Abstract
The aim of this retrospective study was to identify variable factors affecting tacrolimus blood concentration during the switch from continuous intravenous infusion to twice-daily oral administration in allogeneic hematopoietic stem cell transplant recipients (n = 73). The blood concentration/dose ratio of tacrolimus immediately before the change from continuous infusion (C/Div) was compared with that between 3 and 5 days after the change to oral administration (C/Dpo). Median (C/Dpo)/(C/Div) was 0.21 (range 0.04–0.58). Multiple regression analysis showed that concomitant use of oral itraconazole or voriconazole significantly increased the (C/Dpo)/(C/Div) of tacrolimus (p = 0.002), probably owing to the inhibition of enterohepatic cytochrome P450 3A4. In addition, 5 of 18 (28%) patients who had the lowest quartile (C/Dpo)/(C/Div) values developed acute graft-versus-host-disease (GVHD), which was significantly higher than in others [5 of 55 (9%) patients, p = 0.045]. Although the switch from intravenous to oral administration at a ratio of 1:5 appeared to be appropriate, a lower conversion ratio was suitable in patients taking oral itraconazole or voriconazole. In patients whose blood concentration decreases after the switch, the development of GVHD should be monitored and tacrolimus dosage should be readjusted to maintain an appropriate blood concentration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tacrolimus is a widely used immunosuppressant for the prophylaxis of graft-versus-host-disease (GVHD) after allogeneic hematopoietic stem cell transplantation (HSCT) [1–6]. GVHD is the major cause of mortality for patients receiving HSCT [7]; therefore, prevention of severe GVHD is important for successful treatment. Tacrolimus is typically started intravenously a day before HSCT, and is converted to an oral formulation when patients can tolerate oral administration.
Because of its narrow therapeutic range and large inter- and intra-individual variability in pharmacokinetics [8–10], therapeutic drug monitoring of tacrolimus is considered essential in the management of patients. Despite the large variation in tacrolimus pharmacokinetics, the area under the concentration (AUC) time curve versus the trough blood concentration has a nearly linear relationship in HSCT patients [11]. Based on these results, the trough monitoring of tacrolimus blood concentration is strongly recommended in patients receiving HSCT. Previous reports suggest that 10–20 ng/ml may be the optimal trough level of tacrolimus in HSCT patients [12, 13]; lower blood concentrations tend to lead to GVHD, and the risk of kidney injury increases when the blood concentration exceeds 20 ng/mL.
Although the pharmacokinetics of tacrolimus differs between intravenous and oral administration [9, 11], the variable factors affecting tacrolimus blood concentration during route of administration switch have not been fully evaluated. Therefore, it is difficult to determine the dosage adjustment of tacrolimus, especially when switching from continuous intravenous infusion to oral administration in HSCT. In this study, we aimed to identify the variable factors during this switch. In addition, we considered the conversion rate from intravenous to oral administration, and identified associations between the variation of tacrolimus concentration and the occurrence of acute GVHD and kidney injury.
Materials and methods
Patient selection
This study was conducted in accordance with the Declaration of Helsinki and its amendments, and the Ethical Guidelines for Epidemiological Research by the Ministry of Education, Culture, Sports, Science, and Technology, and the Ministry of Health, Labor, and Welfare of Japan. The protocol was approved by the Ethics Committee of Kyushu University Graduate School and Faculty of Medicine (Approval No. 26-26). Eligible subjects were inpatients 20 years or older, diagnosed with hematologic malignancies, and received conditioning regimens followed by HSCT, with GVHD prophylaxis of switching tacrolimus from continuous intravenous infusion to oral administration, at the Department of Hematology, Kyushu University Hospital, between December 2010 and December 2013.
