Abstract
Due to their boom and bust population dynamics and the enormous biomasses they can attain, jellyfish and ctenophores can have a large influence on the cycling of carbon (C), nitrogen (N) and phosphorus (P). This review initially summarises the biochemical composition of jellyfish, and compares and contrasts the mechanisms by which non-zooxanthellate and zooxanthellate jellyfish acquire and recycle C, N and P. The potential influence of elemental cycling by populations of jellyfish on phytoplankton and bacterioplankton production is then assessed. Non-zooxanthellate jellyfish acquire C, N and P predominantly through predation on zooplankton with smaller contributions from the uptake of dissolved organic matter. C, N and P are regenerated via excretion of inorganic (predominantly ammonium (NH4 +) and phosphate (PO4 3−)) and dissolved organic forms (e.g. dissolved free amino acids and dissolved primary amines). Inorganic nutrients excreted by jellyfish populations provide a small but significant proportion of the N and P required for primary production by phytoplankton. Excretion of dissolved organic matter may also support bacterioplankton production but few data are available. In contrast, zooxanthellate medusae derive most of their C from the translocation of photosynthetic products, exhibit no or minimal net release of N and P, and may actively compete with phytoplankton for dissolved inorganic nutrients. Decomposition of jellyfish blooms could result in a large release of inorganic and organic nutrients and the oxygen demand required to decompose their tissues could lead to localised hypoxic or anoxic conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Jellyfish and ctenophore populations are characterised by large and rapid fluctuations in abundances and often represent a substantial proportion of the pelagic consumer biomass. Due to their sheer abundance and boom and bust population dynamics, jellyfish and ctenophores are likely to influence carbon (C), nitrogen (N) and phosphorus (P) cycling in the ecosystems they inhabit.
Jellyfish and ctenophores acquire C, N and P by assimilating organic compounds from ingested prey, taking up small amounts of dissolved organic material, and some species actively take up dissolved inorganic forms. A proportion of the elements ingested become incorporated into their biomass and undigested material is egested as faeces or released via ‘sloppy feeding’. Organic forms of C and N are recycled to the environment as mucus, and both organic and inorganic metabolic products are excreted. During bloom formation, when both individuals and populations are increasing in size, jellyfish and ctenophores act as a net sink for C, N and P; however, they are ephemeral organisms whose populations may decline rapidly. During decomposition, the elements bound within their biomass are regenerated en masse to the water column as dissolved inorganic and organic compounds. Organic C regenerated via mucus production and decomposition may support microbial production, whilst inorganic N and P regenerated by excretion and decomposition may support algal production.
One coronate jellyfish (Linuche unguiculata Schwartz) and many rhizostome species (including members of the genera Cassiopea, Mastigias and Phyllorhiza) form symbioses with zooxanthellae. In zooxanthellate medusae, translocation of photosynthetic products from the zooxanthellae is likely to be the major source of C for the host (Balderston & Claus, 1969; Cates, 1975). Also, inorganic excretory products are often translocated from the host to the zooxanthellae instead of being released to the external environment. Consequently, the way in which zooxanthellate jellyfish cycle C, N and P is likely to be very different to non-zooxanthellate taxa.
The aim of this paper is to review the biochemical composition of jellyfish and ctenophores, detail the processes by which they accumulate and release inorganic and organic C, N and P, compare and contrast the influence of zooxanthellate and non-zooxanthellate medusae on C, N and P cycling and examine the extent to which elemental recycling by jellyfish and ctenophore populations could support algal and microbial production.
Biochemical composition of jellyfish and ctenophores
Due to their large water and salt content, the total organic matter of jellyfish and ctenophores is very small (typically <3% of wet weight: Larson, 1986; Clarke et al., 1992). Proteins are consistently the most abundant organic fraction in both medusae and ctenophores (72 ± 14% of total organic matter (TOM)), followed by lipids (22 ± 12%) and carbohydrates (7 ± 5%; Fig. 1). In jellyfish, proteins serve a variety of functions. Structural proteins occur in the nematocyst capsules (Hessinger & Lenhoff, 1988) and the collagen fibres of the mesoglea. Enzymes (Båmstedt, 1988; Arai, 1997, and references within), toxins (Hessinger & Lenhoff, 1988) and pigments (Blanquet & Phelan, 1987) also largely are composed of proteins. The large protein content in the tissues of medusae and ctenophores is reflected in their low molar C:N (4.5 ± 1.1; Fig. 2). Large quantities of N-rich collagen fibres and inorganic N as NH4 + in the mesoglea most likely drive the low overall C:N (Arai, 1997).
Comparison of the average (±SD) protein, lipid and carbohydrate content (expressed as fraction of total organic content) in jellyfish and ctenophores. Values are taken from Ikeda (1972), Percy & Fife (1981), Hoeger (1983), Schneider (1988), Youngbluth et al. (1988), Arai et al. (1989), Clarke et al. (1992), Malej et al. (1993), Lucas (1994), Bailey et al. (1994a, b, 1995), Finenko et al. (2001), Yousefian & Kideys (2003) and Anninsky et al. (2005). n = sample size of data used in analyses
Average (±SD) C:N (mol:mol) ratios of various families of jellyfish (Hydromedusae, Coronatae, Rhizostomeae, Semaeostomeae) and ctenophores (Cestidae, Lobata, Nuda, Tentaculata). Dotted line represents average C:N for dataset (4.5 ± 1.1 SD, see text for further detail). C:N values are calculated from Kremer (1977, 2005), Ceccaldi et al. (1978), Reeve et al. (1978), Hoeger (1983), Purcell & Kremer (1983), Shenker (1985), Larson (1986), Lutcavage & Lutz (1986), Kremer et al. (1986), Gorsky et al. (1988), Schneider (1988), Youngbluth et al. (1988), Malej (1989), Clarke et al. (1992), Nemazie et al. (1993), Bailey et al. (1994a, b, 1995), Kasuya et al. (2000), Kinoshita et al. (2000), Yousefian & Kideys (2003) and Uye & Shimauchi (2005). n = sample size of data used in analyses
Uptake of C, N and P by jellyfish and ctenophores
Assimilation of C, N and P via predation
Both non-zooxanthellate and zooxanthellate jellyfish acquire C, N and P by ingestion of prey. Predation by jellyfish on zooplankton has been a major focus of research and an extensive literature exists on the types of prey captured as well as their clearance and digestion rates. The literature relating to predation on zooplankton has been reviewed previously (e.g. Mills, 1995; Arai, 1997; Purcell, 1997, 2008) and will not be examined further here. Despite the relative wealth of information about predation, there are few estimates of the efficiencies with which ingested C, N and P are assimilated into jellyfish biomass, even though such measurements are critical for calculating elemental budgets. Assimilation generally is defined as the difference between the quantity of an element ingested and the quantity egested (Conover, 1966). Since most jellyfish do not produce distinct faeces, assimilation efficiencies (i.e. (ingestion − egestion)/ingestion × 100) are difficult to quantify. Egested material (i.e. faeces and material lost via ‘sloppy feeding’) has generally been collected by filtration (e.g. Reeve et al., 1989; Costello, 1991). In siphonophores, however, egested material is produced by the gastrozooids as cohesive particles that can be collected intact (Purcell, 1983).
Assimilation efficiencies of C are typical of carnivores and generally exceed 70% and often exceed 90% (Table 1). The experimental conditions, however, such as the concentration of food supplied and whether food is supplied in a short pulse or continuously appears to greatly influence estimated efficiencies (Table 1) and so care needs to be taken during experiments to ensure experimental conditions reflect natural conditions as closely as possible. The assimilation efficiencies recorded for jellyfish and ctenophores are greater than those measured for doliolids (C assimilation; 20–50%; Katechakis et al., 2004), but are similar to those observed for some copepods (30–80%; Katechakis et al., 2004; generally >70% Acartia tonsa Dana; Besiktepe & Dam, 2002). Assimilation efficiencies for N have only been examined in siphonophores and appear to be similar to those for C (>90%; Table 1; Purcell, 1983).
Increasingly, chemical tracers (e.g. radioisotopes such as 14C) are being used to measure assimilation efficiencies (e.g. Katechakis et al., 2004). These techniques offer some advantages for estimating assimilation efficiencies in jellyfish and ctenophores because food labelled with the tracer (e.g. 14C-labelled copepods) could be fed to the predators and the amount of tracer ingested could then be compared to that remaining in the body after the evacuation of the gut, thus eliminating the need to collect faeces. Early experiments that measured assimilation efficiencies using radioisotope tracers, however, potentially were confounded because they did not account for recycling of elements through excretion or respiration over the period that assimilation was measured (Conover & Francis, 1973). If experiments are carefully designed to account for excretion and respiration, however, net assimilation can be measured (e.g. He & Wang, 2006). Such approaches have not yet been applied to jellyfish or ctenophores.
