Abstract
The mussel Mytilus galloprovincialis is highly invasive worldwide, but displays varying degrees of local and regional coexistence with indigenous mussels through spatial habitat segregation. We investigated the roles of settlement, post-settlement mortality, juvenile growth and recruitment in partial habitat segregation between the invasive M. galloprovincialis and the indigenous mussel Perna perna on the south coast of South Africa. We used two study locations, Plettenberg Bay and Tsitsikamma, 70 km apart, with two sites (separated by 300–400 m) per location, each divided into three vertical zones. There were no significant effects in Tsitsikamma, where daily settlement and monthly recruitment were significantly lower than in Plettenberg Bay. In Plettenberg Bay, settlement (primary and secondary) and recruitment of both species decreased upshore. Post-settlement mortality was measured over two consecutive 6-day periods during a spring tide and a neap tide. For both species mortality was low on the low-shore. High-shore mortality was consistently low for M. galloprovincialis, but increased dramatically for P. perna during spring tide. No data were obtained for growth of P. perna, but juvenile M. galloprovincialis grew more slowly farther upshore. P. perna recruited mainly in spring and summer, with a peak in summer far greater than for M. galloprovincialis. Recruitment of M. galloprovincialis was more protracted, continuing through autumn and winter. Thus local coexistence is due to a combination of pre- and post-recruitment factors differing in importance for each species. P. perna is excluded from the high-shore by recruitment failure (low settlement, high mortality). High survival and slow growth in juveniles may allow large densities of M. galloprovincialis to accumulate there, despite low settlement rates. With no differences between species in settlement or mortality on the low-shore, exclusion of M. galloprovincialis from that zone is likely to be by post-recruitment processes, possibly strengthened by periodic heavy recruitments of P. perna. At larger scales, larval retention and protracted recruitment contribute to the success of M. galloprovincialis at Plettenberg Bay, while recruitment limitation may explain why M. galloprovincialis is less successful at other sites.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The rising number of non-indigenous species being transported across the world’s oceans through shipping and for aquaculture poses a significant threat to marine biodiversity through the elimination or displacement of indigenous species (Carlton and Geller 1993; Occhipinti-Ambrogi 2001). The Mediterranean mussel, Mytilus galloprovincialis Lamarck, is one of the most successful marine invasive species and is now globally distributed throughout the temperate zones of the northern and southern hemispheres (Hilbish et al. 2000). From its origin in the Mediterranean it has spread to the northwest Atlantic coast from North Africa to England (Sanjuan et al. 1994; Comesaña et al. 1998) and has successfully colonised shores in Japan, Hong Kong, Korea, Australia and New Zealand (Wilkins et al. 1983; Lee and Morton 1985; McDonald et al. 1990, 1991). However, the most significant invasions of this species are found on the west coasts of North America and South Africa (McDonald and Koehn 1988; Branch and Steffani 2004; Braby and Somero 2006).
The South African coastline has recently been invaded by the barnacle Balanus glandula Darwin (Laird and Griffiths 2008), but M. galloprovincialis remains the most extensively widespread non-indigenous marine species, now covering over 2,050 km of coastline (Robinson et al. 2005). Since its introduction on the west coast in the late 1970s, M. galloprovincialis has become the dominant intertidal organism from the Cape of Good Hope to southern Namibia, where it has displaced the indigenous mussel Aulacomya ater Molina (Griffiths et al. 1992; Branch and Steffani 2004). It has spread onto the south coast of South Africa as far as East London, probably due to a combination of range expansion from the west coast and an independent introduction to Port Elizabeth harbour for mariculture in 1989 (van Erkom Schurink and Griffiths 1990; McQuaid and Phillips 2000). Here, M. galloprovincialis has come into contact with a different indigenous mussel, Perna perna Linnaeus. It has established large populations at some sites but remains in low densities along most of the coastline (Griffiths et al. 1992; von der Meden unpublished data). It is not infected by parasites that are prevalent in P. perna and reduce its growth and fecundity (Calvo-Ugarteburu and McQuaid 1998a, b), so that it seems likely that M. galloprovincialis has competitive advantages over P. perna. However, where they co-occur at high abundances, the two species show partial vertical segregation. M. galloprovincialis dominates the higher mussel zone, where P. perna is typically sparse, presumably because it has greater tolerance to desiccation (Hockey and van Erkom Schurink 1992; Nicastro et al. 2008). Although it occurs in high densities across the shore this has not been at the expense of P. perna, which remains the dominant species on the low-shore (Bownes and McQuaid 2006). Thus M. galloprovincialis has not displaced P. perna from its preferred habitat and coexistence seems possible.
Where it has become invasive in other parts of the world, M. galloprovincialis exhibits similar local and regional variations in abundance and in its effects on indigenous mussel populations. The same vertical zonation pattern has been described between this species and Perna canaliculus Gmelin in New Zealand (Kennedy 1976). In North America, M. galloprovincialis has displaced the indigenous mussel Mytilus trossulus Gould from most of its former habitat in central and southern California, so that the two species are largely separated regionally (Braby and Somero 2006; Fields et al. 2006). However, it has not displaced another indigenous Californian mussel, Mytilus californianus Conrad, which is predominant on wave-exposed shores, while M. galloprovincialis appears to be confined to more sheltered shores (Martel et al. 1999).
Most studies have examined habitat segregation between M. galloprovincialis and indigenous species from a physiological perspective (Kennedy 1976; Braby and Somero 2006; Fields et al. 2006). In South Africa, differential tolerance to aerial exposure and wave action plays a role in the vertical distributions of M. galloprovincialis and P. perna (Hockey and van Erkom Schurink 1992; Zardi et al. 2006). However, intertidal community structure is influenced by many factors other than physical stress. For organisms with planktonic larvae, recruitment to the shore is one of the most important factors influencing adult populations. In the past, comparative recruitment studies between sympatric mussel species have been hindered by the difficulty in distinguishing between the larval and post-larval stages, particularly amongst closely related congeneric species that hybridise (Garland and Zimmer 2002). This has been improved by the advent of molecular identification techniques (e.g. Johnson and Geller 2006), but these can be impractical for multivariate studies with large sample sizes. The situation on the south coast of South Africa offers a unique opportunity to examine the role of recruitment in habitat segregation as M. galloprovincialis and P. perna do not hybridise and their post-larvae can be distinguished morphologically (Bownes et al. 2008). Recruitment limitation is of particular importance on this coast as mussel recruitment rates are orders of magnitude lower than on most temperate boreal shores and the upwelling-dominated west coast of South Africa (Harris et al. 1998).
Recruitment refers to the number of larvae that have settled and survived for a certain period of time, and so includes settlement and a defined period of post-settlement mortality (Keough and Downes 1982). Settlement involves the initial attachment of larvae to the substratum and can significantly influence adult distribution and abundance. Being partly dependent on the delivery of competent larvae to the shore, factors influencing settlement include large-scale oceanographic processes such as upwelling, currents and hydrodynamics associated with shoreline topography (Roughgarden et al. 1988; Bertness et al. 1996; Archambault and Bourget 1999), while closer to shore tidal movements, wave action, substratum availability and tidal height can be important (Gaines and Roughgarden 1985; Bertness et al. 1992; Vargas et al. 2004). Settlement also includes the additional component of larval behaviour and habitat selection, which is widely documented in marine invertebrates (Raimondi 1988; Osman and Whitlatch 1995; Lemire and Bourget 1996), and may be an important mechanism in avoiding detrimental post-settlement interactions (Petersen 1984; Bushek 1988).