Treatment and study schedule
The initial dose of tacrolimus continuous intravenous infusion was 0.03 mg/kg/day. The dose of tacrolimus was adjusted to maintain the blood concentration level between 10 and 15 ng/mL. When patients could tolerate oral administration, the route of administration of tacrolimus was switched from continuous intravenous infusion (Prograf® injection) to twice-daily oral capsules (Prograf® capsule). Intravenous infusion was stopped just before the first oral administration of medication. After the switch to oral administration, the tacrolimus blood concentration was measured approximately three times a week, using chemiluminescent immunoassay (CLIA; ARCHITECT® system by Abbott, Tokyo, Japan). The dose of tacrolimus was modified at the discretion of each physician.
Data collection and assessment
All data were retrospectively collected from the electronic medical record system. In this study, the blood concentration/dose [(ng/mL)/mg/day, (C/D)] ratio of tacrolimus just before the change from continuous intravenous infusion (C/Div) was compared with that from between 3 and 5 days after the change to oral administration (C/Dpo), when the increase in the tacrolimus blood level was stabilized [14].
The primary endpoint was to identify the variable factors associated with the variation of (C/Dpo) divided by (C/Div) [(C/Dpo)/(C/Div)]. The secondary endpoints were to consider the appropriateness of the conversion rate from intravenous to oral administration of tacrolimus and to identify the associations between the (C/Dpo)/(C/Div) values and the occurrence of acute GVHD and kidney injury during the two weeks after the switch of administration route. Acute GVHD was graded as described earlier [15]. Laboratory variables including serum creatinine, aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin, serum albumin, and hematocrit were evaluated on days 0, 7, and 14. Concomitant medications and laboratory variables that could potentially affect tacrolimus concentration were recorded. With regard to drug interactions between tacrolimus (C/Dpo)/(C/Div) and concomitant use of azole antifungal agents, we divided antifungal agents into the following three groups: without azole antifungal agent (control), fluconazole (FLCZ), and itraconazole (ITCZ) or voriconazole (VRCZ), according to their ability to inhibit the cytochrome P450 (CYP) 3A4 enzyme system.
Statistical analyses
To identify variable factors associated with the variation of (C/Dpo)/(C/Div), patient characteristics, concomitant medications, and clinical laboratory data were analyzed using Chi-square analysis or Fisher’s exact test for categorical variables and the Mann–Whitney U test for continuous variables. Values with borderline significance (p < 0.10) were subjected to multiple regression analysis with backward selection. The comparison of tacrolimus (C/Dpo)/(C/Div) in response to each antifungal agent was evaluated with the Kruskal–Wallis test, followed by Dunn’s multiple comparison test. The correlation between (C/Dpo) and (C/Div) with each antifungal agent was measured using Pearson’s correlation coefficient test. Chi-square analysis or Fisher’s exact test was used to compare the occurrence of acute GVHD. The Mann–Whitney U test was used to compare the serum creatinine.
Data were analyzed using JMP 11.0.2 (SAS Institute Inc., Cary, NC, USA) and GraphPad PRISM, version 6 (GraphPad Software, San Diego, CA, USA). p values of <0.05 were considered statistically significant.
Results
Patient characteristics
Seventy-three HSCT patients receiving oral administration of tacrolimus between December 2010 and December 2013 were enrolled. Patient characteristics are shown in Table 1. Tacrolimus was switched to an oral dose at a median of 3.0 times (range 1.7–4.0) higher than the intravenous dose, which was administered in two divided doses at a median of 45 days (range 21–162).
Tacrolimus blood concentration and C/D ratio during the switch from continuous intravenous infusion to twice-daily oral administration
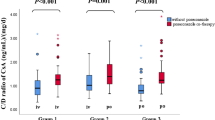
Median tacrolimus concentration just before the change from continuous intravenous infusion was 12.4 ng/mL (range 4.7–18.1), and that after the switch to oral administration was 8.0 ng/mL (range 1.8–18.1) (Fig. 1a). Similarly, median (C/Div) at the time of continuous intravenous infusion was 17.3 [(ng/mL)/(mg/day)] (range 6.7–44.7), and (C/Dpo) was 3.6 [(ng/mL)/(mg/day)] (range 0.6–17.9) (Fig. 1b). Median (C/Dpo)/(C/Div) was 0.21 (range 0.04–0.58) (Fig. 1c).