Relative rates of heterotrophy and autotrophy in zooxanthellate jellyfish
Elemental budgets indicate that the C translocated from the zooxanthellae can exceed the C demands of Mastigias papua (Lesson) (McCloskey et al., 1994), Cassiopea xamachana Bigelow (Verde & McCloskey, 1998) and L. unguiculata (Kremer et al., 1990). Despite this, zooxanthellate scyphomedusae still ingest zooplankton (e.g. Kremer, 2005; Peach & Pitt, 2005). Indeed, when Cassiopea sp. is maintained unfed under wavelengths of light appropriate for photosynthesis, the medusae shrink (Pitt & Welsh, unpublished data) indicating that zooplankton contain nutrients that are essential for normal metabolic function. Only Kremer (2005), however, has estimated the contribution of ingested C, N and P to the elemental budgets of an intact symbiosis. Heterotrophy contributed minor amounts of C (1–9%) relative to photosynthesis in L. unguiculata. Ingestion of prey was an important source of N, but the relative contribution ranged widely as a function of medusa size. For small medusae, ~10 times more N was acquired through predation than by uptake of dissolved sources, but for large medusae, ingestion and uptake of dissolved forms made similar contributions. For large size classes, ingestion of prey was considered to provide relatively small amounts (<30%) of P.
Uptake of dissolved organic matter
Free amino acids (FAA) constitute a major component of the dissolved organic matter (DOM) in seawater. Non-zooxanthellate marine invertebrates can assimilate FAA but rates of uptake suggest that DOM generally supplies <10% of their metabolic requirements (Ferguson, 1982). Uptake of FAAs has been demonstrated using 14C- and 15N-labelled substrates in several hydrozoans (e.g. Sarsia sp. Liriope exiqua) and one scyphozoan (Pelagia cyanella Péron & Lesueur) medusae (Ferguson, 1988). The experiments were done in the oligotrophic Sargasso Sea and the FAA concentrations used (1.48 × 10−3 μmol l−1) were comparable to the small concentrations that occur in the North Atlantic Ocean (Ferguson, 1988). Neritic or estuarine waters, however, generally contain much greater concentrations of DOM (e.g. Minor et al., 2006), and if rates of uptake are proportional to the concentrations available, uptake may be greater in coastal environments. Total DOM concentrations are still small, however, and only about 19% of total DOM in marine environments is labile, and therefore easily utilised and assimilated (Søndergaard & Middelboe, 1995). DOM, therefore, could only ever make a small contribution to the diet. Malej et al. (1993) compared the δ13C of Pelagia noctiluca (Forskål) in the Adriatic Sea with literature-derived values of DOM for neritic systems, and since the medusae were ~2 ppt more enriched in δ13C than DOM, they concluded that DOM was an insignificant source of C. More rigorous approaches (e.g. isotopic labelling experiments), however, are required to reliably resolve the contribution of DOM to jellyfish diets under a range of conditions.
Like non-zooxanthellate taxa, zooxanthellate medusae also can take up DOM. Quantitative rates of uptake of amino acids have been measured for zooxanthellae isolated from C. xamachana (Carroll & Blanquet, 1984) and for the intact symbiosis of L. unguiculata (Wilkerson & Kremer, 1992). In L. unguiculata, rates of uptake were proportional to the concentration of amino acids supplied and although amino acids were incorporated into both the animal and zooxanthellae fractions, greater concentrations occurred within the animal fraction. There is evidence for corals that amino acids may be transferred between the host and zooxanthellae (Swanson & Hoegh-Guldberg, 1998), so the appearance of the label in the zooxanthellae of L. unguiculata also could have occurred via the translocation of dissolved N from the animal. It remains unclear, therefore, as to whether differences in concentrations between the host and zooxanthellae resulted from differences in rates of uptake, a net transfer of amino acids from the zooxanthellae to the host or an internal recycling of N.
Uptake of dissolved inorganic C, N and P by zooxanthellate jellyfish
Unlike their animal hosts, zooxanthellae from medusae can assimilate dissolved inorganic C (Hofmann & Kremer, 1981), N and P (Wilkerson & Kremer, 1992) from the water column. Thus, zooxanthellate medusae can utilise nutrient pools that are not available to non-zooxanthellate taxa. Uptake has been demonstrated by depletion of nutrient pools (e.g. Pitt et al., 2005) as well as by isotopic tracer experiments (e.g. Hofmann & Kremer, 1981; Wilkerson & Kremer, 1992). Although dissolved CO2 could be taken up by zooxanthellae directly, concentrations of aqueous CO2 are small and, at the normal pH of seawater, inorganic C is most abundant as HCO3 −. 14C labelling showed that medusae of Cassiopea andromedea (Forskål) took up HCO3 − from seawater (Hofmann & Kremer, 1981). HCO3 − then is converted to CO2 using carbonic anhydrase (Weis et al., 1989). Since rates of C fixation during photosynthesis are several times greater than C respiration (e.g. Kremer et al., 1990; Verde & McCloskey, 1998), respiration alone could not meet the CO2 demands for photosynthesis. The majority of DIC, therefore, is likely to be obtained from the water column.
Zooxanthellate medusae take up ammonium in preference to NO x (nitrite [NO2 −] + nitrate [NO3 −]) (Muscatine & Marian, 1982; Wilkerson & Kremer, 1992; Pitt et al., 2005). Based on depletion experiments, NO x does not appear to be taken up by Mastigias sp. (Muscatine & Marian, 1982), Phyllorhiza punctata von Lendenfeld (Pitt et al., 2005) or L. unguiculata (Wilkerson & Kremer, 1992). Depletion experiments, however, provide fairly crude information about net uptake/production. Isotopic labelling provides much more sensitivity and information about pathways and fluxes. Wilkerson & Kremer (1992) used 15N labelling of NO x and NH4 + to show that intact symbioses of L. unguiculata took up NH4 + approximately 10 times faster and isolated zooxanthellae took up NH4 + >45 times faster than NO3. When the symbioses were immersed in 15NH4 +, the 15N appeared in both the zooxanthellae and animal fractions, but concentrations were approximately twice as large in the zooxanthellae. There was no indication of a transfer of DIN between the zooxanthellae and host.
Symbiotic, N-fixing cyanobacteria have recently been discovered in anthozoan cnidarians (Lesser et al., 2004). The cyanobacteria may be an important source of N for corals, whose growth is considered to be N-limited. Whether such symbionts occur in scyphozoans is unknown.
Uptake of inorganic P in zooxanthellate scyphomedusae has been examined only for L. unguiculata (Wilkerson & Kremer, 1992) and P. punctata (Pitt et al., 2005). Linuche unguiculata can deplete PO4 3− from seawater and rates of depletion were similar regardless of whether the medusae were fed. Uptake of PO4 3− continued in darkness, however, if starved and retained in the dark, rates of uptake decreased and net excretion was observed after 4 days. These results contrast with those observed for P. punctata. Net excretion of PO4 3− was observed during the day and night even when excess of PO4 3− was available (Pitt et al., 2005). A recent study by West and colleagues (unpublished data) indicates that the duration of retention of N by the zooxanthellate scyphomedusa Cassiopea sp. depends upon the availability of particulate and dissolved sources of N. The differences in P uptake/excretion observed for L. unguiculata and P. punctata may similarly relate to the availability of P. Linuche unguiculata inhabits oligotrophic tropical waters that may have limited availability of P, resulting in retention and active uptake of dissolved P. In contrast, P may not be limited in the coastal lagoons and embayments inhabited by P. punctata, resulting in net excretion by this species. Further data on the effects of availability of P on rates of uptake/excretion and also N:P ratios of the species is required to explain these observations. Interestingly, P. punctata excreted PO4 3− at only 20% of the rate observed in the co-occurring, non-zooxanthellate Catostylus mosaicus (Quoy & Gaimard). This suggests that, like NH4 +, some of the inorganic P generated by the host metabolism is translocated to the zooxanthellae in this species.
