Abstract
The present study (Ishigaki Island, Japan) explored the distance of transmission of chemical cues emitted by live versus dead coral reefs (Exp. 1: High performance liquid chromatography (HPLC) analyses with water sampling station at 0, 1, and 2 km away from the reef) and the potential attraction of these chemical cues by larval fish, crustaceans, and cephalopods (Exp. 2: choice flume experiment conducted on 54 Chromis viridis larvae, 52 Palaemonidae sp larvae, and 16 Sepia latimanus larvae). In the experiment 1, HPLC analyses highlighted that the live coral reef (and not the dead coral reef) produced different and distinct molecules, and some of these molecules could be transported to a distance of at least 2 km from the reef with a reduction of concentration by 14–17-fold. In the experiment 2, C. viridis, Palaemonidae sp, and S. latimanus larvae were significantly attracted by chemical cues from a live coral reef (sampling station: 0 km), but not from a dead coral reef. However, only C. viridis larvae detected the chemical cues until 1 km away from the live coral reef. Overall, our study showed that chemical cues emitted by a live coral reef were transported farthest away in the ocean (at least 2 km) compared to those from a dead coral reef and that fish larvae could detect these cues until 1 km. These results support the assumption of a larval settlement ineffective in degraded coral reefs, which will assist conservationists and reef managers concerned with maintaining biodiversity on reefs that are becoming increasingly degraded.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Understanding how animals make decisions is a fundamental question in behavioral ecology (Dill 1987). While there is a plethora of information about breeding and foraging habitat selection (Blumstein and Bouskila 1996), data on habitat selection for marine species at the time of settlement are relatively scarce (Arvedlund and Kavanagh 2009; Leis et al. 2011). Most marine organisms on coral reefs (i.e., cephalopods, fish, and crustaceans) have stage-structured life histories with two distinct stages: a relatively sedentary benthic stage (usually juveniles and adults) and a pelagic larval stage capable of long-distance dispersal (Kingsford et al. 2002). The replenishment and persistence of most marine species on coral reefs are contingent on dispersing larvae, finding a habitat and becoming established in this habitat (i.e., settlement phase; Leis et al. 2011). Yet, the World Conservation Institute estimates that 20 % of coral reefs are already definitively destroyed, another 25 % are in great immediate threat, and another 25 % will be under threat by 2050 (Wilkinson 2004). Several studies have shown that coral areas experiencing a disturbance often exhibit declines in adult populations, leading to accelerated rates of extirpation compared to “non-degraded” habitats (Hughes et al. 2003; Jones et al. 2004; Munday et al. 2009). The persistence of species in the area then becomes reliant on the “rescue” effect of settlement (Hanski and Gilpin 1997). However, the mechanisms that determine how marine larvae respond to different stages of coral stress and the extent of coral loss during larval settlement are poorly understood (Feary et al. 2007; Gleason et al. 2009; Munday et al. 2009; Lecchini et al. 2012, 2013). We aimed to increase scientific knowledge about the sensory world of crustaceans, cephalopods, and fish during habitat selection, focusing on a vital life history stage (i.e., larval stage) to comprehend the settlement process in the context of coral reef degradation.
Some recent studies have highlighted that marine larvae can orient themselves in the water column and swim directionally either at hatching or soon thereafter (e.g., Gerlach et al. 2007; Dixson et al. 2008; Munday et al. 2009; Simpson et al. 2010; Stanley et al. 2012). Oceanic larvae use swimming behavior, stimulated by chemical or sound cues, to control their position within the water column, increasing the probability that they will be transported to suitable settlement reefs (Leis and McCormick 2002). Once a larva of any taxon has located a reef, a suitable microhabitat must be found for settlement. Recent studies have demonstrated the role of larval sensory mechanisms in patch identification and patch selection at smaller scales, including detection of visual, chemical, and sound cues from conspecifics, predators, or microhabitats (e.g., Jeffs et al. 2005; Igulu et al. 2011; Dixson et al. 2012; Lecchini et al. 2010, 2013; Holles et al. 2013). Overall, evidence is mounting that larval reef fish, cephalopods, and crustaceans are active participants in the process of dispersal and settlement because of their sensory and swimming abilities (Arvedlund and Kavanagh 2009), but the transmission distance of sensory cues remains unknown for coral reefs (see Atema et al. 2002; Mann et al. 2007; Radford et al. 2011 for exceptions). In the present study conducted at Ishigaki Island (Ryukyu Archipelago, Japan), we explored the distance of transmission of chemical cues emitted by live versus dead coral reefs and the potential attraction of these chemical cues by larval fish, cephalopods, and crustaceans. Many reefs on the Ryukyu Islands in Japan have changed in the last 20 years from live coral to dead coral dominate states due to several natural and anthropogenic disturbances (Bruno and Selig 2007). However, the abundance and species richness of dominant corals, sea urchins, parrotfish, and surgeonfish that provide either direct chemical cues or indirect auditory cues vary often according to reef state (live coral vs. dead coral dominance; Shibuno et al. 1999; Sano 2000; Wilson et al. 2006; Lecchini and Tsuchiya 2008, Lecchini et al. 2003, 2012). Therefore, we hypothesized that live coral reefs emit some specific chemical cues in the ocean compared to that of dead coral reefs and that marine larvae will be more attracted by chemical cues from live rather than dead coral reefs.
