Abstract
Behaviour influences individual fitness with effects that can propagate from the individual to the group. Here, we tested for higher-level effects of individual behaviour in the structuring of intertidal populations of two competing ecosystem engineering species. We used the partial habitat segregation exhibited by co-occurring indigenous (Perna perna) and invasive (Mytilus galloprovincialis) mussels in South Africa to test for possible attraction of different size classes of recruits to conspecific adults, using a combination of field and laboratory studies. Each of the two species dominates a particular height on the shore with overlap in the mid-mussel zone, but measurements of settlement and recruitment in the field partially refuted previous findings, generally showing no within-shore pattern of zonation of settlers and recruits. At smaller scales, recruits of both species were found more frequently on adults of Mytilus in natural beds where adults coexist in mixed-species populations. Finally, the results of laboratory choice experiments showed that recruits of all sizes responded to adult cues by movement, but that the smallest recruits showed only minimal movement and never reached adults; only large recruits of Perna responded positively to conspecific Perna adults. This study emphasises how observations made at different scales, from shore (among sites) to mussel bed (within shores), to the individual (field and laboratory), can produce different, or even contrasting, information, highlighting how behavioural traits, like attraction to conspecifics, can differ within the same group of organisms (congeneric species) and change ontogenetically within a species. Incorporating fine-scale responses makes predictions of population dynamics more complex, but identifying the relative strengths of mechanisms that lead to patterns of distribution is necessary for understanding higher-level interactions within a system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patterns in ecology are constantly re-shaped in time and space, reflecting complex interactions that are largely mediated directly or indirectly by species densities (Abrams 1995; Werner and Peacor 2003; Olff et al. 2009; Cole and McQuaid 2010). In benthic systems, most density-mediated processes have been linked to consumer–resource interactions (Wootton 1992; Menge 1995), while less attention has been paid to trait-mediated interactions, which are mostly defined by behavioural changes in one species in relation to another, with (usually) trophic repercussions on the community (Abrams 1995; Trussell et al. 2003). The incorporation of behavioural traits in our understanding of the dynamics of assemblages is important because, under certain circumstances, they may affect population dynamics (Wootton 1992; Siddon and Witman 2004).
Spatial patterns are very important in understanding ecosystem functioning and the role that small-scale interactions can have on structuring communities at larger scales (Hassell et al. 1994; Guichard and Gouhier 2014). For example, spatial patterns, such as self-organised (Liu et al. 2012) and fractal structures (e.g. Kostylev and Erlandsson 2001; Erlandsson et al. 2005), have important repercussions for mussel bed ecosystems by altering recolonisation (Petrović and Guichard 2008), recruitment (Erlandsson and McQuaid 2004; Commito et al. 2014), growth and survival (Petraitis 1995; Liu et al. 2013) and resilience (Liu et al. 2014) and could not be identified if interactions across the appropriate scales were not examined (Guichard et al. 2003; Largaespada et al. 2012). Rocky shore mussel beds in South Africa usually exhibit fractal patchy patterns (Erlandsson and McQuaid 2004; Erlandsson et al. 2005), and on the south coast of South Africa, these patchy, small-scale patterns are context and density dependent and are probably driven by both local recruitment and behavioural processes (Erlandsson et al. 2011; Reaugh-Flower et al. 2011), resulting in patterns that influence local population dynamics.
The marine environment, especially the intertidal system, is very heterogeneous, with high spatio-temporal variability in physical conditions across multiple scales, affecting the dynamics of most populations (Connell 1972; Hewitt et al. 2007). Given the theoretical prediction that long larval dispersal will favour phenotypic plasticity because larvae experience prolonged exposure to environmental heterogeneity (Hollander 2008), the immediate and reversible nature of behavioural responses (Fordyce 2006) could have important repercussions on the dynamics of intertidal systems.
Here, trait-mediated interactions are set in the unusual non-trophic context of distribution and coexistence of organisms controlled by behavioural interactions among conspecifics. We address the small-scale effects of individual behaviour on the structure of marine intertidal assemblages by examining the distribution and interactions of recruits of indigenous (Perna perna) and invasive (Mytilus galloprovincialis) mussels on the South African coast. Strong inter- and intraspecific competition and facilitation drive the dynamics and coexistence of these two species (Erlandsson et al. 2011; McQuaid et al. 2015). In the study area, the two species of mussels show partial habitat segregation, with Mytilus occupying the higher level of the mussel zone, while Perna dominates the low mussel zone. The two species coexist in the mid-mussel zone (Bownes and McQuaid 2006) where they show a patchy pattern and often exhibit non-random conspecific aggregations, which may be caused by both initial facilitation during recruitment and subsequent competition for space (Erlandsson et al., 2011). Settlement of these mussels seems to be regulated by tidal height rather than active attraction to adult beds (Porri et al. 2007; Bownes and McQuaid 2009), with more settlers, regardless of species, arriving on the low shore. We tested the prediction that, as seen previously, settlement would not mirror the distribution of adults, but because settlement is usually followed by a period of high mortality, the pattern of adult distribution would be driven by post-settlement processes and would be evident among recruits. We also tested the hypothesis that recruits in the mid-zone are attracted to adult conspecifics. Settlement and recruitment of the two species of mussels were measured in the field. The occurrence of individual settlers and recruits on conspecific adults and their attraction to conspecific adults were examined at the mussel bed level in the field and tested in the laboratory, respectively, using three size classes of recruits of each species, to possibly explain the patterns observed.
