Abstract
Aurelia spp. is a cosmopolite scyphozoan species and likely the most studied jellyfish in the world. Its pelagic–benthic life cycle is complex, and the benthic asexual reproducing stage (polyp) is acknowledged fundamentally in bloom onset. Despite this, field investigations remain scarce and are mainly restrained to the western Pacific Ocean. Thau lagoon (43°23′59.10″ N 3°36′37.15″ E), a semi-enclosed system that harbours a resident population of Aurelia sp., is in essence a natural laboratory that offers an ideal framework to investigate the life cycle of the species. We here used a non-destructive approach consisting on a field survey over the entire lagoon (ca. 7 ha) and several substrate types by free diving to examine the distribution and habitat use of Aurelia sp. benthic population. We show that polyps were largely distributed over the entire lagoon, settled mainly on artificial hard substrates, thereby stressing the promoting role of anthropogenic perturbations in coastal areas, i.e. habitat modification, for jellyfish proliferations. Therefore, our study suggest a potential increase in Aurelia sp. benthic populations as an outcome of mounting coastal constructions in the near future; the consequences of which ultimately might promote an increase in jellyfish outbreaks.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Jellyfish are important components of ecosystem trophic dynamics and nutrient cycling (e.g. Pitt et al. 2009; Lalande and Fortier 2011) and are known for their sudden and dense aggregations followed by rapid population collapse (Boero et al. 2008). This phenomenon, known as a bloom event, can interfere with several human activities (reviewed in Purcell 2012) and profoundly impact the ecosystem functioning (Richardson et al. 2009; Boero 2013). A large number of jellyfish blooms in coastal areas and semi-enclosed seas are performed by scyphozoan species of the genus Aurelia (Mills 2001). For a long time, Aurelia aurita, the most studied jellyfish species, was considered cosmopolitan, capable of local adaptation due to its phenotypic plasticity (Lucas 2001). However, recent studies have addressed the biogeography of the genus Aurelia and reported that is actually a species complex embracing numerous locally adapted species (Dawson and Jacobs 2001; Dawson and Martin 2001; Schroth et al. 2002; Dawson 2003; Dawson et al. 2005; Ki et al. 2008). The south coast of France, where Thau lagoon is situated, is suggested to be inhabited by Aurelia sp. 1 (Schroth et al. 2002; Dawson 2003; Dawson et al. 2005). However, further molecular studies are required, since isolated populations inhabiting coastal lagoons were already shown as different cryptic species (e.g. Veliko and Malo Jezero lakes, in Mljet, Croatia, is inhabited by Aurelia sp. 5) (Dawson and Jacobs 2001; Dawson et al. 2005).
Although research efforts on jellyfish ecology have substantially increased in the last decade, critical periods of their complex benthic–pelagic life cycle have been little investigated. Research efforts mainly targeted the planktonic medusa stage, while the dynamics of the benthic phase of most jellyfish species (when present) remain elusive. Despite the importance of the benthic population for bloom dynamics, in situ investigations were only recently performed (e.g. Hoover and Purcell 2009; Purcell et al. 2009; Takao et al. 2014; Qiu 2014; Makabe et al. 2014). To our knowledge, direct investigations of benthic population distribution over large spatial scales were only performed in Mikawa Bay, Japan (Toyokawa et al. 2011). These authors showed that the distribution and density of polyps and the subsequent ephyrae production appear fundamental for the pelagic population growth, stressing the key role of polyps as basis for scyphozoan outbreaks. A thorough assessment of polyp ecology is therefore crucial to fully understand and potentially forecast jellyfish blooms (Condon et al. 2014).
Along with the population growth and its concentration near coasts, anthropogenic modifications of productive coastal margins have dramatically increased (Vitousek et al. 1997), i.e. large infrastructures in the coastal landscapes have been developed and continue expanding (Bulleri and Chapman 2010). Such anthropogenic pressures are expected to further spread, as scenarios of global change point towards an enhancement of sea level rise and a greater frequency of climate extremes (Field et al. 2014). Paradoxically, the expansion of breakwaters, jetties and seawalls in coastal areas also promotes large suitable substrates for jellyfish polyp settlement, thereby promoting the foundation of jellyfish outbreaks (Purcell 2012; Duarte et al. 2012; Boero 2013; Makabe et al. 2014). Recent studies showed that a wide variety of structures, including aquaculture, fixed oil and gas production rigs and offshore wind farms, are prone to benefit polyp settlement (Graham 2001; Holst and Jarms 2007; Janßen et al. 2013). Consequently, by expanding the availability of settlement substrates, ocean sprawl is hypothesized as a major cause of jellyfish outbreaks (Duarte et al. 2012).
