Abstract
Several ecological models explain the success of introduced seaweeds by herbivore–prey interactions. The ‘enemy release hypothesis’ states that invaders benefit from a lack of natural enemies compared to the recipient community. The ‘novel weapons hypothesis,’ however, highlights the importance of chemical defense compounds of introduced species that are more effective in invaded regions than native counterparts. In order to explain the tremendous invasion success of the brown alga Sargassum muticum, we compared the palatability and nutritional value of S. muticum individuals from their native and invaded habitat (Japan and North Sea, respectively) with noninvasive congeneric species from Japan (S. fusiforme, S. horneri), and a native competitor from the North Sea (Fucus vesiculosus). Different feeding assays using artificial food with either freeze-dried algae or algal extracts and three dominant North Sea mesograzers were performed to detect feeding preferences. All herbivores preferred the local brown alga F. vesiculosus, followed by North Sea S. muticum, while Sargassum spp. from Japan were the least preferred. Since nutritional value did not correlate with feeding preference and algal extracts had the same effect as algal powder, we could demonstrate a deterrent activity of algal secondary metabolites. The preference of herbivores for the sympatric S. muticum population compared to the allopatric Japanese population could indicate a resource allocation from chemical defense to reproduction and growth. Due to the low palatability of Sargassum spp. from Japan, it might be reasonable to include additional Sargassum species in North Sea monitoring to prevent their establishment in European waters.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Introduction of non-indigenous marine species (NIMS) by anthropogenic influences is one of the key pressures that threaten marine biodiversity and resources of the world’s oceans (Vitousek et al. 1997; Millennium Ecosystem Assessment 2005; Halpern et al. 2008; Carlton 2009; Davidson et al. 2015). From all detected NIMS of all major animal, plant and algal phyla, macroalgae not only constitute a large component of the globally introduced biota, but also cause significant economic and environmental damage over which we have only limited post-invasion control and management options (Ribera and Boudouresque 1995; Thresher 1999; Schaffelke et al. 2006; Anderson 2007; Schaffelke and Hewitt 2007).
To enable a targeted monitoring and adaptive invasion management, the search for specific invasive traits is of increasing interest. Although invasion success has usually been correlated with numerous species traits such as reproduction strategy, growth rate, and dispersal potential (Rejmánek and Richardson 1996; Nyberg and Wallentinus 2005), several studies contradict this assumption (Smith and Knapp 2001; Daehler 2003; Seabloom et al. 2003) and rather highlight the link between the success of marine bioinvasion and the presence of chemical defenses by introduced organisms (Wikström et al. 2006; Cassano et al. 2008). In order to verify the importance of chemical defenses for the invasion success, more investigations are needed.
Since predator–prey interactions deeply shape marine ecosystems, the effectivity of antipredator metabolites might help to explain the success of widespread NIMS. Several theories have been proposed to explain the linkage between invasion success and enemy defense. The ‘enemy release hypothesis’ (ERH) states that introduced species in the new range are less affected by local enemies, as these fail to recognize them as adequate food or hosts (Keane and Crawley 2002). The ‘evolution of increased competitive ability hypothesis‘ (EICA) extends this assumption by assuming that plants experiencing reduced herbivory in a new range should be under strong selection to allocate less resources to defenses and more to growth and reproduction, resulting in an evolution of increased competitive ability in these plant populations (Blossey and Notzold 1995). The ERH has been contradicted by the notion that native generalist herbivores prefer alien to native species because the newcomers have not been selected to resist their new enemies (Parker and Hay 2005; Morrison and Hay 2011). However, recent studies corroborated that aliens may have an advantage over native competitors since they contain potent defense compounds toward which the native consumers are evolutionarily naïve. This so-called novel weapons hypothesis (NWH) is indirectly supported by correlation studies, showing that highly invasive plants are more likely to possess novel chemical defenses compared to noninvasive species (Cappuccino and Carpenter 2005; Cappuccino and Arnason 2006). Enge et al. (2012) corroborated the NWH showing that the low consumption of the successful invader Bonnemaisonia hamifera might be attributed to a chemical defense toward which the indigenous consumers are evolutionarily naïve.
Despite increasing research on traits which promote invasion success and on links between invaders’ secondary metabolites and feeding preference of local herbivores, there is still little direct evidence in support of the NWH or the evolutionary adaptation of chemical defenses to the invaded ecosystem. Most studies investigating correlations between plant invasion and palatability originate from terrestrial organisms and have produced inconsistent results (Dietz and Edwards 2006). In this study, we compared the palatability of an invasive alga with closely related noninvasive species in order to find further support for the ERH or the NWH.
We used the brown algal species Sargassum muticum (Yendo) Fensholt as a model for a highly invasive seaweed. Originating from the Pacific (Chinese and Japanese Sea), it has been non-intentionally introduced to Europe with oysters in the early 1970 s (Critchley 1983). Thereupon, it was reported from various locations along the Atlantic coast of Western Europe from Portugal to Scandinavia. S. muticum was first recorded in the German part of the North Sea in 1988 (Kornmann and Sahling 1994; Karlsson and Loo 1999) and is nowadays present in suitable habitats (i.e., rocky shores and artificial hard substrata) on most European Atlantic coasts (Engelen et al. 2015). It is still extending its range and has recently arrived on the coast of Morocco (Sabour et al. 2013). S. muticum produces several defense compounds effective against bacteria, fungi, algal competitors and herbivores (Plouguerné et al. 2008; Bazes et al. 2009; Plouguerné et al. 2010) and is a rich source of phlorotannins (Tanniou et al. 2014), suggesting that the invasive success of S. muticum might be partly explained by its effective chemical defense. A low herbivore preference for S. muticum has been assumed to give the invader a competitive advantage over the native algal community along the Portuguese coast, thereby contributing to the invasiveness of S. muticum (Monteiro et al. 2009). Several studies evaluated herbivory on invasive algal species in their introduced range, and in most cases, grazers from the introduced range preferred native to non-native algae (Weinberger et al. 2008; Monteiro et al. 2009; Cacabelos et al. 2010; Engelen et al. 2011; Nejrup et al. 2012). However, only few studies compared native and introduced populations of the same—or of closely related algal species. The study of Wikström et al. (2006) revealed that the brown alga Fucus evanescens is less exposed to herbivory in its introduced range in Sweden than in its native range in Iceland, probably due to increased chemical defenses in introduced populations. Similarly, Hammann et al. (2013) found a higher palatability of native compared to invasive Gracilaria vermiculophylla but with high regional variability between the native populations and without considering different life history stages. Thus, differences in palatability might also be explained by different herbivore preferences for haploid, diploid or even polyploid stages (Pereira and da Gama 2008).