However, many species are capable of relocating after initial settlement. In mussels initial attachment (primary settlement) can be followed by detachment and re-attachment (secondary settlement) in different zones or on different substrata (Beukema and de Vlas 1989; Alfaro 2006), which may occur many times before juveniles permanently enter the adult population (Bayne 1964). Mussel larvae frequently settle initially on algae (e.g. McQuaid and Lindsay 2005). While for some species the fate of such larvae is uncertain (Reaugh et al. 2007), settlement onto algae may be followed by secondary settlement to the mussel bed (Bayne 1964; Alfaro 2006). Thus primary and secondary settlement can affect adult population structure in different ways. Mortality in juvenile invertebrates is often determined within the first few days after settlement, due to the physiological stress associated with metamorphosis, and a greater vulnerability to physical stress in smaller individuals (Gosselin and Qian 1997; Garcia-Esquivel et al. 2001). Variations in post-settlement mortality can therefore obscure patterns of settlement, leading to large-scale differences in community structure and composition (Osman and Whitlatch 2004).
Here we examine the factors controlling habitat segregation and coexistence in Perna perna and Mytilus galloprovincialis and whether zonation is a result of primarily pre- or post-recruitment events. We investigate the role of settlement, early post-settlement mortality, juvenile growth and recruitment to test specific hypotheses based on the population structure of P. perna and M. galloprovincialis at two locations with different mussel abundances on the south coast (Bownes and McQuaid 2006): (1) P. perna is excluded from the high-shore by recruitment failure, either through lack of settlement or through post-settlement mortality; (2) high densities of M. galloprovincialis in this zone reflect the accumulation of successive settlements of slow-growing individuals that show good survival; (3) recruitment limitation is responsible for the difference in mussel abundance between these locations, and may delay invasion by M. galloprovincialis at other locations with low recruitment.
Materials and methods
Study sites
Sampling took place at two locations 70 km apart on the south coast of South Africa: Plettenberg Bay (34°05′S; 23°19′E), where M. galloprovincialis is abundant, and Tsitsikamma (33°1′S; 23°53′E), where it is rare. Two randomly chosen sites were selected at each location. The shore at each site was divided into three vertical zones characterised by different taxa and different patterns of mussel cover. Plettenberg Bay is typical of a series of half-heart bays on this coast. It includes a long sandy beach interspersed with patches of sand-swept granite rocks. The two sites, Lookout Beach and Beacon Isle, were ca. 400 m apart near the middle of the bay, and both were exposed in terms of wave action. Mussel beds were multilayered, particularly in the mid and high zones. The indigenous mussel Choromytilus meridionalis Krauss was present only on the low-shore at Lookout Beach. Mussels dominated the lower intertidal zones at this location, while barnacles (Chthamalus dentatus Krauss) were most abundant on the high-shore. Tsitsikamma has sandstone shores and little sand. The two sites, Sandbaai and Driftwood Bay, were ca. 300 m apart and lay in a slight embayment that was moderately exposed or exposed to wave action. Mussels formed fairly continuous monolayered beds on the mid-shore and occurred in isolated patches in the low and high mussel zones. The low-shore was characterised by a band of encrusting coralline algae and the limpet Scutellastra cochlear Born, while barnacles (Octomeris angulosa Sowerby, Tetraclita serrata Darwin) and the snail, Littorina africana knysnaensis Philippi were abundant in the high zone.
Post-larval identification
Post-larvae (0.28–5.0 mm) of the three mussels Perna perna, Mytilus galloprovincialis and Choromytilus meridionalis were identified to species under a dissecting microscopic based on diagnostic morphological features described in Bownes et al. (2008).
Distinguishing primary and secondary settlers
In mussels, there is a clear demarcation between the larval and adult shell regions, allowing size at settlement to be determined (Martel et al. 1995). Settlers were collected from randomly selected settlement pads (see below) retrieved after 24 h on the shore from all zones and both locations. Size at settlement was measured under a light microscope fitted with an ocular micrometer.
Settlement and recruitment
Settlers were collected using plastic scouring pads soaked in sea water for 24 h before use (see Gilg and Hilbish 2003). Six screws were drilled into the rocks in each zone, about 1.0 m apart, and individual pads were attached to each screw using washers and cable ties. All mussels collected from settlement pads on a daily basis will be referred to as settlers (primary or secondary) and those collected at monthly intervals as recruits. Recruitment was measured monthly from July 2000 to July 2001 and daily settlement samples were collected concurrently in April 2001. However, samples could not be collected every day due to sea conditions, preventing estimation of post-settlement mortality. Settlement and post-settlement mortality were therefore measured again in March 2003.
Settlement
Daily settlement was measured from 27 March to 26 April 2001 in Plettenberg Bay and Tsitsikamma. There were three pads per zone at each site that were replaced every 24 h upon collection. The remaining three pads were collected at the end of the month to estimate recruitment. Both settlement and recruitment were very poor in Tsitsikamma, so that the repetition of the experiment was only done at the two sites in Plettenberg Bay. This took place from 17 to 28 March 2003, although rough seas prevented sampling at Beacon Isle after 24 March. Six pads per zone were replaced daily upon collection.
Settlement pads were frozen immediately after collection. In the laboratory, settlers were removed by vigorously shaking the pad in water, which was then filtered through two sieves of 1 mm and 0.15 mm mesh size. This process was repeated three to four times. The contents of each sieve were washed into a Petri dish and examined under a dissecting microscope. Settlers were transferred to a piece of filter paper (using a pipette for the smaller individuals), identified to species and measured using a micrometer. Juveniles were distinguished as either “dead” (no tissue in the valves) or “alive” at the time of collection. For settlement, all mussels were counted. Samples were stored in 70% alcohol.
Post-settlement mortality
Post-settlement mortality was measured over the same period in March 2003, covering two consecutive periods of 6 days over a spring and a neap tide. An additional six pads were attached to each screw giving six pairs per zone. One pad of each pair was replaced daily, while the second was left on the shore for 6 days. In this way we hoped to minimise variation in settlement between the daily and 6-day pads. Due to interrupted sampling, mortality could not be measured at Beacon Isle over neap tide.
Mortality for each pair was calculated by subtracting the total number of “live” recruits in the 6-day pads from the cumulative daily settlement that occurred during that time. Mortality was expressed as a percentage of the cumulative daily settlement. There are shortcomings to this method, but due to the large number of variables being measured it was the only feasible method of measuring mortality in situ, while keeping an adequate sample size.
Recruitment
Recruitment samples were collected monthly from June 2000 to July 2001 at both sites in Plettenberg Bay, and from August 2000 and October 2000 to July 2001 at Sandbaai and Driftwood Bay in Tsitsikamma, respectively. Logistic problems resulted in sampling intervals of 2 weeks on rare occasions and one interval of 6 weeks. The number of recruits in each pad was averaged for the two time periods over which they were in the field. The data were grouped into months and then standardised by converting to recruitment in 30 days.