Result of the switch from immediately before the change from continuous intravenous infusion to between 3 and 5 days after the vhange to oral twice-daily administration of tacrolimus, blood concentration (a), concentration/dose (C/D) (b), and C/D during oral administration (C/Dpo) divided by during continuous infusion (C/Div) [(C/Dpo)/(C/Div)] (c). The bars show the median values
Variable factors associated with the variation of tacrolimus (C/Dpo)/(C/Div)
In the univariate analyses, the (C/Dpo)/(C/Div) of tacrolimus was significantly high in patients with concomitant use of oral ITCZ or VRCZ, (n = 29, p < 0.001) and oral FLCZ (n = 30, p = 0.022). However, the (C/Dpo)/(C/Div) was low in female patients (n = 37, p = 0.069), without concomitant use of an azole antifungal agent (n = 13, p = 0.021), with concomitant use of oral omeprazole (n = 4, p = 0.074), and with concomitant use of an oral calcium channel blocker (n = 7, p = 0.071) (Table 2). Multiple regression analysis showed that concomitant use of oral ITCZ or VRCZ significantly increased the (C/Dpo)/(C/Div) of tacrolimus (p = 0.002) (Table 3).
Influence of azole antifungal agents on (C/Dpo)/(C/Div) of tacrolimus
The patients were divided into the following 3 groups according to the concomitant use of antifungal agent: control [micafungin (MCFG) (n = 9), liposomal amphotericin B (L-AMB) (n = 2), and without concomitant use of antifungal agents (n = 2)], FLCZ [oral fluconazole (n = 30), and intravenous fosfluconazole (n = 1)], and the ITCZ or VRCZ group [oral ITCZ (n = 25), and oral VRCZ (n = 4)]. Upon comparing the three groups, the (C/Dpo)/(C/Div) of tacrolimus in the ITCZ or VRCZ group (median 0.28; range 0.06–0.58) was significantly higher than in the control group (median 0.11; range 0.04–0.52) (p < 0.001) and in the FLCZ group (median 0.19; range 0.07–0.30) (p < 0.01). The (C/Dpo)/(C/Div) of tacrolimus was not statistically significant between in the control group and in the FLCZ group (Fig. 2). In the control group, one of nine patient who received concomitant MCFG showed a high (C/Dpo)/(C/Div) ratio of 0.52. The AST and ALT values in this patient were 70 and 321 IU/L, respectively.
Influence of azole antifungal agents on the (C/Dpo)/(C/Div) of tacrolimus. Patients were divided into the following 3 groups based on the concomitant use of antifungal agent. Control [intravenous micafungin (n = 9), intravenous liposomal amphotericin B (n = 2), and without concomitant use of antifungal agents (n = 2)], FLCZ [oral FLCZ (n = 30) and intravenous fosfluconazole (n = 1)], and ITCZ or VRCZ [oral ITCZ (n = 25) and oral VRCZ (n = 4)]. Closed circles show ITCZ and opened triangle show VRCZ. Bar shows the median value in each group. *p < 0.01, **p < 0.001, between groups
Influence of azole antifungal agents on the correlation between C/Dpo and C/Div of tacrolimus
There was a statistically significant correlation between the C/Dpo and C/Div of tacrolimus in the FLCZ group (p < 0.001) and the ITCZ or VRCZ group (p < 0.001). The slopes of the lines that indicated a role of conversion ratio from intravenous to oral administration of tacrolimus were 0.12 for the control group, 0.21 for the FLCZ group, and 0.39 for the ITCZ or VRCZ group (Fig. 3).