Jellyfish blooms are frequent in some areas of the world including the Mediterranean Sea (Brotz et al. 2012), where this phenomenon has long been reported (Goy et al. 1989). Aurelia spp. inhabits coastal waters of western and central Mediterranean areas, as well as the Black Sea (Mutlu 2001). They are common in the Adriatic Sea and in semi-enclosed ecosystems, such as in Mljet Island, Lake Varano and Berre, Bages-Sigean and Thau lagoons (Bonnet et al. 2012; Boero 2013; Marques et al., unpublished data), showing an increasing trend of recurrence of blooms in recent decades (Kogovšek et al. 2010). The Mediterranean Sea is one of the most sensitive areas to the combined effects of anthropogenic disturbances, i.e. habitat modification and climate change (Lejeusne et al. 2010), which warns on potential favourable conditions for jellyfish blooms. Despite the manifested importance of jellyfish populations in this area, to our knowledge, only a single field study has been performed on Aurelia spp. benthic phase in the Adriatic Sea (Malej et al. 2012). Here we investigated the benthic phase of Aurelia sp. in Thau lagoon, located in the north-western Mediterranean, which offers an ideal framework to study its life cycle. This lagoon is suggested to harbour a resident population of Aurelia sp. (Bonnet et al. 2012), although the presence of the benthic population still requires further confirmation. Thau lagoon is a semi-enclosed system under heavy human pressure, where shellfish farming is one of the most important economical activities, with a production of 15 000 tons of oysters and mussels per year (Mongruel et al. 2013). We here provide a qualitative assessment of the distribution and habitat use of the benthic population of Aurelia sp. and a semi-quantitative evaluation of colony size over their distribution.
Materials and methods
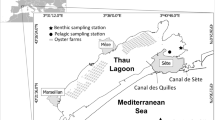
Thau lagoon is a semi-enclosed coastal lagoon (43°23′59.10″ N 3°36′37.15″ E) with 75 km2 and connected to the Mediterranean Sea by three narrow channels (Canal de Sète, Canal des Quilles and the Grau de Pisses-Saumes). It is a shallow environment with mean depth of 4 m and maximum depth of 24 m (Fig. 1).
Although previous studies suggested a resident population of Aurelia sp. (Bonnet et al. 2012), the ecology of the benthic population remains obscure. Hard structures potentially suitable for Aurelia sp. polyp settlement in Thau lagoon were identified based on man-made constructions present in the lagoon (source: IGN—Institut Geographique National) or by local observations and classified by substrate type: concrete, metal, plastics, breakwater rocks, wood and shell bottom (Fig. 1). They were then mapped, using Quantum GIS programme (QGIS-2.2.0—Valmiera version). A structure was defined as a natural or artificial construction, made of one or several types of material. We classified the structures by the main material that could be colonized by polyps, i.e. by substrate type. For instance, a pontoon made of wood and metal, but covered underneath by a plastic sheet (i.e. structure), was classified as the substrate-type plastic. Two structure/substrate types were not identified through QGIS, but were included after visual identification during field survey (moored or submerged boats, which, for the sake of simplicity, we assumed were all made of fibreglass).
