Abstract
Non-native seaweeds constitute a conspicuous component of many benthic coastal communities. Seaweed invaders are known to significantly affect invaded communities, but relatively little is known about the mechanisms underlying their success. In this study, we explored the feeding preferences of three generalist herbivores for the successful non-native red alga Heterosiphonia japonica and native seaweed competitors. The experiments were conducted on the Swedish Skagerrak coast (58°52′N, 11°08′E) from July to August. Additionally, chemical and physical traits of the seaweeds were assessed to mechanistically explain herbivore preferences. The results showed that H. japonica was of low preference to native herbivores and that this was most likely explained by chemical properties of the invader. We were, however, not able to determine whether the low preference was caused by deterrent metabolites or low nutritional quality. We conclude that herbivore avoidance may be important for the survival and success of H. japonica in the introduced range and that efficient means of escaping herbivory may be a common feature of invaders in seaweed communities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Temperate coastal habitats have experienced large changes in species composition and distribution due to human exploitation (Lotze et al. 2006; Worm et al. 2006). The introduction of non-native seaweed species constitutes a significant part of this change (Grosholz 2002; Lotze et al. 2006; Worm et al. 2006) and may affect habitat quality and ecosystem function in invaded communities (Wallentinus and Nyberg 2007; Gribben et al. 2009; Sagerman et al. 2014). Therefore, large efforts have been made to gain a better understanding of the mechanisms that enable non-native species to expand in their new ranges and become dominant constituents of the invaded communities. One factor that can affect the success of non-native species is the degree to which they are attacked by native consumers in the new range (e.g., Elton 1958; Maron and Vilà 2001). Release from co-evolved enemies has been suggested as an important mechanism that can increase invasion success (Elton 1958; Keane and Crawley 2002). However, this requires that the native predators in the new range fail to recognize the invader as a potential prey item, due to lack of mutual evolutionary history, which is unlikely for generalist enemies that attack a large number of prey species. Seaweed communities have a high prevalence of generalist herbivores that are known to include many different food items in their diet (Hay 1991). Herbivory is often intense in these communities, affecting standing biomass and the distribution and abundance of specific species (Duffy and Hay 2000; Poore et al. 2012). Thus, traits that allow for an efficient escape from generalist herbivores in the new range may be the key to success for non-native seaweeds (Keane and Crawley 2002; Wikström et al. 2006). Accordingly, there are several examples where successful non-native seaweeds have been shown to be completely or partly avoided by generalist herbivores in their introduced range (Britton-Simmons 2004; Wikström et al. 2006; Monteiro et al. 2009; Nejrup et al. 2012; Enge et al. 2012). Grazer preference can be driven by the presence of deterrent metabolites, nutritional value, morphological characteristics (e.g., tissue toughness), or a combination of these traits (Mattson 1980; Nicotri 1980).
The northeastern Atlantic has received a number of very successful non-native seaweeds, of which several are known to cause significant ecological impacts (Scheibling and Gagnon 2006; Drouin et al. 2011; Sagerman et al. 2014; Vaz-Pinto et al. 2014). During the past few decades, the filamentous red alga Heterosiphonia japonica has rapidly spread along the European west coast and further advanced to the North American east coast and into the Mediterranean Sea (Sjøtun et al. 2008; Newton et al. 2013). The invader has become a common constituent of seaweed communities and is even dominating at local scale (Husa et al. 2004; Moy and Christie 2012; Newton et al. 2013). The underlying reason for the invasion success of H. japonica has so far not been clarified. Part of the explanation most likely resides in the invader’s rapid growth during the warm summer months (Sagerman et al. 2014). However, it is not known whether H. japonica is consumed by grazers in the new range and/or if herbivore preference contributes to its invasion success. Since H. japonica has established successfully in a system with high grazing pressure from generalist herbivores, we hypothesize that it is less preferred by native herbivores compared to competing native seaweeds in the invaded community.
The aim of this study was to test the preference of native herbivores for H. japonica compared to competing native seaweeds in the invaded community. Thus, we tested the feeding preference of three abundant, generalist herbivores, by assessing their choice between native algal species and H. japonica in pairwise feeding assays. To obtain a more comprehensive picture of the relative palatability of H. japonica, the invader was tested both against native co-occurring algae from shallow sheltered environments (four species) and deep exposed environments (one species). Moreover, to explore the underlying reasons for the observed feeding preferences, the presence of a chemical defense in H. japonica and the influence of structural differences between experimental seaweeds were tested through feeding assays with artificial food. Finally, the nutritional value and the dry matter content of the experimental algae were analyzed to provide an indication of their relative food quality.
