Abstract
The aim of this study was to identify potential environmental controls of the asexual phases of reproduction by measuring the rates of asexual reproduction (budding and strobilation) and mortality in naturally occurring populations of Aurelia sp. scyphistomae at different spatial and temporal scales. The percentage cover, density of colonies of Aurelia sp. scyphistomae, and density of the population of two naturally occurring colonies of Aurelia sp. scyphistomae were examined over 2 years in southern Tasmania. Artificial substrates were also deployed to investigate colony dynamics when density dependent effects were reduced. Clear spatial and temporal differences in the population dynamics of the colonies were observed. Density dependent effects controlled budding and recruitment of new scyphistomae to the substrate when populations were dense and space limiting. In contrast, environmental controls of budding and strobilation were more apparent in a colony with significantly greater area of bare substrate and hence room for expansion. Water temperature and rainfall (as a proxy for salinity) were linked to changes in population size. Annual and seasonal differences in population dynamics were not observed in a colony limited by space but were apparent in a colony where space was not limited. When space was removed as a limiting factor by deploying artificial substrates, a seasonal environmental effect on the rate of growth of the colony was observed. These studies suggest that the growth, survival and reproduction of the sessile colonial phase of Aurelia sp. is regulated by a combination of density dependent factors and environmental conditions, which are consequently important to the formation of jellyfish blooms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Blooms of jellyfish medusae are increasing in many parts of the world and may cause significant economic losses to coastal fisheries (e.g. Brodeur et al. 2002; Lynam et al. 2004) and aquaculture operations (e.g. Båmstedt et al. 1998; Lynam et al. 2004), resulting in a need to better understand the mechanisms driving these blooms and the large interannular variability. However, many jellyfish species have a relatively complex life history involving sexual and asexual reproduction. This makes it difficult to determine which phase of the life cycle is most affected by changing environmental conditions and potentially affecting the presence and size of medusae blooms. Adult medusae reproduce sexually producing planktonic larvae that settle on the seabed and metamorphose into benthic scyphistomae. The scyphistomae reproduce asexually by budding, to form benthic colonies, and by strobilation, to form the juvenile pelagic stage (ephyra) that mature into adult medusae. The benthic colonial phase is an important component of the life cycle because it is perennial, enabling populations to survive through years when recruitment to the medusae sexual phase fails. It also provides the opportunity to greatly increase the population size in preparation for production of the dispersive ephyrae when conditions are suitable (Keen 1991). Factors affecting the growth and survival of scyphistomae colonies are thus highly likely to impact on the distribution and abundance of the bloom forming medusae stage (Watanabe and Ishii 2001; Colin and Kremer 2002; Holst and Jarms 2007).
Scyphistomae abundance is influenced by various factors, including recruitment of larvae to the substrate (Lucas 2001) and predation (Hernroth and Gröndahl 1985b; Keen 1991; Osman and Whitlatch 2004). Those processes that influence the growth of colonies, such as competition for space will affect the density of scyphistomae at a site (Coyne 1973; Watanabe and Ishii 2001). While competition for food (Harper 1985) and food availability (Hernroth and Gröndahl 1985a; Keen and Gong 1989; Purcell et al. 1999) will also affect the density of scyphistomae within a colony. As such it is essential to understand factors affecting the capacity of the polyp colonies to occupy space (measured as site density) and also the density of the polyps (measured as colony density), as both these will ultimately determine the sexual reproductive capacity of the scyphistomae population. The rate and timing of asexual reproduction in scyphistomae is also dependant on environmental conditions such as temperature and salinity (e.g. Hughes and Cancino 1985; Purcell et al. 1999; Willcox et al. 2007). However, the population dynamics of the benthic phase of the life cycle, and links with environmental conditions, remain poorly understood (Purcell et al. 1999; Lucas 2001; Holst and Jarms 2007). Most of what we know is derived from laboratory experiments (e.g. Spangenberg 1965, 1968; Coyne 1973; Kakinuma 1975). Few in situ studies of this small and cryptic benthic stage have been conducted (Doyle et al. 2007) and most have used settling plates (e.g. Hernroth and Gröndahl 1983, 1985a; Watanabe and Ishii 2001; Miyake et al. 2002).