To assess polyp distribution, we used a non-destructive approach consisting of thirty-nine survey dives performed between 14 March and 19 June. Investigations were conducted by free diving in discrete places, selected according to the distribution of previously mapped structures, in order to cover the entire lagoon and different substrate types (Fig. 2). The lagoon was divided in three main zones and chronologically investigated over the surveyed period: the coastal area of small lagoon, oyster-culture tables and coastal area of the big lagoon (Fig. 1). Each site was explored up to 60 min, depending on the quantity of potential substrates promising for investigation at each location. Suitable surfaces for polyp fixation (i.e. shaded areas of natural or artificial surfaces) were surveyed. For all the identified colonies of Aurelia sp., georeferenced location, depth (recorded by dive computer Suunto, Gekko), substrate type and coverage area of each sub-population were recorded and mapped. A sub-population was characterized by one or several colonies of polyps covering the same continuous substrate type. The size of each sub-population was visually estimated according to a semi-quantitative index system (index of polyp coverage, IPC) similar to that used by Toyokawa et al. (2011). Four categories were determined (1: 0.01–0.1 m2; 2: 0.1–0.5 m2; 3: 0.5–1.0 m2; 4: >1.0 m2). The nature of the surface directly colonized was registered, including the diversity of biofouling. When possible, i.e. with small sub-populations (IPC < 3), the colonized biofouling organisms were quantified, since these sub-populations were composed of one or very few isolated colonies, allowing the identification and additional quantification of the colonized biofouling. Although our system appeared to be less accurate than in previous studies (see Purcell et al. 2009; Willcox et al. 2008; Toyokawa et al. 2011), it allowed us to describe distribution and differences in the habitat use of Aurelia sp. benthic population.
At each dive, temperature and salinity were measured in sub-surface at about 0.5 m depth (recorded with the probe EC 300 VWR international/WTW model 350i).
Results
Temperature and salinity showed a typical increasing trend over the study period. Temperature ranged from 12.3 to 24.0 °C, while salinity ranged from 37.0 to 40.1.
Thau lagoon is mainly characterized by muddy bottom with few natural rock formations. Among the different structures identified, the most common was metal structures, representing 92.5 %, while wood was rarely found in the lagoon (0.1 %) (Table 1; Fig. 1). The high percentage of metal structures in the lagoon was due to the high number of vertical metal pillars used as a support for boat moorage, pontoons, small piers and oyster-production structures. The oyster-culture rafts contributed largely to this high value, since they represent 88 % of all metal structures quantified in the lagoon. Four sites were additionally identified as shell bottom due to the accumulation of oyster and mussel shells on the bottom.
Polyps were found on the underside surface of different hard substrate types distributed across the entire lagoon (Fig. 3). The highest polyp densities were present in areas experiencing heavy anthropogenic influence, such as harbours and pontoons, mainly located nearby the most populated areas. It is worth noticing that polyps were not found in natural rocks or shell bottom areas, likely because of the lack of suitable surfaces for downwards settlement. Polyps occured in patches between 0.2 and 6.1 m depth, with most of the population (72 %) present in the first 2 m. Polyps were directly settled on substrates or on other fouling organisms growing on those substrates (i.e. red and brown algae, Ascidia, Porifera, Bryozoa, Bivalvia, Cirripedia, Polychaeta calcareous tubes and Amphipoda muddy tubes). Overall, the population of polyps was mainly composed of many small colonies (55.9 % with 0.01–0.1 m2) with fewer sub-populations covering large areas (9.8 % with >1 m2). Among the different artificial substrates, metal structures were the most colonized, supporting 58.8 % of all sub-populations, followed by plastics, breakwater rocks, concrete, moored or submerged boats (i.e. fibreglass), rubber tires and finally wood (Table 1). However, the vertical metal surfaces were not directly colonized by polyps. Instead, other organisms occupied these substrata and provided favourable surfaces for polyp settlement, as suggested by the high percentage of the polyp population settled on biofouling (86.6 % of the sub-populations settled on biofouling organisms, among which 90.4 % were oysters). The percentage of sub-populations settled on biofouling organisms, considering only colonies with less than 0.1 m2, were smaller for the remaining substrate types (8.3 % of breakwater rocks and 16.7 % of plastics). Larger sub-populations were not included, as the proportion of biofouling and artificial surface directly colonized by polyps was not possible to quantify by visual inspection. However, an exception was made for metal pillars, where populations with size <0.5 m2 were also considered, since each colony was mainly settled on individual biofouling organisms. We acknowledge that the methodology used has limitations, although it is useful to have a first large-scale picture of the distribution and habitat association of polyp in Thau lagoon.