Materials and methods
Study species
Heterosiphonia japonica has a wide depth distribution and grows on hard substrates and gravel in its new range in the northeastern Atlantic (Husa et al. 2004). The spatial distribution of H. japonica varies with exposure level. The species frequently dominates sheltered filamentous seaweed communities from the surface to below 12-m depth. A SCUBA inventory consisting of line transects divided in 1 × 2 m sections showed that H. japonica can reach a mean surface cover of up to 69 % (±21 SD, n = 5) at 4-m depth in this type of environment along the Swedish west coast (Sagerman, unpubl. data). At exposed sites the species only grows abundantly at greater depths (i.e., 10–25 m, pers obs).
We tested the palatability of H. japonica in comparison with five native filamentous seaweeds. The choice of native seaweeds was based on data from diving transects collected in 2009 (Andersson and Engdahl 2009; Andersson et al. 2010). A total of 22 sites were surveyed along the Swedish west coast with H. japonica present at more than half of the sites and abundant at seven sites (having a surface cover of at least 10 % along one section of the transect). We extracted data from these seven sites by dividing the transects into 1 m sections and recording the native seaweed species that co-occurred in each section with H. japonica. Four of the seven sites were sheltered and had H. japonica present in a total of 28 sections (with a mean cover of 10.4 ± 12.7 %, SD) at shallow depths, while three sites were wave exposed with H. japonica present in a total of 29 sections (mean cover 5.8 ± 5.6 %, SD) at greater depths (below 10 m). For both at the sheltered and deep sites, most of the species that co-occurred with H. japonica were red algae (Table 1). We included two of the most common filamentous red algae from the sheltered sites (Polysiphonia fucoides and Ceramium virgatum) and one from the deep site (Brogniartella byssoides). In addition, we included the most common green filamentous taxon (Cladophora sp.) and the second most common filamentous brown alga (Ectocarpus siliculosus). The species of Cladophora used in the experiments could not be identified to species level due to the high degree of morphological variability and overlap in quantitative species characters within the genus (van den Hoek 1982), but it was either one or a mixture of the two species C. sericea and C. albida.
Feeding experiments were performed with three crustacean mesoherbivores, Idotea granulosa (Isopoda), Gammarus locusta (Amphipoda) and Gammarellus angulosus (Amphipoda), which are among the most abundant herbivore species in macroalgal communities in the study area and co-occur with the experimental species (pers. obs.). All three species feed on filamentous seaweeds and are considered to be generalist consumers (Naylor 1955; Hacker and Steneck 1990; Pavia et al. 1999; Kraufvelin et al. 2006). Despite their small size, mesoherbivores can remove a considerable part of seaweed primary production and affect the composition of seaweed communities (e.g., Duffy and Hay 2000; Worm et al. 2001).
The study was conducted during July and August when the grazer density is high and the filamentous seaweed community peaks in terms of biomass (Moy and Christie 2012). The organisms were collected from two sites in the vicinity of the Tjärnö marine laboratory (Sven Lovén Centre for Marine Sciences), located on the Swedish Skagerrak coast (58°52′N, 11°08′E). The seaweeds from the shallow community (including H. japonica) were collected in a sheltered narrow sound at 1–3 m depth, while the seaweeds from the deep community (H. japonica and the native B. byssoides) were collected at an exposed site at 12–20 m depth. Throughout the experiments, the native seaweeds from the shallow community were always compared with H. japonica from the same site and the deep-growing B. byssoides was compared with H. japonica from the deep site. The herbivores I. granulosa and G. angulosus were collected at the exposed site from a mixture of red algae, while G. locusta was collected from Fucus spp. or in drifting seaweeds at sheltered sites. Both algae and animals were kept submerged during transportation to the field station after which the algae were carefully cleaned of fauna and sorted into separate tanks. The organisms were maintained in tanks provided with flow through seawater for a maximum of 10 days prior to their use in experiments. During this time, grazers were fed a diet of Ulva sp. All grazers used in the experiment were 5–15 mm in length.
Feeding experiments with live algae
The three herbivores I. granulosa, G. locusta and G. angulosus were allowed to feed on the non-native seaweed H. japonica and one of the native species Cladophora sp., E. siliculosus, P. fucoides, C. virgatum or B. byssoides, in pairwise choice experiments. Shoots of each alga were carefully dried between paper towels and their fresh weight recorded. For each comparison, one shoot of H. japonica and a native alga (30 ± 5 mg per species) were placed in 200-ml containers filled with 150 ml of seawater (salinity of approximately 27 PSU). One of the grazer species, either 3 I. granulosa, 4 G. locusta or 6–8 G. angulosus (dependent on size), was added to half of the containers. The other half of the containers were used as controls for autogenic changes in the algae. The experiment was replicated 10 times for each algal pair and grazer species. The experiments were run without flow through of seawater since surface diffusion of oxygen was sufficient to sustain the algae and animals within the containers. The experiments were terminated and the remaining algal pieces re-weighed when 25–50 % of the algal biomass was eaten or when at the latest 48 h had elapsed. The experiments were run in a cold room at 16 ± 1 °C with a light/dark cycle of 16:8 h under low light conditions (i.e., 0–5 μmol photons m−2 s−1).