Recruitment samples with exceptionally high numbers were sub-sampled using a plankton splitter. Smaller (<1.0 mm) and larger juveniles were sub-sampled separately, and this was usually only necessary for the smaller sizes. The efficiency of this technique was confirmed by comparing subsamples statistically. Juveniles were again distinguished as either dead or alive upon collection and only live mussels were counted as recruits.
Juvenile growth
Juvenile growth was measured in situ at the two sites in Plettenberg Bay, in November 2003 using the fluorochrome growth marker calcein (Kaehler and McQuaid 1999). Eighteen pads were placed on the low-shore at each location for 48 h to collect settlers. The pads were then removed and immersed in a calcein-sea water solution (200 mg l−1) for 2 h. After immersion, six pads were placed in each zone. Samples were collected after 2 weeks and kept frozen. In the laboratory juveniles were extracted and measured individually under a light microscope fitted with an ocular micrometer. Once measured, each mussel was examined under an Olympus fluorescence microscope and growth was measured as the distance between the growing edge of the shell and the calcein mark (Kaehler and McQuaid 1999).
Analyses
Settlement
Settlement was averaged for each day, and date was used as the replicate. Date was not included as a factor due to poor sample sizes on some days and because substantial variation in daily settlement rates might have masked the effects of those factors that were more relevant to our questions. In 2001, samples could not be collected over the two neap tides due to sea conditions, therefore only dates with pads that had been on the shore for 24 h were used in the analyses (n = 22). We examined the effects of site, zone and/or species on settlement using factorial ANOVA with unbalanced sample sizes, where site was a random factor and zone and species were fixed. Post hoc comparisons were made using Newman–Keuls multiple range tests. The data were log-transformed when the assumptions of parametric analysis were not met. For 2001 no transformation was required and for 2003 all data were transformed except for the comparison of secondary settlement amongst zones at Beacon Isle.
Post-settlement mortality
Mortality was analysed using factorial ANOVA with unbalanced sample sizes and site, zone or species as factors. The data did not require transformation.
Recruitment
The recruitment data were grouped into seasons defined as follows: October–November (spring), December–February (summer), March–May (autumn) and June–July (winter). The months from June to September 2000 were excluded as we did not have data for all four sites. A mixed model Nested ANOVA was performed with season, location, site, zone and species as factors, where site was random and nested within location.
Juvenile growth
Due to poor recovery of marked individuals, virtually no results were obtained for P. perna. There was a strong positive relationship between initial length and growth of M. galloprovincialis, which was generally significant. M. galloprovincialis growth was analysed using a two-way ANCOVA with initial length as a covariate and site and zone as factors. The data did not require transformation.
Results
Categorising primary and secondary settlers
Perna perna and M. galloprovincialis settlers had similar size distributions, generally ranging from 270 to 320 μm, with the largest percentage of larvae settling at approximately 290 μm (Fig. 1). The majority of newly settled post-larvae were <330 μm in length, with a maximum size of 340 μm for P. perna. Primary settlers were therefore categorised as post-larvae of <340 μm in length and all individuals ≥340 μm were regarded as secondary settlers.
Settlement
April 2001
There was no primary settlement in Tsitsikamma. In Plettenberg Bay, primary settlement for P. perna was very low with only a few individuals settling during this time. Therefore, only M. galloprovincialis settlement was analysed and only for 5 April onwards, as there was virtually no settlement before this (n = 16). There was a significant interaction between site and zone (Table 1a), the difference between sites being significant only on the low-shore, with higher settlement at Beacon Isle. M. galloprovincialis also settled in significantly greater numbers on the low-shore than in the upper zones at this site. Although settlement decreased upshore at Lookout Beach, the effect of zone was not significant.
Secondary settlement in Tsitsikamma was very low and intermittent and was only 3% of that in Plettenberg Bay. The Tsitsikamma data were therefore excluded from the analysis so that location effects were not examined in either year. The ANOVA of secondary settlement in Plettenberg Bay revealed significant interactions between site and species and between zone and species (Table 1b). Secondary settlement of M. galloprovincialis was significantly greater than for P. perna at Lookout Beach, with no difference between species at Beacon Isle. M. galloprovincialis settlement was significantly greater at Lookout Beach than at Beacon, Isle while P. perna settlement did not differ between sites. M. galloprovincialis also settled in significantly greater numbers than P. perna on the low-shore across sites (Fig. 2). Both species showed an upshore decrease with similar, low settlement on the high-shore.
March 2003
Primary settlement was generally very low at both Plettenberg Bay sites, except for one peak in settlement of M. galloprovincialis at Lookout Beach on 28 March (low-shore mean 33.5 ± 27.09 settlers/pad). Sites could only be compared for the first 8 days (17–24 March) and there were significant site-zone and site-species interactions (Table 2a). For the site–zone interaction, settlement decreased upshore with a significant difference between the low and high zones at both sites (Fig. 3a). Only mid-shore settlement varied between sites with greater settlement at Lookout Beach. For the site–species interaction there was little difference in primary settlement between species at Lookout Beach, while P. perna had significantly higher settlement than M. galloprovincialis at Beacon Isle (Fig. 3b). Primary settlement of M. galloprovincialis was significantly greater at Lookout Beach than Beacon Isle, with no site differences for P. perna.
For secondary settlement P. perna and M. galloprovincialis showed a significant site-species interaction (Table 2b). Both species had fewer secondary settlers at Beacon Isle and settlement was significantly greater for M. galloprovincialis than P. perna at Lookout Beach (Fig. 4).
Although settlement was measured over relatively short time-scales and direct comparisons between years were not possible, the overall pattern was clear. Both primary and secondary settlement of P. perna and M. galloprovincialis decreased upshore, with no zone-dependent differences between species. This occurred irrespective of site and year in Plettenberg Bay. Settlement also varied among sites and locations. Settlement in Plettenberg Bay was substantially greater than in Tsitsikamma.
Post-settlement mortality
Mortality of P. perna and M. galloprovincialis in March 2003 was compared between zones and sites over spring tide, but there were no significant effects or interactions. There was a marked difference in settlement rates between the two sites in the first 6 days of sampling. We therefore examined post-settlement mortality over spring tide at each site separately.
At Lookout Beach, mortality was significantly greater in the upper zones than the low shore (F2,20 = 3.85, P = 0.038), with no effect of species. The interaction between zone and species was not quite significant (F2,20 = 3.17, P = 0.064), but some zone-specific differences between species were important. Mortality of P. perna and M. galloprovincialis was similar in the two lower zones and significantly higher on the mid-shore than on the low-shore for both species (Fig. 5). However, on the high-shore mortality of P. perna increased dramatically, while mortality of M. galloprovincialis decreased (65 and 17%, respectively). There was no significant difference in mortality between zones or species at Beacon Isle where settlement rates were significantly lower.
Mortality over a neap tide at Lookout Beach showed a significant effect of zone (F2,26 = 4.06, P = 0.029) but not of species or the interaction term. Mortality of both species was significantly greater on the mid-shore than either the low or high-shore. P. perna exhibited different patterns of high-shore mortality between tides, with 65% mortality over spring tide and only 6% over neap tide. In contrast, M. galloprovincialis displayed the same zone mortality pattern during both tides.