Specifically, we estimated the transmission distance of chemical cues emitted by a live coral reef and a dead coral reef from 2 km into the ocean at Ishigaki Island. Subsequently, we tested larval attraction of marine species (fish, cephalopods, and crustaceans) toward chemical cues from a live coral reef (water collected at 0, 1, and 2 km away from the reef, plus control water) and a dead coral reef (0, 1, and 2 km, plus control water) in a 4-channel choice flume.
Materials and methods
Sampling sites
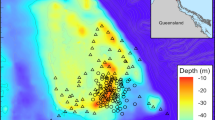
The study was performed on two reefs, spaced 8 km apart, on the northwest coast of Ishigaki Island, southern Ryukyu Islands, Japan (Fig. 1). Oganzaki reef (24°28′08 N, 124°07′01 E; length: 387 m and width: 404 m) is characterized by a high cover percentage of live coral (cover of live coral: 67 ± 4 %, dead coral: 21 ± 9 %, rubble: 12 ± 9 %, and other substrates: 0 %). Kabira reef (24°25′59 N, 124°07′06 E; length: 264 m and width: 545 m) is characterized by a high cover percentage of dead coral (cover of live coral: 1 ± 2 %, dead coral: 98 ± 2 %, rubble: 0 %, and other substrates: 1 ± 2 %). Percent cover estimates (mean ± standard deviation) of dead coral, rubble, live coral, and other substrates (e.g., sand, algae, soft coral, and sponge) were conducted on each reef from six replicate 20-m belt transects using the line transect method (substrate recorded every 1 m) in August 2011. Live coral coverage of the outer reef slope on the northern Ishigaki Island (eastward from Kabira) declined severely after 2011 largely due to an outbreak of crown-of-thorns starfish Acanthaster planci in 2010 while live corals on the western Ishigaki Island (Oganzaki reef) had not been damaged (Suzuki et al. 2012).
Map of Ishigaki Island (Japan) with the location of the Oganzaki and Kabira reefs. Grey line represents the coral reefs along the coast. The white square symbol indicates our visual transect benthic survey station in August 2011. The black circle symbol indicates our water sampling station at each reef. (star): Light trap station (Yoshihara reef). White rectangle: Okinawa Prefectural Fisheries Research and Extension Center
Capture of larval stage marine species
Four light traps were placed on the reef slope of northwest Ishigaki coast (Yoshihara reef; 3.5 km away from Kabira reef—Fig. 1) to capture fish, crustacean, and cephalopod larvae just before they entered the lagoon for settlement (for more description about light traps, see Nakamura et al. 2009a, b). The four traps were separated by 50 m and anchored on the reef slope from 1,700 to 0830 h. One fish species (Chromis viridis: 54 larvae captured), one crustacean species (Palaemonidae sp: 52 larvae), and one cephalopod species (Sepia latimanus: 16 larvae) were captured in August and September 2011.
Water sampling
At each reef (Kabira and Oganzaki), two water samples were collected 3–5 m apart at a depth of 1 m by two plastic containers (10 L and 20 L volumes) at three sampling stations along a line transect perpendicular to the coast using a global positioning system: 1 at the coral reef (chemical cues emitted by marine organisms present on each reef) and 2 in the offshore at different distances from the reef (1 and 2 km into the ocean, Fig. 1). Therefore, we collected a total of 12 water samples over the two reefs in each sampling day. The sampling at Kabira and Oganzaki reefs was conducted at or shortly after sunset on three fair weather days between August and September 2011. All seawater samples (30 L for each station × 3 temporal replicates) were collected under the same meteorological conditions (current: 1 knot; outgoing tide; tidal amplitude: 150 cm). Of the 90 L of water collected at each sampling station (0, 1, and 2 km) of each reef (Kabira and Oganzaki), 10 L were used for chemical analyses and 80 L for a choice flume experiment.
Sample preparation and analysis of seawater chemical fingerprints
High performance liquid chromatography (HPLC) was used to acquire the profiles of seawater metabolites to compare the chemical fingerprints of the different water samples (0, 1, and 2 km) at each reef (Kabira and Oganzaki). Each water sample (10 L) was filtered under vacuum through solid phase extraction (SPE) cartridges containing a C18 silica gel-based bonded phase sorbent, washed with 50 mL of distilled water, and subsequently desorbed with 50 mL of methanol. The organic phase of each sample was then freeze-dried leaving a powdery organic residue. The organic extracts from the different water samples were dissolved in 1 mL of methanol before analysis.
High performance liquid chromatography was performed with a system from Waters including the Alliance separation module 2695, column heater, and 2998 photodiode array detector. The liquid chromatograph was equipped with a 5 µm Phenomenex Gemini C18 column (150 × 3.00 mm). The equipment was controlled, and the data were handled using Empower Chromatography Data software (Waters). The following analytical solvent and gradient systems were used: solvent A, 0.1 % aqueous trifluoroacetic acid; solvent B, acetonitrile–trifluoroacetic acid (99.9:0.1, v:v); linear gradient from 25 to 100 % B within 15 min and then 100 % B for an additional 5 min; the flow rate was 0.5 mL min−1, and injection volume was 20 µL. Column temperature was set to 30 °C, and data collection across the 210–500-nm wavelength range was performed in the following manner: sampling, 1 point/s; resolution, 1.2 nm; and no smoothing.
