Abstract
Juvenile loggerheads (Caretta caretta Linnaeus 1758) exhibit a strong foraging site fidelity to the Río de la Plata estuarine area, as shown by state-space models and kernel density estimations. Six satellite tagged individuals remained in the same 8000-km2 patch during 60 % of their foraging time (7–8 months). After overwintering in coastal, warmer waters off Brazil and Uruguay, individuals move back to the same foraging spot in successive years. The fidelity of juvenile loggerheads to the Rio de la Plata estuarine area encourages site-based conservation tools. Spatial planning may result in special management areas supported by current regional treaties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The loggerhead turtle (Caretta caretta Linnaeus 1758) is listed as Vulnerable on the IUCN Red List due to threats that spread across its geographic range. In their breeding grounds, hatchlings and adult females are affected by the degradation of nesting habitats due to climate change, coastal development, beach erosion, light pollution and invasive predators (Witherington 1992; Bolten and Witherington 2003; Witt et al. 2010; Fuentes et al. 2013). More detrimental, however, is the mortality of juveniles and adults due to bycatch in longline, trawling and gillnet fisheries deployed in foraging grounds (Lewison et al. 2004, 2013; Wallace et al. 2010, 2013). Juveniles are the most robust part of the population, and their increased survival offers the greatest potential for population recovery, contrary to eggs on which most conservation efforts have been afforded to date (Crouse et al. 1987; Heppell 1998). Thus, knowledge of the behavior and spatial distribution of individuals in foraging grounds may help design more effective, local conservation strategies, such as bycatch reduction technologies or time–area closures (McClellan and Read 2009; McClellan et al. 2009, 2011).
Loggerheads forage in neritic (<200 m of depth) and oceanic (>200 m) habitats (Musick and Limpus 1997; Bolten 2003; Wingfield et al. 2011; Mansfield and Putman 2013). Turtles leave the nesting beach soon after hatching, transitioning through neritic waters to reach the open ocean. They can remain in the oceanic realm for decades, moving back to coastal waters as large juveniles until maturity. This habitat shift is usually abrupt and was once to thought to be irreversible (Carr 1986; Musick and Limpus 1997), but some plasticity has been reported: Neritic juveniles can resume an oceanic life for periods of months–years (McClellan and Read 2007; Hawkes et al. 2011), and adults can visit oceanic areas to forage during post-nesting periods (Hatase et al. 2002; Hawkes et al. 2007; Reich et al. 2010). Using multiple foraging habitats exposes loggerheads to various types of fisheries, effects of which accumulate in the populations (Bolten et al. 2011; González Carman et al. 2012a; Maxwell et al. 2013). Besides, these habitats may differ in terms of foraging opportunities and probability of survival. In the neritic habitats, loggerheads may exhibit higher growth and fecundity rates at the expense of less survivorship. On the contrary, oceanic habitats may be safer but energetically less profitable (Peckham et al. 2011). Whether loggerheads choose one or alternate between these two habitats may have an effect on the growth and recovery of depleted populations.
We studied habitat use by juvenile loggerheads at the Río de la Plata estuarine area, which is the southern boundary of the species distribution in the SW Atlantic (Fig. 1). The Río de la Plata is one of the largest (>38,000 km2), and highest productive frontal systems in South America, subject to strong anthropogenic pressure (Mianzan et al. 2001). The area contains artisanal and industrial fishing grounds (FAO 2011), and major metropolitan and industrialized areas are located along its coasts (e.g., Buenos Aires, Montevideo). The management of the area encompasses international waters and national jurisdictions of Argentina and Uruguay and involves a bilaterally managed common fishing zone with multiple regulatory processes and related institutions (González Carman et al. 2012a, 2015).
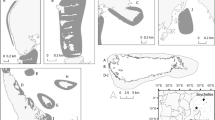
Study area of juvenile loggerhead turtles in the temperate SW Atlantic. ARG Argentina, URU Uruguay, BRA Brazil. Star indicates the San Clemente port, where the turtles were landed by artisanal gillnet fishermen. Black dashed lines show positions of 50, 200 and 1000 m isobaths. Colored full lines illustrate major offshore currents and the annual average shelf currents [adapted from Piola and Matano (2001) and Palma et al. (2008)]
While loggerhead habitat use has been widely studied in the North Atlantic (Mansfield et al. 2009; Hawkes et al. 2011; Arendt et al. 2012; Vander Zanden et al. 2014), the Pacific (Hatase et al. 2002, 2010; Peckham et al. 2011) and the Mediterranean Sea (Cardona et al. 2009; Schofield et al. 2010; Casale et al. 2012a, b), the South Atlantic remains poorly explored.
The Río de la Plata has been identified as a foraging area for loggerheads based on stranding and bycatch data (González Carman et al. 2011; Vélez-Rubio et al. 2013); yet no information on free-ranging individuals has been reported. We assessed the home range and seasonal occurrence of juvenile loggerheads and identified their core foraging areas and routes. We discuss potential implications of our findings in the frame of current conservation efforts and knowledge on the species’ behavior in the region.
Materials and methods
Turtle recovery and measurement
Six loggerhead turtles captured in artisanal gillnets during the period January 2009–December 2012 were used in this study. They were rescued by the Regional Program for Sea Turtle Conservation and Research of Argentina (PRICTMA) and moved to an aquarium to evaluate health status and attach the tag. Individuals were kept in captivity for no more than 2–4 days during which they were fed and responded to stimuli positively. Turtles ranged in size from 46.5 to 66.5 cm (mean ± SD = 59.4 ± 7.1 cm) curved carapace length (CCL, Bolten 2000) and weighed 11.5–34.1 kg (mean ± SD = 24.8 ± 7.3 kg) (Table 1). All turtles were tagged with Inconel tags provided by the Cooperative Marine Turtle Tagging Program (National Marine Fisheries Service) in the second large proximal scale of each rear flipper.