In southern Tasmania, Australia, dense blooms of moon jellyfish (Aurelia sp.) occur in some years, but not others. These blooms have caused major mortalities of caged Atlantic salmon with economic losses valued at several million dollars per annum. As a consequence, the fish farmers have been keen to develop predictive models of bloom occurrence, which requires a better understanding of the ecology of the benthic sessile stage, as well as the medusae, of this jellyfish. Prior to this study, almost nothing was known about the biology of the Tasmanian moon jellyfish and the benthic scyphistomae colonies of this species had not been located or identified.
The aim of this study was to identify potential biotic and abiotic influences on the asexual phases of reproduction by measuring the rates of asexual reproduction (budding and strobilation) and mortality in naturally occurring populations of Aurelia sp. scyphistomae at different spatial and temporal scales. By quantifying changes in polyp dynamics in wild populations at the level of the site and within the colony we could determine the role of inter-specific competition and predation. Artificial substrates were used to study colony dynamics in situ, but in conditions where density dependant effects were reduced from those operating in adjacent naturally occurring colonies and from inter-specific competition. This gave a clearer understanding of environmental controls over colony size, as well as an understanding of how rapidly new habitats might be colonised.
Methods
Study sites
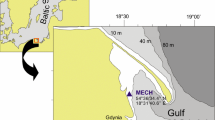
Naturally occurring colonies of Aurelia sp. scyphistomae were monitored at two sites in south east Tasmania from October 2002 to December 2004. The Derwent Estuary site in Hobart (Fig. 1) was the flat horizontal underside of a 300-m long, rigid cement breakwater at a depth of approximately 2.5 m ± calm tidal excursions of ~1 m. The Kettering site in the D’Entrecasteaux Channel (Fig. 1) was on the flat horizontal underside of a 150-m long floating marina at a depth of 0.5 m. Both sites were low energy environments, sheltered from swell surge and tidal flows. Colonies were only located on the horizontal underside of the structures, with no scyphistomae present on the vertical sidewalls of either the breakwater or the floating marina.
Populations on natural substrates
To monitor the population dynamics of the polyp colonies at each site, the sites were visited every 6 weeks. On each visit 15 newly selected, haphazardly placed 0.09 m2 quadrates were photographed (Fig. 2) using a five mega pixel digital camera in an underwater housing operated by a SCUBA diver. The camera was fitted with a framing arm so that it was perpendicular to, and the same distance from, the substrate for all photographs. The camera settings for focal length and zoom were the same on each sampling day. Errors in area measurements due to optical distortion characteristics of the camera and underwater housing were quantified by photographing underwater a grid of known dimensions and calculating the size of each grid square using image analysis software. The mean area error estimate of 2.2 ± 0.4% (SE) was considered to be insignificant and no calibration was used prior to image analysis. However, the distribution of scyphistomae on colony substrates was patchy, with colony size ranging from a few cm2 to >>0.09 m2 (maximum size of images).
To better understand the population dynamics of the polyps the populations was assessed at two levels. At the larger scale the site density (the number of scyphistomae within the photographic field of view divided by the area of the photograph) was examined, while at the smaller scale the colony density (the number of scyphistomae in a colony divided by the area covered by that colony) was examined. A colony was a grouping of polyps where all available space was utilised by polyps.
Populations on artificial substrates
To determine the processes by which colonies expanded to occupy bare space six black acrylic plates,15 cm × 15 cm × 3 mm, were attached at haphazard locations at each site. Plates were lightly abraded with steel wool to roughen the surface and remove any contaminants before being attached to the colony substrate (cement breakwater or cement pontoon) using ‘Z-Spar’ 2-pack epoxy putty. The edges of plates were flush with the substrate ensuring continuity of the surface from natural to artificial. Plates were allowed to ‘acclimatise’ for 6 weeks before being wiped clean of all biofouling organisms using steel wool. Each plate was then photo-sampled at 6-week intervals over three consecutive 18-week periods. Each plate was wiped clean of all biofouling organisms including scyphistomae at the end of each 18-week period. This regime of sampling and cleaning facilitated the study of colony growth during three distinct periods of the year; January–May (summer/autumn), May–August (winter) and August–December (spring). Colonies could expand to occupy available space in several ways. The polyps could first bud and expand to occupy the available habitat at a low density and then continue budding to increase the density within the newly occupied space, or they could bud to increase the density within the existing colony boundary to a maximum and only then start to expand to occupy the adjacent available habitat. We therefore, estimated polyp density both at the level of the plate and also within the colony.