Overall, the data were processed to create a max-plot chromatogram that plotted the maximum spectral absorbance measured at each time point. Max plot enables detection of all detectable UV-absorbing components in the sample. The absorbance of each component (computed as the area of peak and expressed in absorbance units, AU) identified on the chromatograms (water samples at 0, 1, and 2 km at Oganzaki and Kabira reefs) was analyzed in relation to the distance from the reefs and the coral health (live or dead) by principal component analysis (PCA). The “distance from reefs” and “coral health” variables were used as supplemental quantitative and qualitative variables, respectively, to illustrate the graph and were not included when the PCA was calculated.
Choice flume experiments to detect chemical abilities of marine larvae
The marine larvae captured during the night with light traps were transferred in aquaria (0.8 × 0.5 × 0.4 m) by car to the laboratory and subsequently maintained in individual aquariums (0.3 × 0.3 × 0.2 m) at a water temperature of 26–27 °C and supplied with flow-through sand-filtered seawater without any added artificial or natural habitat and food. Source of filtered seawater was pumped from a reef channel at a depth of 13 m connected to the reef edge in front of the research station. Although sand filter used might have not been able to remove the chemical cues of the location, we considered that concentration of the chemical cue was low in the filtered seawater. Moreover, a previous study conducted at the same research station showed that filtered seawater did not repulse or attract marine larvae (Lecchini and Nakamura 2013). Laboratory experiments were performed within 24 h of larval capture under artificial lighting conditions provided by evenly distributed florescent lights (Lecchini et al. 2005, 2013).
The response of the marine larvae to olfactory cues from coral reef water (water samples at 0, 1, and 2 km of each reef + control water) was tested in a 4-channel choice flume (Fig. 2). Four tanks were connected to the four upstream channels (A, B, C, and D) by pipes to create a constant and laminar gravity-driven flow (250 mL min−1) into each channel. A single larva was placed into the central part of the choice flume for 1 min to acclimate for each trial, with a net prohibiting movement into the upstream channels. Before acclimatization, the flume was filled with sand-filtered seawater (control water). After 45 s of acclimatization, the four different water types were discharged into the flume from each channel. The net was removed after 1 min of acclimatization, and the marine larva was free to move to one of the four upstream channels or to stay in the central part of the choice flume.
Photograph of the 4-channel choice flume built with 4 PVC pipes called “upstream channels” (length: 40 cm, width: 4 cm, height: 3 cm) opened in the upper part to see marine larvae and linked together by a central PVC part called the “downstream compartment” (length: 10 cm, width: 4 cm, height: 3 cm). Four plastic tanks (25 × 10 × 8 cm; water depth: 20 cm) were connected to the 4 upstream channels (A, B, C, and D) by plastic pipes to create constant gravity-driven flow into each channel at 250 mL min−1. The water was mixed homogeneously in the downstream compartment and then evacuated by a hole in the bottom of this compartment (total of water drained: 1 L for each test). Dye tests showed laminar flow of water in each channel and subsequently mixing of water only in the downstream compartment. In the wild, marine larvae are always in contact with several chemical cues before selecting one odor that they follow to the source. Therefore, marine larvae in our choice flume were present for mixing of the 4 water types in the downstream compartment and then present in the laminar flow of water in each channel
Preliminary tests with the 4 channels filled only with control water showed that larvae swam spent equal time in each channel (between 10 and 20 s in each channel). Other preliminary tests with the 4 channels filled with water samples at 0, 1, and 2 km of each reef and with control water showed that larvae swam between channels and thus must be able to smell all possibilities before making a decision. If a larva stayed only in the downstream compartment without moving, this individual was removed from the data (2 C. viridis, 6 Palaemonidae sp. and 2 S. latimanus). Therefore, a “choice” was scored as the time (in seconds) spent by the larva in each of the four upstream channels and in the central part of the choice flume. The experiment lasted 1 min. In each trial, two observers were positioned around the aquaria to record the time. We took care to avoid any shadow of observers on the aquarium.
We conducted two similar experiments, but each individual larva was tested only in one of the two experiments. First (Exp. A: live coral reef), we determined the distribution of choices exhibited by a larva presented with live coral reef water in each of the four channels: channel A, water collected on the Oganzaki reef (0 km); channel B, water collected 1 km away from Oganzaki reef; channel C, water collected 2 km away from Oganzaki reef. In the fourth channel, we introduced control water (filtered seawater). Second (Exp. B: dead coral reef), we conducted the same experiment but with dead coral reef water (Kabira reef). Marine larvae were tested in the water samples within 24 h to avoid water quality changes or damage. After each trial, the flume chamber was emptied and washed with freshwater. To exclude a possible side bias of the fish, the order of channels containing each water type was randomized after each trial.
The “no choice” result (i.e., time spent by larvae in the downstream compartment) was not included in the statistical analysis, which is common practice for non-responding animals in behavioral studies (Tolimieri et al. 2004; Huijbers et al. 2012). The mean time spent in the downstream compartment was 21 s (SD = 2.9 s) in the Oganzaki experiment and 23 s (SD = 1.3 s) in the Kabira experiment (for all marine larvae tested). Wilcoxon tests were performed separately for each experiment and for each species to compare the time spent by larvae in a channel filled with 0, 1, or 2 km versus time spent by larvae in the channel filled with control water. This analysis allowed us to determine whether marine larvae were significantly attracted by the chemical cues of live or dead coral reef (sampling point: 0 km) and at what distance they could detect these cues (sampling points: 1 and 2 km).