Satellite tag deployment
Turtles were instrumented with Wildlife Computers SPOT5 (n = 5) and SPLASH (n = 1) platform terminal transmitters (PTT). We ensured that the total weigh of PTT plus the epoxy Tubolit® (Duque de Caxias-RJ, Brazil) used to attach the instrument to the carapace did not exceed 5 % of the turtle’s body weight. Prior to attaching the transmitter with epoxy, we removed epibionts and sanded and cleaned the carapace with acetone. We streamlined attachment materials to reduce effects of drag on the turtle’s swimming ability (Watson and Granger 1998). The anticipated battery life of each PTT was 8 months, and we set the tags with a 24/0-h duty cycle. All tagged turtles were released at or near the place where they were caught, near San Clemente port (36°18.024′S, 56°47.305′W) (Fig. 1). Three out of the six turtles transmitted data on sea temperature, which was captured as time-at-temperature histograms. This information was used to establish the minimum sea temperature experienced by the individuals in different seasons.
Data filtering and analysis
We used the Satellite Tracking and Analysis Tool (STAT; Coyne and Godley 2005) to archive and filter location data. All location classes (0–3, A and B but not Z) were used in the analysis. We also filtered out those that required straight-line travel speeds over 10 km h−1, and a turning angle of <10° (González Carman et al. 2012b). Using ArcGis 10.1® (Copyright© ESRI), we manually removed erroneous points (e.g., those that “zig-zagged” land) and implausible locations that remained after the STAT filtering process.
After filtering, tracking data were analyzed following two complementary methodologies: a switching state-space model (SSM) and fixed kernel density estimation, following Hart et al. (2011). A behaviorally switching SSM was fitted to Argos tracks to infer animal behavioral state from the movement pattern (Breed et al. 2009). Using the free software packages R and WinBUGS, we fit the behaviorally switching SSMs initially developed by Jonsen et al. (2005) and refined by Breed et al. (2009) to each turtle track. We estimated locations and associated credible limits at 5-h intervals; this time interval reflects the average number of Argos locations per day for these individuals (Maxwell et al. 2011). Following Bailey et al. (2008), behavior was discriminated into three states that were nominally referred as: “foraging” (state 1), “transiting” (state 2) and “uncertain” (state 0). Behavioral modes were based on two parameters: mean turning angle and autocorrelation in speed and direction. When animals encounter areas of sufficiently abundant prey or sufficient resources for forage, they often engage in area-restricted searches by decreasing their travel rate and/or increasing their turning frequency and angle. Conversely, animals encountering unsuitable habitat often have fast travel rates and infrequent and small turning angles (Hart and Fujisaki 2010; Turchin 1991). In this study, a lack of overlap between the parameters representing the opposing behavioral states indicated a true differentiation in movement patterns, with slow speeds and high rates of change in direction and turning angle indicative of foraging, and the opposite patterns indicative of transiting.
Once the tracks were fitted using SSM, we used the Spatial Analyst of ArcGis 10.1® (Copyright© ESRI) and a smoothing parameter (h) of 40 km for each kernel density estimation (González Carman et al. 2012b). We differentiated between foraging and transiting locations to construct maps of foraging areas and routes, respectively. We then used all locations regardless of state to construct seasonal density distribution maps (summer: January–March, fall: April–June, winter: July–September and spring: October–December). Density distributions were represented on the maps by the 50, 75, 95 and 100 % utilization distribution (UD) contours, indicating areas within which tracked turtles spent 50, 75, 95 and 100 % of their at-sea time. The 100 and 50 % UD represents overall distribution range and core activity areas of turtles in the SW Atlantic during the tracking period, respectively.
In the seasonal density distribution maps, we also included the isotherm location at 2 °C for February, May, August and November of 2012, representative of the seasonal variation in sea-surface temperature in the region for each studied period. The data were obtained from the satellite MODIS/Aqua, with 9 km resolution via the online PO.DAAC Ocean ESIP Tool (POET) at the Physical Oceanography Distributed Active Archive Center (PO.DAAC), NASA Jet Propulsion Laboratory, Pasadena, CA, USA.
To characterize the habitat use of loggerheads, tracks fitted with SSM were plotted along with bathymetric information (GEBCO Digital Atlas and ETOPO2 Global 2′ Elevations datasets distributed by the British Oceanographic Data Centre and NOAA’s National Geophysical Data Centre, http://www.gebco.net/data_and_products/gridded_bathymetry_data/) and the Exclusive Economic Zones (EEZs, http://www.marineregions.org/downloads.php#eez). To test if there were any seasonal differences between the habitat use (characterized by water depth and distance to shore), we applied a Generalized Linear Mixed Model (McCullagh and Nelder 1989) with a significance level of 0.05, “season” as fixed factor (two levels: summer/fall and winter/spring, according to results of the seasonal density distribution maps) and “individual” as a random factor (six levels: turtles A–F).
Finally, we assessed whether the travel rate of individuals, defined as the number of kilometers moved per day, changed among seasons through a Kruskal–Wallis test (α = 0.05). Post hoc comparisons were then tested using Dunn’s test (Zar 1996).
Results
Satellite tracks and home range
A total of 10,056 locations were recorded during 1475 transmission days. Tags transmitted a mean of 246 days (range 90–506 days). Table 1 summarizes tracking data and information of the individual turtles equipped. Mean travel speed for tracked turtles ranged from 2.4 to 3.5 km h−1 (SD range 0.1–0.5, n = 7434 locations). The net distance traveled by the turtles ranged from 1309 to 7586 km (mean ± SD = 3733 ± 2932 km) (Table 1).
Two out of the six tagged individuals—which exhibited the longest transmission times with more than 400 days—reached southern Brazil (turtles B and F; Fig. 2). The rest of the individuals remained within the estuarine area for at least ca. 100 days. Individual E ventured into the inner, freshwater area of the Río de la Plata.
Most individuals spent a large proportion of their time (93 %) in neritic waters and within the EEZ of Argentina (79 %), Brazil (11 %), Uruguay (7 %) and in international waters (3 %).