Sub-populations covering larger areas were not found on the most common substrate types, but rather on structures with large surface areas facing downwards. Plastic structures (e.g. floating piers, plastic sheet cover on the underside of pontoons and plastic debris) were colonized by 30 and 40 % of the sub-populations with coverage area superior to 0.5 and 1 m2, respectively (Fig. 4). The most important sub-population of Aurelia sp. polyps was found on the underside surface of several submerged boats sunk close to an old industrial concrete pontoon. By contrast, despite their vast presence in Thau lagoon, metal structures were supported mainly small colonies (75.4 % of colonies with coverage less than 0.1 m2 were found in metal structures—Fig. 4). The evaluation of the residence time of the structures supporting big sub-populations (IPC ≥ 3) revealed that 58 % were present in Thau lagoon for more than 20 years, 21 % about 20 years and 21 % were considerably less than 20 years old (Cantou, pers. comm.). Most of the younger structures were classified within the plastics substrate type (67 and 100 % of the structures <20 and ca. 20 years old, respectively), while metal and rock composed the majority of the oldest structures (27 and 36 %, respectively).
Discussion
Polyp distribution
The survey conducted in Thau lagoon revealed that the benthic population of Aurelia sp. is well established in this ecosystem. We found polyps over the entire surveyed area, indicating that the full life cycle of this jellyfish occurs in Thau lagoon, supporting the previous hypothesis (Bonnet et al. 2012). In addition, such results are in line with the lack of evidence regarding potential transport of Aurelia sp. from the near coast to the lagoon (Bonnet et al. 2012), which raises the possibility of the potential isolation of this population from the other populations of the Mediterranean Sea. Furthermore, the broad distribution of ephyrae in Thau lagoon (Bonnet et al. 2012), together with our results, provides additional support to the hypothesis that the spatial patterns of ephyrae in sheltered areas are associated with polyp distribution, as shown in Mikawa Bay, Japan (Toyokawa et al. 2011).
The patchy distribution and settlement on the shaded underside horizontal or oblique surfaces of the surveyed substrates agrees with previous laboratory results (Brewer 1978; Holst and Jarms 2007) and field investigations (Miyake et al. 2002; Makabe et al. 2014; Purcell et al. 2009).
Polyps were found between 0.2 and 6.1 m depth, but more than 70 % of the population was in the first 2 m of the water column, which might be associated with the depth–frequency allocation of the artificial structures, since only a small fraction of the structures (i.e. oyster-culture rafts) are present in deeper waters (see Fig. 1).
Our results suggest a broad and relatively even horizontal distribution of the benthic population over anthropogenic-related hard substrates in Thau lagoon. Indeed, polyps showed a particular occurrence in areas exposed to high anthropogenic influence, i.e. highly populated areas, where human constructions were abundant and offered suitable surfaces for polyp settlement (see Figs. 1 and 3).
The distribution of the benthic population appears mainly determined by recruitment of planulae (Lucas 2001), although spatial patterns at larger scales have been little reported. Such patterns have been related with the distribution of the parental population that influence spatial patterns of planula larvae, and thus the establishment of polyp colonies (Toyokawa et al. 2011). In agreement with previous observations, here the presence of Aurelia sp. medusae over the entire lagoon (Marques et al., unpublished data) may also explain the spread distribution of polyps. Such pattern is also likely favoured by the hydrographic characteristics of Thau lagoon. The relative long residence time of water masses (i.e. 1–4 months), associated with seasonal strong wind events in the lagoon (Fiandrino et al. 2012), may retain and randomly disperse the planula larvae within the lagoon, which can properly settle on the available hard substrates.
Polyp habitat
The long-term survivorship of jellyfish populations relies on the development of benthic stages. A main feature enabling polyps to increase their distribution and abundance is the ability of settling on a great variety of substrate types, including artificial ones (reviewed by Lucas et al. 2012). Laboratory experiments have shown that artificial substrates are suitable for planulae settlement and often preferred by several jellyfish species (Pitt 2000; Holst and Jarms 2007; Hoover and Purcell 2009). In situ, many reports have shown the presence of jellyfish polyps beneath man-made structures (e.g. Miyake et al. 2002; Willcox et al. 2008; Purcell et al. 2009; Ishii and Katsukoshi 2010; Duarte et al. 2012). Here, our results highlight that habitat modification through human constructions appears very important in the expansion of polyp population. Thau lagoon is characterized by sandy or muddy bottoms with scarce natural hard substrates, particularly with suitable surfaces faced downwards. Among the natural substrate types surveyed (shell bottom and some natural rocky formations), none were colonized by Aurelia sp. polyps. This is in agreement with the expected influence that increasing coastal constructions have on promoting jellyfish outbreaks through enlarging settling structures (Duarte et al. 2012; Qiu 2014; Makabe et al. 2014).
