Abstract
The present study aimed to investigate the spatial structure of fish communities at juvenile and adult stages on coral reefs at Kudaka Island (Ryukyu Archipelago, Japan) and to relate spatial patterns in the structure of the fish communities to gradients in environmental variables. Diurnal visual censuses allowed us to record 2,602 juveniles belonging to 60 species and 1,543 adults belonging to 53 species from October to December 2005. The distribution of species highlighted that the juvenile community was organised into three distinct assemblages, rather than exhibiting gradual change in community structure along the cross-reef gradient. Correlations between spatial patterns of juvenile community and environmental variables revealed that the most significant factors explaining variation in community structure were coral rubble and coral slab. In contrast, the adult community was organised into one assemblage, and the most significant variation factors in community structure were depth, live coral in massive form, live coral in branched form, dead coral and sand. Overall, the present study showed that most juvenile and adult coral reef fish at Kudaka Island exhibited striking patterns in their distribution and depth and some biological factors (e.g., abundance of live coral, dead coral and coral rubble) might exert considerable influence on the distribution of fishes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A major goal in ecology and conservation biology is to explain spatial patterns in the distribution of organisms (Caley et al. 1996). In coral reef ecosystems, the settlement of fish and invertebrates to benthic habitats at the end of the larval phase is a critical period in the life cycle of demersal species (for review, see Leis and McCormick 2002). Mortality is high during and immediately after the settlement event (Doherty et al. 2005; Lecchini et al. 2006), and there is strong selection pressure to choose a microhabitat that promotes survival and growth (Jones 1987; Booth 1995). Coral reef fish and invertebrates often show marked selectivity in the habitats they choose based on the presence of specific benthic substrata or the presence of conspecifics or other species (e.g., Öhman et al. 1998; Qian 1999; Lecchini 2005). The literature suggests that initial choices at settlement often determine the patterns of abundance among habitats for the species (for review, see Doherty 2002). These initial distribution patterns established at settlement will be less important, however, when fishes and invertebrates exhibit movement after settlement or are subject to differential mortality related to habitat type, which will modify the spatial distribution patterns of juveniles (e.g., McCormick and Makey 1997; Dahlgren and Eggleston 2000; Lecchini and Galzin 2005). Thus, potential factors that influence fish distributions at juvenile and adult stages are many and varied (for review, see Caley et al. 1996). These factors can be broadly categorised as biological (e.g., settlement, competition, predation), physical (e.g., temperature, salinity) or historical (disturbance events).
The present study aimed to investigate the spatial structure of fish communities at juvenile and adult stage on coral reefs at Kudaka Island (Ryukyu Archipelago, Japan) and to relate spatial patterns in the structure of the fish communities to gradients in environmental variables. Specifically, we addressed the following two questions: (1) Are coral reef fish juveniles and adults distributed uniformly or randomly or do they exhibit striking patterns in their distribution and abundance? (2) What are the physical and/or biological factors explaining the spatial distribution of the juveniles and adults?
Materials and methods
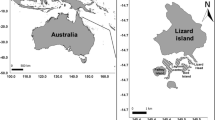
Study area. The present study was conducted on the east coast of Kudaka Island (Ryukyu Archipelago, southern Japan; Fig. 1). This location was divided into six distinct reef zones (Fig. 1) based on depth and substratum composition (cover percentage of sand, coral rubble, coral slab, macro-algae, dead coral with algal turf, live coral in branched form and live coral in massive form; Fig. 2). The cover percentage of different substrate categories was measured on three replicate 25-m belt quadrats within each reef zone by Line Intersect Transect Method (one record every 0.5 m). The percentage cover was analysed along with the depth variable using the non-parametric Kruskal-Wallis test to assess that they can be considered as distinct reef zones (Kruskal-Wallis test, df = 5, P = 0.04).
a Map of Ryukyu Archipelago, Japan, showing the location of Kudaka Island. b Schematic view of the position of the three transects sampled on the east coast of Kudaka Island. c Schematic view of cross-reef profile showing the relative position of reef zones (Rz1–Rz6) along the transects. Rz1 coral slab with small cracks, Rz2 coral slab with high macro-algae cover (>55%), Rz3 mid-flat reef with small coral colony (<1 m large), Rz4 deep-flat reef with rubble and live coral in massive form (depth = 2 m), Rz5 mid-flat reef with large and live coral colony in branched form (>1 m large), Rz6 front flat reef with high algal cover
Data sampling. Diurnal underwater visual counting of fish was conducted along three transects (bands perpendicular to the coast and continuous from the coast to the reef crest), 200 m long and 25 m wide (Fig. 1). Fish were identified to species (all species were recorded except Gobiidae and Blenniidae) and ontogenetic stage (recently settled juvenile vs. adult—distinction based on colour pattern, behaviour and size). For each transect, the abundance of fish was recorded on three replicate 25 × 2 m belt quadrats (orientated parallel to the coast) within each reef zone. Fishes were censused during two passes over each belt quadrat. On the first pass, the diver swam quite quickly (>5 m min−1) to record 'transient' fishes, which swam through the quadrat, but consistently fled at the diver's approach. On the second pass, the diver swam more slowly (<1 m min−1) to record more sedentary species. Fish were counted in October, November and December 2005 (counting centred on new moon with a rhythm of one transect per day), when many reef fish species’ recruits appeared on the shallow reef flats at Kudaka Island.