Palatability relies not only on chemical defensive metabolites but also on the nutritional value or structural characteristics of algal food. Since herbivores are usually nitrogen-limited, they are assumed to prefer algae with a high protein content and a high proportion of nitrogen, i.e., with a low carbon-to-nitrogen (C:N) ratio (Mattson 1980; Cruz-Rivera and Hay 2003). In general, herbivores look for energy-rich compounds and nutrients, such as proteins, nitrogen, and carbohydrates, and the availability of these primary compounds is therefore expected to affect food preference strongly (Mattson 1980).
In this study, we compared the palatability and nutritional value of invasive S. muticum and two Sargassum species from Japan: Sargassum fusiforme (Harvey) Setchell, which is currently not recorded as invasive, and Sargassum horneri (Turner) C. Agardh, which has recently been recorded along the southern region of the Pacific coast but has not yet reached the North Sea (Marks et al. 2015). Feeding preference was also compared to the local dominant North Sea brown alga Fucus vesiculosus Linnaeus 1753. Additionally, we compared intraspecific differences in palatability and chemistry of an invasive S. muticum population from the German North Sea and a native population from Japan and looked for possible adaptations and resulting chemical modification during the invasion process. We performed feeding assays and chemical analyses to test the following hypotheses: (1) the invasive alga S. muticum is less preferred by North Sea herbivores compared to a co-occurring competing brown alga as stated by the NWH; (2) the invasive alga S. muticum is less preferred by North Sea herbivores compared to close relatives which might explain its invasiveness; (3) S. muticum originating from invasive populations is in general less palatable than S. muticum from native populations because it has been selected for resistance against North Sea herbivores during the invasion process and (4) the evolved increased herbivore resistance is due to a different chemistry of invasive and native S. muticum due to changing environmental conditions and grazing pressure.
Materials and methods
Collection of organism and extract preparation
Whole algal thalli of adult Sargassum species were sampled before maturation in Japan and Germany. Three Sargassum species (Phaeophyceae), S. fusiforme, S. horneri and S. muticum were obtained from Japan by Scuba diving at a depth of 1–3 m in the Seto Inland Sea, Oshima, Japan (33°55′04.4″N132°27′42.7″E) during March 2013. The invasive Sargassum muticum from Germany was collected in June 2014 in the sheltered rocky intertidal zone of Heligoland, Germany (54° 11′05.3″N, 7°52′31.4″E). Fucus vesiculosus was collected by hand from Nassau harbor in Wilhelmshaven, Germany (53°30′54.7″N8°08′57.8″E) in April 2012. After collection, all Sargassum specimens were immediately transferred to the laboratory in insulated boxes filled with seawater, where they were gently washed with sterile seawater and cleaned from associated epibiota. Individuals of each algal species were pooled, wet weight (±0.01 g) was measured with a balance, and the volume of each species was determined by water displacement in a graduated cylinder (Table 1). Since we wanted to assess herbivore preferences based on chemical characteristics, the algae were subsequently frozen and freeze-dried to avoid changes in algal chemistry due to transport. Freeze-dried Sargassum spp. from Japan were sent to Germany for further processing and ground into fine powder to increase extraction efficiency. One part of the freeze-dried algal powder from Japan and from Germany was kept for the feeding assays, while the other was used for extraction of secondary metabolites.
Freeze-dried algal powder was extracted three times with ethylacetate (EtOAc)/methanol (MeOH) (AppliChem GmbH) (1:1, v/v) and finally with 100 % methanol. For each gram of algal weight, 20 mL of solvent was used. Each extract was filtered through Watman No. 1 filters, and remaining solvents were removed by rotary evaporation. The obtained extracts were combined, dried in a centrifugal vacuum concentrator, weighed and stored in a freezer at −20 °C until used in feeding assays (Table 1).
Feeding assay
Study organisms
The palatability of the invasive S. muticum from the North Sea, hereafter S. muticum (inv.), was compared with the two noninvasive Sargassum species (S. fusiforme, S. horneri) and native S. muticum from Japan, hereafter S. muticum (nat.), using two common North Sea herbivores: Littorina littorea (Gastropoda), and Psammechinus miliaris (Echinodermata), and the omnivore: Idotea baltica (Isopoda). These species occur abundantly on seaweeds and are considered to be generalist consumers (Toth et al. 2007; Enge et al. 2012; Kelly and Scheibling 2012; Nylund et al. 2012). Isopods were taken from an I. baltica culture of the Alfred Wegener Institute, Bremerhaven, Germany. Isopods were fed with customary fish food and the brown alga Ascophyllum nodosum, and maintained in an aerated 200 L flow-through tank with a 12/12 h light/dark cycle within a constant temperature room of 15 °C. New I. baltica individuals from drift algae collected in the Heligoland Bight were constantly introduced into the culture. During the experimental phase, isopods were maintained on their natural diet A. nodosum to avoid a preconditioning to any of our test algae.
Littorina littorea were collected between June and September 2014 from tide pools in the higher intertidal zone of the North Sea (53°30′46.9″N8°08′39.8″E). Snails were directly transferred to 27 L aerated seawater aquaria with a 12/12 h light/dark cycle and fed with Ulva sp. and A. nodosum up to 2 days before being used in experiments. Although it is known that some animals change their feeding preferences when they are starved, previous studies revealed similar feeding preferences for fed and 2 weeks starved L. littorea (Imrie et al. 1990; unpublished data). We used this fasting period to ensure feeding within 24 h. Water was exchanged manually two times a week, and temperature varied between 18–20 °C.
Psammechinus miliaris were collected around the German North Sea archipelago Heligoland (54°10′57″N 7°53′07″E) in July 2014. Sea urchins were maintained in 27 L aerated flow-through outdoor aquaria with flow-through seawater from the adjacent North Sea. Sea urchins were fed with fresh Ulva sp. and A. nodosum during a 4 weeks acclimation phase and between experiments.
Prior to the experiments, all herbivores were offered artificial food containing agar and pulverized Ulva sp. to get used to the agar-based diet. Snails were left without food 2 days before the experiment to ensure active feeding during the assays. Individual snails and isopods were only used once for the feeding experiments. Due to the smaller stock of sea urchins, they were applied in different feeding assays but with an interval of at least 4 weeks.
Artificial food preparation
Two kinds of artificial algal food were tested to analyze the feeding preferences of L. littorea, I. baltica and P. miliaris: powdered algae and algal extracts. Artificial food pellets containing powdered algae were produced to exclude the morphological characteristics. Consequently, feeding preferences were based on chemical and nutritional algal characteristics only. Food pellets with algal extracts were prepared to exclude both the morphologic structure and the nutritional value of Sargassum species to ensure that feeding preferences were predominantly due to chemical characteristics (Hay et al. 1994).