Feeding experiments with homogenized algae
To test whether feeding preferences of the generalist grazers were affected by differences in algal physical structure (e.g., uniseriate filament vs. multiseriate corticated thallus, tissue toughness and dry matter content), we conducted feeding experiments with the isopod I. granulosa where structural differences between algae were removed. To accomplish this, algae were frozen at −80 °C immediately after collection, freeze-dried, ground, and the resultant powder added to liquid agar at 50 °C (at a concentration of 100 mg seaweed powder to 1 ml agar). Once the agar had coagulated to a solid gel, it was cut into pieces (150 ± 10 mg) and the negatively buoyant gel pieces from different algal species compared in pairwise feeding experiments. The experiments were performed as described for the feeding experiments using intact algae, with the exception that the containers were connected to flow through seawater (at a rate of approximately 0.6–1.0 ml s−1). The reason for this was that the first set of agar feeding experiments failed due to high grazer mortalities, which we suspected were caused by the release of substances from the homogenized tissue of H. japonica.
Measurement of algal nutritional quality
To determine the nutritional value of the seaweeds, ten fronds of each species were freeze-dried. A carbon (C) and nitrogen (N) analysis was conducted on a 4 mg (±0.5) sample from each frond using a Thermo Fischer flash 2000 analyzer. The phosphorus content was measured on a 4.5 mg (±1) sample from each individual frond using segmented flow analysis (Pasquini and De Oliveira 1985). Dry matter content (DMC) was calculated through measuring the wet weight of the seaweed fronds (after careful drying with paper towels) and the dry weight after freeze drying.
Feeding experiments to evaluate the presence of grazer-deterrent metabolites
To test whether the low preference of the native herbivores for H. japonica was due to a chemical defense, we prepared a crude extract by successively extracting 5 g of pooled fresh H. japonica fronds in 200 ml methanol, 200 ml methanol/dichloromethane 1:1, and 200 ml dichloromethane. Each extraction step was conducted for 2 h (Enge et al. 2012). The successive treatment with solvents of different polarities ensures the extraction of a wide array of polar to non-polar compounds in the crude extract. The solvents were evaporated, and the crude extract was re-dissolved in 5 ml methanol/dichloromethane 1:1. For the feeding experiment, a 4 ml of the extract was mixed with 400 mg of freeze-dried and ground C. virgatum. As control food, 400 mg of freeze-dried and ground C. virgatum was treated with the same volume of solvent only. The solvent was evaporated under a stream of nitrogen and the extract-coated and control C. virgatum powders each incorporated into 4 ml agar, which resulted in a natural volumetric concentration of the extract in the artificial food. Ceramium virgatum constitutes a suitable material to apply algal extracts on since it is a species that is readily eaten by the herbivores. The feeding experiment was performed as described for the test of structural resistance against grazing.
Statistical analysis
Since the consumption of two food types in the same experimental unit is not statistically independent, all feeding experiments, both with live algae and artificial food, were analyzed in accordance with Peterson and Renaud (1989). To produce a single independent value for each experimental unit, we calculated the difference in weight change between the two food items in each experimental container. The differences from the experimental units of the grazed treatment were then tested against the differences from the control units. Prior to analysis, the data were inspected for normality and homogeneity of variances. The Welch’s t test was used when data were normally distributed either untransformed or following log or square root transformation. In other cases, a randomized t test was conducted (Manly 1991). The tests and transformations applied to each specific experiment are presented in Figs. 1, 2 and 4.