Materials and methods
Study sites
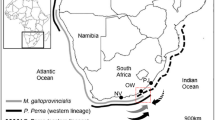
Animals for laboratory experiments and the field study were collected from two adjacent and similarly wave exposed rocky shore sites separated by c. 300 m at Keurbooms, on the south coast of South Africa (34°0′19″S 23°27′20″E), where the mussels Perna perna (indigenous) and Mytilus galloprovincialis (invasive) show distinct partial segregation. P. perna dominates the low mussel zone, and its downward limit is marked by the ascidian Pyura stolonifera and the red alga Hypnea spicifera. M. galloprovincialis occupies the higher level of the mussel zone. Farther upshore, the substratum is occupied by the barnacles Chthamalus dentatus and Octomeris angulosa. The two species of mussels coexist in the mid-mussel zone (Bownes and McQuaid 2006). In the study area, mussel settlers experience high levels of predation through larviphagy by adults (Porri et al. 2008) and recruits experience predation by both benthic and pelagic predators (Plass-Johnson et al. 2010). Predation on adults is, however, believed to be generally low and modelling suggests that the major cause of adult mortality is competition for space (Griffiths and Hockey 1987), though dislodgement by, for example, storm waves and shell damage caused by endolithic cyanobacteria are important causes of mortality (Kaehler 1999; Kaehler and McQuaid 1999; Erlandsson et al. 2006).
Settlement and recruitment on natural beds
Primary settlers (<400 µm) were differentiated from recruits following Porri et al. (2006) for Perna perna and Bownes and McQuaid (2009) for Mytilus galloprovincialis. Settlement and recruitment were monitored in the field in April, June and December 2006 at two sites at Keurbooms. Four haphazardly positioned samples of 10 cm × 10 cm were scraped from each of the low and high mussel zones during each sampling event and frozen at −12 °C until processing. Adults were separated by species and washed thoroughly under a 75-µm sieve, paying particular attention to the byssus, to detect and count the number of settlers and recruits of mussels.
Four 3-way ANOVAs were used to analyse the effects of site (random, 2 levels), time (random, 3 levels) and elevation on the shore (fixed, orthogonal, 2 levels) on settler and recruit abundances of P. perna and M. galloprovincialis, with specific focus on possible different ontogenetic and specific driven patterns. Homogeneity of variances was examined using Cochran’s test. Log(X + 1) transformation achieved homogeneity for the Mytilus dataset, but not for the Perna dataset. Given that heterogeneous variances are not critical when the total degrees of freedom are as high (df = 48) as in this balanced analysis (Underwood 1997; Quinn and Keough 2002), all datasets were analysed following log (X + 1) transformation.
Attachment of recruits on adults in the field
Attachment of settlers and recruits to adults was examined in the field. Ten 12 cm × 17 cm samples haphazardly collected from areas of 100 % mussel cover in mixed-species beds in the mid-mussel zone (Bownes and McQuaid 2006) were scraped from the shore at one of the sites used for investigating settlement. Samples were collected as entire blocks of mussel bed, taking care to minimise disruption of the byssal attachment among individual adults, by ensuring that the removed portions of mussel beds remained intact and that individual adults did not detach from one another. In order to eliminate possible effects of zonation, all samples were collected from the same height on the shore (mid-shore, where both species of mussels co-exist). The samples were kept frozen at −12 °C until processing. In the laboratory, individual mussels were carefully teased apart from the whole frozen samples, with as many of their own byssal threads as possible. All adult mussels (≥20 mm) were identified to species and measured using Vernier callipers. Each mussel was washed individually over a 75-µm sieve, and all attached settlers and recruits were counted and identified to species under a dissecting microscope.
A Chi-square test was used to determine how evenly mixed the natural adult beds were, or whether adult numbers were skewed towards one of the two species therefore departing from a 50/50 distribution. Similarly, a Chi-square test was used to test for attachment of recruits to adult conspecifics as departure from a 50/50 split could also produce skewed patterns of attachment of recruits onto adults.
Movement of recruits towards adults in the laboratory
To complement the information collected from the field study, an experiment was performed in a constant environment room at 20 °C and a 12:12-h light/dark cycle in order to assess active movement behaviour of different size classes (small 2–2.5 mm; medium 4–6 mm; large 8–10 mm) of recruits towards adults of either mussel species.