Foraging areas and routes
Loggerhead turtles expended 60 % of the time foraging in the Río de la Plata, especially within a core area of about 8000 km2 in the Samborombón Bay and adjacent shelf waters of San Antonio Cape (Fig. 3).
Foraging areas and routes of juvenile loggerhead turtles. SB Samborombón Bay, SAC San Antonio Cape. Distribution is represented by the utilization distribution (UD) contours. The 100 and 50 % utilization distribution (UD) represents the overall home range of the turtle and the core activity areas, respectively
Some individuals exhibited fidelity to the estuary, suggesting that this habitat can be visited repeatedly by turtles. Turtles B and F performed a round-trip movement from Río de la Plata to southern Brazil (latitude 28°S) returning to foraging areas separated 25–30 km from the release location 4–6 months later (Fig. 2). Movement of turtles from the Río de la Plata to northern areas occurred along coastal waters of Uruguay and Brazil for individuals B and F (Fig. 3), whereas the return occurred either inshore (turtle F) or offshore (turtle B) waters.
Seasonal distribution
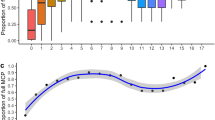
Loggerhead occurrence in the Río de la Plata varied between seasons (Fig. 4). During the austral summer and fall, the individuals used this estuarine area exclusively, whereas during winter and spring they also inhabited shelf waters off southern Brazil and eastern offshore waters. Movement to these warmer waters started during the fall for turtles B and F, concomitant with the northern position of the 18 °C isotherm (Fig. 4). In fact, the minimum sea temperature experienced by individuals C, D and E during the fall—for which transmission stopped early in this season—was around 18–20 °C, in contrast to temperatures of 22–24 °C experienced during the summer (Fig. 5). Unfortunately, transmission of turtles with SST sensors did not last until the winter. The travel rate of all individuals was significantly higher during the summer than during fall, spring and winter (H 3,1365 = 90.1, P < 0.05; Fig. 6).
Seasonal habitat use of six individuals (Table 1). The 100 and 50 % utilization distribution (UD) represents the overall home range of the turtle and the core activity areas, respectively. Isotherms of 18 °C are highlighted in red
Time-at-temperature histograms for three individuals (data combined for individuals C, D and E of Table 1) foraging in the Río de la Plata
Travel rate (km day−1) of six individuals (Table 1). Black square indicates the median, rectangle indicates quartiles of 25th and 75th percentiles and whiskers indicate non-outlier maximum and minimum values. Different letters indicate differences in the median travel rate of turtles between seasons according to post hoc comparisons
Loggerhead turtles occupied waters within a wide range of depths that encompassed neritic (depth <200 m) as well as oceanic waters (depth >200 m, Fig. 4). During the austral summer and fall, they used almost exclusively shallow waters off the coast of Argentina and Uruguay, in contrast to winter and spring, when they occupied shelf waters of Uruguay and Brazil, and even oceanic waters (summer/fall: mean 11.5 m, range 0–63.4 m, 25th–75th percentile = 4.4–20.0 m, n = 4864 locations; winter/spring: mean 662.8 m, range 0–4780.7, 25th–75th percentile = 16.6–155.1 m, n = 2367 locations; F 1,7207 = 1981.6; P < 0.05). Similarly, turtles were closer to shore during the austral summer and fall (summer/fall: mean 40.3 km, range 0–2082 km, 25th–75th percentile = 21.0–52.0 km, n = 5009 locations; winter/spring: mean 94.7 km, range 0–2039 km, 25th–75th percentile = 25.0–99.0 km, n = 2346 locations; F 1,7270 = 487.6; P < 0.05).
Discussion
We confirmed the seasonal occurrence of the loggerhead sea turtle in the Río de la Plata, as previously suggested by stranding and bycatch data (González Carman et al. 2011; Vélez-Rubio et al. 2013). The combined use of satellite tracking and state-space models provided, for the first time, sound evidence that immature turtles exhibit a strong foraging site fidelity to this estuarine area, either by remaining in the same area during a significant portion of their foraging time (Fig. 3), or by moving back to the same foraging spot in successive years (Fig. 2). This last observation is reinforced by the incidental capture of turtle F (whose transmission had stopped at the end of 2012) in waters of the Río de la Plata by a Uruguayan trawler in October 2015. This inter-annual site fidelity to the neritic habitat of the Río de la Plata does not preclude some loggerheads from overwintering in coastal, warmer waters off Brazil and Uruguay, and also in oceanic areas (Fig. 4).
The Río de la Plata is one of the most relevant ecosystems of South America in terms of biological productivity (Mianzan and Guerrero 2000; Mianzan et al. 2001), yet its importance as loggerhead foraging ground has been largely overlooked. In the Río de la Plata, loggerheads likely benefit from high biomasses of their natural prey. This estuarine system sustains extensive benthic habitats (Giberto et al. 2004; Carranza et al. 2008a, b) where loggerheads have access to gastropod mollusks and malacostraca crustaceans, their main prey in the temperate SW Atlantic (Martinez Souza 2009; Carranza et al. 2010). Loggerhead diets also include salps (Martinez Souza 2009) that reach high biomass in adjacent shelf waters (Mianzan and Guerrero 2000; Alvarez Colombo et al. 2003).
The Río de la Plata is also an important foraging ground for other sea turtle species with similar behaviors, such as the green (Chelonia mydas) and leatherback (Dermochelys coriacea) turtles (López-Mendilaharsu et al. 2009; González Carman et al. 2011, 2012b). The three species travel from the Río de la Plata to overwintering areas at lower latitudes, moving away from the low sea temperatures (8–10 °C) characteristic of the area in winter (Lucas et al. 2005), and in some cases return in successive years. Loggerheads seem to take advantage of the seasonal current pattern, as in the case of green turtles (González Carman et al. 2012b). Both species appear to use waters of the Plata plume, which moves northward during winter (Piola et al. 2008), to reach warmer areas. In accordance with this, a closer look at currents in our study area shows strong seasonal variations in the inner shelf circulation caused by changes in local winds (Palma et al. 2008). Shelf circulation is directed to the NE during fall and early winter and to the SW during spring and summer (Palma et al. 2008), a pattern that is concomitant with loggerheads starting to leave the Río de la Plata during the fall to return back in late winter and spring (Fig. 4). On their way back, they travel along shelf and offshore waters, in this last case likely using the southward flow of the warm Brazil Current (Fig. 1 and turtle B in Fig. 2).