Recruitment
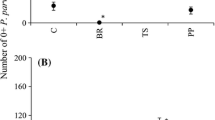
Perna perna and M. galloprovincialis exhibited different patterns of recruitment in Plettenberg Bay (Fig. 6). P. perna recruited mainly in spring and summer (October–March) with very little recruitment in the autumn and winter months. There was a major recruitment peak at Beacon Isle in January, with a smaller peak on the mid-shore only in November and December (spring/early summer) that was observed for M. galloprovincialis as well. However, recruitment of M. galloprovincialis was more protracted, continuing through autumn and winter. Both species had very poor recruitment from July to September 2000. Recruitment rates were reduced higher up the shore.
Recruitment rates were substantially lower in Tsitsikamma. The seasonal patterns of recruitment for P. perna and M. galloprovincialis were similar to those observed in Plettenberg Bay, though there were no obvious peaks (Fig. 7; note difference in scale from that in Fig. 6). The effect of zone was not as apparent here, although recruitment appeared to be reduced on the high-shore.
The Nested ANOVA revealed a significant interaction between season, location, zone and species (F6,575 = 4.819, P < 0.01). Recruitment of both species was significantly greater in Plettenberg Bay than in Tsitsikamma in the lower zones, regardless of season (Fig. 8), with no difference in high-shore recruitment. There were no significant differences in recruitment between seasons, zones or species in Tsitsikamma.
Post hoc comparison of the season × location × zone × species interaction on recruitment of P. perna and M. galloprovincialis in a Plettenberg Bay and b Tsitsikamma. S spring, SU summer, AU autumn, W winter. Letters indicate homogeneous groups (Newman–Keuls test, α < 0.05). All bars in Tsitsikamma are signified by homogeneous group e
In Plettenberg Bay, recruitment decreased higher up the shore and the difference between the low and high zones was generally significant for both species (Fig. 8). High-shore recruitment did not differ significantly between species or seasons. The effect of zone was most apparent for P. perna in summer due to a peak in low-shore recruitment, which was far greater than that of M. galloprovincialis at any other time. Recruitment of P. perna was significantly greater in spring and summer than autumn and winter in the lower zones. There was a smaller peak in recruitment of M. galloprovincialis on the mid-shore in spring, but otherwise recruitment was more similar between seasons. Recruitment in the lower zones was greater than for P. perna in autumn and winter with a significant difference in autumn.
There was also a significant interaction between site (location), season and species (F6,575 = 4.302, P = 0.014). Again there were no significant differences in recruitment between sites in Tsitsikamma. In Plettenberg Bay, the seasonal patterns described for each species were broadly the same, but with some important site-specific differences. P. perna recruited mainly in spring and summer at both sites but the large summer recruitment peak was only observed at Beacon Isle (Fig. 9). At Lookout Beach, recruitment of M. galloprovincialis was significantly greater in spring than the other seasons, while at Beacon Isle, recruitment was similar from spring to autumn, but was lower in winter. The species-specific differences between seasons were only significant at Beacon Isle (i.e. P. perna > M. galloprovincialis in summer and M. galloprovincialis > P. perna in autumn). Both species had significantly greater recruitment at Beacon Isle than at Lookout Beach in summer and for M. galloprovincialis in autumn.
Post hoc comparison of the site (location) × season × species interaction on recruitment of P. perna and M. galloprovincialis at Lookout Beach (LB) and Beacon Isle (BI) in Plettenberg Bay. There were no significant differences between sites in Tsitsikamma. S spring, SU summer, AU autumn, W winter. Letters indicate homogeneous groups (Newman–Keuls test, α < 0.05)
Juvenile growth
No data were obtained for the growth of juvenile P. perna, but post-larval growth of M. galloprovincialis increased with increasing size, although less obviously so on the high-shore where growth of larger juveniles was slower (Fig. 10). Growth appeared to decrease with increasing height on the shore, as seen by the flattening of the growth curve; however, the differences were marginally non-significant (Table 3).
Discussion
Primary settlement is often a strong determinant of adult community structure in intertidal invertebrates, but for mussels its effects can be modified by the dispersal and resettlement of post-larval stages. In this study, there were site effects but the two settlement phases did not appear to differ between tidal heights for either P. perna or M. galloprovincialis; the abundances of both primary and secondary settlers decreased upshore for both species. The effect of site on the abundance of primary and secondary settlers varied between species and in years. Invertebrate post-larvae have been recorded from offshore plankton samples suggesting that large-scale secondary dispersal does occur; however, most evidence indicates that it is a more localised process, often triggered by disturbance (Olivier et al. 1996; Cáceres-Martínez et al. 1994, Cáceres-Martínez and Figueras 1998). In contrast, planktonic larvae can be dispersed over scales of 10s to 100s of km (McQuaid and Phillips 2000; Becker et al. 2007). Primary and secondary settlement are therefore likely to be influenced by different processes. Local physical conditions such as coastal morphology, wind, currents, internal tidal waves and larval patchiness in the water column are likely to result in the differential delivery of larvae among sites 300–400 m apart (Vargas et al. 2004; McQuaid and Lindsay 2005; Porri et al. 2006), while local hydrodynamics and disturbance regimes may be more important to secondary relocation and settlement of post-larvae (Olivier et al. 1996).
In terms of their vertical distributions it is clear that adult populations of M. galloprovincialis do not reflect differential settlement, while vertical patterns of settlement were consistent with the adult distribution of P. perna. This effect of zone has been observed for M. galloprovincialis and P. perna elsewhere (Cáceres-Martínez and Figueras 1997; McQuaid and Lindsay 2005). Intertidal invertebrates often experience reduced settlement at higher shore levels (e.g. Bertness et al. 1992), which may be due to the greater submergence time in the lower zones that allows more time for settlement (Menge 1991; Miron et al. 1995). For P. perna, greater settlement on the low-shore could be interpreted as a response to adult conspecifics (Barnett et al. 1979; Raimondi 1988), but recent manipulative experiments indicate that it is an effect of tidal emersion (Porri et al. 2007).
In California, the invasive M. galloprovincialis settled abundantly where adults were rare, while the indigenous M. californianus settled mostly in its adult habitat (Johnson and Geller 2006). Post-settlement mortality was considered to be responsible for adult distribution of the invasive. Our results showed that post-settlement survival of both species on the low-shore was very good (>80%). Both species also recruited more successfully in the lower zones, indicating that low-shore populations are regulated primarily by adult interactions. However, there were significant variations in seasonal recruitment rates between sites and species on the low-shore. For example, at Beacon Isle, P. perna exhibited strong summer recruitment on the low-shore that was significantly greater than for M. galloprovincialis. This is likely to reflect differences in reproductive season between species (Zardi et al. 2007), but the effects of post-settlement mortality may also be important.