Data analysis
Images were analysed using Sigma Scan Pro 5. image analysis software. The area of non-strobilating scyphistomae, strobilating scyphistomae, bare substrate, algae, other, and obscure categories was measured in each photo (Table 1). The area was digitised and the total area calculated for each image. On the artificial plates, the distance that scyphistomae incurred onto the plate on subsequent sampling times following cleaning (encroachment), was the shortest perpendicular measurement from a plate edge to the scyphistoma attached closest to the centre of the plate.
Water temperature was not different between colony sites during a trial period in summer (F = 0.1, df = 1, 7, P = 0.8) and temperature was subsequently recorded at Kettering only. It was recorded every four hours using an Onset Optic Stowaway temperature data logger that remained in situ from May 2003 to December 2004. Salinity was measured at the depth of the colony at each site on four occasions using a WTW salinity meter. Three samples were on randomly selected dates. The fourth day of sampling targeted the end of a 3-day period of very heavy rainfall. Daily rainfall, recorded by the Bureau of Meteorology at Margate (Fig. 1), was used as a proxy for salinity when examining temporal changes in colony dynamics. Rainfall was assumed to have an immediate and short term impact on salinity based on measurements of salinity during and after rainfall.
Statistical analysis
The effect of year, colony site, and season on the proportion of the substrate occupied by scyphistomae, colony density, and population density was examined by three-way ANOVA. Season was identified by water temperature and a ‘year’ was the 12 month period from the start of one summer period to the start of the next. Tukeys HSD post hoc test was used to determine differences among means when terms in the ANOVAs were significant.
Temporal patterns in scyphistomae density and substrate coverage were explored using multiple regression. Water temperature, rainfall, and the area of bare substrate were included as predictor variables for density. Mean water temperature and rainfall values for the 30-day period prior to each sample day were used in regression analysis. Data were grouped by year allowing inter-annual differences in relationships between predictor variables and dependent variables to be examined.
Univariate repeated measures ANOVA was used to examine the effects of site and season on the rate of encroachment of scyphistomae, changes in the proportion of the substrate covered by scyphistomae, and changes in scyphistomae density on artificial substrates. Heterogeneity of variance was checked using residual plots. Population density was transformed (log 10) for analysis. Pairwise comparisons were made using repeated measures ANOVA to compare rates of change between sites and among seasons when significant effects of site and/or season were found. The Type II error rate was adjusted for the number of tests to α = 0.006.
Results
Scyphistomae colonies
Scyphistomae were found only on the flat horizontal undersides of floating marinas and a breakwater at a depth of 0.5–3.5 m; none were present on the sides of these structures. The scyphistomae were attached to bare substrate and to other biofouling organisms growing on the substrate in discreet ‘patches’ (Fig. 2). Scyphistomae were predominantly attached to barnacles, solitary ascidians and both living and dead oyster and mussel shells. They were also occasionally observed on trapped marine debris including seagrass fronds, drift algae and plastic.
Environmental parameters
Water temperature during the study was 7.7-20.4°C. Mean water temperature during the periods when artificial substrate experiments were conducted was: summer/autumn; 16.2°C, winter; 10.4°C, and spring; 13.4°C. Salinity was typically 31–34, however, salinity dropped suddenly during heavy rainfall and subsequent runoff. The Hobart site was particularly susceptible to short term fluctuations in salinity due to large influxes of fresh water from a nearby stormwater outfall and from the Derwent River. Here the lowest salinity at the depth of the colony was 27.3 following heavy rainfall. The same rainfall event resulted in a salinity of 30.7 at the Kettering colony.
Dynamics of naturally occurring colonies
Differences in the percent cover of the substrate occupied by scyphistomae was a function of significant Year × Site × Season interaction (F = 3.4, df = 3, 238, P = 0.018). Percent cover at Kettering was 1.5–6 times greater than at Hobart (Fig. 3) and did not change during the 2-year study. In contrast there were large seasonal differences at the Hobart site in 2003, but not in 2004 (Fig. 3). The greatest cover of scyphistomae (56%) at Hobart occurred in summer 2003, after which time it declined by 40% (Fig. 3). It then increased slightly the summer 2004 (Fig. 3). During 2004 percent cover was not significantly different from those seen in 2003.