The close resemblance between the seasonal movement pattern of neritic juvenile loggerheads and the variable shelf circulation challenges us to quantitatively evaluate the impact of currents on the trajectories of turtles in future studies. Even though the role of ocean currents has been largely acknowledged in hatchlings, oceanic juveniles and adults of many sea turtle species (e.g., Luschi et al. 2003a, b and references therein; Gaspar et al. 2006; Girard et al. 2006; Hays et al. 2014), little is known for neritic juveniles (Luschi et al. 2003b) and this holds true for loggerheads. Experiences from the Mediterranean Sea showed that the movement pattern of juveniles seems to match prevailing currents, although traveling against them has been observed as well (e.g. Cardona et al. 2005; Bentivegna et al. 2007; Revelles et al. 2007). In fact, the few studies that explicitly consider the effect of currents also showed variable results. The juveniles would be more likely to move in straight line in the presence of diffuse, weak currents (McCarthy et al. 2010), but they also would be able to reach higher than average speeds when swimming upstream (Bentivegna et al. 2007). What is clear from these studies is that juvenile loggerheads are well-accomplished swimmers that can also passively drift in certain circumstances (Cardona et al. 2005). However, since currents definitively distort movement in powerful swimmers such as adult leatherbacks (Gaspar et al. 2006), it is expected that they also affect juvenile loggerheads in a very energetic habitat such as the Rio de la Plata and its surrounding waters. Loggerheads from this study left the Río de la Plata at a slower rate compared to green turtles (see González Carman et al. 2012b). Loggerhead moved faster in the summer while foraging in the Río de la Plata, than during the fall and winter, when migrating to northern waters (Fig. 6). Green turtles, on the contrary, showed the fastest travel rate when leaving the Río de la Plata during the winter (González Carman et al. 2012b). This may be due to differences in body size since loggerheads were double or triple the size of the greens in that study. A larger body size likely enables the loggerheads to withstand lower temperatures than smaller greens translate into the capability to better support low temperatures through “thermal inertia,” allowing loggerheads to leave the Río de la Plata at a slower rate of travel than green turtles. In fact, some extreme temperatures (12–16 °C) were experienced by some of the studied individuals during the fall and winter (Fig. 4).
Caution should be exercised not to over-interpret results due to our relatively small sample size compared to other studies (e.g., McClellan and Read 2007; Mansfield et al. 2009), particularly in winter as tracking data from summer were 2.4 times greater than winter. It is worthy of note, however, that we are confident in our results because loggerheads exhibited almost the same movement pattern as sympatric green and leatherback turtles described above: seasonal travel from the Río de la Plata to overwintering areas at lower latitudes, with returning observed in some cases with the longest transmission times (López-Mendilaharsu et al. 2009; González Carman et al. 2012b).
Loggerhead habitat use in the temperate SW Atlantic was also studied by Barceló et al. (2013) through the satellite tracking of juveniles incidentally captured by pelagic longliners along the continental slope. High-use areas of tracked individuals were over the continental shelf (0–200 m depth) and slope (1000–3000 m depth) within the Argentinean, Uruguayan and Brazilian EEZs, and in adjacent international waters (Barceló et al. 2013). While none of the turtles studied by Barceló et al. (2013) went into the Río de la Plata despite similar body sizes of individuals and the larger sample size (n = 27), our study did show that some loggerheads foraging in the Río de la Plata can overwinter in the oceanic areas used by individuals from Barceló et al. (2013). In addition to this, the combined use of satellite tracking and state-space models performed in our study allowed us to gain quick insight into the specific behavior of loggerheads and to estimate foraging areas and routes with accuracy within the species home range (Fig. 3).
Our findings support the hypothesis that the habitat shift from oceanic to neritic habitats performed by immature loggerheads is not as abrupt and irreversible as previously thought (Witzell 2002; McClellan and Read 2007). This has also been suggested for juvenile green turtles inhabiting the Río de la Plata (González Carman et al. 2012b). Behavior in the temperate SW Atlantic with the juveniles of both species resembles that of loggerheads in the NW Atlantic, where juveniles exhibit fidelity to specific areas during summer months and return to those areas following long-distance movements that, sometimes, include overwintering in oceanic as well as shelf habitats (Avens et al. 2003; McClellan and Read 2007; Mansfield et al. 2009; Arendt et al. 2012). Loggerhead therefore seems to exhibit great plasticity while foraging in the temperate SW Atlantic. This behavior deserves further attention in the frame of current climate change scenarios and their potential effects on the expansion of the species’ geographic range (Witt et al. 2010).
Loggerheads foraging in the Río de la Plata are subject to several threats. This estuarine area concentrates the fishing effort of Uruguayan and Argentinean artisanal gillnet and bottom trawling fleets, and loggerhead bycatch has been reported in the past (Domingo et al. 2006; González Carman et al. 2011, 2012a; Laporta et al. 2012). This type of trawling can severely degrade the sea floor (Thrush and Dayton 2002), although its potential impact on the abundance of loggerhead benthic prey is unknown. The Río de la Plata is also an area where the turtles are vulnerable to plastic pollution affecting other sympatric species such as the green turtles and the Franciscana dolphin (Pontoporia blainvillei) (Denuncio et al. 2011; González Carman et al. 2014, 2015). After leaving the Río de la Plata to overwinter in oceanic areas, loggerheads are exposed to bycatch in the pelagic longline fisheries of Uruguay and Brazil (Sales et al. 2008; González Carman et al. 2012a).