Artificial food preparation was adapted from Hay et al. (1994) and Schupp and Paul (1994). To prepare the artificial food, 1.08 g agar was dissolved in 30 mL distilled H2O and heated in a microwave. Dried and ground algae were added at natural concentrations corresponding to 30 mL volume to obtain the algal powder food pellets. Natural concentration was calculated as algal dry weight per 1 mL algal volume times 30 (Table 1). For the food with algal extracts, lyophilized Ulva sp., a local palatable green alga, served as basis for the artificial food. 3.3 g Ulva sp. powder was added to the microwaved agar and stirred rapidly for even distribution of the algal powder. Crude extracts of Sargassum spp. and F. vesiculosus were redissolved in 5 mL methanol and incorporated into the Ulva-agar mixture at natural volumetric concentration. Most of the methanol evaporates when added to the hot algal mixture, minimizing potential toxic effects. Additionally, the Ulva-agar mixture was treated with the same amount of methanol and consumed readily (data not shown). To achieve pieces of algal food in equal size and thickness, the hot agar-algae mixture was pressed onto a stainless steel window screen for sea urchin and on plastic window screen for snail and isopod food. After cooling, the agar food was firmly attached to the window screen and cut into pieces of 2 × 2 cm for sea urchins and 1 × 1 cm for snails and isopods and immediately used in feeding assays (Hay et al. 1994; Rohde et al. 2004)
Two-choice feeding assays
In a crossed design, Sargassum spp. and F. vesiculosus were tested in two-choice feeding preference tests (n = 20) with each herbivore. Each of the three herbivores was allowed to choose between the same two varieties of artificial agar food, either as algal powder or extract. The assays were conducted in 17 × 13 cm boxes filled with filtered seawater and one herbivore per box. In the case of L. littorea, three snails were inserted in each replicate due to low consumption rates. Herbivores were allowed to feed until half of one agar piece was consumed or until 48 h had passed. Preference was quantified as the number of entirely eaten window screen squares. Replicates where no food was consumed were excluded from statistical analysis.
Algal chemical defense and nutritional characteristics
Algal nutritional value was characterized by measuring the wet/dry weight ratio (ww/dw), the amount of phenolic compounds, proteins and mannitol concentration. All the chemical analyses were run from algal material which was freeze-dried immediately after collection, pulverized and stored at −20 °C until final determination. Such a procedure is advised to preserve the chemical constituents in the best possible way (Waterman and Mole 1994). Phenolic, protein and mannitol content were measured photometrically using a microplate reader (see below, Thermo Fisher Scientific Inc., Waltham, MA., USA).
Quantification of polyphenols
The total phenolic content of all species used in the feeding assays was determined with a microplate-adapted Folin–Cioalteu assay following the procedure described in Zhang et al. (2006). Phloroglucinol (1,3,5-trihydroxybenzene, Sigma-Aldrich, Germany) was used as a standard, and a calibration curve was generated with concentrations of 0, 6.25, 12.5, 25, 50 and 100 µg mL−1. Total phenolic contents (TPCs) were expressed as percentages of phenolic compounds per algal dw. The Folin–Ciocalteu method quantifies non-phenolic hydroxylated aromatic compounds as well, but since these interfering substances make up <5 % of the total reactive compounds, they were neglected (van Alstyne 1995). In the following, the term ‘phlorotannins’ is used for total phenolics, since brown algae are not known to contain others polyphenols (Targett and Arnold 1998).
Protein determination
The protein content was quantified using the microplate-adapted Bradford method (Bradford 1976). For the calibration curve, Bovine serum albumin (BSA) was used as a standard in concentrations of 20, 30, 40, 50, 60, 80 and 100 µg mL−1. Algal samples (0.1 g) were extracted with 1 M sodium hydroxide, and the absorbance was measured at 595 nm.
Mannitol determination
To determine mannitol concentrations, 0.1 g of each algal sample was extracted and analyzed after Vas’kovskii and Isai (1972), with the difference that periodate oxidation was stopped after 10 s. The short reaction time is important since this method is not specific for mannitol and it also stains some other polyols. However, the formation of formaldehyde from mannitol is particularly fast, which allows the quantification of algal mannitol content.
C/N ratio
Nitrogen and carbon contents were determined by using an elemental analyzer (Flash EA 1112, Thermo Fisher, Germany).
Statistics
Statistical calculations were performed using SPSS IBM Statistics version 23, Illinois, USA. Normality and homogeneity of variances were determined using the Kolmogorov–Smirnov test and Levene’s tests, respectively. Since no homoscedasticity was achieved in the feeding preference assays, means of the Wilcoxon signed-ranks paired test have been used to identify significant differences in the feeding preference assay.
A one-way ANOVA was used to assess differences of algal characteristics in terms of phlorotannin, mannitol and protein contents of Sargassum spp. and F. vesiculosus extracts. Pairwise differences were analyzed using Tukey post hoc tests.
Spearman’s rank correlation was performed to examine relationships between feeding preferences of the three herbivore species and determined algal characteristics.
In all cases, the threshold for significance was α = 0.05.
Results
Feeding preferences
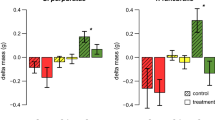
All herbivore species demonstrated very similar feeding preferences in the two-choice feeding assays. All herbivores preferred the local brown algae F. vesiculosus and the invasive population of S. muticum (inv.), while the S. muticum population from Japan, as well as Sargassum fusiforme and Sargassum horneri, were less consumed in all assays. Not all combinations were tested since the assumption that A > C when A > B and B > C seems to be justified when the preferences among five species could be linearly arranged without contradiction between preference results. However, at both extremes of the proposed preference gradient, preferences were not always clear (Fig. 1a–c). Thus, the resulting ranking of the Japanese species should be considered with some caution (Table 2). Especially, the gastropod L. littorea and the echinoderm P. miliaris were overall less selective than the isopod I. baltica and most choices were not significant. Nevertheless, L. littorea significantly preferred F. vesiculosus over every Sargassum species and S. muticum (inv.) over Sargassum spp. from Japan (Fig. 1b, p < 0.05). Surprisingly, both L. littorea and P. miliaris clearly differentiated between the invasive and native S. muticum with 97 and 81 % more consumption of the artificial food containing North Sea S. muticum extracts, respectively (Fig. 1b, c).
Mean consumption (+SD, n = 20) by the North Sea herbivores a Idotea baltica, b Littorina littorea, and c Psammechinus miliaris. Herbivory was tested in two-choice experiments with artificial food containing either algal powder or algal extracts of the species S. fusiforme (SF; striped), S. horneri (SH; dotted), S. muticum (SM (nat)) from Japan (blank), as well as S. muticum (SM (inv.; black) and F. vesiculosus (FV; gray) from the North Sea. Significant consumption differences are indicated by asterisks above the bars (n.s. = nonsignificant, *p < 0.05, **p < 0.01, ***p < 0.001, Wilcoxon signed-ranks test)
Artificial food type (algal powder or extract) and herbivore species had overall no significant influence on the ratio of consumed algae (Table 2). In the case of L. littorea and P. miliaris, the ranking of algal species remained equal with changing food type. Rank four and five as well as two and three changed during assays with I. baltica when offered food pellets containing algal extracts compared to algal powder.