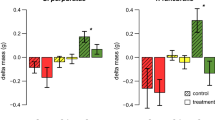
Total consumption of the non-native seaweed Heterosiphonia japonica (H. jap) and the native seaweeds Cladophora sericea/albida (Clad. sp.), Ectocarpus siliculosus (E. sil), Polysiphonia fucoides (P. fuc), Ceramium virgatum (C. vir) and Brongniartella byssoides (B. bys) offered in pairwise feeding trials to the native grazers Idotea granulosa, Gammarus locusta and Gammarellus angulosus. Bars show mean loss in fresh weight of the two algal species during the course of the experiment in the grazed treatments corrected by autogenic changes in the control treatments (±CI95, n = 10–8). Significant preferences are indicated with dark colored bars and P < 0.05 (see text for the statistical analysis of the data). Symbols indicate data transformations and use of randomization tests: [no symbol] untransformed; ‡square root-transformed; †log-transformed; §untransformed, randomization t test
Total consumption of the non-native seaweed Heterosiphonia japonica (H. jap) and the native seaweeds Cladophora sericea/albida (Clad. sp.), Ectocarpus siliculosus (E. sil), Polysiphonia fucoides (P. fuc), Ceramium virgatum (C. vir) and Brongniartella byssoides (B. bys) offered in pairwise feeding trials to the native grazer Idotea granulosa after structural differences between seaweeds had been removed through incorporating freeze-dried homogenized algae into agar. Bars show mean loss in fresh weight of the two food types during the course of the experiment in the grazed treatments corrected by autogenic changes in the control treatments (±CI95, n = 10–8). Significant preferences are indicated with dark colored bars and P < 0.05 (see text for the statistical analysis of the data). Symbols indicate data transformations and use of randomization tests: [no symbol] untransformed; ‡square root-transformed; †log-transformed; §untransformed, randomization t test
Differences in DMC between seaweeds from the shallow range were analyzed with ANOVA after arcsine transformation, while N and P content and C/N ratio were analyzed with randomization tests since assumptions of normality and homogeneity of variance of residuals could not be fulfilled, despite transformation attempts. The differences between groups were assessed with Tukey’s HSD test in the case of DMC and with pairwise randomization tests corrected with the Holm correction for the N and P content and C/N ratio. All nutritional traits of seaweeds from the deep range were compared with randomization tests since the assumptions of normality and homogeneity of variance of residuals could not be fulfilled, despite transformation attempts. All statistical tests were conducted in R 3.0.2 (R Core Team 2013).
Results
In feeding experiments with live seaweeds, the isopod Idotea granulosa and the amphipod Gammarus locusta preferred to feed on the native seaweeds compared to the non-native Heterosiphonia japonica in all pairwise comparisons. The amphipod Gammarellus angulosus preferred the native seaweed in three cases (H. japonica in comparison with Cladophora sp., Ectocarpus siliculosus and Polysiphonia fucoides), but showed no significant preference in two cases (H. japonica versus Brongniartella byssoides and Ceramium virgatum, Fig. 1).
The preference pattern was partly changed after removing structural differences between the seaweeds. The isopod I. granulosa still preferred three of the native seaweeds (E. siliculosus, P. fucoides and B. byssoides) over H. japonica, but in the experiment with the native seaweeds Cladophora sp. and C. virgatum no significant preferences could be detected (Fig. 2).
The measures of nutritional value and tissue toughness indicated that H. japonica constitutes a food of high quality to herbivores. Heterosiphonia japonica showed the lowest C/N ratio of all seaweeds included in the experiments, both from the shallow and the deep communities (Shallow: Tukey’s HSD test after ANOVA, F(4,44) = 18.67, Prand < 0.001; Deep: Welch’s t test, t11 = 14.62, Prand < 0.001, Fig. 3). The invader had a significantly higher N content than all the native seaweeds except Cladophora sp. (Shallow: Tukey’s HSD test after ANOVA, F(4,44) = 18.16, Prand < 0.001; Deep: Welch’s t test, t18 = −13.53, Prand < 0.001, Fig. 3). The P content of H. japonica was within the range of the native seaweeds (Shallow: Tukey’s HSD test after ANOVA, F(4,44) = 22.14, Prand < 0.001; Deep: Welch’s t test, t18 = −22.04, Prand < 0.001, Fig. 3). The DMC of H. japonica was significantly lower compared to the native seaweeds in both depth ranges (Shallow: Tukey’s HSD test after ANOVA, F(4,45) = 39.05, P < 0.001; Deep: Welch’s t test, t16 = 10.74, Prand < 0.001, Fig. 3).
Carbon to nitrogen ratio (C/N), nitrogen (N), phosphorous (P) and dry matter content (DMC) of the native seaweeds Cladophora sericea/albida (Clad. sp.), Ectocarpus siliculosus (E. sil), Polysiphonia fucoides (P. fuc), and Ceramium virgatum (C. vir) and the non-native seaweed Heterosiphonia japonica (H. jap) collected in its shallow range and the native seaweed Brongniartella byssoides (B. bys) and Heterosiphonia japonica (H. jap) from its deeper range. Values are means ± CI95, means with a mutual letter in the figure are not significantly different (P > 0.05) in pairwise post hoc comparisons
No feeding deterrent metabolites could be detected in the crude extract of H. japonica (Fig. 4). On the contrary, I. granulosa fed significantly more on the artificial food with H. japonica extract compared to the solvent control.
Total consumption of agar disks with Ceramium virgatum treated with extract from Heterosiphonia japonica or treated with solvent only. Bars show mean loss of fresh weight of the two food types during the course of the experiment in the grazed treatments corrected by autogenic changes in the control treatments (±CI95, n = 10). The p value represents the outcome of the comparison of the differences in the treatment with the control for autogenic changes (see text for statistical analysis)
Discussion
This study shows that generalist herbivores native to northern European coasts prefer co-occurring native seaweeds to the introduced Heterosiphonia japonica. Low palatability is most likely a favorable trait for an invader in seaweed communities, where the predation pressure exerted by generalist grazers is high (Duffy and Hay 2000; Poore et al. 2012). Unpalatable non-native seaweeds may be favoured by predation on native competitors, as limited resources such as space and light are rendered available. This mechanism has been shown to be important for the invasion success of Bonnemaisonia hamifera (Enge et al. 2013), another highly abundant non-native filamentous red alga that is co-occurring with H. japonica in northern European seaweed communities (Moy and Christie 2012).