Settlement therefore fails to explain the large densities of M. galloprovincialis in the high zone and the vast difference in relative species abundances there. We proposed that slow growth in juveniles would minimise intra-specific competition for space and, combined with good survival, would allow the accumulation of large densities of M. galloprovincialis in this zone despite low rates of settlement, while low settlement and/or post-settlement mortality would limit the abundance of P. perna. Our results support this hypothesis. Growth of juvenile M. galloprovincialis tended to decrease further upshore, particularly in larger individuals (>1.0 mm). Most intertidal bivalves experience slower growth further upshore due to reduced feeding time and increased physiological stress at higher shore levels (Griffiths 1981; Griffiths and Griffiths 1987; Vincent et al. 1994; Bartol et al. 1999). However, the influence of tidal height on juvenile mussel (M. galloprovincialis) growth may not become apparent for several weeks post-settlement (Phillips 2002), which may account for the (marginally) non-significant results in our study.
A shortcoming of the mortality method used is that it cannot distinguish between mortality and emigration of mussels (if any) from the recruitment pads, nor can it account for immigration of larger individuals. Also, mortality estimates could be biased between species, for example, if one species is dislodged more easily after death. As adults, P. perna has greater attachment strength than M. galloprovincialis (Zardi et al. 2007), so that dislodgement of dead M. galloprovincialis juveniles might occur more quickly than for P. perna. We have not measured this, but large numbers of dead individuals of both species were found in the monthly recruitment samples, suggesting that the risk of losing individuals within 24 h of settlement is minimal. Nevertheless, the results should be treated cautiously. Post-settlement mortality of M. galloprovincialis on the high-shore remained low over both the spring and neap tides and at both sites. However, mortality of P. perna increased markedly upshore over the spring tide at Lookout Beach, so that high-shore mortality of P. perna far exceeded that of M. galloprovincialis (65 and 17%, respectively). In other juvenile invertebrates, increased mortality upshore has been attributed to increased desiccation stress with greater aerial exposure (Kennedy 1976; Roegner and Mann 1995; Chan and Williams 2003). Conditions during neap tide were often too rough to sample, indicating that high-shore areas are more wave splashed during neap than spring low tides (personal observation), which presumably reduces desiccation. M. galloprovincialis is more tolerant of desiccation stress than P. perna as adults (Hockey and van Erkom Schurink 1992), and the better post-settlement survival of M. galloprovincialis in the high zone implies the same dissimilarity in the early settlement stages, at least at certain times.
Perna perna recruited mainly in spring and summer, with a strong summer peak on the low-shore that was significantly greater than for M. galloprovincialis at any other time. M. galloprovincialis experienced a small peak in recruitment in spring/early summer, but otherwise recruitment was more protracted for this species, continuing through autumn and winter. Strong pulses of recruitment of P. perna over M. galloprovincialis may contribute to the persistence of P. perna as the dominant species on the low-shore. However, the absence of a significant seasonal peak for M. galloprovincialis could have been an anomaly, as it experiences seasonal recruitment peaks on the west coast of South Africa (Harris et al. 1998) and strong variations in recruitment rates among years are common in many invertebrates (Siegel et al. 2008); but on face value the data suggest a more protracted reproductive season for this species on the south coast. Protracted recruitment can be advantageous for invasive species as the process is repeated under differing conditions (Crawley 1986). In Plettenberg Bay, disturbance caused by winter storms creates free space in the mid-intertidal zone that M. galloprovincialis is able to exploit better than P. perna due to its greater colonisation ability (Erlandsson et al. 2006). This could reflect the prolonged recruitment of M. galloprovincialis through winter, at a time when P. perna recruits are virtually absent. Recovery of P. perna was poor, which raises the concern that M. galloprovincialis may eventually be able to outcompete P. perna at this location (Erlandsson et al. 2006).
Settlement and recruitment were consistently and substantially greater in Plettenberg Bay than Tsitsikamma, supporting our hypothesis that poor settlement and recruitment limitation are responsible for the differences in adult abundances between locations. Wind-induced upwelling is common along the Tsitsikamma coast (Schumann et al. 1982), and may cause larvae to be transported offshore, resulting in poor recruitment rates there (Roughgarden et al. 1988; Connolly and Roughgarden 1998). The south coast in general experiences low, trickle recruitment (Lasiak and Barnard 1995; McQuaid and Phillips 2006), which has been linked to low spawning intensity (McQuaid and Phillips 2006). Larval dispersal and mortality may also result in heavier recruitment losses on the open coast compared to bays, which frequently act as larval retention sites (Archambault and Bourget 1999; Helson and Gardner 2004; McQuaid and Phillips 2006). This has important implications for the success of M. galloprovincialis at certain sites and its spread on the south coast.
In marine environments, many non-indigenous species have disjointed distributions that are restricted to semi-enclosed systems such as harbours, bays and estuaries (Robinson et al. 2005; Wasson et al. 2005). This may be influenced by larval retention, which promotes successful establishment and population expansion of a colonising species. For example, the success of the invasive barnacle Elminius modestus Darwin in Lough Hyne was attributed to a high degree of water retention, which led to it forming a self-perpetuating population (Lawson et al. 2004; Watson et al. 2005). However, larval retention can also limit larval dispersal and hence the spread of non-indigenous species out of these systems. There is likely to be some exchange with the open coast so that high density populations may act as a source for nearby coastal sites (McQuaid and Phillips 2006). This may have affected the increase in abundance of M. galloprovincialis in Tsitsikamma during this study (Bownes and McQuaid 2006). However, this effect diminishes with distance from the source population (McQuaid and Phillips 2000). The rate of spread of M. galloprovincialis on this coast appears to be decreasing (Rius and McQuaid 2006) and the evidence suggests that recruitment limitation plays a role.
Conclusions
Local coexistence between P. perna and M. galloprovincialis on the south coast seems to be due to a combination of pre- and post-recruitment events that differ in importance between species. Our results show that the relative contributions of settlement, post-settlement mortality and recruitment to the determination of adult distributions are complex, and none could explain habitat segregation entirely. As hypothesised, P. perna is largely excluded from the high-shore by recruitment failure. M. galloprovincialis is able to dominate the high intertidal zone, most likely because of the absence of a dominant competitor and the high physiological tolerance of M. galloprovincialis to desiccation stress (Nicastro et al. 2008). In other parts of the world, M. galloprovincialis displays a similar superiority to indigenous species in the upper intertidal zones, which has been explained by its enhanced tolerance of high temperatures and aerial exposure (Kennedy 1976; Petes et al. 2007; Schneider and Helmuth 2007). High survival and slow growth in juveniles may allow large densities of M. galloprovincialis to accumulate there, despite low settlement rates. Both P. perna and M. galloprovincialis had strong settlement and recruitment on the low-shore and high post-settlement survival. Low adult abundance of M. galloprovincialis in this zone must therefore be determined primarily by post-recruitment events, including greater vulnerability to wave action and competitive exclusion by P. perna (Rius and McQuaid 2006; Zardi et al. 2006). At larger scales, larval retention and protracted recruitment are likely to have contributed to the success of M. galloprovincialis in Plettenberg Bay. However, recruitment is strongly limited on the south coast, and this appears to have restricted the spread of M. galloprovincialis and its ability to establish viable populations at most sites.