In order to confirm that all size classes of recruits are capable of local relocation, we ran a preliminary trial. Thirty recruits each of the small, medium and large size classes were tested separately by placing them about 4 cm apart in a grid of 10 × 3 on a smooth substratum. The trial was run twice (5–7/04/2006 and 24–25/04/2006) for the larger two size classes, but only once for small (2–2.5 mm) recruits, due to insufficient numbers of individuals. The trials were monitored continuously for the first hour and then hourly for the next 3 h to record any immediate aggregation response; the final number and size of clumps and solitary individuals were then recorded after 24 h. For the medium and large classes, the number of solitary individuals left at the end of the trial and the maximum number of individuals within a clump were analysed using separate 1-way ANOVAs.
The laboratory experiments on movement of recruits towards adults were therefore run over several assays during two trials, in April 2006 and March 2007, with a total of 15 replicates for each of three size classes.
Adults (30–55 mm shell length) and recruits were collected from Keurbooms, brought back to the laboratory in fresh seawater and kept aerated for 1 day prior to experimentation. The health of the adults was verified by observation of gaping behaviour, while the activity of recruits was confirmed by movement of the foot, observed under a dissecting microscope.
Experimental aquaria (12 cm × 17 cm) were set as controls or treatments, with aeration and circulation controlled using an air stone. Treatments consisted of monospecific clumps of four adults of each species placed on opposite sides (one species on the left side and the other species on the right) of the long side of the aquarium, using wire mesh to prevent them from ranging across the aquarium. Control aquaria were identical, with aeration and mesh, but no adults present. The experiment was run as a series of assays, each running for 4 days and involving a single recruit placed in a control aquarium and a recruit in a treatment aquarium. Each recruit was used in only one assay. After each assay, aquaria were washed in soapy waters and thoroughly rinsed in hot freshwater to avoid deposition of possible traces of chemical cues.
At the beginning of each assay, a single mussel recruit was placed at the centre of each treatment and each control container. The final position (left/right towards adults, or north/south of aquarium) and the straight-line distance travelled by each recruit were recorded after 4 days. If a recruit in a treatment aquarium reached and attached to an adult mussel, the species of that adult was noted. Assays for each of the three size classes of recruits of each species were interspersed in space and time to avoid confounding experimental effects. The experiment was run in 2006 (number of replicates for each size/species combination = 10) and 2007 (n = 5), involving a total of 180 individual recruits. Separate 3-way ANOVAs were used for each year (2006 and 2007) to analyse the effects of size (fixed, three levels: small, medium, large), species of recruit (fixed, two levels: Mytilus galloprovincialis, Perna perna) and treatment (fixed, two levels: presence or absence of adults) on the final distance travelled (dependent variable) by recruits. Both datasets were homogenous (Cochran’s test) after logarithmic (X + 1) transformation. Student–Newman–Keuls post hoc comparisons were used to examine differences among means of groups in the event of significant effects (Underwood 1997; Quinn and Keough 2002).
Due to the small number of expected values for each size class in the Chi-square tests and based on the outcome of ANOVA tests, the medium and large size classes were pooled for the following analyses. To determine whether there was a general response of medium to large recruits to the presence of adult mussels (regardless of which adult species), a Chi-square test was performed comparing movement towards adults versus movement not towards adults (i.e. along the north/south of the aquaria).
Medium and large recruits that joined adults in the laboratory experiment were analysed using a Chi-square test to determine whether they occurred more frequently on conspecific adults.
Results
Settlement and recruitment in the field on natural beds
Generally, settlement and recruitment of Perna perna showed high variability, and there were no clear trends driven by any of the factors considered (Fig. 1). The results for settlers and recruits of Mytilus galloprovincialis exhibited temporal variability, but significant interactions of time and shore elevation revealed higher abundances of Mytilus settlers on the low than on the high shore in December (although this difference between heights on the shore was driven only by Site 1, Fig. 1). A significant interaction between site and shore elevation for Mytilus recruits indicated higher abundance of recruits on the high shore at both sites in April and June (Tables 1, 2).
Attachment of recruits to adults in the field
Large recruits showed a faster clumping response than medium and small recruits, reaching their final positions within the first 2 h of the experiment after which they stopped moving. One-way ANOVAs showed no differences between large and medium recruits in the number of solitary individuals [F(1,2) = 3.449 P > 0.05] or maximum clump size [F(1,2) = 0.023 P > 0.05] after 24 h. As the trial could only be run once for small recruits, no test was performed, but the raw data suggest no obvious difference from the results for medium and large recruits (4, 10.5 and 4 solitary individuals and 11, 8 and 11.5 maximum size of clumps for small, medium and large recruits, respectively).
The Chi-square test on the distribution of adults in the mid-mussel bed was significant (χ2 (9) = 17.932, P < 0.05), indicating a prevalence of Mytilus over Perna. The size range of both species ranged between 30 and 70 mm with 53 % of Mytilus falling between 30 and 40 mm (Fig. 2).
The Chi-square test of the distribution of total recruit abundance was not significant (χ2 (9) = 12.480 for Mytilus and χ2 (9) = 2.692 for Perna recruits, P > 0.05 in both cases), indicating that recruits of both species occurred in similar frequencies on adults of both species, though overall, there were more recruits on Mytilus than Perna (Fig. 3).
