Abstract
Diet items and habitat constitute some of the environmental resources that may be used differently by individuals within a population. Long-term fidelity by individuals to particular resources exemplifies individual specialization, a phenomenon that is becoming increasingly recognized across a wide range of species. Less is understood about the consequences of such specialization. Here, we investigate the effects of differential foraging ground use on reproductive output in 183 loggerhead sea turtles (Caretta caretta) nesting at Wassaw Island, Georgia (31.89°N, 80.97°W), between 2004 and 2011 with resulting possible fitness effects. Stable isotope analysis was used to assign the adult female loggerheads to one of three foraging areas in the Northwest Atlantic Ocean. Our data indicate that foraging area preference influences the size, fecundity, and breeding periodicity of adult female loggerhead turtles. We also found that the proportion of turtles originating from each foraging area varied significantly among the years examined. The change in the number of nesting females across the years of the study was not a result of uniform change from all foraging areas. We develop a novel approach to assess differential contributions of various foraging aggregations to changes in abundance of a sea turtle nesting aggregation using stable isotopes. Our approach can provide an improved understanding of the influences on the causes of increasing or decreasing population trends and allow more effective monitoring for these threatened species and other highly migratory species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Individual differences in foraging strategies within a population occur across a range of species (Bolnick et al. 2003; Araújo et al. 2011). Consistent inter-individual patterns in diet or habitat use represent individual specializations, whereby individual conspecifics use different portions of the population’s ecological niche, irrespective of factors such as age, sex, or morph (Roughgarden 1972). Sustained patterns of differential habitat use or diet may contribute to variation in fitness or performance among individuals. Evolutionary ecologists typically measure the reproductive output of a species, as it relates to fitness. Individual specialization is documented to affect annual and long-term reproductive output in a variety of species (Annett and Pierotti 1999; Norris et al. 2004; McLoughlin et al. 2007; Zbinden et al. 2011; Authier et al. 2012; Hoye et al. 2012).
In the loggerhead sea turtle (Caretta caretta), differential use of foraging areas has previously been identified in the Northwest Atlantic (NWA) (Hawkes et al. 2011; Ceriani et al. 2012; Pajuelo et al. 2012b; Griffin et al. 2013), and these individual foraging site preferences are likely maintained over long time spans (Vander Zanden et al. 2010; Hawkes et al. 2011). Differences in reproductive output may stem from individual foraging preferences that can ultimately affect demographic parameters that are critical for assessing sea turtle population status and trends (National Research Council 2010). Across the NWA, environmental factors such as resource availability, temperature, and oceanic productivity vary among the key biogeographic regions used by loggerheads (Wilkinson et al. 2009; Pajuelo et al. 2012b). Previous studies indicate that sea turtle reproductive output is influenced by the differential utilization of foraging areas. For instance, loggerheads using two foraging areas in the Mediterranean differed in body size and number of eggs laid per clutch (Zbinden et al. 2011), and loggerheads nesting in Japan that used neritic foraging areas exhibited greater cumulative reproductive output than those using oceanic foraging areas (Hatase et al. 2013). Thus, variation in foraging site preference could influence the reproductive output of female loggerheads in the NWA as well.
Individual variation in diet or habitat use can be both difficult to monitor in a free-ranging, migratory animal and difficult to link to reproductive output. First, the foraging strategy, or in this case the foraging area, that is used by each individual must be determined. Carbon and nitrogen stable isotopes are increasingly being used to determine geographic origin and trace movements of migratory marine animals. These isotopes are assimilated through the diet and reflect biogeochemical processes that create spatial gradients in the isotope values of primary producers at the base of the food web (Goericke and Fry 1994; Montoya 2007). This method requires spatial variation in the stable isotope landscape, or isoscape, of the habitat used by the organism as well as knowledge of how these isotopic values vary across the geographic range. Because isoscape coverage is limited in the marine environment (McMahon et al. 2013), a combination of stable isotope data and satellite telemetry or geolocation data can efficiently identify the geographic origin of marine species that utilize isotopically distinct areas, such as sea turtles (Zbinden et al. 2011; Ceriani et al. 2012; Pajuelo et al. 2012b; Seminoff et al. 2012) and marine birds (Phillips et al. 2009; Jaeger et al. 2010; González-Solís et al. 2011).
The second component of understanding the potential effects of alternative foraging strategies requires measuring parameters linked to reproductive output. Individual females must be marked and followed through multiple years with observation of reproductive activities. The Caretta Research Project (CRP) at Wassaw Island, Georgia, USA, has conducted saturation tagging and monitored nesting activity of loggerheads since 1973, thus providing a long-term database to link reproductive output to foraging strategy.
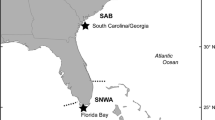
Using satellite telemetry data and isotopic values from epidermal tissue, Pajuelo et al. (2012b) characterized three major biogeographic regions that are used by loggerheads nesting in the southeastern United States: (1) Mid-Atlantic Bight (MAB), (2) South Atlantic Bight (SAB), and (3) Subtropical Northwest Atlantic (SNWA) (Fig. 1). The MAB extends from Cape Cod, MA to Cape Hatteras, NC; the SAB extends from Cape Hatteras, NC to West Palm Beach, FL; the SNWA extends from West Palm Beach, FL to Naples, FL and includes the Florida Keys and Bahamas (Fig. 1). These areas represent well-defined biogeographic regions of the NWA with discrete biotic and abiotic features (Hutchins 1947, Wilkinson et al. 2009) that contribute to distinct isotope values in lower trophic levels that are transferred up the food web to the turtles (Pajuelo et al. 2012a).
Because the relationship between habitat use and tissue stable isotope values have been validated for this population, stable isotope data alone can be successfully applied to a large number of individuals at low cost to determine the foraging area of individuals in a nesting aggregation (Pajuelo et al. 2012b). This approach also has the potential to provide an improved understanding of nesting abundance if evaluation of stable isotopes becomes a component of long-term studies at nesting beaches.
The NWA population of loggerheads is divided into five recovery units defined as geographically and/or genetically distinct subpopulations, and the annual total number of nests in several of these recovery units has declined significantly over the past one to two decades (National Marine Fisheries Service and US Fish and Wildlife Service 2008). In addressing the conservation efforts for this species, it is critical to assess the causes for any change in nesting female abundance. Sea turtle population trends are usually evaluated based on the number of females in nesting aggregations, but because females originate from many foraging areas, this can confound the efforts to understand trends in the nesting aggregation. Therefore, our approach using stable isotope data can also aid in understanding changes in nesting abundance due to variation at particular foraging areas.
The purpose of this study was to: (1) identify the foraging areas used by nesting female loggerheads at Wassaw Island and determine whether foraging site preference affects reproductive output as measured through a suite of ten metrics and (2) examine the trends in the number of females originating from each of three foraging areas in the NWA over multiple years. With respect to the first objective, we expected that variation in environmental conditions and resources available on foraging grounds would contribute to differences in reproductive output. The ten reproductive output metrics examined in this study are known to be important in sea turtles and have the potential to influence an individual’s lifetime reproductive output. Each of the parameters is described in further detail in Materials and Methods. In relation to the second objective, we predicted that the proportion of turtles originating from each foraging ground would vary over the years studied, as the proportion of females originating from different foraging grounds was previously shown to vary between 2 years at Florida’s nesting beaches (Pajuelo et al. 2012b). We also explored how this approach might be used to examine trends in abundance in sea turtle nesting aggregations.