Algal characteristics
Mean phlorotannin concentration of F. vesiculosus and native S. muticum ranged from 3.32 to 5.87 % dw, respectively, and exceeded other Sargassum spp. by up to 6.7 times in the case of S. horneri (Fig. 2a). The protein content of F. vesiculosus (3.58 % dw) was significantly lower compared to Sargassum spp. and was around 33 % below the native S. muticum, which exhibited the highest protein concentration with 10.64 % dw (Fig. 2b). The native S. muticum and S. fusiforme are characterized by elevated protein content, while native and invasive S. muticum exhibited higher mannitol contents (Fig. 2b, c). Mannitol content of the native S. muticum was about 21 times higher compared to S. horneri but was even exceeded by the mannitol concentration of F. vesiculosus which averaged 12.24 % dw (Fig. 2c). The C/N ratio ranged between the lowest ratio for S. fusiforme with 9.33 and the high ratios for the native S. muticum and F. vesiculosus with 19.84 and 22.08, respectively (Fig. 2d).
Tissue contents (means + SD; n = 3) of a phlorotannin, b protein, c mannitol and d C/N ratio for Sargassum spp. from Japan (white bars; S. muticum (SM (nat), S. fusiforme (SF), S. horneri (SH)) and F. vesiculosus (FV) and S. muticum (SM (inv.) from the North Sea (black bars). Different letters above the bars indicate significant differences between tissue concentrations (ANOVA, Tukey post hoc test, p < 0.05)
A Spearman’s rank-order correlation was run to determine the relationship between algal preference ranking and the nutritional value of powdered algal food (Table 3). There was a significant positive correlation between isopod feeding preference, phlorotannin (r s = 0.655, p = 0.008), mannitol content (r s = 0.895, p < 0.001) and C/N ratio (r s = 0.753, p = 0.001). While protein content did not significantly correlate with isopod feeding preference (p = 0.187), it was negatively correlated with the feeding preference of L. littorea (r s = −0.655, p = 0.01) and P. miliaris (r s = −0.862, p < 0.001).
Discussion
Herbivory is especially intense in marine environments, with approximately 70 % of benthic primary production being consumed by herbivores globally (Poore et al. 2012). Therefore, feeding preferences of native generalist herbivores may determine community composition and could influence algal invasion success in two opposing ways: Native generalists could either preferentially attack exotics which are not defended against herbivores in their new range and consequently suppress their abundance (e.g., Lind and Parker 2010; Morrison and Hay 2011), or release less preferred invaders from predation (Wikström et al. 2006; Monteiro et al. 2009; Nejrup et al. 2012).
In our study, the three tested local herbivores clearly preferred the local brown alga F. vesiculosus over S. muticum (inv.), while the three Sargassum species from Japan were less consumed in all assays. The detected preference for a native alga is consistent with the ERH and several studies which quantified grazing on invasive seaweed species in their new range. In most cases, grazers from the invaded region preferred native over non-native algae (Cappuccino and Carpenter 2005; Weinberger et al. 2008; Monteiro et al. 2009; Cacabelos et al. 2010; Engelen et al. 2011; Nejrup et al. 2012). The lower grazing pressure on S. muticum compared to its abundant competitor F. vesiculosus might partly explain the invasive success of S. muticum in the North Sea. Engelen et al. (2011) investigated whether the low food preference for S. muticum reported by Monteiro et al. (2009) also holds when tested with different foods and herbivores. Their results confirmed the avoidance of S. muticum compared to native algae by all tested herbivores. Additionally, the high growth rate of S. muticum was less affected by the grazers compared to native seaweeds which showed a decreased growth rate in the presence of grazers. Similarly as in our study, Johnsen et al. (2013) found an overall tendency for the avoidance of S. muticum compared to native algae by P. miliaris. Nevertheless, the ERH does not only relate to the complete absence of grazing on a non-native species, but also to relative grazing pressure on non-native species when compared with native, co-occurring species. Preference for and higher consumption of the dominant brown alga F. vesiculosus may lower the grazing pressure on the non-native S. muticum and even release it from potential competition with indigenous species, thereby increasing the overall fitness and the space extension of invasive S. muticum within its new range. Since S. muticum (inv.) was not completely refused by the tested herbivores in our study, it can be argued that it was the reduction in herbivore intensity rather than the escape from (native) herbivores that provided the invasive macroalga with an advantage over the local F. vesiculosus (Vermeij et al. 2009). Since there are no publications about herbivory of S. muticum in its native range, the threat of predation by those diverse herbivores inhabiting S. muticum’s native range can merely be assumed (Trowbridge et al. 2009).
Artificial algal food type (powder or extract) had overall no significant influence on the ratio of consumed algae (Table 2), thus indicating that algal chemistry is responsible for herbivore preference. This is concurrent with the NWH, since S. muticum might possess exotic metabolites to which North Sea herbivores are evolutionarily naïve. Additionally, chemical metabolites seem to be efficient against a broad range of generalist herbivores, given that different herbivores did not change the general pattern of preference for the local North Sea algae compared to Sargassum species from Japan.
Low preference of herbivores for S. muticum has been linked to the presence of secondary metabolites, such as the relatively high levels of phenolic compounds (Monteiro et al. 2009). This correlates with our result that the least preferred S. muticum (nat.) exhibited the highest phlorotannin content compared to all other tested species. However, the native S. horneri and S. fusiforme were similarly avoided by herbivores and revealed the lowest phlorotannin contents. Previous studies have shown opposing effects of phlorotannin on herbivores (Targett and Arnold 1998; Deal et al. 2003), suggesting that adaptations to utilize phlorotannin-rich algae for food have evolved among marine herbivores. Especially, I. baltica has been shown to be adapted to feed on phlorotannin-rich species. This herbivore even preferred those algae with the highest phlorotannin content in feeding choice experiments (Jormalainen et al. 2001). Furthermore, we found a positive correlation between preference ranking and phlorotannin content, meaning that preference increases with increasing phlorotannin concentrations, and also suggesting that other metabolites are responsible for feeding deterrence of Japanese Sargassum species. Protein content decreased with increasing feeding preferences of I. baltica, L. littorea and P. miliaris. Thus, the most preferred F. vesiculosus and S. muticum (inv.) exhibited the lowest protein per dry weight. I. baltica feeding preference and C/N ratio is however positively correlated, meaning that most preferred F. vesiculosus exhibited the highest C/N ratio. Herbivores are generally nitrogen-limited and depend on the intake of food with a high protein content and a high proportion of nitrogen, thus with a low (C/N) ratio (Mattson 1980; Cruz-Rivera and Hay 2003). Therefore, the preference for algae with low nitrogen content might be another indication that algal palatability is determined by other algal characteristics, such as the quality and quantity of secondary metabolites. Only algal mannitol content might contribute to feeding preference with a significant positive correlation between I. baltica feeding preference and an increasing amount of mannitol per dry weight. Mannitol-containing food has previously been shown to attract I. baltica (Weinberger et al. 2011) since mannitol food concentration positively co-varied with egg size and therefore plays an important role in the reproductive performance of isopods (Hemmi and Jormalainen 2002, 2004).