Release of C, N and P
Elements assimilated by jellyfish are released via excretion of dissolved inorganic and organic forms, as mucus, and, following death, by decomposition of the tissues. Studies of excretion have mostly focused on inorganic forms and, for most species, the proportions of elements excreted as inorganic and organic forms are unknown. The few studies that have examined excretion of both forms have produced inconsistent results. For example, the ctenophore Mnemiopsis leidyi A. Agassiz, releases about 38% of C as dissolved organic C (DOC), 46% of N as dissolved organic N (DON) and 28% of P as dissolved organic P (DOP) (Kremer, 1977). Similarly, the hydromedusa Cladonema californicum Hyman was estimated to release approximately 50% of N as DON (Costello, 1991). In both Kremer (1977) and Costello (1991), however, handling of animals may have artificially increased rates of excretion of organic matter (Kremer, pers. comm.). These studies contrast with Shimauchi & Uye (2007) who were unable to detect any organic N or P excretia from the scyphomedusae, Aurelia aurita Linnaeus.
Excretion of inorganic N and P by non-zooxanthellate jellyfish
Ammonium and urea are two common inorganic excretory products (note that urea is an inorganic molecule, despite containing C as the C is oxidised, not reduced). Although urea is commonly excreted by other types of zooplankton (e.g. crustaceans; Miller & Glibert, 1998), urea comprises a negligible proportion (<2%) of the excretia of jellyfish (Kremer, 1975). Ammonium is the dominant form of inorganic N that is excreted and so most studies have focused on this form (e.g. Kremer, 1982; Matsakis, 1992; Nemazie et al., 1993). When standardised to dry weight, rates of excretion of NH4 + by pelagic cnidarians are <10% of those of zooplankton (Jawed, 1973; Ikeda, 1974; Smith, 1982). When standardised to C biomass, however, rates of excretion are comparable to other zooplankton, indicating that gelatinous and non-gelatinous zooplankton contribute equally to nutrient recycling in the water column on an elemental weight basis (Schneider, 1990). Rates, however, are highly variable, ranging from 110 to 2319 μmol NH4 + g C−1 d−1 for ctenophores and 77 to 2639 μmol NH4 + g C−1 d−1 for pelagic cnidarians (Schneider, 1990). Small quantities of NO x are also released by medusae (Kremer 1975; Pitt et al., 2005; Shimauchi & Uye, 2007). In all cases, NO x comprised <2% of total DIN that was released. NO x , however, is probably not excreted by the jellyfish but is likely to result from the oxidation of NH4 + by nitrifying bacteria colonising the jellyfish’s surface (Welsh et al., unpublished data), as has been observed for other marine invertebrates (Welsh & Castadelli, 2004; Southwell et al., 2008).
Compared to measures of N excretion, measures of P are rare. P comprises a small component of the particulate organic content of medusae and is, therefore, excreted in small quantities. For example, molar N:P ratios for inorganic excretia range from 6.9 to 11.4 for A. aurita (in Schneider, 1989; Shimauchi & Uye, 2007), 7.5 for P. noctiluca (in Malej, 1991), 8.7 for C. mosaicus (in Pitt et al., 2005) and 7.4 for M. leidyi (in Kremer 1975). The N:P ratios of jellyfish excretia are much lower than the 16N:1P required by phytoplankton, as indicated by the canonical Redfield Ratio (Redfield et al., 1963). The larger quantities of P in the excretia, relative to the body (Kremer, 1977), may indicate that surplus P is available and that N is retained preferentially (Malej, 1989; Kremer, pers. comm.).
Rates of excretion in ctenophores and scyphomedusae are greatly influenced by recent feeding history. For example, faster excretion rates are observed in animals that have fed recently compared to those that are starved, and the rate decreases as the duration of starvation increases (Kremer, 1982; Kremer et al., 1986; Malej, 1991; Table 2). Increasing the duration of starvation from 4 to 16 h also reduces rates of excretion in the scyphozoan, P. noctiluca, by approximately 67% (Malej, 1991). Kremer et al. (1986) observed similar declines in rates of excretion of the ctenophore Bolinopsis vitrea (Agassiz) but over periods of 30 h. In contrast to Malej (1991), Morand et al. (1987) claimed that rates of excretion of NH4 + (reported as NH3) were constant for P. noctiluca that had been starved for periods ranging from a few hours to a few days.
Rates of excretion of inorganic nutrients are also positively correlated with the availability of prey (Table 2). For example, increasing concentrations of copepods from 5 to 500 l−1 caused rates of excretion to more than double in the ctenophore Mnemiopsis mccradyi Mayer (Kremer, 1982). A similar doubling of excretion rates was observed for the ctenophore Bolinopsis vitrea when its food concentration increased from 0 to 100 copepods l−1 (Kremer et al., 1986).
Temperature also has a substantial influence on excretion rates (Table 3). For example, rates of excretion of NH4 + were greater at warmer temperatures in the scyphomedusae A. aurita (Shimauchi & Uye, 2007) and Chrysaora quinquecirrha (Desor) (Nemazie et al., 1993), the hydrozoan Clytia sp. (Matsakis, 1992) and the ctenophore M. leidyi (Kremer, 1977; Nemazie et al., 1993). Similar effects of temperature on rates of excretion of PO4 3− were also observed for A. aurita (Shimauchi & Uye, 2007) and M. leidyi (Kremer, 1977).
Only Matsakis (1992) has examined the interactive effects of food concentration and temperature on rates of excretion of NH4 +. She observed that rates of excretion increased with increasing food concentration (Table 4). Over the same range of food concentrations, rates of excretion were greater at warmer temperatures until the temperature reached 25°C, at which point feeding was inhibited (Matsakis, 1992).
Since rates of excretion are greatly influenced by environmental conditions, direct comparisons amongst taxa from different ecosystems are problematic. Differences in the temperatures at which measurements were made have been standardised using the Q10 law (Ikeda, 1985), but rates still range widely, even within taxa (e.g. Schneider, 1990). Purcell (2008) cautions against adjusting metabolic rates by Q10s determined at experimentally manipulated temperatures. Such variability may reflect the differences in the feeding histories of the animals amongst the studies. More detailed information about how the feeding history influences excretion rates and rigorous reporting of the feeding histories of test animals are required to reliably standardise results. Information about the proportions of nutrients excreted as inorganic and organic forms also are required for robust comparisons amongst taxa. These factors may account for some of the large variability in rates of excretion observed amongst and within taxa.
Excretion of dissolved organic matter
The two primary mechanisms by which jellyfish and ctenophores release DOM are by excretion and mucus production. As proteins constitute the greatest proportion of organic biomass of pelagic coelenterates, products of protein metabolism are likely to constitute the bulk of DON excreted by gelatinous zooplankton. For example, dissolved primary amines (DPA) constitute 21% and 46%, respectively, of the total N and DON excreted by M. leidyi (Kremer, 1977), although handling of the ctenophore during the experiment may have increased rates of excretion of organic metabolites (Kremer, pers. comm.). Likewise, dissolved free amino acids (DFAA) are also excreted in large amounts by “jellyfish” (taxa not stated) (15.0 mg N g dw−1 d−1), with glycine and alanine being the most abundant DFAA species (Webb & Johannes, 1967). The large DPA and DFAA content in the excretory products of jellyfish and ctenophores might be related to rapid turnover of DNA and RNA coupled with fast growth rates (Båmstedt & Skjoldal, 1980). In this case, the metabolism of DNA and RNA could also be a source of DOP. Very few measurements of excretion of DOP, however, have been made. Kremer (1975) reported that 21% of P was excreted in organic form in M. leidyi, but Shimauchi & Uye (2007) could not detect organic P in the excretia of A. aurita. Only Kremer et al. (1986) have examined factors affecting the proportion of inorganic to organic nutrients excreted. They observed that food concentration and starvation had minimal influence on the NH4 +:TDN (Total Dissolved N) of the ctenophore Bolinopsis vitrea.
Glycoproteins are also produced by jellyfish and ctenophores. For example, M. leidyi releases modified aminosugar disaccharide metabolites (Cohen & Forward, 2003). These function as kairomones (molecules that are produced by one organism and that invoke an adventitious change in behaviour or physiology of a second organism; Dicke & Sabelis, 1988) and cause crabs to alter their behaviour to avoid predation. Glycoproteins are also used by zooxanthellate medusae in the formation of pigments that filter injurious solar radiation whilst retaining photosynthetically active wavelengths for zooxanthellae (Blanquet & Phelan, 1987). Release of glycoproteins, either directly or indirectly (e.g. UV breakdown of pigment molecules), would contribute to DOC and DON pools.