Movement of recruits towards adults in the laboratory
The results for the distances moved by recruits during the experiments in 2006 and 2007 showed a significant effect of size in both cases [F(2,108) = 22.976, for 2006; F(2, 48) = 25.572, for 2007, P < 0.00001 in both cases] and of treatment [F(1,108) = 11.573 P < 0.001 for 2006 and F(1,48) 6.243, P < 0.05 for 2007], with no significant interaction, or significant effect of species. In both years, the net distance (i.e. straight line start to finish) travelled under control conditions was shorter than under treatment conditions. The SNK for the effect of size showed that, in 2006, small recruits (which did not move) grouped separately from medium and larger recruits, while in 2007, small recruits moved shorter distances than medium-sized recruits, which, in turn, moved shorter distances than large recruits for both species (Fig. 4; species pooled given the absence of a significant species effect or interaction). It is worth noting that small recruits in 2007 never reached adults, with only 8 out of 20 specimens moving an average distance of 0.4 and 0.8 cm in control and treatment, respectively, and the maximum distance of 2.5 cm travelled by one individual.
Preferential movement of recruits (species pooled) towards adults in the laboratory. Final distance travelled by small, medium and large recruits in the presence (treatment) or absence (control) of adults in 2006 (a) and 2007 (b). Error bars indicate standard errors. Treatments differed significantly in all cases and consistently among species. Letters on histograms indicate significant post hoc groups (Student–Newman–Keuls test on the effect of size)
The Chi-square test of movement of recruits towards adult mussels of either species was not significant (χ2(1) = 2.133, P > 0.05, indicating that medium to large size classes of recruits of both species were equally likely to move towards or away from adults. Of those recruits that did move towards adults, Perna of 4–10 mm moved towards conspecific adults (χ2(1) = 11.909, P < 0.005), while Mytilus recruits moved in similar proportions towards either species of adult.
Discussion
The patterns of settlement observed in the field during this study did not exactly reflect the findings of earlier studies in the same area where higher settlement on the low shore was observed (Porri et al. 2007; Bownes and McQuaid 2009). Only Mytilus settlers were significantly more abundant on the low than on the high shore, during one event (December). The results for recruitment showed no significant effects of zonation for Perna, whereas recruits of Mytilus were significantly more abundant in the high than in the low mussel zone on two out of the three sampled events (April and June), mostly at both sites. We therefore partly (for Perna), but not completely (for Mytilus) rejected the hypothesis that recruits attaching to conspecific adults would determine the zonation of mussels. This type of “uncertain” result, where previously observed patterns do not re-occur, highlights how difficult it is to make generalisations for systems with such high spatio-temporal variability. This is particularly problematic where the typical complexity and dynamism that drive the intertidal assemblages are further entangled by a biphasic life cycle. The inclusion and acknowledgement of such spatial heterogeneity is, however, necessary in order to generate more realistic models of population dynamics (Levin 1976; Siegel et al. 2008) and a better understanding of processes (Underwood and Chapman 1996; Kostylev et al. 2005) as well as a need for more applied fields such as effective management and conservation of living resources (García-Charton and Pérez-Rufaza 1999; García-Charton et al. 2004). Even though there was no consistent pattern of recruitment among shore heights, smaller-scale behavioural interactions between recruits and adult mussels at the individual level were revealed. The greatest abundance of recruits of both species was found on adults of Mytilus in natural mussel beds where adults of the two species coexist, very often in physical contact with one another. Nevertheless, non-random distributions of recruits are not the sole explanation for the non-random patchy pattern of Mytilus and Perna adults often found in the mid-zone, as this seems rather to be context dependent, and also driven by a balance between facilitation and competition (Erlandsson et al. 2011). Adults of Perna have approximately 20 % more byssal threads than similarly sized Mytilus (Zardi et al. 2006), and this presumably provides more habitat for the attachment of recruits. The total abundance of small-sized Mytilus adults (in the size range 30–50 mm) was, however, more than double the abundance of Perna adults, potentially providing increased interstitial space and humidity (Bertness et al. 2006, Lima et al. 2011) and causing recruits to select Mytilus or to survive better among Mytilus than Perna.