References
Alfaro AC (2006) Population dynamics of the green-lipped mussel, Perna canaliculus, at various spatial and temporal scales in northern New Zealand. J Exp Mar Biol Ecol 334:294–315. doi:https://doi.org/10.1016/j.jembe.2006.02.004
Archambault P, Bourget E (1999) Influence of shoreline configuration on spatial variation of meroplanktonic larvae, recruitment and diversity of benthic subtidal communities. J Exp Mar Biol Ecol 238:161–184. doi:https://doi.org/10.1016/S0022-0981(98)00146-4
Barnett BE, Edwards SC, Crisp DJ (1979) A field study of settlement behaviour in Balanus balanoides and Elminius modestus (Cirripedia: Crustacea) in relation to competition between them. J Mar Biol Assoc UK 59:575–580
Bartol IK, Mann R, Luckenbach M (1999) Growth and mortality of oysters (Crassostrea virginica) on constructed intertidal reefs: effects of tidal height and substrate level. J Exp Mar Biol Ecol 237:157–184. doi:https://doi.org/10.1016/S0022-0981(98)00175-0
Bayne BL (1964) Primary and secondary settlement in Mytilus edulis L. (Mollusca). J Anim Ecol 33:513–523. doi:https://doi.org/10.2307/2569
Becker BJ, Levin LA, Fodrie FJ, McMillan PA (2007) Complex larval connectivity patterns among marine invertebrate populations. Ecology 104:3267–3272
Bertness MD, Gaines SD, Stephens EG, Yund PO (1992) Components of recruitment in populations of the acorn barnacle Semibalanus balanoides (Linnaeus). J Exp Mar Biol Ecol 156:199–215. doi:https://doi.org/10.1016/0022-0981(92)90246-7
Bertness MD, Gaines SD, Wahle RA (1996) Wind-driven settlement patterns in the acorn barnacle Semibalanus balanoides. Mar Ecol Prog Ser 137:103–110. doi:https://doi.org/10.3354/meps137103
Beukema JJ, de Vlas J (1989) Tidal-current transport of thread-drifting post-larval juveniles of the bivalve Macoma balthica from the Wadden Sea to the North Sea. Mar Ecol Prog Ser 52:193–200. doi:https://doi.org/10.3354/meps052193
Bownes SJ, McQuaid CD (2006) Will the invasive mussel Mytilus galloprovincialis Lamarck replace the indigenous Perna perna L. on the south coast of South Africa? J Exp Mar Biol Ecol 338:140–151. doi:https://doi.org/10.1016/j.jembe.2006.07.006
Bownes SJ, Barker NP, McQuaid CD (2008) Morphological identification of primary settlers and post-larvae of three mussel species from the coast of South Africa. Afr J Mar Sci 30:233–240. doi:https://doi.org/10.2989/AJMS.2008.30.2.3.553
Braby CE, Somero GN (2006) Following the heart: temperature and salinity effects on heart rate in native and invasive species of blue mussels (genus Mytilus). J Exp Mar Biol Ecol 209:2554–2566
Branch GM, Steffani CN (2004) Can we predict the effects of alien species? A case-history of the invasion of South Africa by Mytilus galloprovincialis (Lamarck). J Exp Mar Biol Ecol 300:189–215. doi:https://doi.org/10.1016/j.jembe.2003.12.007
Bushek D (1988) Settlement as a major determinant of intertidal oyster and barnacle distributions along a horizontal gradient. J Exp Mar Biol Ecol 122:1–18. doi:https://doi.org/10.1016/0022-0981(88)90208-0
Cáceres-Martínez J, Figueras A (1997) Mussel (Mytilus galloprovincialis Lamarck) settlement in the Ria de Vigo (NW Spain) during a tidal cycle. J Shellfish Res 16:83–85
Cáceres-Martínez J, Figueras A (1998) Distribution and abundance of mussel (Mytilus galloprovincialis Lmk.) larvae and post-larvae in the Ria de Vigo (NW Spain). J Exp Mar Biol Ecol 229:227–287. doi:https://doi.org/10.1016/S0022-0981(98)00059-8
Cáceres-Martínez J, Robledo AF, Figueras A (1994) Settlement and post-larvae behaviour of Mytilus galloprovincialis: field and laboratory experiments. Mar Ecol Prog Ser 112:107–117. doi:https://doi.org/10.3354/meps112107
Calvo-Ugarteburu G, McQuaid CD (1998a) Parasitism and invasive species: effects of digenetic trematodes on mussels. Mar Ecol Prog Ser 169:149–163. doi:https://doi.org/10.3354/meps169149
Calvo-Ugarteburu G, McQuaid CD (1998b) Parasitism and invasive species: effects of digenetic trematodes on mussels. Mar Ecol Prog Ser 169:149–163. doi:https://doi.org/10.3354/meps169149
Carlton JT, Geller JB (1993) Ecological roulette: the global transport of non-indigenous marine organisms. Science 261:78–82. doi:https://doi.org/10.1126/science.261.5117.78
Chan BKK, Williams GA (2003) The impact of physical stress and molluscan grazing on the settlement and recruitment of Tetraclita species (Cirripedia: Balanomorpha) on a tropical shore. J Exp Mar Biol Ecol 284:1–23. doi:https://doi.org/10.1016/S0022-0981(02)00475-6
Comesaña AS, Posada D, Sanjuan A (1998) Mytilus galloprovincialis LMK. in northern Africa. J Exp Mar Biol Ecol 223:271–283. doi:https://doi.org/10.1016/S0022-0981(97)00185-8
Connolly SR, Roughgarden J (1998) A latitudinal gradient in Northeast Pacific Intertidal community structure: evidence for an oceanographically based synthesis of marine community theory. Am Nat 151:311–326. doi:https://doi.org/10.1086/286121
Crawley MJ (1986) The population biology of invaders. Philos Trans R Soc Lond B Biol Sci 314:711–731. doi:https://doi.org/10.1098/rstb.1986.0082
Erlandsson J, Pal P, McQuaid CD (2006) Re-colonization rate differs between co-existing indigenous and invasive intertidal mussels following major disturbance. Mar Ecol Prog Ser 320:169–176. doi:https://doi.org/10.3354/meps320169
Fields PA, Rudomin EL, Somero GN (2006) Temperature sensitivities of cystolic malate dehydrogenase from native and invasive species of marine mussels (genus Mytilus): sequence-function linkages and correlations with biogeographic distribution. J Exp Mar Biol Ecol 209:656–667
Gaines S, Roughgarden J (1985) Larval settlement rate: a leading determinant of structure in an ecological community of the marine intertidal zone. Proc Natl Acad Sci USA 82:3707–3711. doi:https://doi.org/10.1073/pnas.82.11.3707
Garcia-Esquivel Z, Bricelj VM, González-Gómez MA (2001) Physiological basis for energy demands and early postlarval mortality in the Pacific oyster, Crassostrea gigas. J Exp Mar Biol Ecol 263:77–103. doi:https://doi.org/10.1016/S0022-0981(01)00300-8
Garland ED, Zimmer CA (2002) Techniques for the identification of bivalve larvae. Mar Ecol Prog Ser 225:299–310. doi:https://doi.org/10.3354/meps225299
Gilg MR, Hilbish TJ (2003) Patterns of larval dispersal and their effect on the maintenance of a Blue Mussel hybrid zone in southwestern England. Evol Int J Org Evol 57:1061–1077