Interestingly, both L. littorea and P. miliaris clearly differentiate between the invasive and native S. muticum with a 97 and 81 % higher consumption of food containing North Sea S. muticum extracts, respectively. This result is contrary to our initial hypothesis that invasive S. muticum populations are in general less palatable than S. muticum from native populations because they have been selected for resistance against herbivores during the invasion process. It seems rather likely that the defensive level of S. muticum decreased after the establishment in the North Sea. Since there is not much known about S. muticum–herbivore interactions in Japan, we only can hypothesize what happened during its invasion process. Japanese shores have a higher density of macroalgae and herbivores, probably resulting in more species interactions compared to other temperate shores (Trowbridge et al. 2009). Thus, Japanese S. muticum might invest more energy in costly defenses against herbivores and competitors to persist in this highly competitive environment. Anti-feeding compounds might have aided the establishment of S. muticum, which benefited from the higher grazing pressure on its strongest competitor F. vesiculosus. These suggestions are in line with the ‘novel weapons hypothesis’ (NWH). Many studies suggest that well-defended introduced species can get a significant advantage over native competitors and rapidly become abundant in communities dominated by generalist consumers (Callaway and Ridenour 2004; Cappuccino and Carpenter 2005; Cappuccino and Arnason 2006; Forslund et al. 2010). After the positive establishment of S. muticum in its new range, it might have come to a resource allocation shift from the production of defense metabolites to reproduction and/or growth (i.e., evolution of increased competitive ability or EICA hypothesis; Blossey and Notzold 1995). A terrestrial metaanalysis by (Hawkes 2007) which compared conspecifics in native and introduced ranges provides further clues for possible evolutionary adaptations of introduced organisms due to altered environmental conditions. Furthermore, the author’s analyses suggested that conspecifics were generally larger and allocated more to reproduction in the introduced range compared with the native range. In fact, S. muticum is one of the smaller Sargassum species in Japan (75–120 cm; Josefsson and Jansson 2011; Yendo 1907), but grows considerably larger when introduced into new areas and reaches a length of >4 m in the German North Sea (Polte and Buschbaum 2008). However, the size of S. muticum in its native range is controversial. The sampled S. muticum used in the present study was about 1–1.5 m long and reached maximum length of 2 m after maturation (pers. communication), but there are recent findings of large specimens of up to 3.2 m (Engelen et al. 2015). It seems that there have been two phases of invasion (Dietz and Edwards 2006) into the North Sea. In phase one, the pre-adapted highly defended S. muticum arrived in the new range where it benefited from grazer avoidance compared to the local dominant brown alga F. vesiculosus. Grazer attacks decreased growth rates of F. vesiculosus and therefore facilitated the rapid establishment of S. muticum in the German North Sea. In phase two, the continuous low grazing pressure resulted in a resource allocation shift from the production of defense metabolites to reproduction or growth, which is reflected by the higher length of S. muticum in the North Sea compared to Japan. This caused a selection of the less defended but more vigorous or competitive genotypes (Blossey and Notzold 1995; Callaway and Ridenour 2004; Cano et al. 2009). However, this mechanism is highly speculative and other studies found opposite results. Non-native populations of the East Asian seaweed Gracilaria vermiculophylla have been shown to be generally less palatable to marine herbivores than native populations, and the increased capacity for activated chemical defense is suggested to have contributed to their invasion success (Hammann et al. 2016). Furthermore, Le Cam et al. (2015) found limited genetic polymorphism of S. muticum populations in the invaded ranges and a perfect match of the invaded genotype in one of the native population, implying a restricted probability of genetic adaptation due to the release from predation. A resource allocation shift might therefore rather result from gene expression regulation than from real adaptation through gene modification.
Additionally, differences in feeding preference might arise from other confounding factors. The more apical and younger areas of the thallus are usually defended more strongly (Pavia et al. 2002), and the sampling of Sargassum populations of different developmental stages might result in more or less feeding deterrence. Phenolic content of S. muticum in Brittany peaked during the reproductive period, which was hypothesized as providing a maximum protection of the fertile receptacles from both grazing and solar radiation (Plouguerné et al. 2006). Thus, different tissue types or developmental stages of native and invasive S. muticum might result in different feeding preferences. We tried to minimize these factors in this study by sampling whole algal thalli of Sargassum spp. North Sea and Japanese populations and before maturation only. Furthermore, algal metabolites can differ among populations over scales of hundreds of kilometers. Phlorotannin concentration of Ascophyllum nodusum populations differed significantly at scales of meters up to one kilometer, whereas only little variations were found among populations located 1000 km apart (Pavia and Åberg 1996). Therefore, general assumptions about invasive and native Sargassum spp. should be handled with caution.
While S. muticum was significantly less preferred to F. vesiculosus by all tested herbivores, there is no clear evidence that it is better defended than its native, non- or weakly-invasive relatives. S. muticum (nat.) has in fact been the least preferred by two out of three tested herbivores. However, this is just a weak indication for explaining the invasion success of S. muticum compared to S. horneri or S. fusiforme. There have been a number of other traits besides, or in conjunction with, herbivore defense (Carpenter and Cappuccino 2005; Engelen et al. 2011) that have been found to be important for the establishment and dispersal of introduced species: Faster growth or higher fecundity (van Kleunen et al. 2010; Engelen et al. 2011), positive response to disturbance (Deysher and Norton 1982), and invasion melt-downs where non-native herbivores selectively suppress native plants and facilitate invasion by non-native plants that have evolved with these invasive herbivores (Parker et al. 2006). S. muticum shows several of these characteristics with its high reproductive output, fast growth and great potential to colonize uninhabited areas (Deysher and Norton 1982; Wernberg et al. 2000; Engelen et al. 2011). Additionally, S. muticum exerts other adverse effects on the local algal community. The introduction of S. muticum in Limfjorden has led to a decline in slow growing perennial species, such as Fucus vesiculosus, Fucus serratus, Saccharina latissima and Codium fragile. It has been suggested that the reduction of these algae is due to competition for space with S. muticum (Stæhr et al. 2000). Furthermore, S. muticum is less affected by herbivory and can compensate grazing losses through high growth rates, while growth of local co-occurring species is reduced due to grazing. On the other hand, it can be argued that the well-defended Japanese S. fusiforme and S. horneri might invade the North Sea in future as well. The high growth rates of S. muticum of 4 cm day−1 under optimal conditions (Jephson and Gray 1977) has not yet been achieved by S. horneri (0.1–0.9 cm day−1 in situ; Gao and Hua 1997) or S. fusiforme (0.2 cm day−1 in the laboratory; Zou 2005). However, S. horneri has recently been detected in California, USA, from where it spread rapidly along the southern region of the Pacific Coast and might achieve higher growth rates with further dispersal (Miller et al. 2007; Marks et al. 2015). It might therefore be reasonable not to focus on S. muticum only, but to include other Sargassum species in North Sea monitoring programs to prevent their establishment in European waters.