Among the artificial structures surveyed in this study, metal was by far the most colonized, supporting 58.8 % of all sub-populations (Table 1). Vertical pillars represented the big majority of metal structures (e.g. support of small pontoons and oyster-production rafts, which alone occupy ca. 20 % of the lagoon surface; Mongruel et al. 2013) and were the most common artificial substrate identified in the lagoon, even when excluding oyster-production rafts. Therefore, the elevated colonization of metal structures appears to be associated with their high accessibility. However, the number of sub-populations present in the different artificial substrates did not follow their availability in the lagoon. For instance, although plastics were one of the rarest substrate types identified, they were the second most colonized, with 11.8 % of all sub-populations (Table 1). Such results suggest some level of preference for plastic materials such as polyethylene, as previously observed in laboratory and field conditions (Holst and Jarms 2007; Hoover and Purcell 2009). Overall, our observations stress the strong association between polyps and hard artificial substrates, which were directly and indirectly colonized by Aurelia sp. polyps. Hard structures provided settling substrates for other biofouling organisms, which are then directly colonized by polyps. In line with this, colonization of natural biofouling was also reported by other authors (Miyake et al. 2002; Willcox et al. 2008; Toyokawa et al. 2011), where bivalves, Porifera, Polychatea and Amphipoda tubes were among the most common colonized organisms. In Thau lagoon, although a quantitative proportion of direct/indirect colonization of polyps was not assessed for all substrate types, similar diversity was observed, with bivalves playing a special role. Indeed, on the common metal structures, 86.6 % of the settled colonies were fixed on biofouling organisms. The vertical metal surfaces do not provide the suitable surfaces for polyps, but offer proper substrate for biofouling fixation. Among the colonized organisms attached to metal surfaces, 90.4 % were oysters. The massive production of oysters in Thau lagoon supplies large amount of larvae to the surrounding pelagic environment that benefit from the available hard substrates. In the adult stage, oysters provide the necessary shaded surfaces for polyp fixation. It is worth noticing, however, that Aurelia sp. colonies were not found on the reared oysters, likely due to their frequent removal from the water, which is carried on to mimic the tides influence (every 6–12 h) and to reduce the survivorship of biofouling organisms on oyster shells. In agreement with our results, the removal of extensive aquaculture rafts was pointed as responsible for the disappearance of Aurelia sp. 1 populations in Taiwan, as an outcome of changes in light, water retention and availability of settling substrates (Lo et al. 2008).
Sub-population size
Although metal structures supported most of the surveyed colonies, their size was usually small (75.4 % <0.1 m2 and only 10 % >1 m2; Fig. 4), likely due to the limited available underside surface area, which was mainly provided by the biofouling, such as oyster shells. By contrast, we found the biggest colonies associated with shaded continuous large surfaces, such as plastics (i.e. polyethylene pontoons) and the surface of submerged boats. Although direct quantitative evaluation of intra- and inter-specific competitions is lacking, reduced space competition may have positively influenced Aurelia sp. benthic population allowing their expansion and enhancing their survivorship (Toyokawa et al. 2011; Watanabe and Ishii 2001; Willcox et al. 2008). In Thau lagoon, 58 % of the structures supporting big sub-populations (IPC ≥ 3) were submerged for more than 20 years (Cantou, pers. comm.), which suggests a potential long-term establishment of the benthic population. After metamorphosis to the polyp phase, the ability of asexual reproduction (i.e. by budding) and the settling of new buds in dense colony formation may provide an inter-specific competitive advantage (Miyake et al. 2002). Such ability allows their long-term establishment and development, while decreasing the available space for fixation of the remaining benthic organisms.