Our new understanding of the fidelity of juvenile loggerheads to the Río de la Plata improves the probability of success of conservation measures implemented in this foraging ground. In particular, the predictability of loggerhead occurrence in time and space can facilitate zoning of human activities within the area. Spatial planning of the estuarine area should take into account key habitats for endangered species such as loggerhead and green turtles. In particular, areas within the Samborombón Bay and the San Antonio Cape should consider special management tools based on our understanding of important turtle areas.
Conservation and management could be promoted in the frame of current regional instruments. The area is under binational administration through the Río de la Plata Bilateral Treaty, established between Argentina and Uruguay. The treaty regulates fishing and coastal development, and attempts to prevent pollution and promote research to evaluate and preserve resources. Since juvenile loggerheads intensively used the area (Figs. 4, 6), this treaty and its enforcement authorities—the Technical Commission of the Maritime Front and the Administrative Commission of the Río de la Plata (in Spanish, Comisión Técnica Mixta del Frente Marítimo and Comisión Administradora del Río de la Plata, respectively)—are proper instruments for the conservation of loggerheads. Future provisions under the bilateral treaty should also focus on: (a) monitoring and reducing interactions between loggerheads and fisheries, particularly the coastal trawling fleet that is not monitored by onboard observers (González Carman et al. 2012a) and which intensively fishes within the core foraging area of loggerheads from January to June; and (b) preventing and reducing marine pollution such as plastic debris disposed from coastal areas as well as from fishing activities. These actions should also be included into the Sea Turtle National Plan of Action currently being developed in Argentina. These initiatives would directly benefit severely exploited nesting grounds of Brazil (Marcovaldi et al. 2005), from where the loggerheads of the temperate SW Atlantic originate (Reis et al. 2010; Prosdocimi et al. 2015).
References
Alvarez Colombo G, Mianzan H, Madirolas A (2003) Acoustic characterization of gelatinous-plankton aggregations: four case studies from the Argentine continental shelf. ICES J Mar Sci 60:650–657
Arendt M, Segars A, Byrd J, Boynton J, Whitaker J, Parker L, Owens D, Blanvillain G, Quattro J, Roberts M (2012) Seasonal distribution patterns of juvenile loggerhead sea turtles (Caretta caretta) following capture from a shipping channel in the Northwest Atlantic Ocean. Mar Biol 159:127–139
Avens L, Braun-McNeill J, Epperly S, Lohmann KJ (2003) Site fidelity and homing behavior in juvenile loggerhead sea turtles (Caretta caretta). Mar Biol 143:211–220
Bailey H, Shillinger G, Palacios D, Bograd S, Spotila J, Paladino F, Block B (2008) Identifying and comparing phases of movement by leatherback turtles using state-space models. J Exp Mar Biol Ecol 356:128–135
Barceló C, Domingo A, Miller P, Ortega L, Giffoni B, Sales G, McNaughton L, Marcovaldi M, Heppell S, Swimmer Y (2013) High-use areas, seasonal movements and dive patterns of juvenile loggerhead sea turtles in the Southwestern Atlantic Ocean. Mar Ecol Prog Ser 479:235–250
Bentivegna F, Valentino F, Falco P, Zambianchi E, Hochscheid S (2007) The relationship between loggerhead turtle (Caretta caretta) movement patterns and Mediterranean currents. Mar Biol 151:1605–1614
Bolten AB (2000) Técnicas para la medición de tortugas marinas. In: Eckert KL, Bjorndal, KA, Abreu-Grobois FA, Donnelly M (eds) Técnicas de Investigación y Manejo para la Conservación de las Tortugas Marinas. Grupo Especialista en Tortugas Marinas UICN/CSE, Publicación 4 (Español). Consolidated Graphic Communications, Blanchard, pp 126–131
Bolten AB (2003) Variation in sea turtle life history patterns: neritic versus oceanic developmental stages. In: Lutz PL, Musick JA, Wyneken J (eds) The biology of sea turtles. CRC Press, Boca Raton, pp 243–257
Bolten AB, Witherington BE (2003) Loggerhead sea turtles. Smithsonian Books, Washington, DC
Bolten AB, Crowder LB, Dodd MG, MacPherson SL, Musick JA, Schroeder BA, Witherington BE, Long KJ, Snover ML (2011) Quantifying multiple threats to endangered species: an example from loggerhead sea turtles. Front Ecol Environ 9:295–301
Breed GA, Jonsen ID, Myers RA, Bowen WDW, Leonard ML (2009) Sex-specific, seasonal foraging tactics of adult grey seals (Halichoerus grypus) revealed by state–space analysis. Ecology 90:3209–3221
Cardona L, Revelles M, Carreras C, San Félix M, Gazo M, Aguilar A (2005) Western Mediterranean immature loggerhead turtles: habitat use in spring and summer assessed through satellite tracking and aerial surveys. Mar Biol 147:583–591
Cardona L, Revelles M, Parga ML, Tomás J, Aguilar A, Alegre F, Raga A, Ferrer X (2009) Habitat use by loggerhead sea turtles Caretta caretta off the coast of eastern Spain results in a high vulnerability to neritic fishing gear. Mar Biol 156:2621–2630
Carr A (1986) Rips, FADS, and little loggerheads. Bioscience 36:92–100
Carranza A, Scarabino F, Brazeiro A, Ortega L, Martínez S (2008a) Assemblages of megabenthic gastropods from Uruguayan and northern Argentinean shelf: spatial structure and environmental controls. Cont Shelf Res 28:788–796