Materials and methods
Sample collection and analysis
A total of 212 skin samples were collected from 192 loggerhead females at Wassaw Island, Georgia, USA, during the nesting season (May–August) in the years 2004, 2005, 2006, 2009, and 2011. Females were sampled at the time they were first encountered during the nesting season, and the majority (73 %) were sampled in the months of May or June, though some were not sampled until July or August, with the latest collection occurring on August 4. Seventeen females were sampled in more than 1 year: 15 females were sampled in 2 years, one was sampled in 3 years, and one was sampled in 4 years. Females were not sampled more than once in the same year. The number of females sampled represented 32–86 % of the observed nesting aggregation in that year (Table 1). Skin was sampled using a sterile 6 mm biopsy punch from the “shoulder” area of each female (between the neck and the front flipper), and samples were stored in 70 % ethanol. Stable isotope values of loggerhead epidermis are not significantly affected by preservation in 70 % ethanol (Barrow et al. 2008).
Prior to stable isotope analysis, the skin biopsies were rinsed with distilled water and cleaned with isopropyl alcohol swabs. Surface epidermis was separated from dermal tissue and homogenized with a scalpel blade, and the epidermis was dried at 60 °C for a minimum of 24 h. Lipids were removed from epidermis samples collected prior to 2011 using an ASE300 accelerated solvent extractor (Dionex) and petroleum ether solvent for three consecutive cycles consisting of 5 min of heating to 100 °C and pressurization to 1,500 PSI, 5 min static, purging, and then flushing with additional solvent.
Samples from 15 loggerhead turtles that nested in 2011 were used to determine whether lipid extraction significantly affects δ13C and δ15N values of loggerhead epidermis. All samples of epidermis were dried and homogenized using the protocol outlined above. Each epidermis sample was subdivided such that half of the sample was lipid-extracted as described above, whereas the other half was not lipid-extracted. Paired t tests were conducted to examine the δ13C and δ15N values before and after lipid extraction.
Isotopic compositions of epidermis samples weighing 0.5–0.6 mg were determined at the Department of Geological Sciences, University of Florida, Gainesville, Florida, using an ECS 4010 elemental analyzer (Costech) interfaced via a ConFlo III to a DeltaPlus XL isotope ratio mass spectrometer (ThermoFisher Scientific). Delta notation was used to express stable isotope abundances, defined as parts per thousand (‰) relative to the standard:
where R sample and R standard are the corresponding ratios of rare to common isotopes (13C/12C and 15N/14N) in the sample and international standard, respectively. Vienna Pee Dee Belemnite was used as the standard for 13C, and atmospheric N2 for 15N. Reference materials were used to normalize all results. N1 (ammonium sulfate) was used for nitrogen isotopes and CH6 (sucrose) was used for carbon isotopes with the 2004 and 2005 samples. The reference material was changed during the study, and USGS40 (l-glutamic acid) was used with the isotopic analysis of the samples from 2006, 2009, and 2011. The standard deviation of N1 + CH6 was 0.19 ‰ for δ13C and 0.08 ‰ δ15N values (n = 13) and the standard deviation of USGS40 was 0.09 ‰ for δ13C and 0.11 ‰ δ15N values (n = 31). Repeated measurements of a laboratory reference material, loggerhead scute, were used to examine consistency in a homogeneous sample with similar isotopic composition to the epidermis samples. The standard deviation of this laboratory reference material was 0.14 ‰ for δ13C values and 0.31 ‰ for δ15N values (n = 10).
Determining foraging area
Because rates of isotopic incorporation have not been measured in adult loggerheads, the precise time period reflected in the skin samples is unknown. Measurements of the isotopic turnover time of epidermis in juvenile loggerheads are approximately 4 mo (Reich et al. 2008), but the time period represented in adult samples is likely longer, as the rate of isotopic incorporation slows with reduced growth rate (Reich et al. 2008) and increasing body mass (Bauchinger and McWilliams 2009). In adult alligators (Alligator mississippiensis), the turnover time in the scute tissue (collagen and keratin-based raised scales) on the tail was estimated to be over a year (Rosenblatt and Heithaus 2013). Therefore, we expect that the epidermis samples from adult loggerheads reflect a foraging period of at least 4 months prior to migration and nesting activity.
Quadratic discriminant function analysis of stable isotope values was used to assign females to one of three foraging areas: (1) Mid-Atlantic Bight (MAB), (2) South Atlantic Bight (SAB), and (3) Subtropical Northwest Atlantic (SNWA). The training data for the quadratic discriminant function analysis consisted of stable isotope values of 60 adult loggerheads from known foraging areas (either through satellite tracking or from sample collection at a foraging site) collected between 2004 and 2011 (Fig S1, Pajuelo et al. 2012b). A uniform prior probability distribution was assumed, meaning it was equally likely the individuals could have originated from each foraging area, rather than weighting the prior probability by sample size from each area. To test the accuracy of assignment, we used leave-one-out cross-validation with the reference group. Fifty-six (93 %) of the 60 turtles in the training data set were assigned to the correct foraging group.
The discriminant analysis was then used to classify 212 nesting female samples from Wassaw Island into one of the three groups. Only assignments with posterior probabilities ≥0.8 were considered, which translates to an eightfold improvement in odds over random assignment (Wunder 2012). A total of 183 of the 212 samples from the nesting females (86 %) had posterior probabilities of assignment ≥0.8. The foraging area assignments for the 20 duplicate samples from 17 females were also compared to examine the consistency in habitat use.
The inter-annual variation in the proportion of turtles originating from each foraging area was compared using a Pearson’s Chi-squared test with a p value computed through Monte Carlo simulation (based on 2,000 replicates) due to the unequal distribution across the foraging sites.
Reproductive parameters
Nocturnal patrols were conducted nightly on Wassaw Island each year from May through August to intercept females during nesting emergences. All females were examined for and, if necessary, fitted with individualized tags (Williams and Frick 2001), and the detection probability of females remained constant through the years of the current study (Pfaller et al. 2013). Each nest was individually marked, monitored throughout incubation, and excavated within 5 days after hatchling emergence.
Ten parameters were analyzed for each female, data permitting. Each female was represented only once. If skin samples were taken in successive years, only data for the most recent nesting year with foraging ground assignment were used in the analysis of reproductive output. Data on body size, arrival date, duration of nesting season, clutch size, clutch frequency, hatching success, and proportion of relocated nests were reported for the same year in which the skin sample was collected. For 23 females, there were no nests deposited on Wassaw Island in the year the sample was collected (i.e., samples were taken during a non-nesting emergence). Information from genetic mark recapture of females (Shamblin and Nairn unpubl data) and tag returns from other beaches present in the CRP database were included when available. Genetic mark recapture incorporating up to 18 microsatellite loci was used to assign clutches laid off-site or on Wassaw Island by unobserved females (Shamblin et al. 2007, 2009, 2011b). Data from these other sources were obtained for 72 females. The ten reproductive output parameters were determined as follows:
-
1.
Body size. Hard-shelled turtles may be limited in the number of eggs they can carry, and clutch size has been demonstrated to positively correlate to linear metrics and body mass (Limpus 1985; Frazer and Richardson 1986; Bjorndal and Carr 1989), which means body size can be indicative of fecundity. Body size was measured as the curved carapace length (CCL notch-to-tip) to the nearest 0.5 cm for the year in which the female was sampled. In some years, the same female was measured more than once. If CCL was measured multiple times in a year, the median was used. If CCL was not available for the year of sample collection, we used CCL measurement(s) from the most recent prior year in which the female was measured because growth is negligible in adult turtles (Bjorndal et al. 1983).
-
2.
Arrival date. The onset of loggerhead nesting is correlated to latitude as well as sea surface temperatures in the Atlantic and Mediterranean basins (Weishampel et al. 2004; Pike et al. 2006; Hawkes et al. 2007b; Mazaris et al. 2013; Lamont and Fujisaki in press). Turtles experience distinct temperature regimes in each of the three foraging areas in the NWA, and if sea surface temperature acts as a cue to initiate migration, foraging ground origin could affect arrival date to the nesting beach. Additionally, migration time may vary depending on the distance to the nesting beach, as not all foraging areas are equidistant to the nesting beach. Significant differences in the arrival date could mean that clutches do not experience equal environmental conditions and sand temperatures through incubation, possibly affecting hatching success and sex ratios among clutches. Arrival date was reported as the Julian calendar day for the first observation of an individual female on the nesting beach during the year, regardless if she nested during that observation (i.e., sometimes the first observation was a non-nesting emergence).