Experimental evidence shows that H. japonica has a very rapid growth rate compared to native co-occurring seaweeds, which was proposed to be an important part in explaining the high abundance of H. japonica in invaded communities (Sagerman et al. 2014). However, large areas of the introduced range maintain temperatures far below the growth optimum for H. japonica during extensive periods of the year (Breeman 1988; Bjærke and Rueness 2004). Being of low preference to native herbivores may provide a competitive advantage that allows H. japonica to persist in the seaweed community under seasons with sub-optimal growth conditions and could thus be a beneficial complement to annual bursts of rapid growth.
Despite different approaches to mechanistically explain the herbivores’ preference, we were not able to draw a firm conclusion on the characteristics that cause grazers to avoid feeding on H. japonica. When the structural differences between the experimental seaweeds were removed through freeze drying and grinding, the preference pattern of the isopod Idotea granulosa was less pronounced (i.e., the native seaweeds were preferred in only three cases out of five). This could indicate that feeding preference is partly dependent on structural differences between H. japonica and the native species. However, it is not obvious how the very thin and delicate structure of H. japonica would deter herbivory. In addition, H. japonica had a lower DMC than the native species, which commonly is associated with high food quality due to the positive correlation between DMC and tissue toughness (Jormalainen et al. 2001; Elger and Willby 2003; Wong et al. 2010; Jormalainen et al. 2011). An alternative explanation is that the weakened preference pattern in the experiment with ground seaweeds was the result of a partial degradation or loss of secondary metabolites during the homogenizing process. Regardless, a preference for the native seaweeds was still present, although less pronounced, in the experiments with homogenized seaweed tissue showing that the grazers respond to some chemical properties of the invader (i.e., secondary metabolites or nutritional value).
Commonly, herbivores are expected to prefer N rich food since N uptake can be a limiting factor for growth (Mattson 1980), although several studies have shown that N content and C/N ratio are often poor predictors of herbivore choice for different seaweed species (Duffy and Hay 1991; Cruz-Rivera and Hay 2001; Jormalainen et al. 2001). H. japonica had a higher N concentration and a lower C/N ratio than four of the five native seaweeds, and an intermediate P concentration, suggesting that macronutrient concentration could not account for the herbivores’ preference pattern. On the other hand, there was no evidence for feeding deterrent metabolites in H. japonica in the experiment with a crude extract. In fact, I. granulosa consumed more of the extract compared to the solvent control, which may have been due to nutritional enrichment of the gel by metabolites from the invader (e.g., sugars, lipids, and amino acids). Thus, we can only speculate about which chemical properties determined the feeding choice for intact seaweeds. It is possible that H. japonica possesses a grazer-deterrent metabolite that we could not detect. Volatile or light sensitive defense compounds may be lost or degraded during the algal extraction process and highly water-soluble compounds in the extract may rapidly diffuse out of the artificial diet. We observed that a red colored compound was released from the homogenized tissue of H. japonica and that the survival of the grazers was low in the presence of such exudates. Thus, the experimental containers had to be connected to flow through seawater during this part of the experiment. If deterrent metabolites were leaking from the artificial diet, it could explain why the pattern in grazer avoidance was less pronounced in the structure test.
The results of this study are in agreement with the majority of the findings from the few previous studies that have explored the palatability of non-native seaweeds. Compared to their native competitors most of the examined seaweed invaders constitute a low preference food for native herbivores; non-native red algae are almost consistently of low palatability (Tomas et al. 2011; Enge et al. 2012; Nejrup et al. 2012; Hammann et al. 2013), while there is somewhat more variation in palatability among non-native brown and green algae (Trowbridge 1995; Thornber et al. 2004; Sumi and Scheibling 2005; Wikström et al. 2006; Cacabelos et al. 2010; Tomas et al. 2011). The conditions under which non-native seaweeds are introduced may explain why being unpalatable tends to be a common feature of seaweed invaders. Firstly, seaweed communities are characterized by intense grazing that is exerted by generalist herbivores that include many different types of seaweeds into their diet (Duffy and Hay 2000; Poore et al. 2012). This makes it highly unlikely that non-native seaweeds will be released from predation because of a lack of recognition by native herbivores, as has been documented for invaders in terrestrial plant–insect systems (Agrawal et al. 2005; Han et al. 2008; Cincotta et al. 2009). Secondly, the majority of non-native seaweeds are unintentionally introduced (Williams and Smith 2007). Although the initial propagule pressure seldom is known, it is likely that many established seaweed invaders originate from a small number of individuals. In these small founder populations, high predation risk could rapidly select for well-defended genotypes. Grazer avoidance has been suggested to contribute to the invasion success of several non-native seaweeds (Gollan and Wright 2006; Monteiro et al. 2009; Nejrup et al. 2012), though it seldom has been explicitly tested (but see Enge et al. 2013). Considering that several of the most successful seaweed invaders are more or less unpalatable, the importance of herbivore defense for invasion success is an interesting topic for further studies.