Gosselin LA, Qian P (1997) Juvenile mortality in benthic marine invertebrates. Mar Ecol Prog Ser 146:265–282. doi:https://doi.org/10.3354/meps146265
Griffiths RJ (1981) Population dynamics and growth of the Bivalve Choromytilus meridionalis (Kr.) at different tidal levels. Estuar Coast Shelf Sci 12:101–118. doi:https://doi.org/10.1016/S0302-3524(81)80120-X
Griffiths CL, Griffiths RJ (1987) Bivalvia. In: Pandian TJ, Vernberg FJ (eds) Animal energetics. Academic Press, New York, pp 1–88
Griffiths CL, Hockey PAR, Van Erkom Schurink C, Le Roux PJ (1992) Marine invasive aliens on South African rocky shores: implications for community structure and trophic functioning. S Afr J Mar Sci 12:713–722
Harris JM, Branch GM, Elliott BL, Currie B, Dye AH, McQuaid CD, Tomalin BJ, Velasquez C (1998) Spatial and temporal variability in recruitment of intertidal mussels around the coast of southern Africa. S Afr J Zool 33:1–11
Helson JG, Gardner JPA (2004) Contrasting patterns of mussel abundance at neighbouring sites: does recruitment limitation explain the absence of mussels on Cook Strait (New Zealand) shores? J Exp Mar Biol Ecol 312:285–298. doi:https://doi.org/10.1016/j.jembe.2004.07.006
Hilbish TJ, Mullinax A, Dolven SI, Meyer A, Koehn RK, Rawson PD (2000) Origin of the antitropical distribution pattern in marine mussels (Mytilus spp.): routes and timing of transequatorial migration. Mar Biol (Berl) 136:69–77. doi:https://doi.org/10.1007/s002270050010
Hockey PAR, Van Erkom Schurink C (1992) The invasive biology of the mussel Mytilus galloprovincialis on the southern African coast. Trans R Soc S Afr 48:123–139
Johnson SB, Geller JB (2006) Larval settlement can explain the adult distribution of Mytilus californianus Conrad but not of M. galloprovincialis Lamarck or M. trossulus Gould in Moss Landing, central California: evidence from genetic differentiation of spat. J Exp Mar Biol Ecol 328:136–145. doi:https://doi.org/10.1016/j.jembe.2005.07.007
Kaehler S, McQuaid CD (1999) Use of the fluorochrome calcein as an in situ growth marker in the brown mussel Perna perna. Mar Biol (Berl) 133:455–460. doi:https://doi.org/10.1007/s002270050485
Kennedy VS (1976) Desiccation, higher temperatures and upper intertidal limits of three species of sea mussels (Mollusca: Bivalvia) in New Zealand. Mar Biol (Berl) 35:127–137. doi:https://doi.org/10.1007/BF00390934
Keough MJ, Downes BJ (1982) Recruitment of marine invertebrates: the role of active larval choices and early mortality. Oecologia 54:348–352. doi:https://doi.org/10.1007/BF00380003
Laird MC, Griffiths CL (2008) Present distribution and abundance of the introduced barnacle Balanus glandula Darwin in South Africa. Afr J Mar Sci 30:93–100. doi:https://doi.org/10.2989/AJMS.2008.30.1.9.459
Lasiak TA, Barnard TCE (1995) Recruitment of the brown mussel Perna perna onto natural substrata: a refutation of the primary/secondary settlement hypothesis. Mar Ecol Prog Ser 120:147–153. doi:https://doi.org/10.3354/meps120147
Lawson J, Davenport J, Whitaker A (2004) Barnacle distribution in Lough Hyne Marine Nature Reserve: a new baseline and an account of invasion by the introduced Australasian species Elminius modestus Darwin. Estuar Coast Shelf Sci 60:729–735. doi:https://doi.org/10.1016/j.ecss.2004.03.011
Lee SY, Morton B (1985) The introduction of the Mediterranean mussel, Mytilus galloprovincialis into Hong Kong. Malacol Rev 18:107–109
Lemire M, Bourget E (1996) Substratum heterogeneity and complexity influence micro-habitat selection of Balanus sp. and Tubularia crocea larvae. Mar Ecol Prog Ser 135:77–87. doi:https://doi.org/10.3354/meps135077
Martel A, Hynes TM, Buckland-Nicks J (1995) Prodissoconch morphology, planktonic shell growth, and size of metamorphosis in Dreissena polymorpha. Can J Zool 73:1835–1844. doi:https://doi.org/10.1139/z95-216
Martel AL, Robles C, Beckenbach K, Smith MJ (1999) Distinguishing early juveniles of Eastern Pacific mussels (Mytilus spp.) using morphology and genomic DNA. Invertebr Biol 118:149–164. doi:https://doi.org/10.2307/3227056
McDonald JH, Koehn RK (1988) The mussels Mytilus galloprovincialis and M. trossulus on the Pacific coast of North America. Mar Biol (Berl) 99:111–118. doi:https://doi.org/10.1007/BF00644984
McDonald JH, Koehn RH, Balakirev ES, Manchenco GP, Pudovkin AI, Sergiyevskii SO, Krutovskii KV (1990) Species identity of the ‘common mussel’ inhabiting the Asiatic coasts of the Pacific Ocean. Biol Mora (Vladivost) 1:13–22
McDonald JH, Seed R, Koehn RK (1991) Allozymes and morphometric characters of three species of Mytilus in the Northern and Southern Hemispheres. Mar Biol (Berl) 3:323–333. doi:https://doi.org/10.1007/BF01319403
McQuaid CD, Lindsay JR (2005) Interacting effects of wave exposure, tidal height and substratum on spatial variation in densities of mussel Perna perna plantigrades. Mar Ecol Prog Ser 301:173–184. doi:https://doi.org/10.3354/meps301173
McQuaid CD, Phillips TE (2000) Limited wind-driven dispersal of intertidal mussel larvae: in situ evidence from the plankton and the spread of the invasive species Mytilus galloprovincialis in South Africa. Mar Ecol Prog Ser 201:211–220. doi:https://doi.org/10.3354/meps201211
McQuaid CD, Phillips TE (2006) Mesoscale variation in reproduction, recruitment and population structure of intertidal mussels with low larval input: a bay/open coast comparison. Mar Ecol Prog Ser 327:193–206. doi:https://doi.org/10.3354/meps327193
Menge BA (1991) Relative importance of recruitment and other causes of variation in rocky intertidal community structure. J Exp Mar Biol Ecol 146:69–100. doi:https://doi.org/10.1016/0022-0981(91)90255-U
Miron G, Boudreau B, Bourget E (1995) Use of larval supply in benthic ecology: testing correlations between larval supply and larval settlement. Mar Ecol Prog Ser 124:301–305. doi:https://doi.org/10.3354/meps124301
Nicastro KR, Zardi GI, McQuaid CD (2008) Movement, behaviour and mortality in invasive and indigenous mussels: resilience and resistance at different spatial scales. Mar Ecol Prog Ser 372:119–126. doi:https://doi.org/10.3354/meps07671
Occhipinti-Ambrogi A (2001) Transfer of marine organisms: a challenge to the conservation of coastal biocoenoses. Aquat Conserv Mar Freshw Ecosyst 11:243–251. doi:https://doi.org/10.1002/aqc.450