Relationships between percent cover of scyphistomae and biotic and abiotic variables differed between the sites and years. At the Hobart site water temperature explained >80% of the variation in percent cover of scyphistomae in 2003, but this relationship was not evident in 2004 (Table 2). The percent cover of other encrusting organisms, rainfall, and the area of bare substrate did not explain any of the variation in scyphistomae abundance at Hobart in either year. In contrast at the Kettering site variation in percent cover of scyphistomae in both 2003 and 2004 was related to the percent cover of encrusting organisms (>75% of variation), but not to water temperature, rainfall, or percent area of bare substrate (Table 2).
The area of bare habitat differed by an order of magnitude between sites (F = 114, df = 1, 238, P < 0.001), with a mean of 2.8 ± 0.4% (SE) at Kettering compared to 20.8 ± 1.7% (SE) at Hobart. The area covered by other encrusting organisms (including algae) also differed significantly between sites (F = 315, df = 1, 238, P < 0.001), with a mean of 6.7 ± 2.1% (SE) at Kettering compared to 51.8 ± 1.8% (SE) at Hobart.
Differences in colony density of scyphistomae was a function of a Year × Site × Season interaction (F = 12.2, df = 3, 186, P < 0.001). Colony density did not change among seasons at Kettering during the two year study; however there were large differences at Hobart (Fig. 4). The differences at Hobart among the season were opposite in the 2-years; in 2003 colony density was 50% greater in spring compared to summer, while in 2004 the reverse was seen (Fig. 4). Scyphistomae colony density at Hobart was not related to water temperature, rainfall or the area of bare substrate in 2003 (Table 2). In contrast nearly 95% of the variability was explained by water temperature in 2004 (Table 2) when colony density increased with water temperature. Rainfall also explained the most variation at Kettering with colony density decreasing as mean daily rainfall increased (Table 2).
Differences in population density were a function of both a Site × Year interaction (F = 4.2, df = 1, 239, P = 0.042), and a Site × Season interaction (F = 3.4, df = 3, 239, P = 0.019). Population density was significantly greater at Kettering (mean = 313,000 individuals ± 13,000 (SE) individuals m−2) than at Hobart (mean = 72,800 individuals ± 6,300 (SE) individuals m−2) in both years. There were no differences at Kettering either between years or among seasons (Fig. 5a, b). However, there were differences at Hobart where population density was 25% greater in 2004 than in 2003, and where population density in summer was more than double the average in the remaining seasons (Fig. 5a, b).
Mean site density (±SE) of scyphistomae for: a the Year × Site interaction (n = 48 for Kettering, n = 72 for Hobart), and b the Season × Site interaction (n = 24 for Kettering, n = 36 for Hobart). Means with different letters were significantly different as determined by Tukey’s HSD tests. The data are for seasons combined for a, and for sampling time combined for b for graphing
Population density at Hobart increased with water temperature, which explained 81% of the variation in 2003 and 66% in 2004 (Table 2) in the temperature range 10–19°C. However, population density at Hobart was not related to rainfall, the area of other encrusting organisms, or the area of bare substrate. At Kettering, population density of scyphistomae decreased as mean daily rainfall increased in the range of 0.4–4.8 mm, with rainfall explaining approximately half of the variation in both years. However, population density at Kettering was not related to water temperature, the area of other encrusting organisms, or the area of bare substrate (Table 2).
The proportion of scyphistomae strobilating varied between sites and between years. At Kettering it differed between years, with 82 ± 14.5% (SE) of scyphistomae strobilating in 2003 compared to 30 ± 11% (SE) in 2004. Strobilation at Hobart occurred at such a low level that it was not detected by the photographic survey in either year, however a thorough visual search found strobilation occurring on a single sampling day in 2003 and again in 2004. Strobilation in 2003 at Kettering occurred over two months from early August to early October. In contrast, in 2004 it was over a shorter six week period from late August to early October (Fig. 6). Strobilation in both years at Kettering began when water temperatures were ca. 10.5°C and finished by ca. 12°C (Fig. 4). Strobilation also occurred among scyphistomae that had colonised the artificial substrates placed at Kettering, but not at Hobart. These plates had been cleaned 3 months prior to this sample day; therefore scyphistomae on the plates were less than 3 months old. The proportion of scyphistomae strobilating on artificial substrates was significantly less than in the naturally occurring colony (F = 35.6, df = 1, 10, P < 0.001); 19.7 ± 9.0% (SE) compared with 30 ± 11% (SE) of scyphistomae in the surrounding colony at the peak of strobilation in 2004.