Associations between tacrolimus (C/Dpo)/(C/Div) values and the occurrence of acute GVHD and kidney injury
(C/Dpo)/(C/Div) values were divided into four quartiles with cutoff values of 0.15, 0.21, and 0.28. The lowest and highest quartiles included the 25% of patients with the lowest and the highest values. Five of 18 (27.8%) patients who had the lowest quartile (C/Dpo)/(C/Div) values had developed acute GVHD after the change in route of administration, which was significantly higher than that in other quartiles [5 of 55 (9.1%) patients, p = 0.045] (Table 4). There was no statistically significant association between the occurrence of acute GVHD and the kind of concomitant use of azole antifungal agent. In addition, there was no statistically significant associations between the (C/Dpo)/(C/Div) value and the occurrence of kidney injury after the change in administration route on day 0 (p = 0.949), day 7 (p = 0.768), or day 14 (p = 0.451), respectively (Table 5).
The associations between tacrolimus blood concentration ratio [(Cpo)/(Civ) values] and the occurrence of acute GVHD and kidney injury, five of 18 (27.8%) patients who had the lowest quartile (Cpo)/(Civ) values had developed acute GVHD after the change in route of administration, which was significantly higher than that in other quartiles [5 of 55 (9.1%) patients, p = 0.045]. In addition, there was no statistically significant associations between the (Cpo)/(Civ) value and the occurrence of kidney injury after the change in administration route on day 0 (p = 0.187), day 7 (p = 0.169), or day 14 (p = 0.072), respectively.
Discussion
This retrospective study revealed following three findings, (1) concomitant use of antifungal agents could affect the (C/Dpo)/(C/Div) of tacrolimus, especially oral ITCZ or VRCZ, as assessed through multiple regression analysis; (2) conversion from intravenous to oral administration of tacrolimus at a ratio of 1:5 was seemingly appropriate; however, a lower conversion ratio such as 1:3 was suitable in patients taking oral ITCZ or VRCZ; and (3) the patients who experienced a rapid decrease in tacrolimus blood concentration developed acute GVHD.
Tacrolimus has many clinically significant drug–drug interactions related to its metabolism by the CYP3A4 isoenzyme system [16]. Drug interactions between tacrolimus and azole antifungal agents including FLCZ, ITCZ, and VRCZ, which interfere with the metabolism of CYP3A4, are well recognized [16–18]. However, there are considerable differences among azole antifungals with regard to their ability to inhibit CYP3A4. Previous studies have reported that ITCZ and VRCZ were more potent inhibitors of CYP3A4 than FLCZ [17, 19, 20]. These reports support our results (Fig. 2). With regard to the route of administration of azoles, in almost all cases in our study, they were administered orally. Therefore, we could not conclude whether the intravenous administration of azole antifungal agents would similarly affect the oral (C/Dpo)/(C/Div) of tacrolimus. Mihara et al. [21] reported that the interaction between FLCZ and tacrolimus was strong when antifungal agents were administered orally, probably owing to the inhibition of gut CYP3A4. From our data, we hypothesized that this strong interaction could be explained by the enterohepatic CYP3A4. In contrast, a mild drug interaction was reported for the control group. MCFG is an echinocandin antifungal agent and a weak inhibitor of CYP3A4 metabolism in vitro [18]; however, no drug interaction was reported between tacrolimus and MCFG in healthy volunteers [22], in those with hematological diseases [23], or HSCT patients [24]. One of nine patient who received concomitant MCFG showed a high (C/Dpo)/(C/Div) ratio of 0.52 (Fig. 2). Because the AST and ALT values in this patient were 70 and 321 IU/L, respectively. Sakaeda et al. [25] reported that amphotericin B had no inhibitory effect on the CYP3A4 metabolic activity in vitro. Two of two patients who received concomitant L-AMB showed a (C/Dpo)/(C/Div) ratio of 0.08 and 0.09, respectively (Fig. 2, data not shown). Therefore, concomitant use of azole antifungal agents could affect the (C/Dpo)/(C/Div) of tacrolimus, especially oral ITCZ or VRCZ, probably via the marked inhibition of enterohepatic CYP3A4.