Lastly, Spearman’s rank correlation test was performed between time spent by larvae in each channel and the absorbance of each component. A generalized linear model was constructed between the rank of time that each marine species spent in every upstream channel and the absorbance of significant components. To highlight the component(s) most important for larval attraction, a stepwise model selection using the Akaike information criterion (AIC) was computed in both directions (backward and forward).
Results
Analysis of seawater chemical fingerprints
High performance liquid chromatography allowed us to identify 6 major peaks on the chemical fingerprints of the different water samples (0, 1, and 2 km) at each reef (Kabira and Oganzaki; Fig. 3a). Two peaks (retention time of 7:30 and 16:10 min; noted as 1 and 5 on the chromatograms) were identified in common on all HPLC chromatographs. These shared patterns were interpreted as background signatures of Ishigaki water (northwest part of island). Analyses of water collected from the dead coral reef (sampling at 0, 1, and 2 km) did not show any other specific peak (Fig. 3a). In contrast, the chromatogram from the live coral reef (sampling: 0 km) showed higher molecular diversity with 4 unique major peaks (retention time: 9:50, 10:15, 11:05, and 19:05 min; noted as 2, 3, 4, and 6 on the chromatograms). These four peaks were present on the chromatogram from samples at 1 km. Peaks noted as 2, 4, and 6 were present on the 2-km chromatogram, but their absorbance was reduced by 14-fold (peak 6) to 17-fold (peak 4) relative to their absorbance at Oganzaki reef (sample at 0 km) (Fig. 3b).
a High performance liquid chromatography chromatograms of seawater collected at 3 sampling stations (0, 1, and 2 km) at Oganzaki (live coral reef) or Kabira (dead coral reef). We deleted the first 3 min of the chromatograms, as they corresponded to the dead volume in the column. The chromatograms were divided into 3 zones: 1 zone where polar compounds were eluted (3–8 min), 1 zone where medium-polar compounds were eluted (8–14 min), and 1 zone where non-polar compounds were eluted (14–25 min). To maximize sensitivity, the data were processed to create max-plot chromatograms, which plots the maximum spectral absorbance measured at each time point. A number was given for each identified peak (1–6). The peaks 2, 3, 4, and 6 are; however, few visible on the chromatograms (see Fig. 3b). b Quantity of absorbance for the peaks 2, 3, 4, and 6 according to the distance to Oganzaki reef in order to highlight the reduction of their concentration. Only the peak 3 disappeared totally at 2 km
Principal component analysis showed that the four peaks present on the chromatograms of live coral reef (noted as 2, 3, 4, and 6) were correlated in the same way along axis 1 (68 % of variance; Fig. 4), whereas the two peaks present on all chromatograms (noted as 1 and 5) were correlated in the opposite way along axis 2 (30 % of variance). A significant regression was highlighted for all six peaks with distance from a reef (0, 1, and 2 km). The absorbance of peaks 1, 2, 3, 4, and 6 decreased significantly (R 2 > 0.93; p < 0.001), whereas the absorbance of peak 5 increased significantly with distance from a reef (R 2 = −0.91; p = 0.01). For example, the absorbance of peaks 3 and 4 was 0.04 and 0.18 AU, respectively, for samples at Oganzaki reef (0 km), then decreased to 0.03 and 0.13 AU at 1 km. Peak 3 disappeared at 2 km, whereas absorbance of peak 4 was 0.01 AU (Fig. 3b).
Correlation circle on the first two axes of the principal component analysis (PCA) conducted on the absorbance of each component identified (peaks 1–6) on the chromatograms (water samples at 0, 1, and 2 km of Oganzaki and Kabira reefs) and the distance from reefs (Dist). Correlation circle showing a projection of the variables in factor space. Variables that are far from the center and are close to each other are significantly and positively correlated (as r approaches 1). The length of arrows represents the strength of the link between variables and the corresponding axis
Overall, the results highlighted that the live coral reef (and not the dead coral reef) produced different and distinct molecules, and some of these molecules could be transported to a distance of at least 2 km from the reef with a reduction of concentration by 14–17-fold.
Choice flume experiment to detect chemical abilities of marine larvae
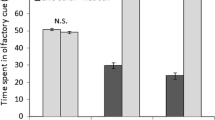
In experiment A (Oganzaki water), the three species spent most of their time in the channel filled with water collected on the live coral reef (sampling: 0 km; C. viridis: 40 ± 9 %, Palaemonidae sp: 48 ± 9 %, and S. latimanus: 51 ± 11 %). The channel filled with control water was chosen the least (C. viridis: 6 ± 3 %, Palaemonidae sp: 14 ± 4 %, and S. latimanus: 13 ± 7 %). The three species used chemical cues to move toward live coral water (Fig. 5a). The distributions of C. viridis, Palaemonidae sp, and S. latimanus larvae were significantly different between the sampling at 0 km and the control sampling (Wilcoxon test; n = 26, z value = 2.8, p = 0.004; n = 24, z value = 2.5, p = 0.01; and n = 7, z value = 1.9, p = 0.04, respectively). Only the distribution of C. viridis larvae was still significantly different from the control at the 1-km sample point (n = 26, z value = 2.6, p = 0.009). The three species did not respond to the chemical cues of live coral waters from the 2-km samples (p > 0.05).
