-administration to prevent the occurrence of adverse events. The reduction rate and the time for the single dosage of tacrolimus were significantly greater and faster than that of cyclosporine. These findings also suggest that the magnitude of the drug interaction between orally administered tacrolimus and ITCZ was significantly greater than that between cyclosporine and ITCZ. Further, the therapeutic window of tacrolimus is very narrow, and its target concentration might be too low for it to be adjusted compared to cyclosporine.
It is important to note that this study was performed with a small number of patients. Therefore, our results must be interpreted within the context of this limitation, and further examination with a larger sample size to corroborate our current findings is necessary.
References
Staatz CE, Tett SE (2004) Clinical pharmacokinetics and pharmacodynamics of tacrolimus in solid organ transplantation. Clin Pharmacokinet 43(10):623–653
Hesselink DA, van Schaik RH, van der Heiden IP, van der Werf M, Gregoor PJ, Lindemans J, Weimar W, van Gelder T (2003) Genetic polymorphisms of the CYP3A4, CYP3A5, and MDR-1 genes and pharmacokinetics of the calcineurin inhibitors cyclosporine and tacrolimus. Clin Pharmacol Ther 74(3):245–254. doi:10.1016/S0009-9236(03)00168-1
Roy JN, Barama A, Poirier C, Vinet B, Roger M (2006) Cyp3A4, Cyp3A5, and MDR-1 genetic influences on tacrolimus pharmacokinetics in renal transplant recipients. Pharmacogenet Genom 16(9):659–665. doi:10.1097/01.fpc.0000220571.20961.dd
Hesselink DA, van Gelder T, van Schaik RH (2005) The pharmacogenetics of calcineurin inhibitors: one step closer toward individualized immunosuppression? Pharmacogenomics 6(4):323–337. doi:10.1517/14622416.6.4.323
Winston DJ, Maziarz RT, Chandrasekar PH, Lazarus HM, Goldman M, Blumer JL, Leitz GJ, Territo MC (2003) Intravenous and oral itraconazole versus intravenous and oral fluconazole for long-term antifungal prophylaxis in allogeneic hematopoietic stem-cell transplant recipients. A multicenter, randomized trial. Ann Intern Med 138(9):705–713
Marr KA, Crippa F, Leisenring W, Hoyle M, Boeckh M, Balajee SA, Nichols WG, Musher B, Corey L (2004) Itraconazole versus fluconazole for prevention of fungal infections in patients receiving allogeneic stem cell transplants. Blood 103(4):1527–1533. doi:10.1182/blood-2003-08-2644
Venkatakrishnan K, von Moltke LL, Greenblatt DJ (2000) Effects of the antifungal agents on oxidative drug metabolism: clinical relevance. Clin Pharmacokinet 38(2):111–180
Niwa T, Shiraga T, Takagi A (2005) Effect of antifungal drugs on cytochrome P450 (CYP) 2C9, CYP2C19, and CYP3A4 activities in human liver microsomes. Biol Pharm Bull 28(9):1805–1808
Koks CH, Meenhorst PL, Bult A, Beijnen JH (2002) Itraconazole solution: summary of pharmacokinetic features and review of activity in the treatment of fluconazole-resistant oral candidosis in HIV-infected persons. Pharmacol Res 46(2):195–201
Isoherranen N, Kunze KL, Allen KE, Nelson WL, Thummel KE (2004) Role of itraconazole metabolites in CYP3A4 inhibition. Drug Metab Dispos: Biol Fate Chem 32(10):1121–1131. doi:10.1124/dmd.104.000315
Buchkowsky SS, Partovi N, Ensom MH (2005) Clinical pharmacokinetic monitoring of itraconazole is warranted in only a subset of patients. Ther Drug Monit 27(3):322–333
Varhe A, Olkkola KT, Neuvonen PJ (1994) Oral triazolam is potentially hazardous to patients receiving systemic antimycotics ketoconazole or itraconazole. Clin Pharmacol Ther 56(6 Pt 1):601–607
Jalava KM, Olkkola KT, Neuvonen PJ (1997) Itraconazole greatly increases plasma concentrations and effects of felodipine. Clin Pharmacol Ther 61(4):410–415. doi:10.1016/S0009-9236(97)90191-0
Huang W, Lin YS, McConn DJ 2nd, Calamia JC, Totah RA, Isoherranen N, Glodowski M, Thummel KE (2004) Evidence of significant contribution from CYP3A5 to hepatic drug metabolism. Drug Metab Dispos: Biol Fate Chem 32(12):1434–1445. doi:10.1124/dmd.104.001313
Yamazaki H, Nakamoto M, Shimizu M, Murayama N, Niwa T (2010) Potential impact of cytochrome P450 3A5 in human liver on drug interactions with triazoles. Br J Clin Pharmacol 69(6):593–597. doi:10.1111/j.1365-2125.2010.03656.x