Jellyfish are renowned for producing large quantities of mucus that is used in feeding and defence (Heeger & Möller, 1987; Shanks & Graham, 1988; Arai, 1997). The mucus produced by jellyfish is colloidal in nature (Wells, 2002), originating in cells in the epidermis (Heeger & Möller, 1987) and gastrodermis (Arai, 1997). Rates of production of mucus have not been quantified and the biochemical composition of mucus has been examined for only one non-zooxanthellate and one zooxanthellate scyphozoan (Ducklow & Mitchell, 1979). In A. aurita, the composition of the mucus resembled the organic composition of the tissues (73% protein, 27% lipid, 5% carbohydrate (total values exceed 100% due to analytical error; H. Ducklow, pers. comm.). In contrast, the mucus of the zooxanthellate medusa Cassiopea sp. contained a similar proportion of carbohydrate (2%) but more lipids (38%) and considerably less protein (10%).
Other sources of DOM released from jellyfish include the leaking of digestive enzymes to DOC and DON pools. Possible enzyme species include trypsin and amylase, which have high activities in A. aurita medusae (Båmstedt, 1988). The release of damaged cell wall and phospholipid components may also contribute to DOC and DOP pools (Arai, 1997). ‘Sloppy feeding’ and egestion of undigested prey are other possible mechanisms by which jellyfish could influence the recycling of DOM in marine systems, but no data exist for these processes for jellyfish.
Recycling of C and N between the host and its symbionts in zooxanthellate jellyfish
A characteristic of symbioses involving zooxanthellae is the tight recycling of inorganic and organic compounds between the zooxanthellae and host. Most studies have been undertaken on anthozoans. Similar physiological mechanisms probably occur in scyphozoans but they mostly remain untested. In anthozoans, zooxanthellae take up dissolved compounds, but some of their C and N requirements are derived from the CO2 and NH4 + produced by their hosts (Odum & Odum, 1955; Rahav et al., 1989; but see Wang & Douglas (1998) for the alternative ‘nitrogen conservation’ hypothesis). Inorganic C is fixed into organic forms by the zooxanthellae and recycled back to the host, predominantly as carbohydrates (Muscatine & Cernichiari, 1969; Lewis & Smith, 1971) and some N may also be translocated to the host as amino acids (Swanson & Hoegh-Guldberg, 1998). This tight coupling results in no net release of either CO2 or NH4 + during periods when medusa–zooxanthellae symbioses are undergoing net photosynthesis. In the dark, a net release of CO2 is observed, but despite the lack of photosynthesis, NH4 + either continues to be taken up rather than excreted (Muscatine & Marian, 1982; Wilkerson & Kremer, 1992) or NH4 + is excreted in very small quantities (at rates that are approximately 7% of those of non-zooxanthellate taxa; Pitt et al., 2005).
Influences of jellyfish and ctenophore blooms on ecosystem level C, N and P cycles
Blooms of jellyfish and ctenophores can attain enormous biomasses and cover extensive areas (Mills, 2001; Brodeur et al., 2002; Hay, 2006). For example, Lynam et al. (2006) reported that blooms of mainly Aequorea forskalea (Forskål, 1775) in the Northern Benguela (~34,000 N mi−2) attained mean biomass densities of 361 ± 22 tonnes N mi−2 with the total biomass of the bloom estimated to be 12.2 million tonnes. Equally impressive blooms also occur at smaller scales in coastal embayments and lagoons. For example, in Lake Illawarra, Australia, abundances of C. mosaicus increased 30-fold over a period of only 6 weeks and attained ~530 tonnes km−2 (Pitt & Kingsford, 2003). During blooms, jellyfish may represent a substantial or even the greatest proportion of the pelagic consumer biomass (Arai, 1997; Pagés et al., 1996; 2001) and the nutrients regenerated by blooms of jellyfish via excretion, mucus production or decomposition may influence plankton production.
Contribution of recycled inorganic N and P to phytoplankton production
Inorganic nutrients regenerated by medusae supply a small but significant proportion of those required for phytoplankton production. For example, in coastal waters, excretion of NH4 + by jellyfish blooms has been estimated to supply 8% of the N requirements for phytoplankton in Lake Illawarra, Australia (Pitt et al., 2005), 11% in the Kiel Bight, Western Baltic (Schneider, 1989), 10% in the Inland Sea of Japan (Shimauchi & Uye, 2007) and up to 4% to net microplankton production in Chesapeake Bay, USA (Nemazie et al., 1993). Similarly, a subset of the same studies estimated that PO4 3− excretion could provide 23% in the Kiel Bight and 21.6% in the Inland Sea of Japan of the phytoplankton’s P requirements. Although the contribution may appear small, in all cases, except for Chesapeake Bay, the excretory products of jellyfish were estimated to be the second most important source of N and P for primary production, after the sediment (Schneider, 1989; Pitt et al., 2005; Shimauchi & Uye, 2007); therefore, they should be considered in nutrient budgets and models. Indeed, recycled nutrients may be particularly important in estuaries where primary production may be temporarily (e.g. after the spring phytoplankton blooms; Kemp et al., 2005) or permanently (Thingstad et al., 2005) N- or P-limited. Excretion by jellyfish would be expected to be even more important in open oligotrophic waters where allochthonous inputs of nutrients from terrestrial sources are insignificant and where thermal stratification can often greatly limit the transfer of nutrients from the nutrient-rich deep water to the productive surface waters for much of the year. This suggestion is supported by Biggs (1977) in the western North Atlantic, where NH4 + excretion by gelatinous zooplankton was estimated to provide between 39% and 63% of the N required by phytoplankton. Whilst some doubts exist about the biomass estimates used to calculate these figures (Nemazie et al., 1993), they do demonstrate the potential role that jellyfish and ctenophores could play in supporting phytoplankton production. Indeed, the estimated contributions of regenerated nutrients are likely to be very conservative since NH4 + may contribute only ~50% of N released by the medusae (Kremer, 1977; Costello, 1991). At least part of the directly excreted DON, in addition to DON that leaches from egested material and occurs in mucus, would be directly available to the phytoplankton (Bronk et al., 2006 and references therein). The remaining DON would subsequently become available to phytoplankton when bacterioplankton mineralise it to NH4 + (Fenchel et al., 1998). Unfortunately, complete N and C budgets are only available for the hydrozoan Cladonema californicum Hyman (Costello, 1991) and the ctenophore Pleurobrachia sp. (Reeve et al., 1978). Budgets for a wider range of species need to be done to enable more rigorous assessment of the potential role of jellyfish in elemental cycling and in supporting primary production.
Contribution of dissolved organic matter to bacterioplankton production
Bacteria are the major consumers of DOM in the oceans (Kirchman, 2000). In most marine systems, the majority of bacterial production is sustained by organic matter derived from primary production or phytoplankton exudates (del Giorgio & Cole, 1998; Kirchman, 2000; Hansell & Carlson, 2002). The bacterial C demand, however, often exceeds rates of primary production (del Giorgio & Peters, 1993; del Giorgio & Cole, 1998) and bulk organic pools contain large concentrations of refractory DOM (Hansell & Carlson, 2002). At such times, the DOM excreted by gelatinous zooplankton may be an important source of C, N and P for bacterioplankton. For example, the excretion of DFAA by “jellyfish” may be a N-rich energy source for bacteria and other marine saprotrophs (Webb & Johannes, 1967). The predominant forms of DOM produced by jellyfish (e.g. DFAAs and DPAs) are labile, suggesting there could be tight coupling between excretion of DOM by jellyfish and bacterioplankton production. Only one study, however, has attempted to correlate abundances of jellyfish and bacterial activity in the field. Periphylla periphylla (Péron & Lesueur) undertakes diel vertical migrations in some Norwegian fjords. Riemann et al. (2006) predicted bacterial activity would be stimulated at different depths throughout the diel cycle in correlation with changes in the depth distribution of the medusae. No diel differences in bacterial activity were observed, but the depth at which the greatest biomass of jellyfish occurred coincided with the depth of maximum bacterial activity integrated over 24 h. Care must be taken, however, not to infer causation from correlative data and sampling at multiple places and over periods longer than 24 h are required before robust conclusions regarding the influence of jellyfish on bacterial activity in the field can be drawn.