Our survey of Aurelia sp. benthic population covered the spring season, which overall corresponds to the period of polyp increase. Indeed, the annual pattern of the development of Aurelia spp. in temperate areas is generally characterized by decreasing abundance of polyps during autumn and winter and an increase during spring and summer (Willcox et al. 2008; Ishii and Katsukoshi 2010). In Thau lagoon, one colony was simultaneously monitored during our survey period, revealing an increasing trend of the density of polyp (Marques et al., unpublished data). Although such information is limited to just one colony and need to consider a larger sample of colonies, this may suggest a slight underestimation of the real polyp density in the first areas surveyed (i.e. small lagoon). Nevertheless, the ranges reported through the index of polyp coverage are wide enough to capture such density variations. It is worth noticing though the high density of polyps is observed in Thau lagoon (32 ind. cm−2, Marques et al., unpublished data). Such values are higher than previous in situ studies reported in Japan (Ishii and Katsukoshi 2010; Makabe et al. 2014) and in accordance with in situ polyp densities reported in the Adriatic Sea (Malej et al. 2012) and in Tasmania (Willcox et al. 2008). Bearing in mind that the small lagoon is the most human-impacted area in Thau lagoon, these results stress that artificial structures greatly promote the development of jellyfish benthic populations.
Concluding remarks
Our results provide empirical support to recent hypothesis regarding the spread of jellyfish blooms in coastal waters (e.g. the ocean sprawl influence on jellyfish, Duarte et al. 2012), thereby stressing the crucial role of man-made constructions in expanding new habitats for polyp development. This, together with an extraordinary ability of polyps to colonize a variety of substrata, favours the ubiquity of Aurelia sp. in Thau lagoon. In the Mediterranean Sea, the ocean sprawl phenomenon might have a larger significance than previously considered to understand the growing jellyfish outbreaks in the last decades along with the anthropogenic modification of Mediterranean coasts.
Perceiving the critical role of polyps in jellyfish outbreaks emergence, understanding the population dynamics and repercussions of ecological interactions on jellyfish benthic populations, such as inter- and intra-specific space and food competition, as well as prey and predator trophic interactions is of greatest importance. Failures to understand the ecology of benthic life stages will constraint the understanding of jellyfish blooms and their eventual forecast.
References
Boero F (2013) Review of jellyfish blooms in the Mediterranean and Black Sea. Stud Rev Gen Fish Comm Mediterr, FAO, Rome
Boero F, Bouillon J, Gravili C, Miglietta MP, Parsons T, Piraino S (2008) Gelatinous plankton: irregularities rule the world (sometimes). Mar Ecol Prog Ser 356:299–310. doi:10.3354/meps07368
Bonnet D, Molinero JC, Schohn T, Daly Yahia MN (2012) Seasonal changes in the population dynamics of Aurelia aurita in Thau lagoon. Cah Biol Mar 53:343–347
Brewer RH (1978) Larval settlement behavior in the jellyfish Aurelia aurita (Linnaeus) (Scyphozoa: Semaeostomeae). Estuaries 1:120–122
Brotz L, Cheung WWL, Kleisner K, Pakhomov E, Pauly D (2012) Increasing jellyfish populations: trends in large marine ecosystems. Hydrobiologia 690:3–20. doi:10.1007/s10750-012-1039-7
Bulleri F, Chapman MG (2010) The introduction of coastal infrastructure as a driver of change in marine environments. J Appl Ecol 47:26–35. doi:10.1111/j.1365-2664.2009.01751.x
Condon RH, Lucas CH, Pitt KA, Uye S (2014) Jellyfish blooms and ecological interactions. Mar Ecol Prog Ser 510:109–110. doi:10.3354/meps10993
Dawson MN (2003) Macro-morphological variation among cryptic species of the moon jellyfish, Aurelia (Cnidaria: Scyphozoa). Mar Biol 143:369–379. doi:10.1007/s00227-003-1070-3