According to the guidelines for GVHD by The Japan Society for Hematopoietic Cell Transplantation, when switching from continuous intravenous infusion to oral administration of tacrolimus in HSCT, a 3- to 4-fold higher dosage range is recommended. However, Yano et al. [26] reported that the 1:4 ratio resulted in a decrease of tacrolimus exposure in 6 out of 10 patients (60%) and required a dose adjustment. Our results showed that the median value of the (C/Dpo)/(C/Div) of tacrolimus was 0.21 (Fig. 1c). Detailed analyses revealed that the concomitant use of antifungal agents could affect the (C/Dpo)/(C/Div) of tacrolimus, especially oral ITCZ or VRCZ (Table 3; Fig. 2). Figure 3c indicates that a 3-fold higher dosing range of oral tacrolimus was needed to maintain the same blood level with intravenous administration in the oral ITCZ or VRCZ group, because the slope of the line was 0.39. Therefore, although the switch from intravenous to oral administration of tacrolimus at a ratio of 1:5 was seemingly appropriate, a lower conversion ratio such as 1:3 was suitable in patients taking oral ITCZ or VRCZ.
When the blood concentration of tacrolimus varies suddenly, we should weigh the relative risks of the development of GVHD and toxicity, such as kidney injury. The increased frequency of kidney injury was indicated in previous studies might be resulted from higher doses of tacrolimus or higher targeted blood concentration ranges [1, 27]. In this study, patients who had the lowest quartile (C/Dpo)/(C/Div) developed acute GVHD after the change in the administration route (Table 4). There were no statistically significant associations between the occurrence of acute GVHD and the concomitant use of azole antifungal agents. Notably, one patient in the lowest quartile group experienced a rapid decrease in (C/Dpo)/(C/Div) of tacrolimus (value was 0.09), immediately after the conversion and developed grade III acute GVHD in the gut. Yano et al. [26] reported that one patient experienced a rapid decrease in the trough concentration of tacrolimus from 9.8 to 3.6 ng/mL immediately after the conversion, and developed grade II acute GVHD on the skin. Therefore, in those patients whose (C/Dpo)/(C/Div) decreased after the switch, GVHD should be monitored carefully, and the dose of tacrolimus should be readjusted, considering its blood level. In addition, the conversion should be performed under close medical supervision. On the other hand, there were no statistically significant associations between (C/Dpo)/(C/Div) value and the occurrence of kidney injury (Table 5). Grade 1–2 renal toxicities were observed, but they were mild and transient.
This study has a limitation. Because this was a retrospective study, we did not examine genetic polymorphisms, notably the CYP3A5 genotype that affect tacrolimus blood concentration [28–30]. CYP3A5 genotyping is not examined in routine work of HSCT in Japan. Therefore, future examinations will be required, including genetic polymorphisms that may affect tacrolimus blood concentration as well as (C/Dpo)/(C/Div).
In conclusion, although the conversion from continuous intravenous infusion to oral administration of tacrolimus at a ratio of 1:5 seemed appropriate, a lower conversion ratio such as 1:3 is suitable in patients taking oral ITCZ or VRCZ. In patients whose tacrolimus blood concentration decrease after switching the route of administration, the development of GVHD should be carefully monitored, and the dosage of tacrolimus should be frequently adjusted, considering its blood level.
References
Ratanatharathorn V, Nash RA, Przepiorka D, Devine SM, Klein JL, Weisdorf D, et al. Phase III study comparing methotrexate and tacrolimus (prograf, FK506) with methotrexate and cyclosporine for graft-versus-host disease prophylaxis after HLA-identical sibling bone marrow transplantation. Blood. 1998;92:2303–14.