In conclusion, the invasion success of S. muticum in the North Sea might have been facilitated by feeding preferences of local herbivores. The invasive S. muticum was less preferred compared to its local competitor F. vesiculosus which might have facilitated the expansion of S. muticum in its new range. The native S. muticum population from Japan was less preferred compared to its allopatric population from the North Sea. This might be the result of a resource allocation shift due to reduced grazing pressure in the North Sea.
More comparisons between invasive and noninvasive related species should be conducted in various environments differing in grazing pressure and competition in order to get a closer insight into invasive traits and evolutionary adaptations.
Since other Sargassum species from Japan are equally defended against grazers like S. muticum from Japan, they should be included in European monitoring programs.
References
Anderson LWJ (2007) Control of invasive seaweeds. Bot Mar 50:418–437. doi:10.1515/BOT.2007.045
Bazes A, Silkina A, Douzenel P et al (2009) Investigation of the antifouling constituents from the brown alga Sargassum muticum (Yendo) Fensholt. J Appl Phycol 21:395–403. doi:10.1007/s10811-008-9382-9
Blossey B, Notzold R (1995) Evolution of increased competitive ability in invasive nonindigenous plants—a hypothesis. J Ecol 83:887–889
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. doi:10.1016/0003-2697(76)90527-3
Cacabelos E, Olabarria C, Incera M, Troncoso JS (2010) Do grazers prefer invasive seaweeds? J Exp Mar Bio Ecol 393:182–187. doi:10.1016/j.jembe.2010.07.024
Callaway RM, Ridenour WM (2004) Novel weapons: invasive success and the evolution of increased competitive ability. Front Ecol Environ 2:436–443. doi:10.1890/1540-9295(2004)002[0436:NWISAT]2.0.CO;2
Cano L, Escarré J, Vrieling K, Sans FX (2009) Palatability to a generalist herbivore, defence and growth of invasive and native Senecio species: testing the evolution of increased competitive ability hypothesis. Oecologia 159:95–106. doi:10.1007/s00442-008-1182-z
Cappuccino N, Arnason JT (2006) Novel chemistry of invasive exotic plants. Biol Lett 2:189–193. doi:10.1098/rsbl.2005.0433
Cappuccino N, Carpenter D (2005) Invasive exotic plants suffer less herbivory than non-invasive exotic plants. Biol Lett 1:435–438. doi:10.1098/rsbl.2005.0341
Carlton JT (2009) Deep invasion ecology and the assembly of communities in historical time. Biol Invasions Mar Ecosyst 13–56. doi:10.1007/978-3-540-79236-9_2
Carpenter D, Cappuccino N (2005) Herbivory, time since introduction and the invasiveness of exotic plants. J Ecol 93:315–321. doi:10.1111/j.1365-2745.2005.00973.x
Cassano V, De-Paula JC, Fujii MT et al (2008) Sesquiterpenes from the introduced red seaweed Laurencia caduciramulosa (Rhodomelaceae, Ceramiales). Biochem Syst Ecol 36:223–226. doi:10.1016/j.bse.2007.07.005
Critchley AT (1983) Sargassum muticum—a taxonomic history including world-wide and western Pacific distributions. J Mar Biol Assoc United Kingdom 63:617–625
Cruz-Rivera E, Hay ME (2003) Prey nutritional quality interacts with chemical defenses to affect consumer feeding and fitness. Ecol Monogr 73:483–506. doi:10.1890/0012-9615(2003)073
Daehler CC (2003) Performance comparisons of co-occurring native and alien invasive plants: implications for conservation and restoration. Annu Rev Ecol Evol Syst 34:183–211. doi:10.1146/132403
Davidson AD, Campbell ML, Hewitt CL, Schaffelke B (2015) Assessing the impacts of nonindigenous marine macroalgae: an update of current knowledge. Bot Mar 58:55–79. doi:10.1515/bot-2014-0079
Deal MS, Hay ME, Wilson D, Fenical W (2003) Galactolipids rather than phlorotannins as herbivore deterrents in the brown seaweed Fucus vesiculosus. Oecologia 136:107–114. doi:10.1007/s00442-003-1242-3
Deysher L, Norton TA (1982) Dispersal and colonization in Sargassum muticum (Yendo) Fensholt. J Exp Mar Bio Ecol 56:179–195. doi:10.1016/0022-0981(81)90188-X
Dietz H, Edwards PJPJ (2006) Recognition that causal processes change during plant invasion helps explain conflicts in evidence. Ecology 87:1359–1367. doi:10.1890/0012-9658(2006)87[1359:RTCPCD]2.0.CO;2
Enge S, Nylund GM, Harder T, Pavia H (2012) An exotic chemical weapon explains low herbivore damage in an invasive alga. Ecology 93:2736–2745. doi:10.1890/12-0143.1
Engelen AH, Henriques N, Monteiro C, Santos R (2011) Mesograzers prefer mostly native seaweeds over the invasive brown seaweed Sargassum muticum. Hydrobiologia 669:157–165. doi:10.1007/s10750-011-0680-x
Engelen AH, Serebryakova A, Ang P, Britton-Simmons K, Mineur F, Pedersen MF, Arenas F et al (2015) Circumglobal invasion by the brown seaweed Sargassum muticum. Ocean Mar Biol Ann Rev 53:81–126
Forslund H, Wikström SA, Pavia H (2010) Higher resistance to herbivory in introduced compared to native populations of a seaweed. Oecologia 164:833–840. doi:10.1007/s00442-010-1767-1
Gao K, Hua W (1997) In Situ growth rates of Sargassum horneri (Fucales, Phaeophyta). Phycol. Res. 45:55–57
Halpern BS, Walbridge S, Selkoe KA et al (2008) A global map of human impact on marine ecosystems. Science 319(80):948–953. doi:10.1126/science.1149345
Hammann M, Wang G, Rickert E et al (2013) Invasion success of the seaweed Gracilaria vermiculophylla correlates with low palatibility. Mar Ecol Prog Ser 486:93–103. doi:10.3354/meps10361
Hammann M, Rempt M, Pohnert G, Wang G, Boo SM, Weinberger F (2016) Increased potential for wound activated production of Prostaglandin E2 and related toxic compounds in non-native populations of Gracilaria vermiculophylla. Harmful Algae 51:81–88. doi:10.1016/j.hal.2015.11.009
Hawkes CV (2007) Are invaders moving targets? The generality and persistence of advantages in size, reproduction, and enemy release in invasive plant species with time since introduction. Am Nat 170:832–843. doi:10.1086/522842
Hay ME, Kappel QE, Fenical W (1994) Synergisms in plant defenses against herbivores: Interactions of chemistry, calcification, and plant quality. Ecology 75:1714–1726