-
3.
Duration of nesting season. Regardless of whether females arrive on the same date, they may spend differing lengths of time at the breeding grounds. Turtles with longer nesting seasons can potentially produce a greater number of clutches, assuming the inter-nesting interval remains constant. However, one study has demonstrated a link between arrival date and duration of the nesting season, such that earlier arrival was correlated with shorter nesting seasons (Pike et al. 2006). Duration of the nesting season was calculated as the number of days between the first and last observation for each female.
-
4.
Clutch size. The number of eggs per clutch is a measure of fecundity, and it is predicted that turtles should maximize clutch size in order to maximize reproductive output (Hays and Speakman 1991). Clutch size was calculated as the mean number of eggs per clutch within a year, though 62 females had only one clutch from which to measure clutch size. Because there may be a physical limit to the number of mature eggs a female can hold, clutch size tends to be greater in larger individuals (Limpus 1985; Frazer and Richardson 1986, Broderick et al. 2003). Therefore, we examined the influence of body size on clutch size by using CCL as a covariate with ANCOVA for the MAB and SAB foraging groups.
-
5.
Estimated clutch frequency. Fecundity within a nesting season depends on the number of eggs per clutch and the number of clutches each female produces. However, observing every clutch a sea turtle lays during the season is difficult, and the number of observed nests is typically an underestimate of the actual number of nests laid (Hays and Speakman 1991). Therefore, clutch frequency estimates are often adjusted to include probable missed nests (Frazer and Richardson 1985). Because all nests laid in a season are rarely observed, we accounted for probable missed nests when the time between nesting events exceeded the mean inter-nesting interval, which is 13 days for loggerheads in this nesting aggregation (Pfaller et al. 2013). One nest was added to the observed number of nests if ≥18 days separated two nests; two nests were added if ≥36 days separated two nests; and three nests were added if ≥54 days separated two nests. Additionally, nests were added when a non-nesting emergence preceded or followed the first or last nest by at least 8 days; additional nests were added using the same temporal limits set for gaps between two nests. A maximum of three nests was added to our estimates of clutch frequency, and the mean ± SD number of nests added was 0.47 ± 0.68. Females with zero observed nests were not included in the analysis. The estimated clutch frequency is likely to still be an underestimate of the actual number of nests, as clutch frequency is more accurately determined through satellite telemetry (Tucker 2010). However, we expect that the rate of nest detection does not vary among females from different foraging grounds.
-
6.
Mean hatching success. The successful incubation and hatching of eggs deposited by a female depends on a number of factors, including distance to the water, temperature, humidity, and sand characteristics (Miller et al. 2003; Foley et al. 2006). The placement of the nest can significantly affect hatching success, thus nest-site selection could lead to differences in reproductive output for females originating from different foraging areas. Loggerheads exhibit variation in nest-site placement (Pfaller et al. 2009), and the spatial distribution of nests is not uniform (Hays and Speakman 1993). Hatching success is the percentage of eggs in a clutch that produce live hatchlings. The CRP relocates all nests placed below the high water line (=“doomed nests”). Hatching success was calculated as the mean hatching success from all nests for each female, and a hatching success of 0 % was assigned to relocated nests.
-
7.
Proportion of relocated nests. Nests sites too close to the water are vulnerable to tidal inundation and are relocated by CRP personnel. In relation to the hatching success parameter above, we wanted to determine whether the number of “doomed” nests differed among females from the three foraging areas. The proportion of relocated nests was calculated as the number of nests that were relocated divided by the total number of nests that were observed during the year for each female.
-
8.
Mean remigration interval. Loggerheads rarely nest in consecutive years (Dodd 1988). The remigration interval, or time period between successive nesting seasons, may reflect conditions encountered at the foraging area, with productive conditions leading to shorter remigration intervals (Hays 2000; Solow et al. 2002). The mean remigration interval in loggerheads in southeastern USA ranges from about 2.5 to 3.0 years (Schroeder et al. 2003). We determined the remigration interval for females that had previously nested on Wassaw Island or nearby beaches by using the number of years between consecutive seasons sighted and calculating the mean of all remigration intervals available for each female. The number of remigration intervals, when available, ranged from one to nine.
-
9.
Breeding frequency. Females that breed most frequently typically display higher fecundity during their reproductive lifetime. Breeding frequency was calculated as the number of years a female was observed nesting at Wassaw between 1973 and 2011, and only females that were observed in more than 1 year were included in this metric.
-
10.
Cumulative reproductive output. To integrate a portion of the reproductive output parameters above, a cumulative reproductive output value was calculated by determining the number of hatchlings produced per female throughout the survey period. This value was a product of four parameters and is similar to the metric used by Hatase et al. (2013), whereby cumulative reproductive output = clutch size × estimated clutch frequency × mean hatching success × breeding frequency. This metric was calculated only for females that were observed nesting for more than 1 year, thus reducing the sample size for comparison.
Differences in reproductive parameters among turtles originating from the three foraging areas were identified with ANOVA with Type II Sums of Squares (due to the unequal sample sizes among foraging grounds) followed by Tukey’s HSD tests where applicable. All data were analyzed using program R version 3.0 (R Development Core Team 2013) with an α level of 0.05.
Results
There was no significant difference in δ13C values (t 14 = −0.17, p = 0.87) or δ15N values (t 14 = −1.10, p = 0.29) of epidermis following lipid extraction (Fig. 2). Therefore, the samples did not contain sufficient lipids to affect the stable isotope values of the tissue, and we are confident that loggerhead epidermis samples that were not lipid-extracted are comparable to those that were lipid-extracted in this study and in other studies.
A total of 183 of the 212 nesting females (86 %) were assigned to one of three foraging areas in the NWA with posterior probabilities of group membership ≥0.8 (Fig. 3). The 29 females that could not be assigned to a foraging area fell between the MAB and SAB groups (Fig. 3). There is some overlap in the distributions of the stable isotope values of these two foraging areas, making it difficult to assign turtles with values that fall in the overlapping region of the isotopic distributions (Fig. S1). Because the isotope values change along a latitudinal gradient (Pajuelo et al. 2012a), these individuals were likely foraging near the border of these two foraging areas.
Stable carbon and nitrogen isotope values of 212 loggerhead females nesting at Wassaw Island, Georgia between 2004 and 2011. Dark points (circles, triangles, and squares) represent 183 loggerhead females that were determined to have originated from one of three foraging areas in the Northwest Atlantic through discriminant analysis with posterior probability of group membership ≥0.8. Gray points (diamonds) represent 29 females with probability of group membership <0.8 that could not be assigned to a foraging area. MAB Mid-Atlantic Bight, SAB South Atlantic Bight, SNWA Subtropical Northwest Atlantic
Multiple samples were obtained from 17 turtles during two or more years. Though duplicate samples were not used to compare reproductive parameters, they were useful in examining foraging area utilization by individual females over numerous years. Eleven of the 17 turtles sampled over multiple years had foraging ground assignment probabilities ≥0.8 in all years sampled. These turtles were assigned to the same foraging area in all years, including a single female that was sampled in four different seasons spanning a time period of 7 years. Six turtles sampled over multiple years, however, had foraging ground assignment probabilities <0.8, and could not be reliably assigned to the same foraging area for each season they were sampled, suggesting that these females were feeding near the boundaries of the defined foraging areas (Table S1).