Hustert E, Haberl M, Burk O, Wolbold R, He YQ, Klein K, Nuessler AC, Neuhaus P, Klattig J, Eiselt R, Koch I, Zibat A, Brockmoller J, Halpert JR, Zanger UM, Wojnowski L (2001) The genetic determinants of the CYP3A5 polymorphism. Pharmacogenetics 11(9):773–779
Nara M, Takahashi N, Miura M, Saitoh H, Kagaya H, Sawada K (2010) Effect of oral itraconazole on the pharmacokinetics of tacrolimus in a hematopoietic stem cell transplant recipient with CYP3A5*3/*3. Am J Hematol 85(8):634–635. doi:10.1002/ajh.21759
Wallemacq P, Maine GT, Berg K, Rosiere T, Marquet P, Aimo G, Mengozzi G, Young J, Wonigeit K, Kretschmer R, Wermuth B, Schmid RW (2010) Multisite analytical evaluation of the Abbott ARCHITECT cyclosporine assay. Ther Drug Monit 32(2):145–151. doi:10.1097/FTD.0b013e3181d46386
Amann S, Parker TS, Levine DM (2009) Evaluation of 2 immunoassays for monitoring low blood levels of tacrolimus. Ther Drug Monit 31(2):273–276. doi:10.1097/FTD.0b013e318196dfed
Miura M, Takahashi N, Nara M, Fujishima N, Kagaya H, Kameoka Y, Saitoh H, Tagawa H, Sawada K (2010) A simple, sensitive high-performance liquid chromatography-ultraviolet method for the quantification of concentration and steady-state pharmacokinetics of itraconazole and hydroxyitraconazole. Ann Clin Biochem 47(Pt 5):432–439. doi:10.1258/acb.2010.010029
Fukuen S, Fukuda T, Maune H, Ikenaga Y, Yamamoto I, Inaba T, Azuma J (2002) Novel detection assay by PCR-RFLP and frequency of the CYP3A5 SNPs, CYP3A5*3 and *6, in a Japanese population. Pharmacogenetics 12(4):331–334
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, Thomas ED (1995) 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant 15(6):825–828
Santoro A, Felipe CR, Tedesco-Silva H, Medina-Pestana JO, Struchiner CJ, Ojopi EB, Suarez-Kurtz G (2011) Pharmacogenetics of calcineurin inhibitors in Brazilian renal transplant patients. Pharmacogenomics 12(9):1293–1303. doi:10.2217/pgs.11.70
Anglicheau D, Legendre C, Beaune P, Thervet E (2007) Cytochrome P450 3A polymorphisms and immunosuppressive drugs: an update. Pharmacogenomics 8(7):835–849. doi:10.2217/14622416.8.7.835
Leather H, Boyette RM, Tian L, Wingard JR (2006) Pharmacokinetic evaluation of the drug interaction between intravenous itraconazole and intravenous tacrolimus or intravenous cyclosporin A in allogeneic hematopoietic stem cell transplant recipients. Biol Blood Marrow Transplant 12(3):325–334. doi:10.1016/j.bbmt.2005.10.022
Uesugi M, Masuda S, Katsura T, Oike F, Takada Y, Inui K (2006) Effect of intestinal CYP3A5 on postoperative tacrolimus trough levels in living-donor liver transplant recipients. Pharmacogenet Genomics 16(2):119–127
Templeton IE, Thummel KE, Kharasch ED, Kunze KL, Hoffer C, Nelson WL, Isoherranen N (2008) Contribution of itraconazole metabolites to inhibition of CYP3A4 in vivo. Clin Pharmacol Ther 83(1):77–85. doi:10.1038/sj.clpt.6100230
Amundsen R, Asberg A, Ohm IK, Christensen H (2012) Cyclosporine A- and tacrolimus-mediated inhibition of CYP3A4 and CYP3A5 in vitro. Drug Metab Dispos: Biol Fate Chem 40(4):655–661. doi:10.1124/dmd.111.043018
Keogh JP, Kunta JR (2006) Development, validation and utility of an in vitro technique for assessment of potential clinical drug-drug interactions involving P-glycoprotein. Eur J Pharm Sci 27(5):543–554. doi:10.1016/j.ejps.2005.11.011
Shon JH, Yoon YR, Hong WS, Nguyen PM, Lee SS, Choi YG, Cha IJ, Shin JG (2005) Effect of itraconazole on the pharmacokinetics and pharmacodynamics of fexofenadine in relation to the MDR1 genetic polymorphism. Clin Pharmacol Ther 78(2):191–201. doi:10.1016/j.clpt.2005.04.012
Thiebaut F, Tsuruo T, Hamada H, Gottesman MM, Pastan I, Willingham MC (1987) Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc Natl Acad Sci USA 84(21):7735–7738
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nara, M., Takahashi, N., Miura, M. et al. Effect of itraconazole on the concentrations of tacrolimus and cyclosporine in the blood of patients receiving allogeneic hematopoietic stem cell transplants. Eur J Clin Pharmacol 69, 1321–1329 (2013). https://doi.org/10.1007/s00228-013-1471-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-013-1471-2