The preliminary movement trials showed that all size classes are capable of aggregating with similarly sized conspecifics, while the main experiment indicates a response to adults over the time scales used. The lack of a size x treatment interaction in the main experiment indicates that all size classes of recruits responded to conspecific adults (though minimal for small recruits which never reached adult beds). We do, however, see an ontogenetic difference in the degree of responsiveness to adults. In the laboratory choice experiments, medium and large recruits (4–10 mm in length) responded to adult cues, while small recruits (2–2.5 mm) showed very little movement, less than in the preliminary experiment where they clumped with similarly sized recruits, suggesting an ontogenetic shift/gradient of responsiveness. Moreover, the only class of recruits to show clear movement towards adult conspecifics in the laboratory was large recruits of Perna. While size-dependent mobility constraints cannot be entirely excluded, preliminary tests to set the appropriate temporal and spatial scales of the experiments for all size classes indicated the potential ability of small recruits to cover distances similar to those offered in the experimental trials in a shorter time than the duration of these experiments. Fine-scale ontogenetic shifts in the selection of settlement substratum are also known for different size classes of recruits of the same species of mussels tested in this study (von der Meden et al. 2010). Considering that Mytilus is invasive in this region, the lack of choice towards conspecifics could be interpreted as a greater capacity for behavioural plasticity, as generally suggested in invasion biology (Hazlett et al. 2002; Richards et al. 2006), though we did not test this here and the results indicating increased recruitment (by both species) on Mytilus adults in the field seems to contradict the lack of a conspecific attraction in the laboratory. These seemingly contradictory results from laboratory and field studies may indicate better survival of recruits associated with Mytilus under field conditions through indirect additional benefits provided by natural Mytilus beds, such as increased interstices, not present in the laboratory. Ontogenetic changes in behaviour, or more generally flexible behaviours, are important as they can allow fine-scale detection and response to environmental cues (Hazlett 1988; Sexton et al. 2002), possibly influencing patterns in intertidal habitats (Chapman 2000), such as distribution (Rochette and Dill 2000). Our results provide one example, together with the growing literature in marine systems (e.g. Williams and Morritt 1995; Crowe and Underwood 1998; Underwood et al. 2000; Wootton 2001; Commito et al. 2014), on how behaviourally mediated intra- and inter-specific interactions (direct and indirect) can influence patterns and dynamics on rocky shores. A species-specific, ontogenetically dependent attraction to conspecifics could provide a basic mechanism for early habitat choice and influence the patterns of adult distribution for both sessile and very mobile species (Gutiérrez 1998; Mundy and Babcock 1998). In the laboratory component of this study, we have only demonstrated direct behavioural interactions (either within species across ontogenetic stages or among congeneric species), but given the key role of mussels on the functioning of rocky shores, such interactions are likely to have indirect cascading effects on other species or life history stages (Dill et al. 2003).
This study shows how the investigation of spatial patterns or behaviour through different scale domains (sensu Wiens 1989), from shore to bed (natural patterns of settlement and recruitment on the shore to within-bed increased occurrence of individuals), to individual level (frequency of occurrence in the laboratory), can produce different, sometimes even contrasting, information. The different results observed at fine individual scales for the two species also highlight how different dynamic traits, like the attraction towards conspecifics shown in the laboratory, can potentially change across scales of time and space within the same group of organisms, as well as changing ontogenetically. Different behaviours may be further contingent on the state of the individual (Patterson et al. 2008), the presence and abundance of conspecifics (Sardiña et al. 2009) and the taxonomic scale at which the study is conducted (Díaz and McQuaid 2014). Our study confirms this and indicates that the concept of contingency extends to the ontogenetic stage within a species. Disentangling the dynamics that determine patterns and identifying the relative strength of which features and pathways are relevant and operate at different scales is, however, necessary for understanding higher level of interactions within a system. Unlike other studies, we did not analyse the spatial structure of mussel beds (Erlandsson and McQuaid 2004; van de Koppel et al. 2005, 2008; Erlandsson et al. 2011), but rather tried to explain the distribution of two mussel species within mussel beds and understand whether the patchy pattern of Mytilus and Perna observed in the mid-zone of these shores can be explained by recruitment processes and the subsequent behaviour of mussel recruits. Our scaling approach of studying patterns from shore to bed and individual behavioural attraction to conspecific adults could, nevertheless, be used in fine-scale landscape contexts, to help explain empirically/experimentally, the mechanisms that drive patterns in intertidal systems (Williams 1994; Burrows and Hawkins 1998; Kostylev et al. 2005; Commito et al. 2006, 2014).