In conclusion, the current study shows an example of a very successful seaweed invader that is escaping predation by being of low palatability. We suggest that the low palatability of H. japonica may be an important property both for the survival and success of the invader in its new range. The results adds to the picture that many successful non-native seaweeds are avoided by native grazers and calls for more research on the effects of herbivory on seaweed invasion success.
References
Agrawal AA, Kotanen PM, Mitchell CE, Power AG, Godsoe W, Klironomos J (2005) Enemy release? An experiment with congeneric plant pairs and diverse above-and belowground enemies. Ecology 86:2979–2989. doi:10.1890/05-0219
Andersson S, Engdahl A (2009) Mätkampanj 2009. Gullmarsfjorden, Askeröfjorden-Marstrandsfjorden. Marine Monitoring AB, Lysekil
Andersson S, Engdahl A, Asplund M (2010) Bohuskustens vattenvårdsförbund Makroalger i Brofjorden 2009. Marine Monitoring AB, Lysekil
Bjærke MR, Rueness J (2004) Effects of temperature and salinity on growth, reproduction and survival in the introduced red alga Heterosiphonia japonica (Ceramiales, Rhodophyta). Bot Mar 47:373–380. doi:10.1515/BOT.2004.055
Breeman AM (1988) Relative importance of temperature and other factors in determining geographic boundaries of seaweeds: experimental and phenological evidence. Helgoländer Meeresunters 42:199–241. doi:10.1007/BF02366043
Britton-Simmons KH (2004) Direct and indirect effects of the introduced alga Sargassum muticum on benthic, subtidal communities of Washington State, USA. Mar Ecol Prog Ser 277:61–78
Cacabelos E, Olabarria C, Incera M, Troncoso JS (2010) Do grazers prefer invasive seaweeds? J Exp Mar Biol Ecol 393:182–187. doi:10.1016/j.jembe.2010.07.024
Cincotta CL, Adams JM, Holzapfel C (2009) Testing the enemy release hypothesis: a comparison of foliar insect herbivory of the exotic Norway maple (Acer platanoides L.) and the native sugar maple (A. saccharum L.). Biol Invasions 11:379–388. doi:10.1007/s10530-008-9255-9
Core Team R (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Cruz-Rivera E, Hay ME (2001) Macroalgal traits and the feeding and fitness of an herbivorous amphipod: the roles of selectivity, mixing, and compensation. Mar Ecol Prog Ser 218:249–266. doi:10.3354/meps218249
Drouin A, McKindsey CW, Johnson LE (2011) Higher abundance and diversity in faunal assemblages with the invasion of Codium fragile ssp. fragile in eelgrass meadows. Mar Ecol Prog Ser 424:105–117. doi:10.3354/meps08961
Duffy JE, Hay ME (1991) Food and shelter as determinants of food choice by an herbivorous marine amphipod. Ecology 72:1286–1298. doi:10.2307/1941102
Duffy JE, Hay ME (2000) Strong impacts of grazing amphipods on the organization of a benthic community. Ecol Monogr 70:237–263. doi:10.2307/2657176
Elger A, Willby NJ (2003) Leaf dry matter content as an integrative expression of plant palatability: the case of freshwater macrophytes. Funct Ecol 17:58–65. doi:10.1046/j.1365-2435.2003.00700.x
Elton C (1958) The ecology of invasions by animals and plants. Methuen, London
Enge S, Nylund GM, Harder T, Pavia H (2012) An exotic chemical weapon explains low herbivore damage in an invasive alga. Ecology 93:2736–2745. doi:10.1890/12-0143.1
Enge S, Nylund GM, Pavia H (2013) Native generalist herbivores promote invasion of a chemically defended seaweed via refuge-mediated apparent competition. Ecol Lett 16:487–492. doi:10.1111/ele.12072
Gollan JR, Wright JT (2006) Limited grazing pressure by native herbivores on the invasive seaweed Caulerpa taxifolia in a temperate Australian estuary. Mar Freshw Res 57:685–694. doi:10.1071/MF05253
Gribben PE, Byers JE, Clements M, McKenzie LA, Steinberg PD, Wright JT (2009) Behavioural interactions between ecosystem engineers control community species richness. Ecol Lett 12:1127–1136. doi:10.1111/j.1461-0248.2009.01366.x
Grosholz E (2002) Ecological and evolutionary consequences of coastal invasions. Trends Ecol Evol 17:22–27. doi:10.1016/S0169-5347(01)02358-8