Percentage of fish, crustacean, and cephalopod larval preferences for water samples collected at Oganzaki (live coral reef—a) or Kabira (dead coral reef—b) in the 4-channel choice flume. The four channels were filled with water collected on the reef (0 km); at 1 or 2 km away of the reef, or with filtered ocean seawater (control). The number of larvae tested for each species is given in parenthesis. Stars indicate a significant difference (p < 0.05) in time spent by larvae in the channel filled with 0, 1, or 2 km versus time spent by larvae in the channel filled with control water by Wilcoxon’s test
In experiment B (Kabira water; Fig. 5b), C. viridis larvae (26 individuals tested) spent most of their time in the channel filled with water collected 2 km away from the dead coral reef (30 ± 9 %). Palaemonidae sp. larvae (22 individuals tested) showed greatest presence in control water (31 ± 8 %). S. latimanus larvae (7 individuals tested) showed greatest presence in water collected 1 km away from the dead coral reef (38 ± 18 %). Their distributions were not significantly different between the samples at 0, 1, or 2 km and the control sample (p > 0.05). Therefore, the three species did not respond to chemical cues of dead coral waters.
Overall, significant correlations were observed between the absorbance of peaks 2, 3, 4, and 6 that were characteristic of the chemical fingerprint of the live coral reef (Oganzaki) and larval attraction in the choice flume (Spearman’s rank correlation: rho value > 0.1, p < 0.04 for all peaks). Nevertheless, the stepwise model selection using the AIC showed that the peaks 2 and 4 were defined as the main variables fitting the time spent by the larvae in each channel (F value = 4.42, p = 0.03 for peak 2; F value = 7.29, p = 0.007 for peak 4).
Discussion
Transmission distance of chemical cues from coral habitats
Because it is unlikely that successful habitat selection by coral reef animals at settlement is solely a matter of chance (Doherty 2002), one of the greatest challenges faced by a marine species with pelagic larval stages is the relocation of the relatively rare patches of suitable habitat on which they settle and ultimately reside as adults (Myrberg and Fuiman 2002; Montgomery et al. 2006). Olfactory and acoustic cues play an important role in directing pelagic larval stage of fish, crustaceans, and cephalopods to a suitable settlement reef (Arvedlund and Kavanagh 2009; Leis et al. 2011). However, the chemical or acoustic cue transmission distance remains relatively unknown (Moore and Crimaldi 2004).
Some studies have estimated that the distances that marine larvae could potentially detect coastal reef noise range from 500 m to ~5 km (Mann et al. 2007; Wright et al. 2010; Radford et al. 2011). Atema et al. (2002) estimated chemical cue transmission distance from ebb tide plumes of lagoon water based on temperature measurements and on visual observations of turbidity. They estimated that the transmission distance of the odor plume from a coral lagoon could be 1.6–3 km into the ocean. Although low spatial and temporal replication of water samples limit the strength of inference drawn from our results, the HPLC analyses showed that chemical cues emitted by the live coral reef were different and were transported farther away in the ocean than chemical cues of the dead coral reef (Fig. 3a). Thus, the transmission distance of chemical cues emitted by the live coral reef (Oganzaki site) was at least 2 km in the ocean, while the dead coral reef (Kabira site) did not produce any specific chemical cues detectable in HPLC. Atema et al. (2002) suggested that water mixing from coral reefs into the ocean is a slow process, allowing lagoon water to remain identifiable in larger and then in smaller patches for long periods of time and long distances. In our study, the four peaks characterizing the chemical fingerprints of live coral reef (peaks 2, 3, 4, and 6) had their concentration reduced from one- to twofold at 1 km away of the reef, and from 14–17-fold at 2 km, except for peak 3, which disappeared at 2 km (Fig. 3b). While identity of compounds among the water samples remains speculation, our results are in accordance with results of Atema et al. (2002). However, as chemical cues are not uniformly dissolved in the water, it is possible that the water sample that was taken did not have the chemical cues emitted from the dead coral reef in high concentration, whereas as the sample from the live coral reef happened to be taken from a part of the water column with a patch of chemical cues. Although we cannot completely deny the possibility of such sampling limitation, similar behavioral response of marine larvae to respective reef water after each water sampling day suggests distinct difference in the distribution of chemical stimuli between live and dead coral reefs.
Future studies should be, nevertheless, conducted under different current directions and with different meteorological conditions to generalize chemical cue transmission distances from live or dead coral reefs. Understanding the interaction between a chemical signal and the environment requires a better knowledge of the physical processes that transport chemicals through environments creating different structures in the form of spatial and temporal signal information (Zimmer and Zimmer 2008).