Hemmi A, Jormalainen V (2002) Nutrient enhancement increases performance of a marine herbivore via quality of its food alga. Ecology 83:1052–1064. doi:10.1890/0012-9658(2002)083[1052:NEIPOA]2.0.CO;2
Hemmi A, Jormalainen V (2004) Geographic covariation of chemical quality of the host alga Fucus vesiculosus with fitness of the herbivorous isopod Idotea baltica. Mar Biol 145:759–768. doi:10.1007/s00227-004-1360-4
Imrie DW, McCrohan CR, Hawkins SJ (1990) Feeding behaviour in Littorina littorea: a study of the effects of ingestive conditioning and previous dietary history on food preference and rates of consumption. Hydrobiologa 193:191–198
Jephson NA, Gray PWG (1977) Aspects of the ecology of Sargassum muticum (Yendo) Fensholt, in the Solent Region of the British Isles. 1. The growth cycle and epiphytes. In: Keegan BF, Boaden PJS, Ceidigh PO (eds) Biology of benthic organisms. 11th European symposium on marine biology. Pergamon Press, Oxford, pp 367–375
Johnsen KL, Halle LL, Karling ND (2013) Can grazer avoidance explain the invasiveness of the brown alga Sargassum muticum in Limfjorden, Denmark? Thesis, Roskilde Universitet, pp 12–17
Jormalainen V, Honkanen T, Heikkilä N (2001) Feeding preferences and performance of a marine isopod on seaweed hosts: cost of habitat specialization. Mar Ecol Prog Ser 220:219–230. doi:10.3354/meps220219
Josefsson M, Jansson K (2011) NOBANIS—invasive Alien Species fact sheet—Sargassum muticum. In: Online Database Eur. Netw. Invasive Alien Species—NOBANIS www.nobanis.org
Karlsson J, Loo LO (1999) On the distribution and continuous expansion of the Japanese seaweed—Sargassum muticum—in Sweden. Bot Mar 42:285–294. doi:10.1515/BOT.1999.032
Keane RM, Crawley MJ (2002) Exotic plant invasions and the enemy release hypothesis. Trends Ecol Evol 17:164–170. doi:10.1016/S0169-5347(02)02499-0
Kelly JR, Scheibling RE (2012) Fatty acids as dietary tracers in benthic food webs. Mar Ecol Prog Ser 446:1–22. doi:10.3354/meps09559
Kornmann P, Sahling PH (1994) Meeresalgen von Helgoland: Zweite Ergänzung. Helgoländer Meeresuntersuchungen 48:365–406. doi:10.1007/BF02366253
Le Cam S, Thiebaut CD, Bouchemousse S, Viard F (2015) Elucidating unresolved invasion history with genome-wide sequencing approach: the case of the global invader Sargassum muticum. Eur J Phycol 50:24–25. doi:10.1080/09670262.2015.1069489
Lind EM, Parker JD (2010) Novel weapons testing: are invasive plants more chemically defended than native plants? PLoS ONE. doi:10.1371/journal.pone.0010429
Marks LM, Salinas-Ruiz P, Reed DC et al (2015) Range expansion of a non-native, invasive macroalga Sargassum horneri (Turner) C. Agardh, 1820 in the eastern Pacific. BioInvasions Rec 4:243–248
Mattson WJJ (1980) Herbivory in relation to plant nitrogen content. Annu Rev Ecol Syst 11:119–161
Millennium Ecosystem Assessment (2005) Ecosystems and human well-being: synthesis. Island Press, Washington
Miller KA, Engle JM, Uwai S, Kawai H (2007) First report of the Asian seaweed Sargassum filicinum Harvey (Fucales) in California, USA. Biol Invasions 9:609–613. doi:10.1007/s10530-006-9060-2
Monteiro CA, Engelen AH, Santos ROP (2009) Macro- and mesoherbivores prefer native seaweeds over the invasive brown seaweed Sargassum muticum: a potential regulating role on invasions. Mar Biol 156:2505–2515. doi:10.1007/s00227-009-1275-1
Morrison WE, Hay ME (2011) Herbivore preference for native vs. exotic plants: generalist herbivores from multiple continents prefer exotic plants that are evolutionarily naïve. PLoS ONE. doi:10.1371/journal.pone.0017227
Nejrup LB, Pedersen MF, Vinzent J (2012) Grazer avoidance may explain the invasiveness of the red alga Gracilaria vermiculophylla in Scandinavian waters. Mar Biol 159:1703–1712. doi:10.1007/s00227-012-1959-9
Nyberg CD, Wallentinus I (2005) Can species traits be used to predict marine macroalgal introductions? Biol Invasions 7:265–279. doi:10.1007/s10530-004-0738-z
Nylund GMN, Pereyra RT, Wood HL, Johannesson K (2012) Increased resistance towards generalist herbivory in the new range of a habitat-forming seaweed. Ecosphere 3:1–13. doi:10.1890/ES12-00203.1
Parker JD, Hay ME (2005) Biotic resistance to plant invasions? Native herbivores prefer non-native plants. Ecol Lett 8:959–967. doi:10.1111/j.1461-0248.2005.00799.x
Parker JD, Burkepile DE, Hay ME (2006) Opposing effects of native and exotic herbivores on plant invasions. Science 311(80):1459–1461
Pavia H, Åberg P (1996) Spatial variation in polyphenolic content of Ascophyllum nodosum (Fucales, Phaeophyta). Hydrobiologia 326:199–203
Pavia H, Toth GB, Åberg P (2002) Optimal defense theory: elasticity analysis as a tool to predict intraplant variation in defenses. Ecology 83:891–897. doi:10.2307/3071898
Pereira RC, da Gama BAP (2008) Macroalgal chemical defenses and their roles in structuring tropical marine communities. In: Amsler CD (ed) Algal chemical ecology. Springer, Berlin, Heidelberg, pp 25–49
Plouguerné E, Le Lann K, Connan S, Jechoux G, Deslandes E, Stiger-Pouvreau V (2006) Spatial and seasonal variation in density, reproductive status, length and phenolic content of the invasive brown macroalga Sargassum muticum (Yendo) Fensholt along the coast of Western Brittany (France). Aquat Bot 85:337–344
Plouguerné E, Hellio C, Deslandes E et al (2008) Anti-microfouling activities in extracts of two invasive algae: Grateloupia turuturu and Sargassum muticum. Bot Mar 51:202–208. doi:10.1515/BOT.2008.026
Plouguerné E, Ioannou E, Georgantea P et al (2010) Anti-microfouling activity of lipidic metabolites from the invasive brown alga Sargassum muticum (Yendo) Fensholt. Mar Biotechnol (NY) 12:52–61. doi:10.1007/s10126-009-9199-9
Polte P, Buschbaum C (2008) Native pipefish Entelurus aequoreus are promoted by the introduced seaweed Sargassum muticum in the northern Wadden Sea, North Sea. Aquat Biol 3:11–18. doi:10.3354/ab00071
Poore AGB, Campbell AH, Coleman RA et al (2012) Global patterns in the impact of marine herbivores on benthic primary producers. Ecol Lett 15:912–922. doi:10.1111/j.1461-0248.2012.01804.x