a Daily water temperature measured at Kettering and b mean daily rainfall measured at Margate. Arrows above the line (pointing down) indicate the timing of the first and last observation of strobilation at Kettering in 2003 and arrows below the line (pointing up) indicate the timing at Kettering in 2004. Strobilation at Hobart was only noted on one sample day each year and is indicated by stars (above the line 2003, below the line 2004)
Dynamics of scyphistomae colonising artificial substrates
The percent cover of scyphistomae on artificial substrates increased through time, however there was a significant Sample Time × Site × Season interaction (F = 4.0, df = 4, 71, P = 0.006). At Kettering the percent cover at the end of spring was 68% greater than at the end of winter; however, there were no differences among seasons at Hobart (Fig. 7). Percent cover at Kettering was 130 times greater than at Hobart by the end of the summer/autumn season and 10 times greater at the end of the spring season. There was no difference between sites in the winter season at the adjusted α level of α = 0.006 (F = 4.1, df = 2, 23, P = 0.04).
The pattern of increase in the percent cover (±SE) of scyphistomae on artificial substrates following plate cleaning in three different seasons at Kettering and at Hobart (n = 6). Letters indicate where seasonal patterns were different between sites and among seasons as determined by repeated measures ANOVA
Colony density
Changes through time in the colony density of scyphistomae on the artificial substrates did not differ between sites (F time×site = 0.4, df = 2, 71, P = 0.70), however there were differences among seasons (Ftime×season = 2.8, df = 4, 71, P = 0.036), with density in spring up to 40% greater than in winter across the sampling period (Fig. 8).
Site density
The site density of scyphistomae increased through time, however there was a significant time × site × season interaction (F = 4.1, df = 4, 71, P = 0.006). At Kettering the site density at the end of winter was half that at the end of spring. In contrast, no differences among seasons were observed at Hobart. All seasons were significantly different between sites, with site density 11-21 times greater at Kettering than at Hobart by the end of each sample season (Fig. 9).
The pattern of increase in site density (±SE) of scyphistomae on artificial substrates following plate cleaning in three different seasons at Kettering and Hobart (n = 6). Letters indicate where seasonal patterns were different between sites and among seasons as determined by repeated measures ANOVA
Encroachment
The distance scyphistomae moved onto plates (encroachment) increased through time and the rate of encroachment varied between sites (F Time×Site = 30, df = 2, 71, P < 0.001), with encroachment three times greater at Kettering than at Hobart (Fig. 10a). This equated to a mean rate of encroachment during each experimental season of 1.5 mm day−1 at Kettering and 0.5 mm day−1 at Hobart. The rate of encroachment also depended on season (F Time×Season = 3.1, df = 4, 71, P = 0.021), where encroachment in spring was 40% greater than in winter (Fig. 10b).
The distance (±SE) scyphistomae moved onto artificial substrates (encroachment) following plate cleaning for a the time × site interaction (n = 18) with seasons combined, and b the time × season interaction (n = 12) with the sites combined. Letters indicate where seasonal patterns were different as determined by repeated measures
Discussion
There was clear spatial differences in the factors that affected the growth, survival and reproduction of Aurelia sp. scyphistomae populations with one site (Kettering) providing the most favourable conditions. The substantial differences in site densities were primarily due to dense colonies of scyphistomae occupying a greater proportion of the substrate. Furthermore, the proportion of scyphistomae strobilating were also far greater at this site, with 80% of the scyphistomae strobilating over a 6–8-week period in spring.
Competition for space was thought to have limited colony growth, given the inverse linear relationship between the area covered by scyphistomae and the area covered by other encrusting organisms at Kettering. Greater rates of mortality of A. aurita scyphistomae are been attributed to competition for space from other encrusting organisms (Watanabe and Ishii 2001). Interspecific competition for space is also a major controlling process of density in Cyanea spp. scyphistomae (Colin and Kremer 2002). Similarly, rates of colony growth and therefore density of A. aurita scyphistomae can change according to environmental conditions and density dependant effects (Coyne 1973; Watanabe and Ishii 2001; Fischer and Hofmann 2004). In contrast, at Hobart competition for space did not appear evident as there was no relationship between the area covered with scyphistomae and the area covered with other encrusting organisms, and >20% of the substrate was bare. Therefore, given the abundance of space other factors must have were acting to limit the ability of Aurelia sp. scyphistomae to use the available habitat.