Females originating from the three foraging areas differed in three of the ten reproductive parameters examined (Table 2). Turtles originating from SAB were significantly smaller than those from the MAB or SNWA (Fig. 4a), and females from the SAB also laid significantly fewer eggs per clutch than females from the MAB or SNWA (Fig. 4b). The effect of body size (CCL) on clutch size was further investigated between MAB and SAB turtles (SNWA females were not included in this analysis, due to the small sample size). Clutch size was positively related to CCL for both groups of females, and there was no difference in the two regression lines (F (1,121) = 0.11, p = 0.75, Fig. 5). Therefore, clutch size was not significantly different between the two foraging areas after accounting for body size. We believe the significant difference in clutch size between SAB and SNWA turtles is also a result of body size because body size and clutch size were not significantly different between MAB and SNWA turtles.
a Body length (CCL curved carapace length) differed significantly among loggerheads originating from the different foraging areas. MAB Mid-Atlantic Bight, SAB South Atlantic Bight, SNWA Subtropical Northwest Atlantic. SAB females were significantly smaller than MAB and SNWA turtles. b Clutch size also differed among the females originating from different foraging areas with SAB females depositing significantly fewer eggs per clutch than MAB or SNWA females. c Mean remigration interval was significantly shorter for MAB females than SNWA females
The relationship between body size (CCL curved carapace length) and clutch size was not significantly different between females originating from MAB and SAB foraging areas, and thus the regression line is plotted for both areas together (slope = 2.1, intercept = −92.0, r 2 = 0.32, F 1,122 = 59.6, p < 0.001). MAB Mid-Atlantic Bight, SAB South Atlantic Bight
The remigration interval varied significantly among the foraging areas, with fewer years between nesting seasons for females originating from the MAB than those from the SNWA (Fig. 4c). The remigration interval for females originating from the SAB was not significantly different from the other two areas. We caution that the sample size from the SNWA was only two females for remigration intervals, thus limiting the ability to make a robust comparison.
A trend in arrival date approached significance as MAB females tended to arrive earlier than SAB females, and both tended to arrive earlier than SNWA females (Table 2). While SAB females tended to have fewer relocated nests, there were no significant differences. Despite differences in the foraging areas utilized by turtles, there were no significant differences in the remaining reproductive parameters examined (estimated clutch frequency, duration of the nesting season, adjusted hatching success, breeding frequency, and cumulative reproductive output; Table 2).
The sampling protocol allowed for examination of the trends in abundance from each of the foraging areas between 2004 and 2011 (excluding 2007, 2008, and 2010). Among the years sampled, the proportion of turtles originating from each foraging area varied significantly (χ 214 = 18.4, p = 0.02, Table 1). The largest proportion of females originating from the SAB (0.29) and SNWA (0.21) occurred in 2004, while the largest proportion of females originating from the MAB occurred in 2006 and 2009 (0.89 in both years). Over the time period of the study, the nesting population size corrected for imperfect detection (double counting, misidentifying, or overlooking individuals) ranged from 24 to 103 females (Fig. 6). Visual inspection of Fig. 6 reveals that differences in abundance of nesting turtles is primarily the result of changes in the number of turtles originating from MAB, not of equal changes from all foraging areas.
Estimated number of loggerheads nesting at Wassaw Island, Georgia, assigned to each of the three foraging areas. The estimated total is the number of females nesting during the year, corrected for imperfect detection (Pfaller et al. 2013). The number of females from each foraging area was calculated as the proportion of individuals from each foraging area in a given year multiplied by the estimated total. Thus, the number of females in all foraging areas in a particular year sums to the estimated total. Skin samples were not collected in 2007, 2008, and 2010, and points between non-consecutive sampling years for each foraging area are connected by dotted lines. MAB Mid-Atlantic Bight, SAB South Atlantic Bight, SNWA Subtropical Northwest Atlantic
Discussion
Reproductive output
We found that female loggerheads that forage in the MAB contributed a disproportionate number of offspring to the population because they were larger, had larger clutch sizes, and had shorter remigration intervals than individuals foraging in other areas of the NWA. Individual differences in foraging strategies, such as differences in foraging site preference by loggerheads in this study, can affect reproductive performance both on an annual and long-term basis. A skew in reproductive performance as a result of differential foraging can lead to individuals contributing disproportionately to the population (Annett and Pierotti 1999). Additionally, the “decisions” made by an animal may not have immediate consequences, but rather such processes may operate across seasons, resulting in significant “carry-over effects” on performance in subsequent time periods (Norris 2005; Harrison et al. 2011). In many cases, carry-over effects are a consequence of variation in the use and access to resources among individuals (Harrison et al. 2011).
In the case of nesting loggerheads in the NWA, females utilize three distinct foraging areas that differ in a variety of biotic and abiotic characteristics, though the majority of females nesting at Wassaw Island, GA, forage in the MAB. Based on the repeated samples from individuals in our study, adult loggerheads appear to consistently use the same foraging area across multiple years. This supports the high degree of foraging site fidelity that has been suggested for loggerheads in the NWA (Vander Zanden et al. 2010; Hawkes et al. 2011) and in other regions (Broderick et al. 2007; Schofield et al. 2010; Marcovaldi et al. 2010; Thomson et al. 2012). Individuals may pass through other regions as they migrate to the nesting beach, and turtles foraging in the MAB move south seasonally (Hawkes et al. 2011; Griffin et al. 2013), yet our results indicate that the majority of the nutrients consumed and assimilated reflect consistent foraging in the same area.
The cause for differential foraging area use may be due to many reasons. Nothing is known about the heritability of foraging location. Almost all of the hundreds of the females nesting at Wassaw Island that have been examined to date have the same mtDNA haplotype: CC-A1.1 (Shamblin et al. 2011a; Shamblin and Nairn unpubl data). Therefore, the lack of genetic structure among these females does not support the possibility that genetic differences contribute to the choice in foraging area. No genotypic or haplotype differences were observed among loggerhead females using distinct foraging areas in Japan either (Watanabe et al. 2011). Significant, though shallow, genetic haplotype population structure does exist among neritic juvenile foraging areas in the NWA, which was correlated to the haplotype frequencies of adjacent nesting aggregations, supporting the hypothesis that juveniles recruit to neritic foraging areas near their natal rookery (Bowen et al. 2004). Further investigation into the genetic relatedness of individuals may help to elucidate whether foraging location may be heritable and is a direction for future research. It is also possible that adult female dispersion from the nesting beach may be linked with the drift scenarios that hatchlings would have experienced when first leaving the natal beach (Fossette et al. 2010; Hays et al. 2010), as the Gulf Stream would first carry them northward along the coast before veering east into the oceanic waters of the northern Atlantic. Additionally, body size could contribute to the dispersal of adults to different foraging areas based on the ability to endure water temperature extremes, such that larger turtles could be expected to withstand larger temperature ranges (Abecassis et al. 2013).
In this study, we demonstrate that fidelity to foraging areas among individual loggerheads is linked to reproductive output. The primary difference observed in loggerheads using distinct foraging areas is body size: females using the SAB are significantly smaller than those foraging in the MAB or SNWA. SAB females also produce smaller clutches. While smaller clutch size is a corollary of smaller body size, it may ultimately influence the lifetime reproductive output. Clutch frequency and remigration intervals do not differ between MAB and SAB turtles, but because SAB turtles do not reach the same maximum size as those from the MAB (Fig. 4), the lifetime reproductive output of SAB turtles will be significantly reduced if reproductive lifespan does not vary between turtles originating from these areas.
We were unable to calculate a lifetime reproductive output in this study, but MAB turtles tended to have a higher cumulative reproductive output than SAB turtles over the study period, although no significant differences were observed due to the wide variation observed among individuals. If the lifetime reproductive output does indeed differ, not all foraging aggregations will contribute equally to the nesting aggregation over the long term, and the proportion of females using the MAB could continue to increase if foraging area choice is heritable.