Dawson MN, Jacobs DK (2001) Molecular evidence for cryptic species of Aurelia aurita (Cnidaria, Scyphozoa). Biol Bull 200:92–96
Dawson MN, Martin LE (2001) Geographic variation and ecological adaptation in Aurelia (Scyphozoa, Semaeostomeae): some implications from molecular phylogenetics. Hydrobiologia 451:259–273
Dawson MN, Gupta AS, England MH (2005) Coupled biophysical global ocean model and molecular genetic analyses identify multiple introductions of cryptogenic species. Proc Natl Acad Sci USA 102:11968–11973. doi:10.1073/pnas.0503811102
Duarte CM, Pitt KA, Lucas CH et al (2012) Is global ocean sprawl a cause of jellyfish blooms? Front Ecol Environ 11:91–97. doi:10.1890/110246
Fiandrino A, Giraud A, Robin S, Pinatel C (2012) Validation d’une méthode d’estimation des volumes d’eau échangés entre la mer et les lagunes et définition d’indicateurs hydrodynamiques associés. 3211472:96
Field CB, Barros VR, Mach KJ, et al. (2014) Technical summary. In: Field CB, Barros VR, Dokken DJ, et al (ed) Clim Change 2014: impacts, adaptation vulnerability. Part A: global and sectoral aspects contribution of working group II to the fifth assessment report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, pp 35–94
Goy J, Morand P, Etienne M (1989) Long-term fluctuations of Pelagia noctiluca (Cnidaria, Scyphomedusa) in the western Mediterranean Sea. Prediction by climate variables. Deep Sea Res Part I Oceanogr Res Pap 36:269–279
Graham WM (2001) Numerical increases and distributional shifts of Chrysaora quinquecirrha (Desor) and Aurelia aurita (Linné) (Cnidaria: Scyphozoa) in the northern Gulf of Mexico. Hydrobiologia 451:97–111
Holst S, Jarms G (2007) Substrate choice and settlement preferences of planula larvae of five Scyphozoa (Cnidaria) from German Bight, North Sea. Mar Biol 151:863–871. doi:10.1007/s00227-006-0530-y
Hoover RA, Purcell JE (2009) Substrate preferences of scyphozoan Aurelia labiata polyps among common dock-building materials. Hydrobiologia 616:259–267. doi:10.1007/s10750-008-9595-6
Ishii H, Katsukoshi K (2010) Seasonal and vertical distribution of Aurelia aurita polyps on a pylon in the innermost part of Tokyo Bay. J Oceanogr 66:329–336. doi:10.1007/s10872-010-0029-5
Janßen H, Augustin CB, Hinrichsen HH, Kube S (2013) Impact of secondary hard substrate on the distribution and abundance of Aurelia aurita in the western Baltic Sea. Mar Pollut Bull 75:224–234. doi:10.1016/j.marpolbul.2013.07.027
Ki JS, Hwang DS, Shin K, Yoon WD, Lim D, Kang YS, Lee Y, Lee JS (2008) Recent moon jelly (Aurelia sp. 1) blooms in Korean coastal waters suggest global expansion: examples inferred from mitochondrial COI and nuclear ITS-5. 8S rDNA sequences. ICES J Mar Sci 65:443–452
Kogovšek T, Bogunović B, Malej A (2010) Recurrence of bloom-forming scyphomedusae: wavelet analysis of a 200-year time series. Hydrobiologia 645:81–96. doi:10.1007/s10750-010-0217-8
Lalande C, Fortier L (2011) Downward particulate organic carbon export and jellyfish blooms in southeastern Hudson Bay. J Mar Syst 88:446–450. doi:10.1016/j.jmarsys.2010.12.005
Lejeusne C, Chevaldonné P, Pergent-Martini C, Boudouresque CF, Pérez T (2010) Climate change effects on a miniature ocean: the highly diverse, highly impacted Mediterranean Sea. Trends Ecol Evol 25:250–260. doi:10.1016/j.tree.2009.10.009
Lo WT, Purcell JE, Hung JJ, Su HM, Hsu PK (2008) Enhancement of jellyfish (Aurelia aurita) populations by extensive aquaculture rafts in a coastal lagoon in Taiwan. ICES J Mar Sci 65:453–461
Lucas CH (2001) Reproduction and life history strategies of the common jellyfish, Aurelia aurita, in relation to its ambient environment. Hydrobiologia 451:229–246
Lucas CH, Graham WM, Widmer C (2012) Jellyfish life histories: role of polyps in forming and maintaining scyphomedusa populations. In: Lesser M (ed) Adv Mar Biol, 1st edn. Academic Press, Amsterdam, pp 133–196