References
Abrams PA (1995) Implications of dynamically variable traits for identifying, classifying, and measuring direct and indirect effects in ecological communities. Am Nat 146:112–134
Bertness MD, Crain CM, Silliman BR et al (2006) T he community structure of western Atlantic Patagonian rocky shores. Ecol Monogr 76:439–460. doi:10.1890/0012-9615(2006)076[0439:TCSOWA]2.0.CO;2
Bownes SJ, McQuaid CD (2006) Will the invasive mussel Mytilus galloprovincialis Lamarck replace the indigenous Perna perna L. on the south coast of South Africa? J Exp Mar Bio Ecol 338:140–151. doi:10.1016/j.jembe.2006.07.006
Bownes SJ, McQuaid CD (2009) Mechanisms of habitat segregation between an invasive and an indigenous mussel: settlement, post-settlement mortality and recruitment. Mar Biol 156:991–1006. doi:10.1007/s00227-009-1143-z
Burrows MT, Hawkins SJ (1998) Modelling patch dynamics on rocky shores using deterministic cellular automata. Mar Ecol Prog Ser 167:1–13. doi:10.3354/meps167001
Chapman MG (2000) Poor design of behavioural experiments gets poor results: examples from intertidal habitats. J Exp Mar Bio Ecol 250:77–95. doi:10.1016/S0022-0981(00)00180-5
Cole VJ, McQuaid CD (2010) Bioengineers and their associated fauna respond differently to the effects of biogeography and upwelling. Ecology 91:3549–3562. doi:10.1890/09-2152.1
Commito JA, Dow WE, Grupe BM (2006) Hierarchical spatial structure in soft-bottom mussel beds. J Exp Mar Biol Ecol 330:27–37
Commito JA, Commito AE, Platt R V., et al (2014) Recruitment facilitation and spatial pattern formation in soft-bottom mussel beds. Ecosphere 5. art160. doi:10.1890/ES14-00200.1
Connell JH (1972) Community interactions on marine rocky intertidal shores. Annu Rev Ecol Syst 3:169–192
Crowe TP, Underwood AJ (1998) Testing behavioural “preference” for suitable microhabitat. J Exp Mar Biol Ecol 225:1–11. doi:10.1016/S0022-0981(97)00187-1
Díaz E, McQuaid C (2014) Short-term spatial stability in trophic interactions. J Ecol 102:1138–1149. doi:10.1111/1365-2745.12272
Dill LM, Heithaus MR, Walters CJ (2003) Behaviorally mediated indirect interactions in marine communities and their conservation implications. Ecology 84:1151–1157. doi:10.1890/0012-9658(2003)084[1151:BMIIIM]2.0.CO;2
Erlandsson J, McQuaid CD (2004) Spatial structure of recruitment in the mussel Perna perna at local scales: effects of adults, algae and recruit size. Mar Ecol Prog Ser 267:173–185. doi:10.3354/meps267173
Erlandsson J, McQuaid CD, Kostylev VE (2005) Contrasting spatial heterogeneity of sessile organisms within mussel (Perna perna L.) beds in relation to topographic variability. J Exp Mar Biol Ecol 314:79–97
Erlandsson J, Pal P, McQuaid CD (2006) Re-colonisation rate differs between co-existing indigenous and invasive intertidal mussels following major disturbance. Mar Ecol Prog Ser 320:169–176. doi:10.3354/meps320169
Erlandsson J, McQuaid CD, Sköld M (2011) Patchiness and co-existence of indigenous and invasive mussels at small spatial scales: the interaction of facilitation and competition. PLoS ONE 6(11):e26958. doi:10.1371/journal.pone.0026958
Fordyce J (2006) The evolutionary consequences of ecological interactions mediated through phenotypic plasticity. J Exp Biol 209:2377–2383. doi:10.1242/jeb.02271
Garcia Charton JA, Perez Ruzafa A (1999) Ecological heterogeneity and the evaluation of the effects of marine reserves. Fish Res 42:1–20
García-Charton JA, Pérez-Ruzafa A, Sánchez-Jerez P et al (2004) Multi-scale spatial heterogeneity, habitat structure, and the effect of marine reserves on Western Mediterranean rocky reef fish assemblages. Mar Biol 144:161–182. doi:10.1007/s00227-003-1170-0
Griffiths CL, Hockey PAR (1987) A model describing the interactive roles of predation, competition and tidal elevation in structuring mussel populations. S Afr J Mar Sci 5:547–556. doi:10.2989/025776187784522496
Guichard F, Gouhier TC (2014) Non-equilibrium spatial dynamics of ecosystems. Math Biosci 255:1–10
Guichard F, Halpin PM, Allison GW et al (2003) Mussel disturbance dynamics: signatures of oceanographic forcing from local interactions. Am Nat 161:889–904. doi:10.1086/375300
Gutiérrez L (1998) Habitat selection by recruits establishes local patterns of adult distribution in two species of damselfishes: Stegastes dorsopunicans and S. planifrons. Oecologia 115:268–277. doi:10.1007/s004420050516
Hassell MP, Comins HN, May RM (1994) Species coexistence and self-organizing spatial dynamics. Nature 370:290–292. doi:10.1038/370290a0
Hazlett BA (1988) Behavioural plasticity as an adaptation to a variable environment. In Behavioral adaptation to intertidal life. NATO ASI Series. Series A, life sciences, vol 151. Springer, pp 317–332