While adult loggerheads in the NWA and Mediterranean use distinct coastal, or neritic, foraging areas (Hawkes et al. 2011; Zbinden et al. 2011; Ceriani et al. 2012; Pajuelo et al. 2012a; Schofield et al. 2013), loggerheads in other regions have alternative foraging strategies that include both neritic and oceanic habitats. This oceanic/neritic dichotomy has been observed for loggerheads nesting in Cape Verde and Japan, whereby females using oceanic foraging areas are smaller than those that forage in neritic areas with reduced reproductive output, likely due to differences in nutrient availability in the distinct habitats (Hatase et al. 2002, 2013; Hawkes et al. 2006; Eder et al. 2012).
The three neritic foraging areas used by loggerheads in the NWA vary in productivity. The MAB is one of the most productive marine regions of the world (Wilkinson et al. 2009), but this area is used only on a seasonal basis, as loggerheads that forage above 35°N migrate to the south or east in the winter to areas with more suitable water temperatures (Hawkes et al. 2007a, 2011; Griffin et al. 2013). The SAB is moderately productive, with more patchy production due to short-lived plankton blooms associated with upwelling along the Gulf Stream front (Wilkinson et al. 2009). The SNWA contains a complex physiography with areas of reef supporting a rich biota and overall moderate productivity in the region (Wilkinson et al. 2009). Loggerheads using the SAB and SNWA do so year-round, as the water temperatures do not necessitate seasonal migrations (Hawkes et al. 2011; Griffin et al. 2013). If productivity is the main driver of body size, the inconsistent productivity in the SAB may contribute to the smaller body sizes observed in females using that area, as has been observed for leatherbacks foraging in less productive areas (Wallace et al. 2006). Furthermore, the high seasonal productivity of the MAB and consistent moderate productivity with warmer water temperatures in the SNWA, respectively, may contribute to the larger body size and clutch size of females from those areas. We cannot rule out the possibility that body size drives the dispersal ability of the turtles and the areas where they can forage, but given that differences in productivity have been correlated to loggerhead body size in other regions (Hatase et al. 2002, 2013; Hawkes et al. 2006; Eder et al. 2012), we expect that it is also the case in the NWA.
Future examination of the energy requirements for migration and reproduction among the foraging areas may help to explain the variation in reproductive output parameters. Oceanic (and smaller) loggerhead females in Japan breed less frequently than those that are neritic foragers (and larger), likely due to the time needed to accumulate sufficient energy for reproduction (Hatase and Tsukamoto 2008; Hatase et al. 2013). The differences in distance to the nesting beach from each foraging area in the NWA are not as substantial as the differences in distance for the oceanic and neritic habitats in Japan, though mean remigration interval was observed to differ among the three foraging grounds in this study. In this case, distance to the nesting beach may be less important than the currents encountered during migration or habitat quality differences (including food availability) among the foraging areas, as it would be expected that SAB turtles would nest more frequently if distance was the main factor influencing remigration interval.
However, caution is warranted in the interpretation of these results. First, we have only measured the remigration intervals using the data collected at Wassaw Island, but females may nest unobserved at other beaches. Therefore, we are unable to distinguish whether a long remigration interval and short breeding frequency are a result of low fidelity to the nesting beach or a true absence of nesting activity during that period. Second, we had an extremely limited sample size from the SNWA, as only two of the eight SNWA females were observed across multiple years at Wassaw Island, thus limiting our ability to make robust comparisons for remigration interval, breeding frequency, and cumulative reproductive output.
Further investigation of these parameters should be pursued at a beach where the proportion of females originating from the SNWA is higher, for example, at a more southerly beach in Florida (Pajuelo et al. 2012b). If the pattern we observed truly reflects a longer remigration interval rather than low nest-site fidelity, then this would be a reproductive consequence of using distinct foraging areas. MAB turtles that nest nearly twice as frequently as those from the SNWA will exhibit a higher lifetime reproductive output if reproductive life spans are similar.
Finally, the difference in arrival date to the nesting beach approached significance, with the MAB turtles arriving first in the season. If it were an effect of migration differences, we might expect that SAB females would arrive first, as they would have the shortest migration distance, with MAB and SNWA arriving later. More likely, this is an effect of sea surface temperature influencing the initiation of migration (Weishampel et al. 2004; Hawkes et al. 2007b; Lamont and Fujisaki in press), as we also observed inter-annual variation in the onset of nesting. While Pike et al. (2006) reported that earlier nesting contributed to a shorter nesting season, we found no difference in the duration of the nesting season among foraging areas. However, a later arrival to the nesting beach can potentially result in nests experiencing higher sand temperatures, thus influencing the sex ratio and hatching success (Matsuzawa et al. 2002; Reece et al. 2002).
Trends in abundance
By examining the foraging area origin of nesting females over a span of 7 years, we found that the change in the nesting aggregation was not a result of equal increases from each foraging area. Stable isotope analysis is increasingly being used to determine the origin of nesting sea turtles when persistent isotopic differences can be used to discriminate among foraging grounds (Hatase et al. 2002; Zbinden et al. 2011; Ceriani et al. 2012; Pajuelo et al. 2012b; Seminoff et al. 2012). This method requires initial tracking efforts to validate the isotopic data—and though it may not be useful in regions where the stable isotope values of turtles from different foraging areas exhibit high overlap (e.g., Vander Zanden et al. 2013)—it holds great promise for monitoring sea turtle foraging aggregations from the nesting beach in many regions by scaling up the knowledge gained from tracking a limited number of individuals. This study is the first time stable isotope data from a sea turtle nesting aggregation have been used to monitor inter-annual variability in abundance and trends among foraging aggregations. This technique has important conservation value for sea turtle populations, as it may aid in the development of more specific management approaches that address the threats and trends for each foraging aggregation.
Long-term monitoring of nesting aggregations with this approach can be used to determine whether fluctuations in the number of nesting females are due to disproportionate trends in foraging aggregations. In this study, foraging area was determined for 183 nesting loggerheads, and the proportion of females originating from each of the three foraging areas varied over the 5 years of the study. The annual number of nesting females at Wassaw Island, corrected for imperfect detection, has not exhibited a significant trend from 1973 to 2011 (Pfaller et al. 2013). However, the years included in this study represent near minimum (20 in 2004) and maximum (93 in 2011) number of females observed over the long-term population monitoring at Wassaw Island (Pfaller et al. 2013). From female counts or nest counts alone, it is impossible to determine whether the nearly fivefold increase in the number of nesting females between these years resulted from a uniform increase in females from all foraging areas. Our approach offers the advantage of examining trends among the three major foraging aggregations in addition to the nesting aggregation.
Our data indicate that the increase in nesting females between 2004 and 2011 is not a consequence of a uniform increase from all foraging areas, but rather the difference is driven by turtles originating from the MAB. This study does not address possible causes for fluctuations in the nesting aggregation, though anthropogenic threats and regional ocean temperatures may be major drivers of loggerhead nesting population dynamics (Chaloupka et al. 2008; Witherington et al. 2009; Mazaris et al. 2013). Further investigation is needed to examine whether there is a relationship between sea surface temperature at each of the foraging areas and the number of nesting females originating from each area.
Continued monitoring of this population with stable isotope analysis can be used to develop an understanding of the long-term patterns in the composition of the nesting aggregation and could be complemented by monitoring other nesting subpopulations in the NWA, as this information is important for interpreting nesting abundance as a proxy for population dynamics. An improved understanding of variation in foraging aggregations can also aid in the geospatial prioritization of threat reduction. If this approach becomes a component of long-term studies, it can be a valuable tool not only to monitor sea turtle demographic trends in other parts of the globe, but it could be useful for other animal taxa that exhibit a similar dispersion phenomenon whereby reproductive areas are geographically separated from foraging areas (e.g., salmonids, seabirds, and marine mammals).