Water temperature and rainfall (as a proxy for salinity) both explained significant amounts of variation in the site density of scyphistomae. In the laboratory growth rates of colonies and of individuals of this species significantly increase with temperature, but were not affected by salinity (Willcox et al. 2007). Similarly, rates of asexual reproduction in A. aurita in experiments increase with water temperature (Keen 1991; Omori et al. 1995; Miyake et al. 2002). However, the effect of salinity on colony dynamics is not clear. Salinity has little direct effect on scyphistomae reproduction in A. aurita (Halisch 1933; Watanabe and Ishii 2001), but may affect mortality of A. aurita (Watanabe and Ishii 2001) and asexual budding of Chrysaora quinquecirrha (Purcell et al. 1999). The perceived effect of rainfall on site density may be a function that rainfall is correlated with other variables, such as food availability, which is positively correlated with rates of budding in scyphistomae (e.g. Hernroth and Gröndahl 1985b; Lucas 2001).
Predation was not thought to be a process affecting the colonies at either site at any time as populations of predators were not observed in the colony photographs. Predator populations numerically increase in response to large populations of scyphistomae, resulting in a rapid reduction in the number of scyphistomae (Gröndahl and Hernroth 1987; Keen 1991), and would have been observed if they had been present in significant numbers. The absence of medusae in the Derwent Estuary during the study, along with the observed presence of the colony a year prior to, and it’s persistence a year following this study (pers. obs.) suggests that colonies in the Estuary had survived for at least 4 years. This is not unrealistic given that A. aurita scyphistomae colonies have persisted in the laboratory for 4 years (Gong 2001). Given the nature of asexual reproduction, it is likely that colonies could survive indefinitely, with a continual turnover of new individuals replacing those dying (Jackson 1985). However, if there was differential survival of colonies it is possible that this may eventually limit genetic diversity in the population (Keen and Gong 1989; Keen 1991; Gong 2001).
Annual and seasonal differences in the percent cover, colony density, and site density of scyphistomae were not observed in the naturally occurring colony at Kettering where all available space was occupied throughout this time. However, where space was not limiting, i.e. at the Hobart site, there were substantial temporal changes in the colony cover, colony density and density of scyphistomae. Interestingly at the Kettering site when new space was generated through the deployment of artificial substrates, effectively removing space as a limiting factor, seasonal differences in rates of budding and mortality became apparent. This suggests that other density dependent factors such as food availability and water quality may be limiting colony growth (Harper 1985). Growth rates of colonies on artificial substrates were greatest in spring coinciding with the local annual phytoplankton bloom (Harris et al. 1991; Cheshuck 2001) suggesting food availability is a key factor in population growth.
The fast rate of increase in site density in spring corresponds to the period immediately following strobilation. Scyphistomae are likely to be small with depleted body reserves following strobilation and colonies may have experienced more than normal mortality at this time (Watanabe and Ishii 2001). The fast growth rates of colonies seen in spring suggested that conditions during this period would be ideal for colonies to rebuild following strobilation. In addition, with strobilation having just occurred, newly released ephyrae will have access to the same productive bloom conditions benefiting the scyphistomae (Harris et al. 1991; Cheshuck 2001).
Strobilation in Aurelia sp. scyphistomae occurred as water temperatures began to rise following six weeks of stable low temperature during the winter. Strobilation in A. aurita also occurs following minimum winter water temperatures (Palmen 1953; Custance 1964; Rasmussen 1973). Although strobilation occurred as water temperatures began increasing in spring, it began when temperatures were still near their annual average low but before a spring bloom would be expected (Harris et al. 1991; Cheshuck 2001). This suggests that spring warming of the water column was not the cue for strobilation to commence, however, water temperature may have some control over the proportion of the population strobilating. Strobilation started later, ended sooner, and fewer scyphistomae strobilated in 2004 when winter water temperatures were one degree cooler than the previous year. Similarly, A. aurita delays strobilation in cooler years (Purcell et al. 1999; Lucas 2001). However, strobilation at Kettering started and finished when water temperature was the same in both years, suggesting that strobilation may be tightly constrained by water temperature.