Hazlett B, Acquistapace P, Gherardi F (2002) Differences in memory capabilities in invasive and native crayfish. J Crustac Biol 22:439–448. doi:10.1163/20021975-99990251
Hewitt JE, Thrush SF, Dayton PK, Bonsdorff E (2007) The effect of spatial and temporal heterogeneity on the design and analysis of empirical studies of scale-dependent systems. Am Nat 169:398–408. doi:10.1086/510925
Hollander J (2008) Testing the grain-size model for the evolution of phenotypic plasticity. Evolution (N Y) 62:1381–1389. doi:10.1111/j.1558-5646.2008.00365.x
Kaehler S (1999) Incidence and distribution of phototrophic shell-degrading endoliths of the brown mussel Perna perna. Mar Biol 135:505–514
Kaehler S, McQuaid CD (1999) Lethal and sub-lethal effects of phototrophic endoliths attacking the shell of the intertidal mussel Perna perna. Mar Biol 133:497–503
Kostylev V, Erlandsson J (2001) A fractal approach for detecting spatial hierarchy and structure on mussel beds. Mar Biol 139:497–506
Kostylev VE, Erlandsson J, Mak YM, Williams GA (2005) The relative importance of habitat complexity and surface area in assessing biodiversity: fractal application on rocky shores. Ecol Complex 2:272–286. doi:10.1016/j.ecocom.2005.04.002
Largaespada C, Guichard F, Archambault P (2012) Meta-ecosystem engineering: nutrient fluxes reveal intraspecific and interspecific feedbacks in fragmented mussel beds. Ecology 93:324–333. doi:10.1890/10-2359.1
Levin SA (1976) Population dynamic models in heterogeneous environments. Annu Rev Ecol Syst 7:287–310. doi:10.1146/annurev.es.07.110176.001443
Lima FP, Nicholas P. Burnett NP, Brian Helmuth B, Nicole Kish N, Kyle Aveni-Deforge K, Wethey DS (2011) Monitoring the intertidal environment with biomimetic devices, biomimetic based applications. In: Cavrak M (ed). ISBN: 978-953-307-195-4
Liu Q-X, Weerman EJ, Herman PMJ et al (2012) Alternative mechanisms alter the emergent properties of self-organization in mussel beds. Proc R Soc B Biol Sci 279:2744–2753. doi:10.1098/rspb.2012.0157
Liu Q-X, Doelman A, Rottschafer V et al (2013) Phase separation explains a new class of self-organized spatial patterns in ecological systems. Proc Natl Acad Sci USA 110:11905–11910. doi:10.1073/pnas.1222339110
Liu Q-X, Herman PMJ, Mooij WM et al (2014) Pattern formation at multiple spatial scales drives the resilience of mussel bed ecosystems. Nat Commun 5:5234. doi:10.1038/ncomms6234
McQuaid CD, Porri F, Nicastro KR, Zardi GI (2015) Simple, scale-dependent patterns emerge from very complex effects: an example from the intertidal mussels Mytilus galloprovincialis and Perna perna. Oceanogr Mar Biol 53:127–156
Menge BA (1995) Indirect effects in marine rocky intertidal interaction webs: patterns and importance. Ecol Monogr 65:21. doi:10.2307/2937158
Mundy CN, Babcock RC (1998) Role of light intensity and spectral quality in coral settlement: implications for depth-dependent settlement? J Exp Mar Biol Ecol 223:235–255. doi:10.1016/S0022-0981(97)00167-6
Olff H, Alonso D, Berg MP et al (2009) Parallel ecological networks in ecosystems. Philos Trans R Soc Lond B Biol Sci 364:1755–1779. doi:10.1098/rstb.2008.0222
Patterson TA, Thomas L, Wilcox C et al (2008) State-space models of individual animal movement. Trends Ecol Evol 23:87–94
Petraitis PS (1995) The role of growth in maintaining spatial dominance by mussels (Mytilus edulis). Ecology 76:1337–1346. doi:10.2307/1940940
Petrović F, Guichard F (2008) Scales of Mytilus spp population dynamics: importance of adult displacement and aggregation. Mar Ecol Prog Ser 356:203–214
Plass-Johnson JG, McQuaid CD, Porri F (2010) Top–down effects on intertidal mussel populations: assessing two predator guilds in a South African marine protected area. Mar Ecol Prog Ser 411:149–159. doi:10.3354/meps08681
Porri F, McQuaid CD, Radloff S (2006) Spatio-temporal variability of larval abundance and settlement of Perna perna: differential delivery of mussels. Mar Ecol Prog Ser 315:141–150. doi:10.3354/meps315141
Porri F, Zardi GI, McQuaid CD, Radloff S (2007) Tidal height, rather than habitat selection for conspecifics, controls settlement in mussels. Mar Biol 152:631–637. doi:10.1007/s00227-007-0716-y
Porri F, Jordaan T, McQuaid CD (2008) Does cannibalism of larvae by adults affect settlement and connectivity of mussel populations? Estuar Coast Shelf Sci 79:687–693. doi:10.1016/j.ecss.2008.06.010
Quinn GP, Keough MJ (2002) Experimental design and data analysis for biologists. Cambridge University Press, Cambridge
Reaugh-Flower K, Branch GM, Harris JM, McQuaid CD, Currie B, Dye A, Robertson B (2011) Scale-dependent patterns and processes of intertidal mussel recruitment around southern Africa. Mar Ecol Prog Ser 434:101–119. doi:10.3354/meps09169