Przepiorka D, Khouri I, Ippoliti C, Ueno NT, Mehra R, Körbling M, et al. Tacrolimus and minidose methotrexate for prevention of acute graft-versus-host disease after HLA-mismatched marrow or blood stem cell transplantation. Bone Marrow Transpl. 1999;24:763–78.
Nash RA, Antin JH, Karanes C, Fay JW, Avalos BR, Yeager AM, et al. Phase 3 study comparing methotrexate and tacrolimus with methotrexate and cyclosporine for prophylaxis of acute graft-versus-host disease after marrow transplantation from unrelated donors. Blood. 2000;96:2062–8.
Hiraoka A, Ohashi Y, Okamoto S, Moriyama Y, Nagao T, Kodera Y, et al. Japanese FK506 BMT(Bone Marrow Transplantation) Study Group. Phase III study comparing tacrolimus (FK506) with cyclosporine for graft-versus-host disease prophylaxis after allogeneic bone marrow transplantation. Bone Marrow Transpl. 2001;28:181–5.
Yanada M, Emi N, Naoe T, Sakamaki H, Takahashi S, Hirabayashi N, et al. Tacrolimus instead of cyclosporine used for prophylaxis against graft-versus-host disease improves outcome after hematopoietic stem cell transplantation from unrelated donors, but not from HLA-identical sibling donors: a nationwide survey conducted in Japan. Bone Marrow Transpl. 2004;34:331–7.
Murata M. Prophylactic and therapeutic treatment of graft-versus-host disease in Japan. Int J Hematol. 2015;101:467–86.
Gooley TA, Chien JW, Pergam SA, Hingorani S, Sorror ML, Boeckh M, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010;363:2091–101.
Christians U, Strom T, Zhang YL, Steudel W, Schmitz V, Trump S, et al. Active drug transport of immunosuppressants: new insights for pharmacokinetics and pharmacodynamics. Ther Drug Monit. 2006;28:39–44.
Jacobson P, Ng J, Ratanatharathorn V, Uberti J, Brundage RC. Factors affecting the pharmacokinetics of tacrolimus (FK506) in hematopoietic cell transplant (HCT) patients. Bone Marrow Transplant. 2001;28:753–8.
Venkataramanan R, Swaminathan A, Prasad T, Jain A, Zuckerman S, Warty V, et al. Clinical pharmacokinetics of tacrolimus. Clin Pharmacokinet. 1995;29:404–30.
Boswell GW, Bekersky Fay J, Wingard J, Antin J, Weisdorf D, et al. Tacrolimus pharmacokinetics in BMT patients. Bone Marrow Transpl. 1998;21:23–8.
Wingard JR, Nash RA, Przepiorka D, Klein JL, Weisdorf DJ, Fay JW, et al. Relationship of tacrolimus (FK506) whole blood concentrations and efficacy and safety after HLA-identical sibling bone marrow transplantation. Biol Blood Marrow Transpl. 1998;4:157–63.
Przepiorka D, Nash RA, Wingard JR, Zhu J, Maher RM, Fitzsimmons WE, et al. Relationship of tacrolimus whole blood levels to efficacy and safety outcomes after unrelated donor marrow transplantation. Biol Blood Marrow Transpl. 1999;5:94–7.
The Japanese Society of Therapeutic Drug Monitoring and the Japanese Society of Transplantation (eds). Guidelines on TDM of immunosuppressive drugs in organ transplantation, 1st edn. Kanehara Publishing Ltd: Japan, 2014, p 31.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus conference on acute GVHD grading. Bone Marrow Transpl. 1995;15:825–8.
Glotzbecker B, Duncan C, Alyea E 3rd, Campbell B, Soiffer R. Important drug interactions in hematopoietic stem cell transplantation: what every physician should know. Biol Blood Marrow Transpl. 2012;18:989–1006.
Dodds-Ashley E. Management of drug and food interactions with azole antifungal agents in transplant recipients. Pharmacotherapy. 2010;30:842–54.