Statistical analysis. Distribution of fish species was analysed by using correspondence analysis (CA; length of gradient from the initial detrended CA screening step was over 3; Benzecri 1973), which compared the relative abundance of different fish species among reef zones of each transect. To assess the influence of environmental variables on the distribution of fish species, a multivariate analysis of correlations between fish communities and the seven biological (i.e., seven substratum categories) and two physical variables (i.e., water depth and distance from the shoreline of each reef zone) was conducted using Canonical Correspondence Analysis (CCA, ter Braak 1986). The nine environmental variables were selected using a permutation test. During this stepwise procedure (analogous to backward elimination in multiple regression), various combinations of the nine variables were tested to determine which combination best explained observed variation in the structure of fish communities. After the best combination was selected, the CCA was run to determine the degree to which the model would best explain the structure of coral reef fish communities. The robustness of the final analysis was then determined using the Monte Carlo permutation test (ter Braak 1986).
Results
Spatial pattern in juvenile assemblages. A total of 2,602 juveniles belonging to 60 species were recorded in the Kudaka sector, corresponding to a density of 1.03 juveniles m−2 (Fig. 3). Mean densities of fishes varied almost eight-fold among reef zones, ranging from 0.24 (±0.03 SE) fishes m−2 at reef zone 1 to 1.90 (±0.31 SE) fishes m−2 at reef zone 5 (Fig. 3). The distribution of species from a CA highlighted that the juvenile community was organised into three distinct assemblages rather than exhibiting a gradual change in community structure along the cross-reef gradient (Fig. 4). The first juvenile assemblage (coordinates superior at 0.5 on axis 1) was characterised by species mainly present on reef zones 1 and 2 of the three transects (e.g., Chrysiptera leucopoma, Halichoeres margaritaceus, Halichoeres trimaculatus, Rhinecanthus aculeatus). The second assemblage (coordinates inferior at 0.5 on axis 1 and inferior at 0.5 on axis 2) was characterised by species present in high abundance on reef zones 3, 4, 5 and 6 of transects B and C (e.g., Abudefduf sexfasciatus, Coris aygula, Stegastes nigricans, Ctenochaetus striatus). The third assemblage (coordinates superior at 0.5 on axis 2) was characterised by species present in high abundance on reef zones 3, 4 and 5 of transects A (e.g., Chaetodon ornatissimus, Chromis viridis, Epinephelus merra, Naso lituratus). No fish was counted on reef zone 6 of transect A. At the family level, the variation in density of Acanthuridae, Chaetodontidae, Labridae and Pomacentridae relative to “assemblages” identified in CA showed that the majority of juvenile fishes recorded at Kudaka were from the families Pomacentridae and Labridae, with a dominance of Labridae on assemblage 1 and Pomacentridae on assemblages 2 and 3 (Fig. 5).
Correspondence analysis (CA) showing spatial structures of juvenile community. Plots of the CA show ordination along the first two axes (the inertia of each axis is given). Stations are coded by the abbreviation of reef zones (Rz1–6) and by the letter of transect (A, B or C). Species are coded by the first two letters of name of genus and species: Abudefduf vaigiensis, Abudefduf sexfasciatus, Abudefduf sordidus, Acanthurus lineatus, Acanthurus triostegus, Amphiprion clarkia, Apogon angustatus, Balistapus undulatus, Chaetodon auriga, Cheilinus chlorourus, Chaetodon citrinellus, Chrysiptera leucopoma, Chaetodon lunula, Chaetodon ornatissimus, Chaetodon pelewensis, Chaetodon vagabundus, Chromis viridis, Cheilodipterus quinquelineatus, Coris aygula, Coris gaimard, Ctenochaetus striatus (Ct st1), Ctenochaetus strigosus (Ct st2), Dascyllus aruanus, Epinephelus merra, Gomphosus varius, Halichoeres hortulanus, Halichoeres margaritaceus (Ha ma1), Halichoeres marginatus (Ha ma2), Halichoeres trimaculatus, Heniochus chrysostomus, Labroides dimidiatus, Naso brevirostris, Naso lituratus, Naso unicornis, Neoniphon opercularis, Ostracion meleagris, Parupeneus barberinus, Paracirrhites forsteri, Parapercis clathrata, Pomacanthus imperator, Pomacentrus coelensis, Pomacentrus pavo, Pomacentrus lepidogenys, Pseudocheilinus hexataenia, Pterois volitans, Rhinecanthus aculeatus, Saurida gracilis, Sargocenton microstoma, Scarus psittacus, Siganus spinus, Stegastes albifasciatus, Stegastes nigricans, Stethojulis bandanensis, Synodus binotatus, Thalassoma amblycephalum, Thalassoma hardwicke, Thalassoma lutescens, Zanclus cornutus, Zebrasoma veliferum, Zebrasoma scopas
Correlations between spatial patterns of juvenile community and environmental variables revealed that the most significant factors explaining variation in community structure were coral rubble and coral slab. These two variables explained 29.7% of variance in the species matrix for juvenile community (Table 1). The addition of other environmental variables did not increase the proportion of variation that could be explained by these two variables. A high cover percentage of coral slab would explain the distinction of the first juveniles assemblage (reef zones 1 and 2 of the three transects). A high cover percentage of coral rubble would explain the distinction of the third juveniles assemblage (reef zones 3, 4 and 5 of transects A).