Richards CL, Bossdorf O, Muth NZ et al (2006) Jack of all trades, master of some? On the role of phenotypic plasticity in plant invasions. Ecol Lett 9:981–993. doi:10.1111/j.1461-0248.2006.00950.x
Rochette R, Dill LM (2000) Mortality, behavior and the effects of predators on the intertidal distribution of littorinid gastropods. J Exp Mar Bio Ecol 253:165–191. doi:10.1016/S0022-0981(00)00253-7
Sardiña P, Cataldo DH, Boltovskoy D (2009) Effects of conspecifics on settling juveniles of the invasive golden mussel, Limnoperna fortunei. Aquat Sci 71:479–486. doi:10.1007/s00027-009-0103-5
Sexton JP, McKay JK, Sala A (2002) Plasticity and genetic diversity may allow saltcedar to invade cold climates in North America. Ecol Appl 12:1652–1660. doi:10.1890/1051-0761(2002)012[1652:PAGDMA]2.0.CO;2
Siddon CE, Witman JD (2004) Behavioral indirect interactions: multiple predator effects and prey switching in the rocky subtidal. Ecology 85:2938–2945. doi:10.1890/03-0519
Siegel D, Mitarai S, Costello CJ et al (2008) The stochastic nature of larval connectivity among nearshore marine populations. Proc Natl Acad Sci USA 105:8974–8979. doi:10.1073/pnas.0802544105
Trussell GC, Ewanchuk PJ, Bertness MD (2003) Trait-mediated effects in rocky intertidal food chains: predator risk cues alter prey feeding rates. Ecology 84:629–640. doi:10.1890/0012-9658(2003)084[0629:TMEIRI]2.0.CO;2
Underwood AJ (1997) Experiments in ecology: their logical design and interpretation using analysis of variance. Cambridge University Press, Cambridge, pp 1–522
Underwood A, Chapman M (1996) Scales of spatial patterns of distribution of intertidal invertebrates. Oecologia 107:212–224. doi:10.1007/BF00327905
Underwood AJ, Chapman MG, Connell SD (2000) Observations in ecology: you can’t make progress on processes without understanding the patterns. J. Exp. Mar. Bio. Ecol. 250:97–115
van de Koppel J, Rietkerk M, Dankers N, Herman PMJ (2005) Scale-dependent feedback and regular spatial patterns in young mussel beds. Am Nat 165:E66–E77. doi:10.1086/428362
van de Koppel J, Gascoigne JC, Theraulaz G et al (2008) Experimental evidence for spatial self-organization and its emergent effects in mussel bed ecosystems. Science (80) 322:739–742. doi:10.1126/science.1163952
Von Der Meden CEO, Porri F, McQuaid CD et al (2010) Fine-scale ontogenetic shifts in settlement behaviour of mussels: changing responses to biofilm and conspecific settler presence in Mytilus galloprovincialis and Perna perna. Mar Ecol Prog Ser 411:161–171. doi:10.3354/meps08642
Werner EE, Peacor SD (2003) A review of trait-mediated indirect interactions in ecological communities. Ecology 84:1083–1100. doi:10.1890/0012-9658(2003)084[1083:AROTII]2.0.CO;2
Wiens JA (1989) Spatial scaling in ecology. Funct Ecol 3:385–397. doi:10.2307/2389612
Williams GA (1994) The relationship between shade and molluscan grazing in structuring communities on a moderately-exposed tropical rocky shore. J Exp Mar Bio Ecol 178:79–95. doi:10.1016/0022-0981(94)90226-7
Williams GA, Morritt D (1995) Habitat partitioning and thermal tolerance in a tropical limpet, Cellana grata. Mar Ecol Prog Ser 124:89–103
Wootton JT (1992) Indirect effects, prey susceptibility, and habitat selection: impacts of birds on limpets and algae. Ecology 73:981–991
Wootton JT (2001) Local interactions predict large-scale pattern in empirically derived cellular automata. Nature 413:841–844. doi:10.1038/35101595
Zardi GI, Nicastro KR, McQuaid CD et al (2006) Hydrodynamic stress and habitat partitioning between indigenous (Perna perna) and invasive (Mytilus galloprovincialis) mussels: constraints of an evolutionary strategy. Mar Biol 150:79–88. doi:10.1007/s00227-006-0328-y
Acknowledgments
We thank C.E.O von der Meden and T. Jordaan for assistance during the field collections and F.K. McQuaid and I. McQuaid and E. Yeruham for help in processing samples in the laboratory. Many thanks also go to V.J. Cole for advice on the statistical analyses. This study was funded by the Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning (FORMAS) and SIDA/Sarec, Sweden. This contribution is based upon research supported by the South African Research Chairs Initiative of the Department of Science and Technology and National Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Additional information
Responsible Editor: M.G Chapman.
Reviewed by Undisclosed experts.
Rights and permissions
About this article
Cite this article
Porri, F., McQuaid, C.D. & Erlandsson, J. The role of recruitment and behaviour in the formation of mussel-dominated assemblages: an ontogenetic and taxonomic perspective. Mar Biol 163, 157 (2016). https://doi.org/10.1007/s00227-016-2932-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-016-2932-9