The spatial variability in strobilation suggests that unfavourable environmental conditions can result in a reduction or absence of strobilation in some years. This is contrary to reproductive theory that suggests production of dispersive rametes (ephyrae) will occur when conditions are least favourable in an effort to ensure survival of the genotype (Roff 1992). There is evidence from laboratory experiments that photoperiod is a trigger for strobilation, and that light intensity can alter the rates of strobilation (Purcell 2007). Alternately, an inverse relationship between density and the timing of onset of strobilation (Silverstone et al. 1977; Keen 1991) may explain the delay in the strobilation that was observed at Hobart relative to strobilation at Kettering. If onset of strobilation is density dependant then there is the possibility that density at Hobart was below the threshold necessary for strobilation to occur at this site. Poor nutrition can also affect the number of ephyrae produced (Spangenberg 1968), but there are no reports of whole colonies failing to reproduce, although some clone types can fail to strobilate in the laboratory (Gong 2001).
Laboratory experiments with Aurelia sp. suggest that the reproductive strategy to maximise production by increasing the size of scyphistomae colonies via asexual budding when conditions are good (Willcox et al. 2007). During the cold winter period leading up to strobilation budding activity slows but the size of scyphistomae increases, resulting in the production of a greater number of ephyrae (Willcox et al. 2007). In the natural environment we observed greater growth rates of benthic polyp colonies in spring and summer compared with winter, but did not measure the size of scyphistomae. These field and laboratory studies suggest that the sessile colonial phase of Aurelia sp. is regulated by a combination of environmental conditions and density dependent factors, which are important determinants of ephyrae production and hence bloom formations.
References
Båmstedt U, Fossa JH, Martinussen MB, Fosshagen A (1998) Mass occurrence of the physonect siphonophore Apolemia uvaria (Leseur) in Norwegian waters. Sarsia 83:79–85
Brodeur RD, Sugisaki H, Hunt GLJ (2002) Increases in jellyfish biomass in the Bering Sea: implications for the ecosystem. Mar Ecol Prog Ser 233:89–103
Cheshuck B (2001) The potential of integrated open water mussel (Mytilus planulatus) and Atlantic samon (Salmo salar) culture in North West Bay, Tasmania. PhD Thesis, Department of Zoology, University of Tasmania, Tasmania, p 263
Colin SP, Kremer P (2002) Population maintenance of the scyphozoan Cyanea sp. settled planulae and the distribution of medusae in the Niantic River, Connecticut, USA. Estuaries 25:70–75
Coyne JA (1973) An investigation of the dynamics of population growth and control in scyphistomae of the scyphozoan Aurelia aurita. Chesapeake Sci 14:55–58
Custance D (1964) Light as an inhibitor of strobilation in Aurelia aurita. Nature 204:1219–1220
Doyle TK, Houghton JDR, Buckley SM, Hays GC, Davenport J (2007) The broad-scale distribution of five jellyfish species across a temperate coastal environment. Hydrobiologia 579:29–37
Fischer BA, Hofmann KD (2004) Budding, bud morphogenesis, and regeneration in Carybdea marsupialis Linnaeus, 1758 (Cnidaria: Cubozoa). Hydrobiologia 530/531:331–337
Gong A (2001) Allocation to clonal replication in a Scyphozoan (Aurelia), PhD Thesis, University of California, California
Gröndahl F, Hernroth L (1987) Release and growth of Cyanea capilata (L.) ephyrae in the Gullmar Fjord, western Sweden. J Exp Mar Biol Ecol 106:91–101
Halisch W (1933) Brobachtungen an Scyphopolypen. Zool Anzeigischer 104:206–304
Harper J (1985) Modules, branches and capture of resources. In: Jackson J, Buss W, Cook R (eds) Population biology and evolution of clonal organisms. Yale University Press, New Haven, pp 1–33
Harris G, Griffiths FB, Clementson LA, Lyne VD, Van der Doe H (1991) Seasonal and interannual variability in physical processes, nutrient cycling and the structure of the food chain in Tasmanian shelf waters. J Plankton Res 13:109–131
Hernroth L, Gröndahl F (1983) On the biology of Aurelia aurita (L.) 1. Release and growth of Aurelia aurita (L.) Ephyrae in the Gullmar Fjord, Western Sweden, 1982–1983. Ophelia 22:189–199