In conclusion, we found that persistent differences in foraging strategies among loggerheads can have carry-over effects on reproductive output, as significant differences were observed in body size, clutch size, and remigration interval. We also observed that fluctuations in the nesting aggregation were related to variation in the contribution of females from the three foraging areas in the NWA. Our approach to assess foraging area origins of nesting loggerheads through stable isotope analysis can be useful to interpret trends in abundance at nesting beaches and to monitor trends in abundance of other sea turtle species, thus becoming a valuable tool for sea turtle conservation. An improved understanding of the influences on demographic parameters and trends among distinct foraging areas, such as reproductive differences among foraging aggregations, can aid in more effectively managing these populations.
References
Abecassis M, Senina I, Lehodey P, Gaspar P, Parker D, Balazs G, Polovina J (2013) A model of loggerhead sea turtle (Caretta caretta) habitat and movement in the oceanic North Pacific. PLoS ONE 8:e73274. doi:10.1371/journal.pone.0073274
Annett CA, Pierotti R (1999) Long-term reproductive output in western gulls: consequences of alternate tactics in diet choice. Ecology 80:288–297. doi:10.2307/176997
Araújo MS, Bolnick DI, Layman CA (2011) The ecological causes of individual specialisation. Ecol Lett 14:948–958. doi:10.1111/j.1461-0248.2011.01662.x
Authier M, Dragon A-C, Richard P, Cherel Y, Guinet C (2012) O’ mother where wert thou? Maternal strategies in the southern elephant seal: a stable isotope investigation. Proc R Soc B 279:2681–2690. doi:10.1098/rspb 2012.0199
Barrow LM, Bjorndal KA, Reich KJ (2008) Effects of preservation method on stable carbon and nitrogen isotope values. Physiol Biochem Zool 81:688–693. doi:10.1086/588172
Bauchinger U, McWilliams S (2009) Carbon turnover in tissues of a passerine bird: allometry, isotopic clocks, and phenotypic flexibility in organ size. Physiol Biochem Zool 85:541–548. doi:10.1086/605548
Bjorndal KA, Carr A (1989) Variation in clutch size and egg size in the green turtle nesting population at Tortuguero, Costa Rica. Herpetologica 45:181–189. doi:10.2307/3892160
Bjorndal KA, Meylan AB, Turner BJ (1983) Sea turtles nesting at Melbourne Beach, Florida, I. Size, growth and reproductive biology. Biol Conserv 26:65–77. doi:10.1016/0006-3207(83)90049-6
Bolnick DI, Svanback R, Fordyce JA, Yang LH, Davis JM, Hulsey CD, Forister ML (2003) The ecology of individuals: incidence and implications of individual specialization. Am Nat 161:1–28. doi:10.1086/343878
Bowen BW, Bass AL, Chow SM, Mostrom M, Bjorndal KA, Bolten AB, Okuyama T, Bolker BM, Epperly S, Lacasella E, Shaver E, Dodd M, Hopkins-Murphy SR, Musick JA, Swingle M, Rankin-Baransky K, Teas W, Witzell WN, Dutton PH (2004) Natal homing in juvenile loggerhead turtles (Caretta caretta). Mol Ecol 13:3797–3808. doi:10.1111/j.1365-294X.2004.02356.x
Broderick AC, Glen F, Godley BJ, Hays GC (2003) Variation in reproductive output of marine turtles. J Exp Mar Biol Ecol 288:95–109. doi:10.1016/S0022-0981(03)00003-0
Broderick AC, Coyne MS, Fuller WJ, Glen F, Godley BJ (2007) Fidelity and over-wintering of sea turtles. Proc R Soc B 274:1533–1539. doi:10.1098/rspb 2007.0211
Ceriani SA, Roth JD, Evans DR, Weishampel JF, Ehrhart LM (2012) Inferring foraging areas of nesting loggerhead turtles using satellite telemetry and stable isotopes. PLoS ONE 7:e45335. doi:10.1371/journal.pone.0045335
Chaloupka M, Kamezaki N, Limpus C (2008) Is climate change affecting the population dynamics of the endangered Pacific loggerhead sea turtle? J Exp Mar Biol Ecol 356:136–143. doi:10.1016/j.jembe.2007.12.009
R Development Core Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Dodd CK (1988) Synopsis of the biological data on the loggerhead sea turtle Caretta (Linnaeus 1758). US Fish Wild Services Biological Report, Washington, DC
Eder E, Ceballos A, Martins S, Pérez-García H, Marn I, Marco A, Cardona L (2012) Foraging dichotomy in loggerhead sea turtles Caretta caretta off northwestern Africa. Mar Ecol Prog Ser 470:113–122. doi:10.3354/meps10018
Foley AM, Peck SA, Harman GR (2006) Effects of sand characteristics and inundation on the hatching success of loggerhead sea turtle (Caretta caretta) clutches on low-relief mangrove islands in southwest Florida. Chelonian Cons Biol 5:32. doi: 10.2744/1071-8443(2006)5[32:EOSCAI]2.0.CO;2
Fossette CG, López-Mendilaharsu M, Miller P, Domingo A, Evans D, Kelle L, Plot V, Prosdocimi L, Verhage C, Gaspar P, Georges JY (2010) Leatherback migratory paths and temporary residence areas. PLoS ONE 5:e13908. doi:10.1371/journal.pone.0013908
Frazer NB, Richardson JI (1985) Annual variation in clutch size and frequency for loggerhead turtles, Caretta caretta, nesting at Little Cumberland Island, Georgia, USA. Herpetologica 41:246–251. doi:10.2307/3892269
Frazer NB, Richardson JI (1986) The relationship of clutch size and frequency to body size in loggerhead turtles, Caretta caretta. J Herpetol 20:81–84. doi:10.2307/1564129
Goericke R, Fry B (1994) Variations of marine plankton δ13C with latitude, temperature, and dissolved CO2 in the world ocean. Glob Biogeochem Cycle 8:85–90. doi:10.1029/93GB03272
González-Solís J, Smyrli M, Milito T, Gremillet D, Tveraa T, Phillips RA, Boulinier T (2011) Combining stable isotope analyses and geolocation to reveal kittiwake migration. Mar Ecol Prog Ser 435:251–261. doi:10.3354/meps09233
Griffin DB, Murphy SR, Frick MG, Broderick AC, Coker JW, Coyne MS, Dodd MG, Godfrey MH, Godley BJ, Hawkes LA, Murphy TM, Williams KL, Witt MJ (2013) Foraging habitats and migration corridors utilized by a recovering subpopulation of adult female loggerhead sea turtles: implications for conservation. Mar Biol 160:3071–3086. doi:10.1007/s00227-013-2296-3
Harrison XA, Blount JD, Inger R, Norris DR, Bearhop S (2011) Carry-over effects as drivers of fitness differences in animals. J Anim Ecol 80:4–18. doi:10.1111/j.1365-2656.2010.01740.x
Hatase H, Tsukamoto K (2008) Smaller longer, larger shorter: energy budget calculations explain intrapopulation variation in remigration intervals for loggerhead sea turtles (Caretta caretta). Can J Zool 86:595–600. doi:10.1139/z08-035
Hatase H, Takai N, Matsuzawa Y, Sakamoto W, Omuta K, Goto K, Arai N, Fujiwara T (2002) Size-related differences in feeding habitat use of adult female loggerhead turtles Caretta caretta around Japan determined by stable isotope analyses and satellite telemetry. Mar Ecol Prog Ser 233:273–281. doi:10.3354/meps233273
Hatase H, Omuta K, Tsukamoto K (2013) A mechanism that maintains alternative life histories in a loggerhead sea turtle population. Ecology 94:2583–2594. doi:10.1890/12-1588.1