Contrasting roles of zooxanthellate and azooxanthellate medusae
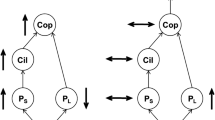
The nutrient dynamics of zooxanthellate medusae are dominated by the internal recycling of organic and inorganic compounds between the jellyfish tissues and their algal symbionts (Fig. 3). Consequently, zooanthellate species, in contrast to non-zooanthellate taxa, recycle relatively small quantities of dissolved nutrients to the water column under dark conditions and can be a net sink for inorganic N and P in the light (Muscatine & Marian, 1982; Wilkerson & Kremer, 1992; Pitt et al., 2005; Todd et al., 2006). Similarly, the mucus secreted by at least some zooanthellate species contains much less N than that of non-zooanthellate species (Ducklow & Mitchell, 1979). Zooxanthellate species, therefore, can be considered as a “bottle-neck” in the N and P cycles, as organic nutrients ingested as food are actively retained by the jellyfish and their symbionts, and the majority of accumulated nutrients would become available to other parts of the ecosystem mainly through predation upon the jellyfish or when the medusae die and decompose. Therefore, whilst both non-zooxanthellate and zooxanthallate jellyfish are able to exert top-down pressure on the zooplankton community through predation, only non-zooxanthellate taxa are likely to simultaneously exert substantial ‘bottom-up’ influences on plankton production via the recycling of nutrients (Fig. 3). Indeed, since zooxanthellate taxa can assimilate dissolved nutrients from the surrounding water, they may even, at times, actively compete with the phytoplankton for dissolved nutrients.
Conceptual models of the contributions of non-zooxanthellate (A) and zooxanthellate (B) medusae to nutrient cycling during growth of jellyfish blooms. The width of the arrows represents their relative contributions. Dotted arrows indicate the contribution has not been confirmed. Microbial feedback loops involving bacteria, phytoplankton and zooplankton are not included. POM = particulate organic matter, DOM = dissolved organic matter and DIM = dissolved inorganic matter
Decomposition
Jellyfish and ctenophores can form dense populations and, therefore, represent large environmental stocks of nutrients. These stocks will become available and could have significant influences on ecosystem nutrient and oxygen dynamics when the populations die and decompose. Although some authors have proposed that bloom declines and moribund jellyfish biomass could exert significant effects on the environment (e.g. Kingsford et al., 2000; Miyake et al., 2005), decomposition of jellyfish has been examined only twice (Titelman et al., 2006; West et al., 2008). Depending on sinking rates and the depth of the water, jellyfish carcasses may decompose within the water column or on the benthos. Rates of decomposition vary from 4–7 days for P. periphylla in the water column (10–12°C) (Titelman et al., 2006) to ~9 days for C. mosaicus decomposing on sediments in a coastal lagoon (~25°C; West et al., 2008). The decay of P. periphylla involved both direct leaching of DOC from the jellyfish tissues and mineralisation by bacteria, with a large accumulation of PO4 3− and NH4 + recorded in the water column at the end of the 50-h incubation. Nitrogenous compounds appeared to be hydrolysed faster than C compounds, resulting in an initial increase in the C:N ratio of decaying medusae. Rates of release of total organic carbon were more than an order of magnitude greater (~0.36 mg C g wet weight−1 d−1) than that produced by living medusae (A. aurita ~0.012 mg C g wet weight−1 d−1; Hansson & Norman, 1995).
To our knowledge only West et al. (2008) have studied the influence of jellyfish decomposition on sediment biogeochemistry and benthic fluxes. In that study, dead C. mosaicus were added to continuous flow mesocosms and benthic fluxes of oxygen and dissolved nutrients were followed for 9 days and compared with control mesocosms. Initially, there were significant peaks of organic nutrient release, but bacterial processes gradually dominated and inorganic nutrient fluxes became more important, with NH4 + efflux reaching 2 mmol m−2 h−1, which was close to an order of magnitude greater than the controls.
DOC released during decomposition of gelatinous zooplankton may support bacterioplankton production. Shipboard mesocosm experiments using homogenised P. periphylla tissue demonstrated variable results on bacterial communities, with some mesocosms having higher, and some lower, bacterial abundances than those recorded in controls (Titelman et al., 2006). Microscopic observations showed that increases in bacterial abundances in some treatments resulted from growth of specific bacterial phylotypes, indicating that whilst jellyfish tissues stimulated the growth of some bacteria, the growth of others was potentially inhibited. Subsequent experiments found that the degree of inhibition depended on both the concentration of jellyfish homogenate and the types of jellyfish tissues that were used to prepare the homogenates (Titelman et al., 2006).
Despite the paucity of direct experimental data, decomposition of medusae is also likely to have significant effects on oxygen dynamics. The oxygen required to decompose medusae can be calculated from estimates of the carbon biomass. For example, Titelman et al. (2006) observed that 0.36 mg C (g wet weight−1 d−1), equivalent to 30 μmol C (g wet weight−1 d−1), was released from P. periphylla as DOC. Complete oxidation of the DOC alone, depending on its biochemical composition, would require around 30 μmol of oxygen or approximately 11% of the oxygen present in a litre of oxygen-saturated seawater (Solubility = 276.0 μM at 11°C and 35 salinity). Complete oxidation of the tissues (i.e. C other than that released as DOC), would require considerably more oxygen than this and could result in local hypoxia if the decomposing biomass was large. Whilst the effects of jellyfish decomposition in the water column may be significant, decomposition on the benthos could be more severe. Recently, large accumulations of moribund scyphomedusae, Crambionella orsini (Vanhöffen), were observed at depths of 300–3300 m in the Arabian Sea (Billett et al., 2006). The standing stock of C in the decomposing jellyfish was estimated to vary between 1.5 and 78 g C m−2, equivalent to 0.125–6.5 mol C m−2. Based on C:N ratios of 4.5, the medusae also contained ~0.028–1.44 mol N m−2. Complete oxidation of the C alone would require all the oxygen in 367–19,101 l of seawater (solubility of O2 = 340.4 μM at 2°C and 35 salinity), leading to potential anoxia in the boundary layer. Thus, oxygen demands for the mineralisation of C during decomposition of jellyfish could potentially cause or contribute to hypoxic events, particularly in areas with limited water exchange (e.g. coastal embayments) or where stratification inhibits mixing (e.g. some fjords).
Overall, the effects of jellyfish bloom declines and the associated decomposition processes could be similar to those caused by macro-algal blooms, which show similar “boom and bust” population dynamics. Decomposition of these blooms results in massive nutrient recycling and increases in oxygen demand can cause hypoxia, anoxia, and even dystrophy (accumulation of free sulphides in the water column), and decimation of benthic faunal communities (Viaroli et al., 1995; Valiela et al., 1997; Raffealli et al., 1998). Thus, decomposition of jellyfish blooms could have similar impacts, albeit less severe, as algal blooms can achieve significantly higher biomass densities than those of jellyfish.
Conclusions and future directions for research
This review has highlighted several deficiencies in our understanding about the way in which jellyfish and ctenophores contribute to nutrient cycling. The calculation of elemental budgets is a primary requirement for a complete understanding of nutrient cycling. Whilst elemental budgets have been calculated for several zooxanthellate taxa (e.g. McCloskey et al., 1994; Kremer, 2005), very few budgets have been completed for non-zooxanthellate coelenterates (but see Kremer & Reeve, 1989; Costello, 1991). Moreover, assimilation efficiencies have been estimated for relatively few taxa, probably due to the difficulties in quantifying egestion rates, but are fundamental to the completion of elemental budgets. The use of chemical tracers, such as heavy- and radio-isotopes may make assimilation efficiencies easier to quantify. Research on recycling of nutrients has largely focused on excretion of inorganic forms, although these may contribute only ~50% of the total metabolic products (Kremer, 1977; Costello, 1991). Indeed, greater focus on excretion of organic nutrients is required due to their potential importance in supporting bacterioplankton production. The area perhaps most lacking in information, however, is the influence of decomposing medusae on the ecology and nutrient and oxygen dynamics of the water column and benthos.
References
Anninsky, B. E., 1988. The rate and efficiency of copepod assimilation by scyphoid medusa Aurelia aurita L. Ekologiya Morya 28: 58–64.
Anninsky, B. E., G. A. Finenko, G. I. Abolmasova, E. S. Hubareva, L. S. Svetlichny, L. Bat & A. E. Kideys, 2005. Effect of starvation on the biochemical compositions and respiration rates of ctenophores Mnemiopsis leidyi and Beroe ovata in the Black Sea. Journal of the Marine Biological Association United Kingdom 85: 549–561.