Hernroth L, Gröndahl F (1985a) On the biology of Aurelia aurita (L.): 2. Major factors regulating the occurence of ephyrae and young medusae in the Gullmar Fjord, western Sweden. Bull Mar Sci 37:567–576
Hernroth L, Gröndahl F (1985b) On the biology of Aurelia aurita (L.): 3. predation by Coryphella verrucosa (Gastropoda, Opisthobranchia), a major factor regulating the development of Aurelia populations in the Gullmar Fjord, western Sweden. Ophelia 24:37–45
Holst S, Jarms G (2007) Substrate choice and settlement preferences of planula larvae of five Scyphozoa (Cnidaria) from German Bight, North Sea. Mar Biol 151(3):863–871
Hughes RN, Cancino JN (1985) An ecological overview of cloning in metazoa. In: Jackson JBC, Buss LW, Cook RE (eds) Population biology and evolution of clonal organisms. Yale University Press, New Haven, pp 153–186
Jackson JSC (1985) Distribution and ecology of clonal and aclonal benthic invertebrates. In: Jackson JSC, Buss LW, Cook R (eds) Population biology and evolution of clonal organisms. Yale University Press, New Haven
Kakinuma Y (1975) An experimental study of the life cycle and organ differentiation of Aurelia aurita (Lamark). Bull Mar Biol Stn Asamushi 15:101–116
Keen S (1991) Clonal dynamics and life history evolution in the jellyfish Aurelia aurita, PhD Thesis, University of California, California
Keen S, Gong A (1989) Genotype and feeding frequency affect clone formation in a marine cnidarian (Aurelia aurita Lamark 1816). Funct Ecol 3:735–745
Lucas C (2001) Reproduction and life history strategies of the common jellyfish, Aurelia aurita, in relation to its ambient environment. Hydrobiologia 451:229–246
Lynam CP, Hay SJ, Brierley AS (2004) Interannual variability in abundance of North Sea jellyfish and links to the North Atlantic Oscillation. Limnol Oceanog 49:637–643
Miyake H, Terazaki M, Kakinuma Y (2002) On the polyps of the common jellyfish Aurelia aurita in Kagoshima Bay. J Oceanog 58:451–459
Omori M, Ishii H, Fujinaga A (1995) Life history strategy of Aurelia aurita (Cnidaria, Scyphomedusae) and its impact on the zooplankton community of Tokyo Bay. ICES J Mar Sci 52:597–603
Osman RW, Whitlatch RB (2004) The control of the development of a marine benthic community by predation on recruits. J Exp Mar Biol Ecol 311:117–145
Palmen E (1953) Seasonal occurence of ephyrae and subsequent instars of Aurelia aurita (L.) in the shallow waters of Tvärminne, S. Finland. Soc Zool Bot Fenn Vanamo 8:122–131
Purcell JE, White JR, Nemazie DA, Wright DA (1999) Temperature, salinity and food effects on asexual reproduction and abundance of the scyphozoan Chrysaora quinquecirrha. Mar Ecol Prog Ser 180:187–196
Purcell JE (2007) Environmental effects on asexual reproduction rates of the scyphozoan Aurelia labiate. Mar Ecol Prog Ser 348:183–196
Rasmussen E (1973) Systematics and ecology of the Isefjord marine fauna (Denmark). Ophelia 11:1–507
Roff DA (1992) The evolution of life histories. Chapman & Hall, London
Silverstone M, Tosteson T, Cutress C (1977) The effect of iodine and various iodocompounds on initiation of strobilation in Aurelia. Gen Comp Endocrinol 32:108–113
Spangenberg D (1965) Cultivation of the life stages of Aurelia aurita under controlled conditions. J Exp Zool 159:303–318
Spangenberg D (1968) Recent studies of strobilation in jellyfish. Oceanogr Mar Biol Ann Rev 6:231–247
Watanabe T, Ishii H (2001) An in situ estimation of the number of ephyrae liberated from polyps of Aurelia aurita on settling plates in Tokyo Bay. Hydrobiologia 451:247–258
Willcox S, Moltschaniwskyj N, Crawford C (2007) Asexual reproduction in scyphistomae of Aurelia sp.: effects of temperature and salinity in an experimental study. J Exp Mar Biol Ecol 353(1):107–114
Acknowledgments
We thank the initially four, and after amalgamations, two salmon farming companies, Tassal Ltd and Huon Aquaculture Pty. Ltd for their financial support, sharing of information and logistical support on the water. This research was funded by an Australian Research Council Linkage grant to C.M.C and N.A.M.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by U. Sommer.
Rights and permissions
About this article
Cite this article
Willcox, S., Moltschaniwskyj, N.A. & Crawford, C.M. Population dynamics of natural colonies of Aurelia sp. scyphistomae in Tasmania, Australia. Mar Biol 154, 661–670 (2008). https://doi.org/10.1007/s00227-008-0959-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-008-0959-2