Niwa T, Shiraga T, Takagi A. Drug-drug interaction of antifungal drug. Yakugaku Zasshi. 2005;125:795–805.
Hisaka A, Ohno Y, Yamamoto T, Suzuki H. Prediction of pharmacokinetic drug-drug interaction caused by changes in cytochrome P450 activity using in vivo information. Pharmacol Ther. 2010;125:230–48.
Kawazoe H, Takiguchi Y, Tanaka H, Fukuoka N, Ohnishi H, Ishida T, et al. Change of the blood concentration of tacrolimus after the switch from fluconazole to voriconazole in patients receiving allogeneic hematopoietic stem cell transplantation. Biol Pharm Bull. 2006;29:2528–31.
Mihara A, Mori T, Aisa Y, Yamazaki R, Iketani O, Tanigawara Y, et al. Greater impact of oral fluconazole on drug interaction with intravenous calcineurin inhibitors as compared with intravenous fluconazole. Eur J Clin Pharmacol. 2008;64:89–91.
Hebert MF, Blough DK, Townsend RW, Allison M, Buell D, Keirns J, et al. Concomitant tacrolimus and micafungin pharmacokinetics in healthy volunteers. J Clin Pharmacol. 2005;45:1018–24.
Shimoeda S, Ohta S, Kobayashi H, Saitou H, Kubota A, Yamato S, et al. Analysis of the blood level of micafungin involving patients with hematological diseases: new findings regarding combination therapy with tacrolimus. Biol Pharm Bull. 2005;28:477–80.
Fukuoka N, Imataki O, Ohnishi H, Kitanaka A, Kubota Y, Ishida T, et al. Micafungin does not influence the concentration of tacrolimus in patients after allogeneic hematopoietic stem cell transplantation. Transpl Proc. 2010;42:2725–30.
Sakaeda T, Iwaki K, Kakumoto M, Nishikawa M, Niwa T, Jin JS, et al. Effect of micafungin on cytochrome P450 3A4 and multidrug resistance protein 1 activities, and its comparison with azole antifungal drugs. J Pharm Pharmacol. 2005;57:759–64.
Yano S, Mori S, Saito T, Yokoyama H, Machishima T, Shimada T, et al. Pharmacokinetics for once-daily modified release formulation of tacrolimus hydrate in unrelated hematopoietic stem cell transplantation. Ann Hematol. 2015;94:491–6.
The US. Multicenter FK506 Liver Study Group. A comparison of tacrolimus (FK 506) and cyclosporine for immunosuppression in liver transplantation. N Engl J Med. 1994;331:1110–5.
Masuda S, Inui K. An up-date review on individualized dosage adjustment of calcineurin inhibitors in organ transplant patients. Pharmacol Ther. 2006;112:184–98.
Anglicheau D, Legendre C, Beaune P, Thervet E. Cytochrome P450 3A polymorphisms and immunosuppressive drugs: an update. Pharmacogenomics. 2007;8:835–49.
Onizuka M, Kunii N, Toyosaki M, Machida S, Ohgiya D, Ogawa Y, et al. Cytochrome P450 genetic polymorphisms influence the serum concentration of calcineurin inhibitors in allogeneic hematopoietic SCT recipients. Bone Marrow Transpl. 2011;46:1113–7.
Acknowledgements
This work was supported in part by a Grant-in-Aid for Scientific Research (KAKENHI) from the Ministry of Education, Science, Culture, Sports, and Technology of Japan (MEXT) (Grant Nos. 26929032, 15H00573, 15H04666, 16K08404, 16K18946).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
About this article
Cite this article
Suetsugu, K., Ikesue, H., Miyamoto, T. et al. Analysis of the variable factors influencing tacrolimus blood concentration during the switch from continuous intravenous infusion to oral administration after allogeneic hematopoietic stem cell transplantation. Int J Hematol 105, 361–368 (2017). https://doi.org/10.1007/s12185-016-2135-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-016-2135-7