Rejmánek M, Richardson DM (1996) What attributes make some plant species more invasive? Ecology 77:1655–1661. doi:10.2307/2265768
Ribera M, Boudouresque C (1995) Introduced marine plants, with special reference to macroalgae: mechanisms and impact. Prog Phycol Res 11:187–268
Rohde S, Molis M, Wahl M (2004) Regulation of anti-herbivore defence by Fucus vesiculosus in response to various cues. J Ecol 92:1011–1018. doi:10.1111/j.0022-0477.2004.00936.x
Sabour B, Reani A, Magouri HEL, Haroun R (2013) Sargassum muticum (Yendo) Fensholt (Fucales, Phaeophyta) in Morocco, an invasive marine species new to the Atlantic coast of Africa. Aquat Invasions 8:97–102. doi:10.3391/ai.2013.8.1.11
Schaffelke B, Hewitt CL (2007) Impacts of introduced seaweeds. Bot Mar 50:397–417. doi:10.1515/9783110211344
Schaffelke B, Smith JE, Hewitt CL (2006) Introduced macroalgae—a growing concern. J Appl Phycol 18:529–541. doi:10.1007/s10811-006-9074-2
Schupp PJ, Paul VJ (1994) Calcium carbonate and secondary metabolites in tropical seaweeds: variable effects on herbivorous fishes. Ecology 75:1172–1185
Seabloom EW, Harpole WS, Reichman OJ, Tilman D (2003) Invasion, competitive dominance, and resource use by exotic and native California grassland species. Proc Natl Acad Sci USA 100:13384–13389. doi:10.1073/pnas.1835728100
Smith MD, Knapp AK (2001) Physiological and morphological traits of exotic, invasive exotic, and native plant species in tallgrass prairie. Int J Plant Sci 162:785–792. doi:10.1086/320774
Stæhr PA, Pedersen MF, Thomsen MS et al (2000) Invasion of Sargassum muticum in Limfjorden (Denmark) and its possible impact on the indigenous macroalgal community. Mar Ecol Prog Ser 207:79–88. doi:10.3354/meps207079
Tanniou A, Vandanjon L, Incera M et al (2014) Assessment of the spatial variability of phenolic contents and associated bioactivities in the invasive alga Sargassum muticum sampled along its European range from Norway to Portugal. J Appl Phycol 26:1–16. doi:10.1007/s10811-013-0198-x
Targett NM, Arnold TM (1998) Predicting the effects of brown algal phlorotannins on marine herbivores in tropical and temperate oceans. J Phycol 34:195–205. doi:10.1046/j.1529-8817.1998.340195.x
Thresher RE (1999) Key threats from marine bioinvasions: a review of current and future issues. In: Pederson J (ed) Marine bioinvasions: proceedings of the first national conference. Massachussetts Institute of Technology, Sea Grant College Program, Boston, pp 24–36
Toth GB, Karlsson M, Pavia H (2007) Mesoherbivores reduce net growth and induce chemical resistance in natural seaweed populations. Oecologia 152:245–255. doi:10.1007/s00442-006-0643-5
Trowbridge CD, Hirano YM, Hirano YJ (2009) Interaction webs of marine specialist herbivores on Japanese shores. J Mar Biol Assoc United Kingdom 89:277. doi:10.1017/S002531540900318X
van Alstyne KL (1995) Comparison of three methods for quantifying brown algal polyphenolic compounds. J Chem Ecol 21:45–58. doi:10.1007/BF02033661
van Kleunen M, Weber E, Fischer M (2010) A meta-analysis of trait differences between invasive and non-invasive plant species. Ecol Lett 13:235–245. doi:10.1111/j.1461-0248.2009.01418.x
Vas’kovskii VE, Isai SV (1972) Determination of the amount of mannitol in brown seaweeds. Chem Nat Compd 8:596–600. doi:10.1007/BF00564297
Vermeij MJA, Smith TB, Dailer ML, Smith CM (2009) Release from native herbivores facilitates the persistence of invasive marine algae: a biogeographical comparison of the relative contribution of nutrients and herbivory to invasion success. Biol Invasions 11:1463–1474. doi:10.1007/s10530-008-9354-7
Vitousek PM, Dantonio CM, Loope LL et al (1997) Introduced species: a significant component of human-caused global change. N Z J Ecol 21:1–16
Weinberger F, Buchholz B, Karez R, Wahl M (2008) The invasive red alga Gracilaria vermiculophylla in the Baltic Sea: adaptation to brackish water may compensate for light limitation. Aquat Biol 3:251–264. doi:10.3354/ab00083
Weinberger F, Rohde S, Oschmann Y et al (2011) Effects of limitation stress and of disruptive stress on induced antigrazing defense in the bladder wrack Fucus vesiculosus. Mar Ecol Prog Ser 427:83–94. doi:10.3354/meps09044
Wernberg T, Thomsen MS, Stæhr PA, Pedersen MF (2000) Comparative phenology of Sargassum muticum and Halidrys siliquosa (Phaeophyceae: Fucales) in Limfjorden, Denmark. Bot Mar 44:31–39. doi:10.1515/BOT.2001.005
Wikström SA, Steinarsdóttir MB, Kautsky L, Pavia H (2006) Increased chemical resistance explains low herbivore colonization of introduced seaweed. Oecologia 148:593–601
Yendo K (1907) The Fucaceae of Japan. J Coll Sci Tokyo Imp Univ 21(12):1–174
Zhang Q, Zhang J, Shen J et al (2006) A Simple 96-well microplate method for estimation of total polyphenol content in seaweeds. J Appl Phycol 18:445–450. doi:10.1007/s10811-006-9048-4
Zou D (2005) Effects of elevated atmospheric CO2 on growth, photosynthesis and nitrogen metabolism in the economic brown seaweed, Hizikia fusiforme (Sargassaceae, Phaeophyta). Aquaculture 250:726–735. doi:10.1016/j.aquaculture.2005.05.014
Acknowledgments
We would like to thank Fabian Heinke and Johanna Meinecke (ICBM, Germany) for algal nutrients extraction as well as two anonymous reviewers and Jutta Nietzer for valuable comments. NS was supported by the research-orientated teaching program at the University of Oldenburg.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no potential conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the collection, care and use of organisms were followed.
Additional information
Responsible Editor: F. Weinberger.
Reviewed by undisclosed experts.
This article is part of the Topical Collection on Invasive Species.
Rights and permissions
About this article
Cite this article
Schwartz, N., Rohde, S., Hiromori, S. et al. Understanding the invasion success of Sargassum muticum: herbivore preferences for native and invasive Sargassum spp. Mar Biol 163, 181 (2016). https://doi.org/10.1007/s00227-016-2953-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-016-2953-4