Carranza A, Scarabino F, Ortega L (2008b) Distribution of large benthic gastropods in the Uruguayan continental shelf and Río de la Plata Estuary. J Coast Res 24(1A):161–168
Carranza A, Estrades A, Scarabino F, Segura A (2010) Loggerhead turtles Caretta caretta (Linnaeus) preying on the invading gastropod Rapana venosa (Valenciennes) in the Río de la Plata Estuary. Mar Ecol 32(2):1–6
Casale P, Affronte M, Scaravelli D, Lazar B, Vallini C, Luschi P (2012a) Foraging grounds, movement patterns and habitat connectivity of juvenile loggerhead turtles (Caretta caretta) tracked from the Adriatic Sea. Mar Biol 159:1527–1535
Casale P, Broderick AC, Freggi D, Mencacci R, Fuller WJ, Godley BJ, Luschi P (2012b) Long-term residence of juvenile loggerhead turtles to foraging grounds: a potential conservation hotspot in the Mediterranean. Aquat Conserv Mar Freshw Ecosyst 22(2):144–154
Coyne MS, Godley BJ (2005) Satellite tracking and analysis tool (STAT): an integrated system for archiving, analyzing and mapping animal tracking data. Mar Ecol Prog Ser 301:1–7
Crouse DT, Crowder LB, Caswell H (1987) A stage-based population model for loggerhead sea turtles and implications for conservation. Ecology 68:1412–1423
Denuncio P, Bastida R, Dassis M, Giardino G, Gerpe M, Rodríguez D (2011) Plastic ingestion in Franciscana dolphins, Pontoporia blainvillei (Gervais and d’Orbigny, 1844), from Argentina. Mar Pollut Bull 62:1836–1841
Domingo A, Bugoni L, Prosdocimi L, Miller P, Laporta M, Monteiro DS, Estrades A, Albareda D (2006) The impact generated by fisheries on sea turtles in the Southwestern Atlantic. WWF Programa Marino para Latinoamérica y el Caribe, San José
FAO (2011) Review of the state of world marine fishery resources. FAO fisheries and aquaculture technical paper no. 569. Food and Agriculture Organization of the United Nations, Rome. http://firms.fao.org/firms/resource/13329/en. Accessed 19 May 2015
Fuentes MMPB, Pike DA, DiMatteo A, Wallace BP (2013) Resilience of marine turtle regional management units to climate change. Glob Chang Biol 19:1399–1406
Gaspar P, Georges J-Y, Fossette S, Lenoble A, Ferraroli S, Le Maho Y (2006) Marine animal behaviour: neglecting ocean currents can lead us up the wrong track. Proc R Soc Lond B Biol Sci 273:2697–2702
Giberto DA, Bremec CS, Acha EM, Mianzan H (2004) Large-scale spatial patterns of benthic assemblages in the SW Atlantic: the Río de la Plata estuary and adjacent shelf waters. Estuar Coastal Shelf Sci 61:1–13
Girard C, Sudre J, Benhamou S, Roos D, Luschi P (2006) Homing in green turtles Chelonia mydas: oceanic currents act as a constraint rather than as an information source. Mar Ecol Prog Ser 322:281–289
González Carman V, Álvarez K, Prosdocimi L, Inchaurraga MC, Dellacasa RF, Faiella A, Echenique C, González R, Andrejuk J, Mianzan H, Campagna C, Albareda DA (2011) Argentinian coastal waters: a temperate habitat for three species of threatened sea turtles. Mar Biol Res 7:500–508
González Carman V, Machain N, Albareda D, Mianzan H, Campagna C (2012a) Legal and institutional tools to mitigate marine turtle bycatch: Argentina as a case study. Mar Policy 36:1265–1274
González Carman V, Falabella V, Maxwell S, Albareda D, Campagna C, Mianzan H (2012b) Revisiting the ontogenetic shift paradigm: the case of juvenile green turtles in the SW Atlantic. J Exp Mar Biol Ecol 429:64–72
González Carman V, Acha EM, Maxwell SM, Albareda D, Campagna C, Mianzan H (2014) Young green turtles, Chelonia mydas, exposed to plastic in a frontal area of the SW Atlantic. Mar Pollut Bull 78:56–65
González Carman V, Machain N, Campagna C (2015) Legal and institutional tools to mitigate plastic pollution affecting marine species: Argentina as a case study. Mar Pollut Bull 92:125–133
Hart KM, Fujisaki I (2010) Satellite tracking reveals habitat use by juvenile green sea turtles Chelonia mydas in the Everglades, Florida, USA. Endanger Species Res 11:221–232
Hart KM, Lamont MM, Fujisaki I, Tucker AD, Carthy RR (2011) Common coastal foraging areas for loggerheads in the Gulf of Mexico: opportunities for marine conservation. Biol Conserv 145:185–194
Hatase H, Takai N, Matsuzawa Y, Sakamoto W, Omuta K, Goto K, Arai N, Fujiwara T (2002) Size-related differences in feeding habitat use of adult female loggerhead turtles Caretta caretta around Japan determined by stable isotope analyses and satellite telemetry. Mar Ecol Prog Ser 233:273–281
Hatase H, Omuta K, Tsukamoto K (2010) Oceanic residents, neritic migrants: a possible mechanism underlying foraging dichotomy in adult female loggerhead turtles (Caretta caretta). Mar Biol 157:1337–1342
Hawkes LA, Broderick AC, Coyne MS, Godfrey MH, Godley BJ (2007) Only some like it hot—quantifying the environmental niche of the loggerhead sea turtle. Divers Distrib 13:447–457
Hawkes LA, Witt MJ, Broderick AC, Coker JW, Coyne MS, Dodd M, Frick MG, Godfrey MH, Griffin DB, Murphy SR, Murphy TM, Williams KL, Godley BJ (2011) Home on the range: spatial ecology of loggerhead turtles in Atlantic waters of the USA. Divers Distrib 17:624–640
Hays GC, Christensen A, Fossette S, Schofield G, Talbot J, Mariani P (2014) Route optimisation and solving Zermelo’s navigation problem during long distance migration in cross flows. Ecol Lett 17:137–143