Hacker SD, Steneck RS (1990) Habitat architecture and the abundance and body-size-dependent habitat selection of a phytal amphipod. Ecology 71:2269–2285. doi:10.2307/1938638
Hammann M, Wang G, Rickert E, Boo SM, Weinberger F (2013) Invasion success of the seaweed Gracilaria vermiculophylla correlates with low palatability. Mar Ecol Prog Ser 486:93–103
Han X, Dendy SP, Garrett KA, Fang L, Smith MD (2008) Comparison of damage to native and exotic tallgrass prairie plants by natural enemies. Plant Ecol 198:197–210. doi:10.1007/s11258-008-9395-0
Hay ME (1991) Marine-terrestrial contrasts in the ecology of plant chemical defenses against herbivores. Trends Ecol Evol 6:362–365. doi:10.1016/0169-5347(91)90227-O
Husa V, Sjøtun K, Lein TE (2004) The newly introduced species Heterosiphonia japonica Yendo (Dasyaceae, Rhodophyta): geographical distribution and abundance at the Norwegian southwest coast. Sarsia 89:211–217. doi:10.1080/00364820410006600
Jormalainen V, Honkanen T, Heikkilä N (2001) Feeding preferences and performance of a marine isopod on seaweed hosts: cost of habitat specialization. Mar Ecol Prog Ser 220:219–230
Jormalainen V, Koivikko R, Ossipov V, Lindqvist M (2011) Quantifying variation and chemical correlates of bladderwrack quality—herbivore population makes a difference. Funct Ecol 25:900–909. doi:10.1111/j.1365-2435.2011.01841.x
Keane RM, Crawley MJ (2002) Exotic plant invasions and the enemy release hypothesis. Trends Ecol Evol 17:164–170. doi:10.1016/S0169-5347(02)02499-0
Kraufvelin P, Salovius S, Christie H et al (2006) Eutrophication-induced changes in benthic algae affect the behaviour and fitness of the marine amphipod Gammarus locusta. Aquat Bot 84:199–209. doi:10.1016/j.aquabot.2005.08.008
Lotze HK, Lenihan HS, Bourque BJ et al (2006) Depletion, degradation, and recovery potential of estuaries and coastal seas. Science 312:1806–1809. doi:10.1126/science.1128035
Manly BF (1991) Randomization and Monte Carlo methods in biology. Chapman and Hall, London
Maron JL, Vilà M (2001) When do herbivores affect plant invasion? Evidence for the natural enemies and biotic resistance hypothesis. Oikos 95:361–373
Mattson WJ (1980) Herbivory in relation to plant nitrogen content. Annu Rev Ecol Syst 11:119–161
Monteiro CA, Engelen AH, Santos ROP (2009) Macro- and mesoherbivores prefer native seaweeds over the invasive brown seaweed Sargassum muticum: a potential regulating role on invasions. Mar Biol 156:2505–2515. doi:10.1007/s00227-009-1275-1
Moy FE, Christie H (2012) Large-scale shift from sugar kelp (Saccharina latissima) to ephemeral algae along the south and west coast of Norway. Mar Biol Res 8:309–321. doi:10.1080/17451000.2011.637561
Naylor E (1955) The diet and feeding mechanism of Idotea. J Mar Biol Assoc UK 34:347–355
Nejrup LB, Pedersen MF, Vinzent J (2012) Grazer avoidance may explain the invasiveness of the red alga Gracilaria vermiculophylla in Scandinavian waters. Mar Biol 159:1–10. doi:10.1007/s00227-012-1959-9
Newton C, Bracken MES, McConville M, Rodrigue K, Thornber CS (2013) Invasion of the red seaweed Heterosiphonia japonica spans biogeographic provinces in the western North Atlantic Ocean. PLoS ONE 8:e62261. doi:10.1371/journal.pone.0062261
Nicotri ME (1980) Factors involved in herbivore food preference. J Exp Mar Biol Ecol 42:13–26. doi:10.1016/0022-0981(80)90163-X
Pasquini C, De Oliveira WA (1985) Monosegmented system for continuous flow analysis. Spectrophotometric determination of chromium (VI), ammonia and phosphorus. Anal Chem 57:2575–2579. doi:10.1021/ac00290a033
Pavia H, Carr H, Åberg P (1999) Habitat and feeding preferences of crustacean mesoherbivores inhabiting the brown seaweed Ascophyllum nodosum (L.) Le Jol. and its epiphytic macroalgae. J Exp Mar Biol Ecol 236:15–32. doi:10.1016/S0022-0981(98)00191-9
Peterson CH, Renaud PE (1989) Analysis of feeding preference experiments. Oecologia 80:82–86. doi:10.1007/BF00789935
Poore AGB, Campbell AH, Coleman RA et al (2012) Global patterns in the impact of marine herbivores on benthic primary producers. Ecol Lett 15:912–922. doi:10.1111/j.1461-0248.2012.01804.x