Detection distance of coral habitat chemical cues by marine larvae
Several studies have shown that marine larvae can chemically detect desirable water from appropriate islands or reefs (e.g., Gerlach et al. 2007; Dixson et al. 2008; Lecchini and Nakamura 2013). For example, Gerlach et al. (2007) showed that settling fish larvae are not only capable of olfactory discrimination among reefs but also prefer the waterborne odors of their home reefs. However, no study has explored the detection distance for chemical cues from coral reefs. Our study showed that C. viridis, Palaemonidae sp, and S. latimanus larvae were significantly attracted by chemical cues from a live coral reef (sampling station: 0 km), but not from a dead coral reef (Fig. 5). Similarly, Lecchini et al. (2013) showed that 7 of 10 fish species studied at Rangiroa Atoll (French Polynesia) preferred water from reefs dominated by coral compared to reefs dominated by algae (e.g., Aulostomus chinensis, C. viridis, Ptereleotris microlepis, Sargocentron spiniferum). Moreover, our study showed for the first time that chemical cues emitted by a live coral reef were transported farthest away in the ocean (at least 2 km) compared to those from a dead coral reef (Fig. 3). Nevertheless, the detection distance of these chemical cues was only 1 km and varied highly according to marine species (Fig. 5). Indeed, only C. viridis larvae detected the chemical cues until 1 km away from the live coral reef when their concentration was reduced. The main peaks responsible for larval attraction of C. viridis until 1 km away from the live coral reef would be peaks 2 and 4 (Fig. 3a, b). These two peaks were some medium-polar compounds. In a previous study, Lecchini et al. (2005) showed that C. viridis larvae are attracted by chemical cues of conspecifics and that the peak responsible for larval attraction was a non-polar compound. Although we did not attempt to identify the structural composition of these organic compounds that provided cues from the live coral reef (in the present study) or from conspecifics (Lecchini et al. 2005), workers studying other marine species have suggested that such substances may reflect neurotransmitter-like chemicals produced specifically for communication (Painter et al. 1998; Browne and Zimmer 2001) or metabolic by-products such as fish mucus, urine, or feces (Sola et al. 1993; Baker and Montgomery 2001).
Overall, our results (Figs. 3, 5) emphasize a probable difference in the detection threshold of molecules according to marine species; C. viridis larvae would have better chemical sensitivity than that of Palaemonidae sp. and S. latimanus larvae. Although Kingsford et al. (2002) suggested that fish larvae had better sensory abilities than crustaceans and cephalopods, the detection threshold of molecules for marine species in coral reefs has not been studied sufficiently to validate this hypothesis (see Wright et al. 2005, 2008 with the technique of electro-olfactogram for an exception). Generally marine animal odor detection thresholds can be very low: A common threshold for amino acids lies between 10−7 M and 10−9 M in fish and crustaceans (Fuzessery and Childress 1975; Fuzessery et al. 1978; Hara 1994). Nevertheless, due to their much smaller and less developed olfactory organs, this is probably orders of magnitude lower than we might expect in larvae of reef fish, crustacean, and cephalopods (Kingsford et al. 2002). In addition, odor in nature is typically distributed in patches of various concentrations. Patches provide signal contrast with the natural background for typically adapting receptors (Atema 1996). When humans sample water, these odor patches are homogenized, reducing their peak concentration and eliminating contrast needed for adapting receptors. For HPLC detection, the samples are greatly concentrated. It may thus not be surprising to see HPLC detection at greater distance than correspond to animal responses. Moreover, marine larvae tested in our 4-channel choice flume may still detect but choose not to respond behaviorally to any signal provided (Zimmer-Faust and Case 1983).
Conclusion
At present, little is known about how specific biochemical compounds produced by conspecifics or live coral reefs potentially interact to shape the preferences, and in turn the settlement patterns of marine larvae; therefore, we suggest that this may be a fruitful avenue for future research. Thus, our study is the first to determine the chemical cue transmission distance emitted by live versus dead coral reefs and the potential attraction of these chemical cues by fish, cephalopod, and crustacean reef larvae. The choice flume experiments showed that the larval attraction of C. viridis, Palaemonidae sp, and S. latimanus to the chemical cues varied significantly according to reef state (live vs. dead coral dominance) and that the chemical cues of the live coral reef could be detected by C. viridis until 1 km away from the reef. These results raise the issue of coral reef degradation. Indeed, Jones et al. (2004) showed that the decline in adult coral reef fish populations in a degraded habitat has more to do with settlement failure than adult mortality. They also suggested that the settlement rescue effect may be completely ineffective in a degraded habitat. The results acquired from Ishigaki Island (in the present paper) and those acquired from Rangiroa Atoll (Lecchini et al. 2013) support the assumption of more efficient larval settlement in non-degraded reefs (live coral dominance) than that in degraded coral reefs (dead coral or algal dominance). Thus, if the settlement potential of French Polynesian or Ryukyu reefs has decreased, the populations of reef organisms (fish, cephalopods, and crustaceans) will continue their rapid decline, as larval settlement will not help to replace and sustain the adult populations on the degraded reefs. Understanding the relationship between reef state and settlement potential will allow management planning for the maintenance of coral cover and biodiversity on reefs that are increasingly degraded.