Hawkes LA, Broderick AC, Coyne MS, Godfrey MH, Lopez-Jurado LF, Lopez-Suarez P, Merino SE, Varo-Cruz N, Godley BJ (2006) Phenotypically linked dichotomy in sea turtle foraging requires multiple conservation approaches. Curr Biol 16:990–995. doi:10.1016/j.cub.2006.03.063
Hawkes LA, Broderick AC, Coyne MS, Godfrey MH, Godley BJ (2007a) Only some like it hot—quantifying the environmental niche of the loggerhead sea turtle. Divers Distrib 13:447–457. doi:10.1111/j.1472-4642.2007.00354.x
Hawkes LA, Broderick AC, Godfrey MH, Godley BJ (2007b) Investigating the potential impacts of climate change on a marine turtle population. Glob Change Biol 13:923–932. doi:10.1111/j.1365-2486.2007.01320.x
Hawkes LA, Witt MJ, Broderick AC, Coker JW, Coyne MS, Dodd M, Frick MG, Godfrey MH, Griffin DB, Murphy SR, Murphy TM, Williams KL, Godley BJ (2011) Home on the range: spatial ecology of loggerhead turtles in Atlantic waters of the USA. Divers Distrib 17:624–640. doi:10.1111/j.1472-4642.2011.00768.x
Hays GC (2000) The implications of variable remigration intervals for the assessment of population size in marine turtles. J Theor Biol 206:221–227. doi:10.1006/jtbi 2000.2116
Hays GC, Speakman JR (1991) Reproductive investment and optimum clutch size of loggerhead sea turtles (Caretta caretta). J Anim Ecol 60:455–462. doi:10.2307/5290
Hays GC, Speakman JR (1993) Nest placement by loggerhead turtles, Caretta caretta. Anim Behav 45:47–53. doi:10.1006/anbe 1993.1006
Hays GC, Fossette S, Katselidis KA, Mariani P, Schofield G (2010) Ontogenetic development of migration: Lagrangian drift trajectories suggest a new paradigm for sea turtles. J R Soc Interface 7:1319–1327. doi:10.1098/rsif.2010.0009
Hoye BJ, Hahn S, Nolet BA, Klaassen M (2012) Habitat use throughout migration: linking individual consistency, prior breeding success and future breeding potential. J Anim Ecol 81:657–666. doi:10.1111/j.1365-2656.2011.01948.x
Hutchins LW (1947) The bases for temperature zonation in geographical distribution. Ecol Monogr 17:325–335. doi:10.2307/1948663
Jaeger A, Lecomte VJ, Weimerskirch H, Richard P, Cherel Y (2010) Seabird satellite tracking validates the use of latitudinal isoscapes to depict predators’ foraging areas in the Southern Ocean. Rapid Commun Mass Spectrom 24:3456–3460. doi:10.1002/rcm.4792
Lamont M, Fujisaki I (in press) Effects of ocean temperature on nesting phenology and fecundity of the loggerhead sea turtle (Caretta caretta). J Herpetol. http://journalofherpetology.org/doi/abs/10.1670/12-217
Limpus CJ (1985) A study of the loggerhead sea turtle, Caretta caretta, in eastern Australia. Dissertation, The University of Queensland, Brisbane
Marcovaldi MA, Lopez GG, Soares LS, Lima EHSM, Thom JCA, Almeida AP (2010) Satellite-tracking of female loggerhead turtles highlights fidelity behavior in northeastern Brazil. Endanger Species Res 12:263–272. doi:10.3354/esr00308
Matsuzawa Y, Sato K, Sakamoto W, Bjorndal K (2002) Seasonal fluctuations in sand temperature: effects on the incubation period and mortality of loggerhead sea turtle (Caretta caretta) pre-emergent hatchlings in Minabe, Japan. Mar Biol 140:639–646. doi:10.1007/s00227-001-0724-2
Mazaris AD, Kallimanis AS, Pantis JD, Hays GC (2013) Phenological response of sea turtles to environmental variation across a species’ northern range. Proc R Soc B 280:20122397. doi:10.1098/rspb.2012.2397
McLoughlin PD, Gaillard J-M, Boyce MS, Bonenfant C, Messier F, Duncan P, Delorme D, Moorter BV, Saïd S, Klein F (2007) Lifetime reproductive success and composition of the home range in a large herbivore. Ecology 88:3192–3201. doi:10.1890/06-1974.1
McMahon KW, Ling Hamady L, Thorrold SR (2013) A review of ecogeochemistry approaches to estimating movements of marine animals. Limnol Oceanogr 58:697–714. doi:10.4319/lo.2013.58.2.0697
Miller JD, Limpus CJ, Godley MH (2003) Nest site selection, oviposition, eggs, development, hatching, and emergence of loggerhead turtles. In: Witherington BE, Bolten AB (eds) Loggerhead sea turtles. Smithsonian Institution, Washington, DC, pp 125–153
Montoya JP (2007) Natural abundance of 15N in marine planktonic ecosystems. In: Michener R, Lajtha K (eds) Stable isotopes in ecology and environmental science. Blackwell Publishing Ltd, Malden, pp 176–201
National Marine Fisheries Service and US Fish and Wildlife Service (2008) Recovery plan for the Northwest Atlantic population of the loggerhead sea turtle (Caretta caretta), Second Revision. National Marine Fisheries Service, Silver Spring, MD
National Research Council (2010) Assessment of sea-turtle status and trends: integrating demography and abundance. National Academies Press, Washington, DC
Norris DR (2005) Carry-over effects and habitat quality in migratory populations. Oikos 109:178–186. doi:10.1111/j.0030-1299.2005.13671.x
Norris DR, Marra PP, Kyser TK, Sherry TW, Ratcliffe LM (2004) Tropical winter habitat limits reproductive success on the temperate breeding grounds in a migratory bird. Proc R Soc Lond B 271:59–64. doi:10.1098/rspb 2003.2569
Pajuelo M, Bjorndal KA, Reich KJ, Arendt MA, Bolten AB (2012a) Distribution of foraging habitats of male loggerhead turtles (Caretta caretta) as revealed by stable isotopes and satellite telemetry. Mar Biol 159:1255–1267. doi:10.1007/s00227-012-1906-9
Pajuelo M, Bjorndal KA, Reich KJ, Vander Zanden HB, Hawkes LA, Bolten AB (2012b) Assignment of nesting loggerhead turtles to their foraging areas in the Northwest Atlantic using stable isotopes. Ecosphere 3:art89. doi:10.1890/ES12-00220.1
Pfaller JB, Limpus CJ, Bjorndal KA (2009) Nest-site selection in individual loggerhead turtles and consequences for doomed-egg relocation. Conserv Biol 23:72–80. doi:10.1111/j.1523-1739.2008.01055.x
Pfaller JB, Bjorndal KA, Chaloupka M, Williams KL, Frick MG, Bolten AB (2013) Accounting for imperfect detection is critical for inferring marine turtle nesting population trends. PLoS ONE 8:e62326. doi:10.1371/journal.pone.0062326
Phillips RA, Bearhop S, Mcgill R, Dawson D (2009) Stable isotopes reveal individual variation in migration strategies and habitat preferences in a suite of seabirds during the nonbreeding period. Oecologia 160:795–806. doi:10.1007/s00442-009-1342-9
Pike DA, Antworth RL, Stiner JC (2006) Earlier nesting contributes to shorter nesting seasons for the loggerhead sea turtle, Caretta caretta. J Herpetol 40:91–94. doi:10.1670/100-05N.1
Reece SE, Broderick AC, Godley BJ, West SA (2002) The effects of incubation environment, sex and pedigree on the hatchling phenotype in a natural population of loggerhead turtles. Evol Ecol Res 4:737–748