Makabe R, Furukawa R, Takao M, Uye S (2014) Marine artificial structures as amplifiers of Aurelia aurita s.l. blooms: a case study of a newly installed floating pier. J Oceanogr 70:447–455. doi:10.1007/s10872-014-0249-1
Malej A, Kogovšek T, Ramšak A, Catenacci L (2012) Blooms and population dynamics of moon jellyfish in the northern Adriatic. Cah Biol Mar 53:337–342
Mills CE (2001) Jellyfish blooms: are populations increasing globally in response to changing ocean conditions? Hydrobiologia 451:55–68
Miyake H, Terazaki M, Kakinuma Y (2002) On the polyps of the common jellyfish Aurelia aurita in Kagoshima Bay. J Oceanogr 58:451–459
Mongruel R, Vanhoutte-Brunier A, Fiandrino A, Valette F, Ballé-Béganton J, Agúndez JAP, Gallai N, Derolez V, Roussel S, Lample M, Laugier T (2013) Why, how, and how far should microbiological contamination in a coastal zone be mitigated? An application of the systems approach to the Thau lagoon (France). J Environ Manag 118:55–71. doi:10.1016/j.jenvman.2012.12.038
Mutlu E (2001) Distribution and abundance of moon jellyfish (Aurelia aurita) and its zooplankton food in the Black Sea. Mar Biol 138:329–339
Pitt KA (2000) Life history and settlement preferences of the edible jellyfish Catostylus mosaicus (Scyphozoa: Rhizostomeae). Mar Biol 136:269–279
Pitt KA, Welsh DT, Condon RH (2009) Influence of jellyfish blooms on carbon, nitrogen and phosphorus cycling and plankton production. Hydrobiologia 616:133–149. doi:10.1007/s10750-008-9584-9
Purcell JE (2012) Jellyfish and ctenophore blooms coincide with human proliferations and environmental perturbations. Ann Rev Mar Sci 4:209–235. doi:10.1146/annurev-marine-120709-142751
Purcell JE, Hoover RA, Schwarck NT (2009) Interannual variation of strobilation by the scyphozoan Aurelia labiata in relation to polyp density, temperature, salinity, and light conditions in situ. Mar Ecol Prog Ser 375:139–149. doi:10.3354/meps07785
Qiu J (2014) Coastal havoc boosts jellies. Nature 514:545
Richardson AJ, Bakun A, Hays GC, Gibbons MJ (2009) The jellyfish joyride: causes, consequences and management responses to a more gelatinous future. Trends Ecol Evol 24:312–322. doi:10.1016/j.tree.2009.01.010
Schroth W, Jarms G, Streit B, Schierwater B (2002) Speciation and phylogeography in the cosmopolitan marine moon jelly, Aurelia sp. BMC Evol Biol 2:1–10
Takao M, Okawachi H, Uye SI (2014) Natural predators of polyps of Aurelia aurita s.l. (Cnidaria: Scyphozoa: Semaeostomeae) and their predation rates. Plankton Benthos Res 9:105–113
Toyokawa M, Aoki K, Yamada S, Yasuda A, Murata Y, Kikuchi T (2011) Distribution of ephyrae and polyps of jellyfish Aurelia aurita (Linnaeus 1758) sensu lato in Mikawa Bay, Japan. J Oceanogr 67:209–218. doi:10.1007/s10872-011-0021-8
Vitousek PM, Mooney HA, Lubchenco J, Melillo JM (1997) Human domination of earth’s ecosystems. Science 277:494–499
Watanabe T, Ishii H (2001) In situ estimation of ephyrae liberated from polyps of Aurelia aurita using settling plates in Tokyo Bay, Japan. Hydrobiologia 451:247–258
Willcox S, Moltschaniwskyj NA, Crawford CM (2008) Population dynamics of natural colonies of Aurelia sp. scyphistomae in Tasmania, Australia. Mar Biol 154:661–670. doi:10.1007/s00227-008-0959-2
Acknowledgments
The authors are grateful to Laure Paradis from the Center for Bio-Archeology and Ecology, UMR5059, for her assistence with the GIS software and map editing. The study was funded by EC2CO «Ecosphère Continentale et Côtière» through the DYNAMO programme (2012–2014) (PI: D. Bonnet). The research of JCM is a contribution to the EU project OCEAN-CERTAIN (European Commission, OCEAN-CERTAIN, FP7-ENV-2013-6.1-1; No: 603773).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Purcell.
Rights and permissions
About this article
Cite this article
Marques, R., Cantou, M., Soriano, S. et al. Mapping distribution and habitats of Aurelia sp. polyps in Thau lagoon, north-western Mediterranean Sea (France). Mar Biol 162, 1441–1449 (2015). https://doi.org/10.1007/s00227-015-2680-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-015-2680-2