Heppell SS (1998) Application of life-history theory and population model analysis to turtle conservation. Copeia 2:367–375
Jonsen ID, Flemming MJ, Myers RA (2005) Robust state-space modelling of animal movement data. Ecology 86:2874–2880
Laporta M, Miller P, Domingo A (2012) Captura incidental de tortugas marinas en la pesquería de arrastre Uruguaya. In: Zaldua-Mendizabal N, Egaña-Callejo A (eds) Marine turtles of the North East Atlantic. Contributions for the first regional conference, Munibe monographs, nature series no. 1, Aranzadi Society of Sciences, San Sebastian, pp 43–50
Lewison RL, Crowder LB, Read AJ, Freeman SA (2004) Understanding impacts of fisheries bycatch on marine megafauna. Trends Ecol Evol 19:598–604
Lewison R, Wallace B, Alfaro-Shigueto J, Mangel JC, Maxwell SM, Hazen EL (2013) Fisheries bycatch of marine turtles: lessons learned from decades of research and conservation. In: Wyneken J, Lohmann KJ, Musick JA (eds) The biology of sea turtles. CRC Press, Boca Raton, pp 329–352
Linnaeus C (1758) Systema naturae per regna tria naturae, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. Ed. 10, Tomus 1. L. Salvii, Stockholm, Sweden
López-Mendilaharsu M, Rocha CFD, Miller P, Domingo A, Prosdocimi L (2009) Insights on leatherback turtle movements and high use areas in the Southwest Atlantic Ocean. J Exp Mar Biol Ecol 378:31–39
Lucas AJ, Guerrero RA, Mianzan HW, Acha EM, Lasta CA (2005) Coastal oceanographic regimes of the northern Argentine continental shelf (34–43°S). Estuar Coast Shelf Sci 65:405–420
Luschi P, Hays GC, Papi F (2003a) A review of long-distance movements by marine turtles, and the possible role of ocean currents. Oikos 103:293–302
Luschi P, Sale A, Mencacci R, Hughes GR, Lutjeharms JRE, Papi F (2003b) Current transport of leatherback sea turtles (Dermochelys coriacea) in the ocean. Proc R Soc Lond B 270(Suppl. 2):S129–S132
Mansfield KL, Putman NF (2013) Oceanic habits and habitats: Caretta caretta. In: Wyneken J, Lohmann KJ, Musick JA (eds) The biology of sea turtles. CRC Press, Boca Raton, pp 189–210
Mansfield KL, Saba VS, Keinath JA, Musick JA (2009) Satellite tracking reveals a dichotomy in migration strategies among juvenile loggerhead turtles in the Northwest Atlantic. Mar Biol 156:2555–2570
Marcovaldi M, Patiri V, Thomé J (2005) Projeto TAMAR-IBAMA: twenty-five years protecting Brazilian sea turtles through a community-based conservation programme. Marit Stud 3:39–62
Martinez Souza G (2009) Ecologia alimentar da tartaruga marinha cabeçuda (Caretta caretta) no oceano Atlantico sul Ocidental, Uruguai. Dissertation, Universidade Federal do Rio Grande, Rio Grande do Sul
Maxwell SM, Breed GA, Nickel BA, Makanga-Bahouna J, Pemo-Makaya E, Parnell RJ, Formia A, Ngouessono S, Godley BJ, Costa DP, Witt MJ, Coyne MS (2011) Using satellite tracking to optimize protection of long-lived marine species: olive ridley sea turtle conservation in Central Africa. PLoS One 6:e19905
Maxwell SM, Hazen EL, Bograd SJ, Halpern BS, Breed GA, Nickel B, Teutschel NM, Crowder LB, Benson S, Dutton PH, Bailey H, Kappes MA, Kuhn CE, Weise MJ, Mate B, Shaffer SA, Hassrick JL, Henry RW, Irvine L, McDonald BI, Robinson PW, Block BA, Costa DP (2013) Cumulative human impacts on marine predators. Nat Commun 4:2688
McCarthy AL, Heppell S, Royer F, Freitas C, Dellinger T (2010) Identification of likely foraging habitat of pelagic loggerhead sea turtles (Caretta caretta) in the North Atlantic through analysis of telemetry track sinuosity. Prog Oceanogr 86:224–231
McClellan CM, Read AJ (2007) Complexity and variation in loggerhead sea turtle life history. Biol Lett 3:592–594
McClellan CM, Read AJ (2009) Confronting the gauntlet: understanding incidental capture of green turtles through fine-scale movement studies. Endanger Species Res 10:165–179
McClellan CM, Read AJ, Price BA, Cluse WM, Godfrey MH (2009) Using telemetry to mitigate the bycatch of long-lived marine vertebrates. Ecol Appl 19:1660–1671
McClellan CM, Read AJ, Cluse WM, Godfrey MH (2011) Conservation in a complex management environment: the by-catch of sea turtles in North Carolina’s commercial fisheries. Mar Policy 35:241–248
McCullagh P, Nelder JA (1989) Generalized linear models. Chapman & Hall, London
Mianzan HW, Guerrero RA (2000) Environmental patterns and biomass distribution of gelatinous macrozooplankton. Three study cases in the South-western Atlantic Ocean. Sci Mar 64:215–224
Mianzan HW, Lasta C, Acha E, Guerrero R, Macchi G, Bremec C (2001) The Rio de la Plata Estuary, Argentina–Uruguay. Ecol Stud 144:185–204
Musick JA, Limpus CJ (1997) Habitat utilization and migration in juvenile sea turtles. In: Lutz PL, Musick JA (eds) The biology of sea turtles. CRC Press, Boca Rat0n, pp 137–163
Palma ED, Matano RP, Piola AR (2008) A numerical study of the Southwestern Atlantic shelf circulation: stratified ocean response to local and offshore forcing. J Geophys Res 113:C11010
Peckham SH, Maldonado-Diaz D, Tremblay Y, Ochoa R, Polovina J, Balazs G, Dutton PH, Nichols WJ (2011) Demographic implications of alternative foraging strategies in juvenile loggerhead turtles Caretta caretta of the North Pacific Ocean. Mar Ecol Prog Ser 425:269–280
Piola AR, Matano RP (2001) Brazil and Falklands (Malvinas) currents. In: Steele JH, Thorpe SA, Turekian KK (eds) Encyclopedia of ocean sciences. Academic Press, London, pp 340–349