Sagerman J, Enge S, Pavia H, Wikström SA (2014) Divergent ecological strategies determine different impacts on community production by two successful non-native seaweeds. Oecologia 175:937–946. doi:10.1007/s00442-014-2938-2
Scheibling RE, Gagnon P (2006) Competitive interactions between the invasive green alga Codium fragile ssp. tomentosoides and native canopy-forming seaweeds in Nova Scotia(Canada). Mar Ecol Prog Ser 325:1–14
Sjøtun K, Husa V, Peña V (2008) Present distribution and possible vectors of introductions of the alga Heterosiphonia japonica (Ceramiales, Rhodophyta) in Europe. Aquat Invasions 3:377–394. doi:10.3391/ai.2008.3.4.3
Sumi CB, Scheibling RE (2005) Role of grazing by sea urchins Strongylocentrotus droebachiensis in regulating the invasive alga Codium fragile ssp. tomentosoides in Nova Scotia. Mar Ecol Prog Ser 292:203–212
Thornber CS, Kinlan BP, Graham MH, Stachowicz JJ (2004) Population ecology of the invasive kelp Undaria pinnatifida in California: environmental and biological controls on demography. Mar Ecol Prog Ser 268:69–80
Tomas F, Box A, Terrados J (2011) Effects of invasive seaweeds on feeding preference and performance of a keystone Mediterranean herbivore. Biol Invasions 13:1559–1570. doi:10.1007/s10530-010-9913-6
Trowbridge CD (1995) Establishment of the green alga Codium fragile ssp. tomentosoides on New Zealand rocky shores: current distribution and invertebrate grazers. J Ecol 83:949–965. doi:10.2307/2261177
Van den Hoek C (1982) A taxonomic revision of the American species of Cladophora (Chlorophyceae) in North-Atlantic Ocean and their geographic distribution. North-Holland Publishing Company, Amsterdam
Vaz-Pinto F, Olabarria C, Arenas F (2014) Ecosystem functioning impacts of the invasive seaweed Sargassum muticum (Fucales, Phaeophyceae). J Phycol 50:108–116. doi:10.1111/jpy.12136
Wallentinus I, Nyberg CD (2007) Introduced marine organisms as habitat modifiers. Mar Pollut Bull 55:323–332. doi:10.1016/j.marpolbul.2006.11.010
Wikström SA, Steinarsdóttir MB, Kautsky L, Pavia H (2006) Increased chemical resistance explains low herbivore colonization of introduced seaweed. Oecologia 148:593–601. doi:10.1007/s00442-006-0407-2
Williams S, Smith J (2007) A global review of the distribution, taxonomy, and impacts of introduced seaweeds. Annu Rev Ecol Evol Syst 38:327–359. doi:10.1146/annurev.ecolsys.38.091206.095543
Wong PK, Liang Y, Liu NY, Qiu J-W (2010) Palatability of macrophytes to the invasive freshwater snail Pomacea canaliculata: differential effects of multiple plant traits. Freshw Biol 55:2023–2031. doi:10.1111/j.1365-2427.2010.02458.x
Worm B, Lotze HK, Sommer U (2001) Algal propagule banks modify competition, consumer and resource control on Baltic rocky shores. Oecologia 128:281–293. doi:10.1007/s004420100648
Worm B, Barbier EB, Beaumont N et al (2006) Impacts of biodiversity loss on ocean ecosystem services. Science 314:787–790. doi:10.1126/science.1132294
Acknowledgments
We thank G. Cervin, G. M. Nylund, E. Bergvall and F. Baumgartner for field assistance. We also want to thank the staff at the Tjärnö Laboratory (Sven Lovén Centre for Marine Sciences) for their hospitality and practical assistance. We thank F. Baumgartner and two anonymous reviewers for helpful comments that helped improve the manuscript. The work was supported by grants from the Royal Swedish Academy of Sciences (to J. S.) and the Swedish Research Council Formas through contract no. 217-2007-534 (to S. A. W.) and by the Swedish Research Council VR through contract no. 621-2011-5630 (to H. P.).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: F. Weinberger.
Reviewed by M.F. Pedersen and undisclosed experts.
This article is part of the Topical Collection on Invasive Species.
Rights and permissions
About this article
Cite this article
Sagerman, J., Enge, S., Pavia, H. et al. Low feeding preference of native herbivores for the successful non-native seaweed Heterosiphonia japonica . Mar Biol 162, 2471–2479 (2015). https://doi.org/10.1007/s00227-015-2730-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-015-2730-9