References
Arvedlund M, Kavanagh K (2009) The senses and environmental cues used by marine larvae of fish and decapods crustaceans to find tropical coastal ecosystems. In: Nagelkerken I (ed) Ecological connectivity among tropical coastal ecosystems. Springer, New York, pp 135–185
Atema J (1996) Eddy chemotaxis and odor landscapes: exploration of nature with animal sensors. Biol Bull 191:129–138
Atema J, Kingsford MJ, Gerlach G (2002) Larval reef fish could use odour for detection, retention and orientation to reefs. Mar Ecol Prog Ser 241:151–160
Baker CF, Montgomery JC (2001) Species-specific attraction of migratory banded kokopu juveniles to adult pheromones. J Fish Biol 58:1221–1229
Blumstein DT, Bouskila A (1996) Assessment and decision making in animals: a mechanistic model underlying behavioural flexibility can prevent ambiguity. Oikos 77:569–577
Browne KA, Zimmer R (2001) Controlled field release of a waterborne chemical signal stimulates planktonic larvae to settle. Biol Bull 200:87–91
Bruno JF, Selig ER (2007) Regional decline of coral cover in the Indo-Pacific: timing, extent, and subregional comparisons. PLoS ONE 2(8):e711
Dill LM (1987) Animal decision making and its ecological consequences: the future of aquatic ecology and behavior. Can J Zool 65:803–811
Dixson DL, Jones GP, Munday PL, Planes S, Pratchett MS, Srinivasan M, Syms C, Thorrold SR (2008) Coral reef fish smell leaves to find island homes. Proc Biol Sci 275:2831–2839
Dixson DL, Pratchett MS, Munday PL (2012) Reef fishes innately distinguish predators based on olfactory cues associated with recent prey items rather than individual species. Anim Behav 84:45–51
Doherty PJ (2002) Variable replenishment and the dynamics of reef fish populations. In: Sale PF (ed) Coral reef fishes: dynamics and diversity in a complex ecosystem. Academic Press, San Diego, pp 327–358
Feary DA, Almany GR, McCormick MI, Jones GP (2007) Habitat choice, recruitment and the response of coral reef fishes to coral degradation. Oecologia 153:727–737
Fuzessery ZM, Childress JJ (1975) Comparative chemosensitivity to amino acids and their role in the feeding activity of bathypelagic and littoral crustaceans. Biol Bull 149:522–538
Fuzessery ZM, Carr WES, Ache BW (1978) Antennular chemosensitivity in the spiny lobster, Panulirus argus: studies of taurine sensitive receptors. Biol Bull 154:226–240
Gerlach G, Atema J, Kingsford MJ, Black KP, Miller-Sims V (2007) Smelling home can prevent dispersal of reef fish larvae. Proc Natl Acad Sci 104:858–863
Gleason DF, Danilowicz BS, Nolan CJ (2009) Reef waters stimulate substratum exploration in planulae from brooding Caribbean corals. Coral Reefs 28:549–554
Hanski I, Gilpin ME (1997) Metapopulation biology: ecology, genetics and evolution. Academic Press, London
Hara TJ (1994) The diversity of chemical stimulation in fish olfaction and gestation. Rev Fish Biol Fish 4:1–35
Holles S, Simpson SD, Radford AN, Berten L, Lecchini D (2013) Boat noise disrupts orientation behavior in a coral reef fish. Mar Ecol Prog Ser 485:295–300
Hughes TP, Baird AH, Bellwood DR, Card M, Connolly SR (2003) Climate change, human impacts, and the resilience of coral reefs. Science 301:929–933
Huijbers CM, Nagelkerken I, Lossbroek PC, Schulten IE, Seigenthaler A, Holderied MW, Simpson SD (2012) A test of the senses: fish select novel habitats by responding to multiple cues. Ecology 93:46–55
Igulu M, Nagelkerken MI, Fraaije R, van Hintum R, Ligtenberg H, Mgaya YD (2011) The potential role of visual cues for microhabitat selection during the early life phase of a coral reef fish (Lutjanus fulviflamma). J Exp Mar Biol Ecol 401:118–125
Jeffs A, Montgomery JC, Tindle C (2005) How do spiny lobster postlarvae find the coast? N Z J Mar Freshw Res 39:605–617
Jones GP, McCormick MI, Srinivasan M, Eagle JV (2004) Coral decline threatens fish biodiversity in marine reserves. Proc Natl Acad Sci 101:8251–8253
Kingsford MJ, Leis JM, Shanks A, Lindeman K, Morgan S, Pineda J (2002) Sensory environments, larval abilities and local self-recruitment. Bull Mar Sci 70:309–340
Lecchini D, Nakamura Y (2013) Use of chemical cues by coral reef animal larvae for habitat selection. Aquat Biol 19:231–238
Lecchini D, Tsuchiya M (2008) Spatial structure of coral reef fish communities at Kudaka Island (Ryukyu Archipelago—Japan). Ichthyol Res 55:321–327
Lecchini D, Pratchett M, Adjeroud M, Cadoret L, Galzin R (2003) Spatial distribution of coral reef fish communities of Ryukyu Islands (Japan). Oceanol Acta 26:537–547
Lecchini D, Shima J, Banaigs B, Galzin R (2005) Larval sensory abilities and mechanisms of habitat selection of a coral reef fish during settlement. Oecologia 143:326–334
Lecchini D, Mills SC, Brié C, Maurin R, Banaigs B (2010) Ecological determinants and sensory mechanisms in habitat selection of crustacean larvae. Behav Ecol 21:599–607
Lecchini D, Carassou L, Frédérich B, Nakamura Y, Mills S, Galzin R (2012) Effects of alternate reef states on coral reef fish habitat associations. Environ Biol Fishes 94:421–429