Piola AR, Romero SI, Zajaczkovski U (2008) Space–time variability of the Plata plume inferred from ocean color. Cont Shelf Res 28:1556–1567
Prosdocimi L, Bugoni L, Albareda D, Remis MI (2015) Are stocks of immature loggerhead sea turtles always mixed? J Exp Mar Biol Ecol 466:85–91
Reich KJ, Bjorndal KA, Frick MG, Witherington BE, Johnson C, Bolten AB (2010) Polymodal foraging in adult female loggerheads (Caretta caretta). Mar Biol 157:113–121
Reis EC, Soares LS, Vargas SM, Santos FR, Young RJ, Bjorndal KA, Bolten AB, Lôbo-Hajdu G (2010) Genetic composition, population structure and phylogeography of the loggerhead sea turtle: colonization hypothesis for the Brazilian rookeries. Conserv Genet 11:1467–1477
Revelles M, Isern-Fontanet J, Cardona L, Félix MS, Carreras C, Aguilar A (2007) Mesoscale eddies, surface circulation and the scale of habitat selection by immature loggerhead sea turtles. J Exp Mar Biol Ecol 347:41–57
Sales G, Giffoni BB, Barata PCR (2008) Incidental catch of sea turtles by the Brazilian pelagic longline fishery. J Mar Biol Assoc UK 88:853–864
Schofield G, Hobson VJ, Fossette S, Lilley MKS, Katselidis KA, Hays GC (2010) Fidelity to foraging sites, consistency of migration routes and habitat modulation of home range by sea turtles. Divers Distrib 16:840–853
Thrush SF, Dayton PK (2002) Disturbance to marine benthic habitats by trawling and dredging: implications for marine biodiversity. Annu Rev Ecol Syst 33:449–473
Turchin P (1991) Translating foraging movements in heterogeneous environments into the spatial distribution of foragers. Ecology 72:1253–1266
Vander Zanden H, Pfaller J, Reich K, Pajuelo M, Bolten A, Williams K, Frick M, Shamblin B, Nairn C, Bjorndal K (2014) Foraging areas differentially affect reproductive output and interpretation of trends in abundance of loggerhead turtles. Mar Biol 161:585–598
Vélez-Rubio GM, Estrades A, Fallabrino A, Tomás J (2013) Marine turtle threats in Uruguayan waters: insights from 12 years of stranding data. Mar Biol 160:1–15
Wallace BP, Lewison RL, McDonald SL, McDonald RK, Kot CY, Kelez S, Bjorkland RK, Finkbeiner EM, Sr Helmbrecht, Crowder LB (2010) Global patterns of marine turtle bycatch. Conserv Lett 3:369–381
Wallace BP, Kot CY, DiMatteo AD, Lee T, Crowder LB, Lewison RL (2013) Impacts of fisheries bycatch on marine turtle populations worldwide: toward conservation and research priorities. Ecosphere 4(3):40. doi:10.1890/ES12-00388.1
Watson KP, Granger RA (1998) Hydrodynamic effect of a satellite transmitter on a juvenile green turtle (Chelonia mydas). J Exp Biol 201:2497–2505
Wingfield DK, Peckham SH, Foley DG, Palacios DM, Lavaniegos BE, Durazo R, Nichols WJ, Croll DA, Bograd SJ (2011) The making of a productivity hotspot in the coastal ocean. PLoS One 6:e27874
Witherington BE (1992) Behavioral responses of nesting sea turtles to artificial lighting. Herpetologica 48:31–39
Witt MJ, Hawkes LA, Godfrey MH, Godley BJ, Broderick AC (2010) Predicting the impacts of climate change on a globally distributed species: the case of the loggerhead turtle. J Exp Biol 213:901–911
Witzell WN (2002) Immature Atlantic loggerhead turtles (Caretta caretta): suggested changes to the life history model. Herpetol Rev 33:266–269
Zar JH (1996) Biostatistical analysis. Prentice Hall, Upper Saddle River
Acknowledgments
We dedicate this work to Dr. Hermes Mianzan, who passed away in 2014, thanking him for generously sharing the initial thoughts that made this article possible. We are also grateful to Lic. Sergio Rodriguez-Heredia, Gastón Delgado y Gabriela Palacios of Fundación Mundo Marino, Sr. Roberto Ubieta of Aquamarina—CECIM and Téc. Edith Corradini of Unión Argentina de Pescadores Artesanales for their assistance during fieldwork activities and a special thanks to Lic. Valeria Falabella of Wildlife Conservation Society and Dr. Daniel Hernández for their advices on data presentation and statistics, respectively. Thanks to fishermen from San Clemente del Tuyú that provided information on sea turtle occurrence and to the wildlife agencies of Buenos Aires and the National Wildlife Agency of Argentina that issued permits and supported our research. A special thanks to Dr. Manjula Tiwari of NOAA Southwest Fisheries Science Center, and Dr. Alberto Piola for their financial support. Funding was provided by the Buenos Aires Zoo to DA, the Wildlife Conservation Society to CC, the Inter-American Institute for Global Change Research (IAI) CRN 2076 sponsored by the US National Science Foundation grant GEO-0452325 to Hermes Mianzan, the Fondo para la Conservación Ambiental from Banco Galicia, the Cleveland Metroparks Zoo–Scott Neotropical Fund and Agencia Nacional de Promoción Científica y Tecnológica FONCyT PICT 2013-2099 Prestamo BID to VGC. This study adhered to the legal requirements of Argentina and to all institutional guidelines. This is INIDEP Contribution No. 1955.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: J. Houghton.
Reviewed by undisclosed experts.
Rights and permissions
About this article
Cite this article
González Carman, V., Bruno, I., Maxwell, S. et al. Habitat use, site fidelity and conservation opportunities for juvenile loggerhead sea turtles in the Río de la Plata, Argentina. Mar Biol 163, 20 (2016). https://doi.org/10.1007/s00227-015-2795-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-015-2795-5