Arai, M. N., 1997. A Functional Biology of Scyphozoa. Chapman & Hall, London.
Arai, M. N., J. A. Ford & J. N. C. Whyte, 1989. Biochemical composition of fed and starved Aequorea victoria (Murbach et Shearer, 1902) (Hydromedusa). Journal of Experimental Marine Biology and Ecology 127: 289–299.
Bailey, T. G., J. J. Torres, M. J. Youngbluth & G. P. Owen, 1994a. Effect of decompression on mesopelagic gelatinous zooplankton: a comparison of in situ and shipboard measurements of metabolism. Marine Ecology Progress Series 113: 13–27.
Bailey, T. G., M. J. Youngbluth & G. P. Owen, 1994b. Chemical composition and oxygen consumption rates of the ctenophore Bolinopsis infundibulum from the Gulf of Maine. Journal of Plankton Research 16: 673–689.
Bailey, T. G., M. J. Youngbluth & G. P. Owen, 1995. Chemical composition and metabolic rates of gelatinous zooplankton from midwater and benthic boundary layer environments off Cape Hatteras, North Carolina, USA. Marine Ecology Progress Series 122: 121–134.
Balderston, W. L. & G. Claus, 1969. A study of the symbiotic relationship between Symbiodinium microadriaticum Freudenthal, a zooxanthella, and the upside down jellyfish Cassiopea sp. Nova Hedwigia 17: 373–382.
Båmstedt, U., 1988. Interspecific, seasonal and diel variations in zooplankton trypsin and amylase activities in Kosterfjorden, western Sweden. Marine Ecology Progress Series 44: 15–24.
Båmstedt, U. & H. R. Skjoldal, 1980. RNA concentration of zooplankton: relationship with size and growth. Limnology and Oceanography 25: 304–316.
Besiktepe, S. & H. G. Dam, 2002. Coupling of ingestion and defecation as a function of diet in the calanoid copepod Acartia tonsa. Marine Ecology Progress Series 229: 151–164.
Biggs, D., 1977. Respiration and ammonium excretion by open ocean gelatinous zooplankton. Limnology and Oceanography 22: 108–117.
Billett, D. S. M., B. J. Bett, C. L. Jacobs, I. P. Rouse & B. D. Wigham, 2006. Mass deposition of jellyfish in the deep Arabian Sea. Limnology and Oceanography 51: 2077–2083.
Blanquet, R. S. & M. A. Phelan, 1987. An unusual blue mesogleal protein from the mangrove jellyfish Cassiopea xamachana. Marine Biology 94: 423–430.
Brodeur, R., H. Sugisaki & G. J. Hunt, 2002. Increases in jellyfish biomass in the Bering Sea: implications for the ecosystem. Marine Ecology Progress Series 233: 89–103.
Bronk, D. A., J. H. See, P. Bradley & L. Killberg, 2006. DON as a source of bioavailable nitrogen for phytoplankton. Biogeosciences Discussions 3: 1247–1277.
Carroll, S. & R. S. Blanquet, 1984. Alanine uptake by isolated zooxanthellae of the mangrove jellyfish, Cassiopea xamachana. I. Transport mechanisms and utilization. Biological Bulletin 166: 409–418.
Cates, N., 1975. Productivity and organic consumption in Cassiopea and Condylactus. Journal of Experimental Marine Biology and Ecology 18: 55–59.
Ceccaldi, H. J., A. Kanazawa & S.-I. Teshima, 1978. Chemical composition of some Mediterranean macroplanktonic organisms. 1. Proximate analysis. Tethys 8: 295–298.
Clarke, A., L. J. Holmes & D. J. Gore, 1992. Proximate and elemental composition of gelatinous zooplankton from the Southern Ocean. Journal of Experimental Marine Biology and Ecology 155: 55–68.
Cohen, J. H. & R. B. Forward, 2003. Ctenophore kairomones and modified aminosugar disaccharides alter the shadow response in a larval crab. Journal of Plankton Research 25: 203–213.
Conover, R. J., 1966. Assimilation of organic matter by zooplankton. Limnology and Oceanography 11: 338–345.
Conover, R. J. & V. Francis, 1973. The use of radioactive isotopes to measure the transfer of materials in aquatic food chains. Marine Biology 18: 272–283.
Costello, J., 1991. Complete carbon and nitrogen budgets for the hydromedusa Cladonema californicum (Anthomedusa: Cladonemidae). Marine Biology 108: 119–128.
del Giorgio, P. A. & J. J. Cole, 1998. Bacterial growth efficiency in natural aquatic systems. Annual Review of Ecology and Systematics 29: 503–541.
del Giorgio, P. A. & R. H. Peters, 1993. Balance between phytoplankton production and plankton respiration in lakes. Canadian Journal of Fisheries and Aquatic Science 50: 282–289.
Dicke, M. & M. W. Sabelis, 1988. Infochemical terminology: based on cost-benefit analysis rather than compound origins. Functional Ecology 2: 131–139.
Ducklow, H. W. & R. Mitchell, 1979. Composition of mucus released by coral reef coelenterates. Limnology and Oceanography 24: 706–714.
Fenchel, T., G. M. King & T. H. Blackburn, 1998. Bacterial Biogeochemistry: The Ecophysiology of Mineral Cycling. Academic Press, San Diego: 307 pp.
Ferguson, J. C., 1982. A comparative study of the net metabolic benefits derived from the uptake and release of free amino acids by marine invertebrates. Biological Bulletin 162: 1–17.
Ferguson, J. C., 1988. Autoradiographic demonstration of the use of free amino acid by Sargasso Sea zooplankton. Journal of Plankton Research 10: 1225–1238.
Finenko, G. A., B. E. Anninsky, Z. A. Romanova, G. I. Abolmasova & A. E. Kideys, 2001. Chemical composition, respiration and feeding rates of the new alien ctenophore, Beroe ovata, in the Black Sea. Hydrobiologia 451: 177–186.
Gorsky, G., S. Dallot, J. Sardou, R. Fenaux, C. Carre & I. Palazzoli, 1988. C and N composition of some northwestern Mediterranean zooplankton and micronekton species. Journal of Experimental Marine Biology and Ecology 124: 133–144.
Hansell, D. A. & C. A. Carlson, 2002. Biogeochemistry of marine dissolved organic matter. Academic Press, San Diego.
Hansson, L. J. & B. Norman, 1995. Release of dissolved organic carbon (DOC) by the scyphozoan jellyfish Aurelia aurita and its potential influence on the production of planktonic bacteria. Marine Biology 121: 527–532.
Hay, S., 2006. Marine ecology: gelatinous bells may bring change in marine ecosystems. Current Biology 16: R679–R682.
He, X. & W.-X. Wang, 2006. Releases of ingested phytoplankton carbon by Daphnia magna. Freshwater Biology 51: 649–665.
Heeger, T. & H. Möller, 1987. Ultrastructural observations on prey capture and digestion in the scyphomedusa Aurelia aurita. Marine Biology 96: 391–400.
Hessinger, D. A. & H. M. Lenhoff, 1988. The Biology of Nematocysts. Academic Press, San Diego.
Hoeger, U., 1983. Biochemical composition of ctenophores. Journal of Experimental Marine Biology and Ecology 72: 251–261.
Hofmann, D. K. & P. Kremer, 1981. Carbon metabolism and strobilation in Cassiopea andromedea (Cnidaria: Scyphozoa): significance of endosymbiotic dinoflagellates. Marine Biology 65: 25–33.
Ikeda, T., 1972. Chemical composition and nutrition of zooplankton in the Bering Sea. In Takenouti, A. Y. (ed.), Biological Oceanography of the Northern Pacific Ocean. Idemitsu Shoten, Tokyo: 433–442.
Ikeda, T., 1974. Nutritional ecology of marine zooplankton. Memoirs of the Faculty of Fisheries Hokkaido University 22: 1–97.
Ikeda, T., 1985. Metabolic rates of epipelagic marine zooplankton as a function of body mass and temperature. Marine Biology 85: 1–11.
Jawed, M., 1973. Ammonia excretion by zooplankton and its significance to primary productivity during summer. Marine Biology 23: 115–120.
Kasuya, T., T. Ishimaru & M. Murano, 2000. Metabolic characteristics o the lobate ctenophore Bolinopsis mikado (Moser). Plankton Biology and Ecology 47: 114–121.