Lecchini D, Waqalevu VP, Parmentier E, Radford CA, Banaigs B (2013) Fish larvae prefer coral above algal water cues: implications of coral reef degradation. Mar Ecol Prog Ser 475:303–307
Leis JM, McCormick MI (2002) The biology, behaviour, and ecology of the pelagic, larval stage of coral reef fishes. In: Sale PF (ed) Coral reef fishes: dynamics and diversity in a complex ecosystem. Academic Press, San Diego, pp 171–199
Leis JM, Siebeck U, Dixson DL (2011) How nemo finds home: the neuroecology of dispersal and of population connectivity in larvae of marine fishes. Integr Comp Biol 51:826–843
Mann DA, Casper BM, Boyle KS, Tricas TC (2007) On the attraction of larval fishes to reef sound. Mar Ecol Prog Ser 338:307–310
Montgomery JC, Jeffs A, Simpson SD, Meekan M, Tindle C (2006) Sound as an orientation cue for the pelagic larvae of reef fishes and decapod crustaceans. Adv Mar Biol 51:143–196
Moore P, Crimaldi J (2004) Odor landscapes and animal behavior: tracking odor plumes in different physical worlds. J Mar Syst 49:55–64
Munday PL, Dixson DL, Donelson JM, Jones GP, Pratchett MS, Devitsin GV, Døving KB (2009) Ocean acidification impairs olfactory discrimination and homing ability of a marine fish. Proc Natl Acad Sci 21:1–8
Myrberg AA, Fuiman LA (2002) The sensory world of coral reef fishes. In: Sale PF (ed) Coral reef fishes: dynamics and diversity in a complex ecosystem. Academic Press, San Diego, pp 123–148
Nakamura Y, Shibuno T, Lecchini D, Watanabe Y (2009a) Habitat selection by emperor fish larvae. Aquat Biol 6:61–65
Nakamura Y, Shibuno T, Lecchini D, Kawamura T, Watanabe Y (2009b) Spatial variability in habitat associations of pre- and post-settlement stages of coral reef fishes at Ishigaki Island, Japan. Mar Biol 156:2413–2419
Painter SD, Clough B, Garden RW, Sweedler JV, Nagle GT (1998) Characterization of Aplysia attractin, the first water-borne peptide pheromone in invertebrates. Biol Bull 194:120–131
Radford CA, Tindle CT, Montgomery JC, Jeffs AG (2011) Modeling a reef as an extended sound source increases the predicted range at which reef noise may be heard by fish larvae. Mar Ecol Prog Ser 438:167–174
Sano M (2000) Stability of reef fish assemblages: responses to coral recovery after catastrophic predation by Acanthaster planci. Mar Ecol Prog Ser 198:121–130
Shibuno T, Hashimoto K, Abe O, Takada Y (1999) Short-term changes in the structure of a fish community following coral bleaching at Ishigaki Island, Japan. Galaxia JCRS 1:51–58
Simpson SD, Meekan MG, Larsen NJ, McCauley RD, Jeffs A (2010) Behavioral plasticity in larval reef fish: orientation is influenced by recent acoustic experiences. Behav Ecol 117:1098–1105
Sola C, Spampanato A, Tosi L (1993) Behavioural responses of glass eels (Anguilla anguilla) towards amino acids. J Fish Biol 42:683–691
Stanley JA, Radford CA, Jeffs AG (2012) Location, location, location: finding a suitable home among the noise. Proc Biol Sci 279:3622–3631
Suzuki G, Kai S, Yamashita H (2012) Mass standing of crown-of-thorns starfish. Coral Reefs 31:821
Tolimieri N, Haine O, Jeffs A, McCauley RD, Montgomery JC (2004) Directional orientation of pomacentrid larvae to ambient reef sound. Coral Reefs 23:184–191
Wilkinson C (2004) Status of coral reefs of the world. Australian Institute of Marine Science, Townsville, Australia
Wilson SK, Graham NJ, Pratchett MS, Jones GP, Polunin NVC (2006) Multiple disturbances and the global degradation of coral reefs: are reef fishes at risk or resilient? Glob Change Biol 12:2220–2234
Wright KJ, Higgs DM, Belanger AJ, Leis JM (2005) Auditory and olfactory abilities of larvae and juveniles of a coral reef damselfish. Mar Biol 147:1425–1434
Wright KJ, Higgs DM, Belanger AJ, Leis JM (2008) Auditory and olfactory abilities of larvae of coral trout at settlement. J Fish Biol 72:2543–2556
Wright KJ, Higgs DM, Leis JM (2010) Auditory sensitivity in settlement stage larvae of coral reef fishes. Coral Reefs 29:235–243
Zimmer RK, Zimmer CA (2008) Dynamic scaling in chemical ecology. J Chem Ecol 34:822–836
Zimmer-Faust RK, Case JF (1983) A proposal dual role of odor in foraging by the California spiny lobster, Panulirus interruptus (Randall). Biol Bull 164:341–353
Acknowledgments
We are grateful to Y. Akita, K. Inoue, K. Kishimoto, M. Sasaki, and the Okinawa Prefectural Fisheries Research and Extension Center for assistance with the fieldwork and laboratory experiments. This study was supported by grants from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (No. 21780178) and the Japan Science and Technology Agency/Japan International Cooperation Agency, Science and Technology Research Partnership (SATREPS).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. A. Peck.
Rights and permissions
About this article
Cite this article
Lecchini, D., Miura, T., Lecellier, G. et al. Transmission distance of chemical cues from coral habitats: implications for marine larval settlement in context of reef degradation. Mar Biol 161, 1677–1686 (2014). https://doi.org/10.1007/s00227-014-2451-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-014-2451-5