Reich KJ, Bjorndal KA, Martínez del Rio C (2008) Effects of growth and tissue type on the kinetics of 13C and 15N incorporation in a rapidly growing ectotherm. Oecologia 155:651–663. doi:10.1007/s00442-007-0949-y
Rosenblatt AE, Heithaus MR (2013) Slow isotope turnover rates and low discrimination values in the American alligator: implications for interpretation of ectotherm stable isotope data. Physiol Biochem Zool 86:137–148. doi:10.1086/668295
Roughgarden J (1972) Evolution of niche width. Am Nat 106:683–718. doi:10.1086/282807
Schofield G, Hobson VJ, Fossette S, Lilley MKS, Katselidis KA, Hays GC (2010) Fidelity to foraging sites, consistency of migration routes and habitat modulation of home range by sea turtles. Divers Distrib 16:840–853. doi:10.1111/j.1472-4642.2010.00694.x
Schofield G, Dimadi A, Fossette S, Katselidis KA, Koutsoubas D, Lilley MKS, Luckman A, Pantis JD, Karagouni AD, Hays GC (2013) Satellite tracking large numbers of individuals to infer population level dispersal and core areas for the protection of an endangered species. Divers Distrib 19:834–844. doi:10.1111/ddi.12077
Schroeder BA, Foley AM, Bagley DA (2003) Nesting patterns, reproductive migrations, and adult foraging areas of loggerhead turtles. In: Witherington BE, Bolten AB (eds) Loggerhead sea turtles. Smithsonian Institution, Washington, DC, pp 114–124
Seminoff JA, Benson SR, Arthur KE, Eguchi T, Dutton PH, Tapilatu RF, Popp BN (2012) Stable isotope tracking of endangered sea turtles: validation with satellite telemetry and δ15N analysis of amino acids. PLoS ONE 7:e37403. doi:10.1371/journal.pone.0037403
Shamblin BM, Faircloth BC, Dodd M, Wood-Jones A, Castleberry SB, Carroll JP, Nairn CJ (2007) Tetranucleotide microsatellites from the loggerhead sea turtle (Caretta caretta). Mol Ecol Notes 7:784–787. doi:10.1111/j.1471-8286.2007.01701.x
Shamblin BM, Faircloth BC, Dodd MG, Bagley DA, Ehrhart LM, Dutton PH, Frey A, Nairn CJ (2009) Tetranucleotide markers from the loggerhead sea turtle (Caretta caretta) and their cross-amplification in other marine turtle species. Conserv Genet 10:577–580. doi:10.1007/s10592-008-9573-6
Shamblin BM, Dodd MG, Bagley DA, Ehrhart LM, Tucker AD, Johnson C, Carthy RR, Scarpino RA, McMichael E, Addison DDS, Williams KL, Frick MG, Ouellette S, Meylan AB, Godfrey MH, Murphy SR, Nairn CJ (2011a) Genetic structure of the southeastern United States loggerhead turtle nesting aggregation: evidence of additional structure within the peninsular Florida recovery unit. Mar Biol 158:571–587. doi:10.1007/s00227-010-1582-6
Shamblin BM, Dodd MG, Williams KL, Frick MG, Bell R, Nairn CJ (2011b) Loggerhead turtle egg shells as a source of maternal nuclear genomic DNA for population genetic studies. Mol Ecol Resour 11:110–115. doi:10.1111/j.1755-0998.2010.02910.x
Solow AR, Bjorndal KA, Bolten AB (2002) Annual variation in nesting numbers of marine turtles: the effect of sea surface temperature on re-migration intervals. Ecol Lett 5:742–746. doi:10.1046/j.1461-0248.2002.00374.x
Thomson JA, Heithaus MR, Burkholder DA, Vaudo JJ, Wirsing AJ, Dill LM (2012) Site specialists, diet generalists? Isotopic variation, site fidelity, and foraging by loggerhead turtles in Shark Bay, Western Australia. Mar Ecol Prog Ser 453:213–226. doi:10.3354/meps09637
Tucker AD (2010) Nest site fidelity and clutch frequency of loggerhead turtles are better elucidated by satellite telemetry than by nocturnal tagging efforts: implications for stock estimation. J Exp Mar Biol Ecol 383:48–55. doi:10.1016/j.jembe.2009.11.009
Vander Zanden HB, Bjorndal KA, Reich KJ, Bolten AB (2010) Individual specialists in a generalist population: results from a long-term stable isotope series. Biol Lett 6:711–714. doi:10.1098/rsbl 2010.0124
Vander Zanden HB, Arthur KE, Bolten AB, Popp BN, Lagueux CJ, Harrison E, Campbell CL, Bjorndal KA (2013) Trophic ecology of a green turtle breeding population. Mar Ecol Prog Ser 476:237–249. doi:10.3354/meps10185
Wallace BP, Kilham SS, Paladino FV, Spotila JR (2006) Energy budget calculations indicate resource limitation in Eastern Pacific leatherback turtles. Mar Ecol Prog Ser 318:263–270. doi:10.3354/meps318263
Watanabe KK, Hatase H, Kinoshita M, Omuta K, Bando T, Kamezaki N, Sato K, Matsuzawa Y, Goto K, Nakashima Y, Takeshita H, Aoyama J, Tsukamoto K (2011) Population structure of the loggerhead turtle Caretta caretta, a large marine carnivore that exhibits alternative foraging behaviors. Mar Ecol Prog Ser 424:273–283. doi:10.3354/meps08989
Weishampel JF, Bagley DA, Ehrhart LM (2004) Earlier nesting by loggerhead sea turtles following sea surface warming. Glob Change Biol 10:1424–1427. doi:10.1111/j.1529-8817.2003.00817.x
Wilkinson TA, Wiken EB, Bezaury-Creel J, Hourigan TF, Agardy T, Herrmann H, Janishevski L, Madden CJ, Morgan LE, Padilla M (2009) Marine ecoregions of North America. Commission for Environmental Cooperation, Montreal, Canada
Williams KL, Frick MG (2001) Results from the long-term monitoring of nesting loggerhead sea turtles (Caretta caretta) on Wassaw Island, Georgia: 1973–2000. NOAA Tech Memo NFS-SEFSC-446. http://www.sefsc.noaa.gov/turtles/TM_446_Williams_Frick.pdf. Accessed 25 July 2013
Witherington B, Kubilis P, Brost B, Meylan A (2009) Decreasing annual nest counts in a globally important loggerhead sea turtle population. Ecol Appl 19:30–54. doi:10.1890/08-0434.1
Wunder MB (2012) Determining geographic patterns of migration and dispersal using stable isotopes in keratins. J Mammal 93:360–367. doi:10.1644/11-MAMM-S-182.1
Zbinden JA, Bearhop S, Bradshaw P, Gill B, Margaritoulis D, Newton J, Godley BJ (2011) Migratory dichotomy and associated phenotypic variation in marine turtles revealed by satellite tracking and stable isotope analysis. Mar Ecol Prog Ser 421:291–302. doi:10.3354/meps0887
Acknowledgments
The authors thank J. Curtis for stable isotope analysis, the US Fish and Wildlife Service Savannah Costal Refuges, the staff, volunteers, and supporters of the Caretta Research Project, as well as J.Y. Georges and one anonymous reviewer for comments that improved the manuscript. Samples were collected and processed in compliance with the Georgia Department of Natural Resources and the Institutional Animal Care and Use Committee at the University of Florida.
Conflict of interest
The authors declare they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R. Lewison.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Vander Zanden, H.B., Pfaller, J.B., Reich, K.J. et al. Foraging areas differentially affect reproductive output and interpretation of trends in abundance of loggerhead turtles. Mar Biol 161, 585–598 (2014). https://doi.org/10.1007/s00227-013-2361-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-013-2361-y