Katechakis, A., H. Stirbor, U. Sommer & T. Hansen, 2004. Feeding selectivities and food niche separation of Acartia clausi, Penilia avirostris (Crustacea) and Doliolum denticulatum (Thaliacea) in Blanes Bay (Catalan Sea, NW Mediterranean). Journal of Plankton Research 26: 589–603.
Kemp, W. M., W. R. Boynton, J. E. Adolf, D. F. Boesch, W. C. Boicourt, G. Brush, J. C. Cornwell, T. R. Fisher, P. M. Glibert, J. D. Hagy, L. W. Harding, E. D. Houde, D. G. Kimmel, W. D. Miller, R. I. E. Newell, M. R. Roman, E. M. Smith & J. C. Stevenson, 2005. Eutrophication of Chesapeake Bay: historical trends and ecological interactions. Marine Ecology Progress Series 303: 1–29.
Kingsford, M. J., K. A. Pitt & B. M. Gillanders, 2000. Management of jellyfish fisheries with special reference to the order Rhizostomeae. Oceanography and Marine Biology: An Annual Review 38: 85–156.
Kinoshita, J., J. Hiromi & Y. Nakamura, 2000. Feeding of the scyphomedusa Cyanea nozakii on mesozooplankton. Plankton Biology and Ecology 47: 43–47.
Kirchman, D. L., 2000. Microbial Ecology of the Oceans. Wiley-Liss, New York.
Kremer, P., 1975. Excretion and body composition of the ctenophore Mnemiopsis leidyi (A. Agassiz): comparisons and consequences. In 10th European Symposium on Marine Biology, Ostend, Belgium: 351–362.
Kremer, P., 1977. Respiration and excretion by the ctenophore Mnemiopsis leidyi. Marine Biology 44: 43–50.
Kremer, P., 1982. Effect of food availability on the metabolism of the ctenophore Mnemiopsis mccradyi. Marine Biology 71: 149–156.
Kremer, P., 2005. Ingestion and elemental budgets for Linuche unguiculata, a scyphomedusa with zooxanthellae. Journal of the Marine Biological Association of the United Kingdom 85: 613–625.
Kremer, P., M. F. Canino & R. W. Gilmer, 1986. Metabolism of epipelagic tropical ctenophores. Marine Biology 90: 403–412.
Kremer, P., J. Costello, J. Kremer & M. Canino, 1990. Significance of photosynthetic endosymbionts to the carbon budget of the scyphomedusa Linuche unguiculata. Limnology and Oceanography 35: 609–624.
Kremer, P. & M. R. Reeve, 1989. Growth dynamics of a ctenophore (Mnemiopsis) in relation to variable food supply. 2. Carbon budgets and growth model. Journal of Plankton Research 11: 553–574.
Larson, R. J., 1986. Water content, organic content, and carbon and nitrogen composition of medusae from the northeast Pacific. Journal of Experimental Marine Biology and Ecology 99: 107–120.
Lesser, M. P., C. H. Mazel, M. Y. Gorbunov & P. G. Falkowski, 2004. Discovery of symbiotic nitrogen-fixing cyanobacteria in corals. Science 305: 997–1000.
Lewis, D. H. & D. C. Smith, 1971. The autotrophic nutrition of symbiotic marine coelenterates with special reference to hermatypic corals. I. Movement of photosynthetic products between the symbionts. Proceedings of the Royal Society of London Series B 178: 111–129.
Lucas, C. H., 1994. Biochemical composition of Aurelia aurita in relation to age and sexual maturity. Journal of Experimental Marine Biology and Ecology 183: 179–182.
Lutcavage, M. & P. L. Lutz, 1986. Metabolic rate and food energy requirements of the leatherback sea turtle, Dermochelys coriacea. Copeia 1986: 796–798.
Lynam, C. P., M. J. Gibbons, E. A. Bjørn, C. A. J. Sparks, B. G. Heywood & A. S. Brierley, 2006. Jellyfish take over fish in a heavily fished system. Current Biology 16: R492–R493.
Malej, A., 1989. Respiration and excretion rates of Pelagia noctiluca (Semaeostomeae, Scyphozoa). In Proceedings of the 21st EMBS. Polish Academy of Sciences, Institute of Oceanology, Gdansk: 107–113.
Malej, A., 1991. Rates of metabolism of jellyfish as related to body weight, chemical composition and temperature UNEP: jellyfish blooms in the Mediterranean. In Proceedings of the II Workshop on Jellyfish in the Mediterranean. UNEP, Athens: 253–259.
Malej, A., J. Faganeli & J. Pezdic, 1993. Stable isotope and biochemical fractionation in the marine pelagic foodchain: the jellyfish Pelagia noctiluca and net zooplankton. Marine Biology 116: 565–570.
Matsakis, S., 1992. Ammonia excretion rate of Clytia spp. hydromedusae (Cnidaria Thecata): effects of individual dry weight, temperature and food availability. Marine Ecology Progress Series 84: 55–63.
McCloskey, L. R., L. Muscatine & F. P. Wilkerson, 1994. Daily photosynthesis, respiration, and carbon budgets in a tropical marine jellyfish (Mastigias sp.). Marine Biology 119: 13–22.
Miller, C. & P. Glibert, 1998. Nitrogen excretion by the calanoid copepod Acartia tonsa: results of mesocosm experiments. Journal of Plankton Research 20: 1767–1780.
Mills, C. E., 1995. Medusae, siphonophores, and ctenophores as planktivorous predators in changing global ecosystems. ICES Journal of Marine Science 52: 575–581.
Mills, C. E., 2001. Jellyfish blooms: are populations increasing globally in response to changing ocean conditions? Hydrobiologia 451: 55–68.
Minor, E. C., J.-P. Simjouw & M. R. Mulholland, 2006. Seasonal variation in dissolved organic carbon concentrations and characteristics in a shallow coastal bay. Marine Chemistry 101: 166–179.
Miyake, H., D. J. Lindsay, M. Kitamura & S. Nishida, 2005. Occurrence of the scyphomedusa Parumbrosa polylobata Kishinouye, 1910 in Suruga Bay, Japan. Plankton Biology and Ecology 52: 58–66.
Morand, P., C. Carré & D. C. Biggs, 1987. Feeding and metabolism of the jellyfish Pelagia noctiluca (scyphomedusae, semaeostomeae). Journal of Plankton Research 9: 651–665.
Muscatine, L. & E. Cernichiari, 1969. Assimilation of photosynthetic products of zooxanthellae by a reef coral. Biological Bulletin 137: 506–523.
Muscatine, L. & R. E. Marian, 1982. Dissolved inorganic nitrogen flux in symbiotic and nonsymbiotic medusae. Limnology and Oceanography 27: 910–917.
Nemazie, D. A., J. E. Purcell & P. M. Glibert, 1993. Ammonium excretion by gelatinous zooplankton and their contribution to the ammonium requirements of microplankton in Chesapeake Bay. Marine Biology 116: 451–458.
Odum, H. T. & E. P. Odum, 1955. Trophic structure and productivity of a windward coral reef community on Eniwetok Atoll. Ecological Monographs 25: 291–319.
Pagés, F., H. E. González & S. R. González, 1996. Diet of the gelatinous zooplankton in Hardangerfjord (Norway) and potential predatory impact by Aglantha digitale (Trachymedusae). Marine Ecology Progress Series 139: 69–77.
Pagés, F., H. E. Gonzalez, M. Ramon, M. Sobarzo & J.-M. Gili, 2001. Gelatinous zooplankton assemblages associated with water masses in the Humboldt Current System, and potential predatory impact by Bassia bassensis (Siphonophora: Calycophorae). Marine Ecology Progress Series 210: 13–24.
Peach, M. B. & K. A. Pitt, 2005. Morphology of the nematocysts of the medusae of two scyphozoans Catostylus mosaicus and Phyllorhiza punctata (Rhizostomeae): implications for capture of prey. Invertebrate Biology 124: 98–108.
Percy, J. A. & F. J. Fife, 1981. The biochemical composition and energy content of arctic marine macrozooplankton. Arctic 34: 307–313.
Pitt, K. A. & M. J. Kingsford, 2003. Temporal variation in the virgin biomass of the edible jellyfish, Catostylus mosaicus (Scyphozoa, Rhizostomeae). Fisheries Research 63: 303–313.