Olivier F, Desroy N, Retière C (1996) Habitat selection and adult–recruit interactions in Pectinaria koreni (Malmgren) (Annelida: Polychaeta) post-larval populations: results of flume experiments. J Sea Res 36:217–226. doi:https://doi.org/10.1016/S1385-1101(96)90791-1
Osman RW, Whitlatch RB (1995) The influence of resident adults on larval settlement: experiments with four species of ascidians. J Exp Mar Biol Ecol 190:199–220. doi:https://doi.org/10.1016/0022-0981(95)00036-Q
Osman RW, Whitlatch RB (2004) The control of the development of a marine benthic community by predation on recruits. J Exp Mar Biol Ecol 311:117–145. doi:https://doi.org/10.1016/j.jembe.2004.05.001
Petersen JH (1984) Larval settlement behaviour in competing species: Mytilus californianus Conrad and M. edulis L. J Exp Mar Biol Ecol 82:147–159. doi:https://doi.org/10.1016/0022-0981(84)90100-X
Petes LE, Menge BA, Murphy GD (2007) Environmental stress decreases survival, growth, and reproduction in New Zealand mussels. J Exp Mar Biol Ecol 351:83–91. doi:https://doi.org/10.1016/j.jembe.2007.06.025
Phillips NE (2002) Effects of nutrition-mediated larval condition on juvenile performance in a marine mussel. Ecology 83:2562–2574
Porri F, McQuaid CD, Radloff S (2006) Spatio-temporal variability of larval abundance and settlement of Perna perna: differential delivery of mussels. Mar Ecol Prog Ser 315:141–150. doi:https://doi.org/10.3354/meps315141
Porri F, Zardi GI, McQuaid CD, Radloff S (2007) Tidal height, rather than habitat selection for conspecifics, controls settlement in mussels. Mar Biol (Berl) 152:631–637. doi:https://doi.org/10.1007/s00227-007-0716-y
Raimondi PT (1988) Settlement cues and determination of the vertical limit of an intertidal barnacle. Ecology 69:400–407. doi:https://doi.org/10.2307/1940438
Reaugh KE, Harris JM, Branch GM (2007) Further refutation of the primary–secondary settlement hypothesis for the brown mussel Perna perna. Afr J Mar Sci 29:545–549. doi:https://doi.org/10.2989/AJMS.2007.29.3.20.350
Rius M, McQuaid CD (2006) Wave action and competitive interaction between the invasive mussel Mytilus galloprovincialis and the indigenous Perna perna in South Africa. Mar Biol 150:69–78
Robinson TB, Griffiths CL, McQuaid CD, Rius M (2005) Marine alien species of South Africa—status and impacts. Afr J Mar Sci 27:297–306
Roegner GC, Mann R (1995) Early recruitment and growth of the American oyster Crassostrea virginica (Bivalvia: Ostreidae) with respect to tidal zonation and season. Mar Ecol Prog Ser 117:91–101. doi:https://doi.org/10.3354/meps117091
Roughgarden J, Gaines S, Possingham H (1988) Recruitment dynamics in complex life cycles. Science 241:1460–1466. doi:https://doi.org/10.1126/science.11538249
Sanjuan A, Zapata C, Alvarez G (1994) Mytilus galloprovincialis and M. edulis on the coasts of the Iberian Peninsula. Mar Ecol Prog Ser 113:131–146. doi:https://doi.org/10.3354/meps113131
Schneider KR, Helmuth B (2007) Spatial variability in habitat temperature may drive patterns of selection between invasive and native mussel species. Mar Ecol Prog Ser 339:157–167. doi:https://doi.org/10.3354/meps339157
Schumann EH, Perrins LA, Hunter IT (1982) Upwelling along the South Coast of the Cape Province, South Africa. S Afr J Sci 78:238–242
Siegel DA, Mitarai S, Costello CJ, Gaines SD, Kendall BE, Warner RR, Winters KB (2008) The stochastic nature of larval connectivity among nearshore marine populations. Proc Natl Acad Sci USA 105:8974–8979. doi:https://doi.org/10.1073/pnas.0802544105
Van Erkom Schurink C, Griffiths CL (1990) Marine mussels of southern Africa—their distribution patterns, standing stocks, exploitation and culture. J Shellfish Res 9:75–85
Vargas CA, Narváez DA, Piñones A, Venegas RM, Navarrete SA (2004) Internal tidal bore warm fronts and settlement of invertebrates in central Chile. Estuar Coast Shelf Sci 61:603–612. doi:https://doi.org/10.1016/j.ecss.2004.07.006
Vincent B, Joly D, Harvey M (1994) Spatial variation in growth of the bivalve Macoma balthica (L.) on a tidal flat: effects of environmental factors and intraspecific competition. J Exp Mar Biol Ecol 181:223–238. doi:https://doi.org/10.1016/0022-0981(94)90130-9
Wasson K, Fenn K, Pearse JS (2005) Habitat differences in marine invasions of central California. Biol Invasions 7:935–948. doi:https://doi.org/10.1007/s10530-004-2995-2
Watson DI, O’Riordan RM, Barnes DKA, Cross T (2005) Temporal and spatial variability in the recruitment of barnacles and the local dominance of Elminius modestus Darwin in SW Ireland. Estuar Coast Shelf Sci 63:119–131. doi:https://doi.org/10.1016/j.ecss.2004.10.015
Wilkins NP, Fujino K, Gosling EM (1983) The Mediterranean mussel Mytilus galloprovincialis Lmk. in Japan. Biol J Linn Soc Lond 20:365–374. doi:https://doi.org/10.1111/j.1095-8312.1983.tb01597.x
Zardi GI, Nicastro KR, McQuaid CD, Rius M, Porri F (2006) Hydrodynamic stress and habitat partitioning between indigenous (Perna perna) and invasive (Mytilus galloprovincialis) mussels: constraints of an evolutionary strategy. Mar Biol (Berl) 150:79–88. doi:https://doi.org/10.1007/s00227-006-0328-y
Zardi GI, McQuaid CD, Nicastro KR (2007) Balancing survival and reproduction: seasonality of wave action, attachment strength and reproductive output in indigenous Perna perna and invasive Mytilus galloprovincialis mussels. Mar Ecol Prog Ser 334:155–163. doi:https://doi.org/10.3354/meps334155
Acknowledgments
We would like to extend special thanks to the Tsitsikamma National Park for allowing us to sample within the reserve and specifically J. Allen, S. Brouwer and E. Bester for organising permits and for their hospitality during field trips. In addition, thank you to Dr. S. Kaehler, Dr. J. Erlandsson, N. Wilson, Dr. G. Zardi and B. Hayward for their help in the field; Dr. M. Villet, Prof. S. Radloff and Dr. F. Porri for their help and advice with statistics and Mr. and Mrs. R.S. Bownes for their support. Our gratitude also goes to the National Research Foundation for funding this research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by P. Kraufvelin.
Rights and permissions
About this article
Cite this article
Bownes, S.J., McQuaid, C.D. Mechanisms of habitat segregation between an invasive and an indigenous mussel: settlement, post-settlement mortality and recruitment. Mar Biol 156, 991–1006 (2009). https://doi.org/10.1007/s00227-009-1143-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-009-1143-z