Pitt, K. A., K. Koop & D. Rissik, 2005. Contrasting contributions to inorganic nutrient recycling by the co-occurring jellyfishes, Catostylus mosaicus and Phyllorhiza punctata (Scyphozoa, Rhizostomeae). Journal of Experimental Marine Biology and Ecology 315: 71–86.
Purcell, J. E., 1983. Digestion rates and assimilation efficiencies of siphonophores fed zooplankton prey. Marine Biology 73: 257–261.
Purcell, J. E., 1997. Pelagic cnidarians and ctenophores as predators: selective predation, feeding rates and effects on prey populations. Annales de l’Institut océanographique, Paris 73: 125–137.
Purcell, J. E., 2008. Extension of methods for jellyfish and ctenophore trophic ecology to large-scale research. Hydrobiologia (this volume). doi:10.1007/s10750-008-9585-8.
Purcell, J. E. & P. Kremer, 1983. Feeding and metabolism of the siphonophore Sphaeronectes gracilis. Journal of Plankton Research 5: 95–106.
Raffealli, D. G., R. A. Raven & L. J. Poole, 1998. Ecological impact of green macroalgal blooms. Oceanography and Marine Biology: An Annual Review 36: 97–125.
Rahav, B. O., Z. Dubinsky, Y. Achituv & P. G. Falkowski, 1989. Ammonium metabolism in the zooxanthellate coral, Stylophora pistillata. Proceedings of the Royal Society of London Series B 236: 325–337.
Redfield, A. C., B. H. Ketchum & F. A. Richards, 1963. The influence of organisms on the composition of sea-water. In Hill, M. N. (ed.), The Sea, Vol. 2. Interscience, New York: 26–77.
Reeve, M. R., M. A. Syms & P. Kremer, 1989. Growth dynamics of a ctenophore (Mnemiopsis) in relation to variable food supply. I. Carbon biomass, feeding, egg production, growth and assimilation efficiency. Journal of Plankton Research 11: 535–552.
Reeve, M. R., M. A. Walter & T. Ikeda, 1978. Laboratory studies of ingestion and food utilization in lobate and tentaculate ctenophores. Limnology and Oceanography 24: 740–751.
Riemann, L., J. Titelman & U. Båmstedt, 2006. Links between jellyfish and microbes in a jellyfish dominated fjord. Marine Ecology Progress Series 325: 29–42.
Schneider, G., 1988. Chemische zusammensetzung und biomasseparameter der ohrenqualle Aurelia aurita. Helgolander Meeresuntersuchungen 42: 319–327.
Schneider, G., 1989. The common jellyfish Aurelia aurita: standing stock, excretion and nutrient regeneration in the Kiel Bight, Western Baltic. Marine Biology 100: 507–514.
Schneider, G., 1990. A comparison of carbon based ammonia excretion rates between gelatinous and non-gelatinous zooplankton: implications and consequences. Marine Biology 106: 219–225.
Shanks, A. L. & W. M. Graham, 1988. Chemical defense in a scyphomedusa. Marine Ecology Progress Series 45: 81–86.
Shenker, J. M., 1985. Carbon content of the neritic scyphomedusa Chrysaora fuscescens. Journal of Plankton Research 7: 169–173.
Shimauchi, H. & S. Uye, 2007. Excretion and respiration rates of the scyphomedusa Aurelia aurita from the Inland Sea of Japan. Journal of Oceanography 63: 27–34.
Smith, K. L. J., 1982. Zooplankton of a bathyal benthic boundary layer: in situ rates of oxygen consumption and ammonium excretion. Limnology and Oceanography 27: 461–471.
Søndergaard, M. & M. Middelboe, 1995. A cross-system analysis of labile dissolved organic carbon. Marine Ecology Progress Series 118: 283–294.
Southwell, M. W., B. N. Popp & C. S. Martens, 2008. Nitrification controls on fluxes and isotopic composition of nitrate from Florida Keys sponges. Marine Chemistry 108: 96–108.
Swanson, R. & O. Hoegh-Guldberg, 1998. Amino acid synthesis in the symbiotic sea anemone Aiptasia pulchella. Marine Biology 131: 89–93.
Thingstad, T. F., M. D. Krom, R. F. C. Mantoura, G. A. F. Flaten, S. Groom, B. Herut, N. Kress, C. S. Law, A. Pasternak, P. Pitta, S. Psarra, F. Rassoulzadegan, T. Tanaka, A. Tselepides, P. Wassmann, E. M. S. Woodward, C. W. Riser, G. Zodiatis & T. Zohary, 2005. Nature of phosphorus limitation in the ultraoligotrophic eastern Mediterranean. Science 309: 1068–1071.
Titelman, J., L. Riemann, T. A. Sørnes, T. Nilsen, P. Griekspoor & U. Båmstedt, 2006. Turnover of dead jellyfish: stimulation and retardation of microbial activity. Marine Ecology Progress Series 325: 43–58.
Todd, B. D., D. J. Thornhill & W. K. Fitt, 2006. Patterns of inorganic phosphate uptake in Cassiopea xamachana: a bioindicator species. Marine Pollution Bulletin 52: 515–521.
Uye, S. & H. Shimauchi, 2005. Population biomass, feeding, respiration and growth rates, and carbon budget of the scyphomedusa Aurelia aurita in the Inland sea of Japan. Journal of Plankton Research 27: 237–248.
Valiela, I., J. McClelland, J. Hauxwell, P. J. Behr, D. Hersh & K. Foreman, 1997. Macroalgal blooms in shallow estuaries: controls and ecophysiological and ecosystem consequences. Limnology and Oceanography 42: 1105–1118.
Verde, E. A. & L. R. McCloskey, 1998. Production, respiration, and photophysiology of the mangrove jellyfish Cassiopea xamachana symbiotic with zooxanthellae: effect of jellyfish size and season. Marine Ecology Progress Series 168: 147–162.
Viaroli, P., M. Bartoli, C. Bondavalli & M. Naldi, 1995. Oxygen fluxes and dystrophy in a coastal lagoon colonized by Ulva rigida (Sacca di Goro, Po River Delta, Northern Italy). Fresenius Environmental Bulletin 4: 381–386.
Wang, J.-T. & A. E. Douglas, 1998. Nitrogen recycling or nitrogen conservation in an alga-invertebrate symbiosis. Journal of Experimental Biology 201: 2445–2453.
Webb, K. L. & R. E. Johannes, 1967. Studies of the release of dissolved free amino acids by marine zooplankton. Limnology and Oceanography 12: 376–382.
Weis, V. M., G. J. Smith & L. Muscatine, 1989. A “CO2 supply” mechanism in zooxanthellate cnidarians: role of carbonic anhydrase. Marine Biology 100: 195–202.
Wells, M. L., 2002. Marine colloids and trace metals. In Hansell, D. A. & C. A. Carlson (eds), Biogeochemistry of Marine Dissolved Organic Matter. Academic Press, San Diego: 367–404.
Welsh, D. T. & G. Castadelli, 2004. Bacterial nitrification activity directly associated with isolated benthic marine animals. Marine Biology 144: 1029–1037.
Wilkerson, F. P. & P. Kremer, 1992. DIN, DON and PO4 flux by a medusa with algal symbionts. Marine Ecology Progress Series 90: 237–250.
West, E. J., D. T. Welsh & K. A. Pitt, 2008. Influence of decomposing jellyfish on sediment oxygen demand and nutrient dynamics. Hydrobiologia (this volume). doi:10.1007/s10750-008-9586-7.
Youngbluth, M. J., P. Kremer, T. G. Bailey & C. A. Jacoby, 1988. Chemical composition, metabolic rates and feeding behavior of the midwater ctenophore Bathocyroe fosteri. Marine Biology 98: 87–94.
Yousefian, M. & A. E. Kideys, 2003. Biochemical composition of Mnemiopsis leidyi in the southern Caspian Sea. Fish Physiology and Biochemistry 29: 127–131.
Acknowledgements
We thank P. Kremer and an anonymous reviewer who provided valuable feedback on the manuscript. Funding was provided by grant HSF 04-10 from the Hermon Slade Foundation to K. Pitt & D. Welsh.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: K. A. Pitt & J. E. Purcell
Jellyfish Blooms: Causes, Consequences, and Recent Advances
Rights and permissions
About this article
Cite this article
Pitt, K.A., Welsh, D.T. & Condon, R.H. Influence of jellyfish blooms on carbon, nitrogen and phosphorus cycling and plankton production. Hydrobiologia 616, 133–149 (2009). https://doi.org/10.1007/s10750-008-9584-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-008-9584-9