Spatial pattern in adult assemblages. A total of 1,543 adults belonging to 53 species were recorded in the Kudaka sector, corresponding to a density of 0.66 adults m−2 (Fig. 3). Mean densities of fishes varied almost 40-fold among reef zones, ranging from 0.03 (±0.02 SE) fishes m−2 at reef zone 1 to 1.55 (±0.15 SE) fishes m−2 at reef zone 4 (Fig. 3). The distribution of species from a CA (i.e., 39 species analysed—only selected fish species recorded at both juvenile and adult stages) revealed no apparent separation between reef zones or transects (Fig. 6). Correlations between spatial patterns of adult community and environmental variables revealed that the most significant factors were depth, live coral in massive form, live coral in branched form, dead coral and sand. These five variables explained 27.2% of variance in the species matrix for the adult community (Table 2). At the family level, the variation in density of fish families relative to “assemblages” identified in CA (on juveniles data) showed that the majority of adult fishes were from the family Pomacentridae (Fig. 5).
Correspondence analysis showing spatial structures of adult community (see Fig. 4)
Discussion
The present study sought to explain the spatial structure of coral reef fish communities in the Ryukyu Archipelago, southern Japan. The community of fish juveniles was organised into three distinct assemblages, rather than exhibiting gradual change in community structure along the cross-reef gradient. The adult community was organised into one assemblage. Spatial variation in fish communities was then compared to changes in biological and physical factors.
Canonical Correspondence Analysis showed that the structure of the juvenile community varied in relation to the abundance of coral rubble and coral slab. These micro-habitats are generally utilized by transient fishes (e.g., Halichoeres margaritaceus, Halichoeres trimaculatus, Siganus spinus, Stethojulis bandanensis, Rhinecanthus aculeatus; Fig. 4), perhaps because they are the most suitable to their size and their behaviour. In contrast, reef zones 3, 4 and 5, characterized by a high cover percentage of dead and live coral, are most suitable to the presence of resident fishes (Pomacentridae spp.; Figs. 4, 5). These differences in habitat use according to the behavior and size of recently settled juveniles could be due to the high mortality during and immediately after the settlement event (60% in the first day of settlement and 90% after three post-settlement months; Doherty et al. 2005). At adult stage, the structure of the fish community varied in relation to depth, live coral in massive form, live coral in branched form, dead coral and sand. These five significant factors for the adult community are totally different from those of the juvenile community (i.e., coral rubble and coral slab). Such differences frequently are explained as mechanisms to reduce intra- and inter-species competition between ontogenetic stages (for review, see Caley et al. 1996). Indeed, one of the forms of resource partitioning between juveniles and adults that occurs commonly is segregation in the use of micro-habitat. Thus, the difference in significant factors explaining spatial variation of fish communities would be a result of the selection of micro-habitat by recently settled juveniles and adults under different ecological settings to balance the needs to minimize the predation risk while meeting forage uptake demands (for review, see Gillanders et al. 2003). However, the significant factors highlighted in CCA accounted for only a relatively small portion of spatial variation in the structure of fish communities (29.7% for juveniles and 27.2% for adults). The remaining “unexplained” portion of the variation is attributable to pure spatial variation (the amount of spatial structuring in the species matrix) and variation due to environmental factors not measured in this study. Other factors could thus influence where a juvenile settles and where the adults fish live, such as morphological/functional components of the species, components involving the food they can exploit and ecological factors (e.g., facilitation, intra/interspecific competition and predation).
Overall, while understanding where species occur is a fundamental ecological requirement, prediction of occurrence is essential for much conservation and population management. The present study provides important baseline data that will enable subsequent investigation of temporal changes in fish communities, as coral reefs in the Ryukyu Archipelago are subject to considerable pressure from anthropogenic impacts and natural disturbances (Sano 2001). It would be important, therefore, to conduct a follow-up study to assess whether there have been any further changes in coral reef fish communities of the Ryukyu Islands, indicative of ongoing impacts on these reef systems.
References
Benzecri JP (1973) L’analyse des correspondances. Dunod, Paris
Booth DJ (1995) Juvenile groups in a coral-reef damselfish: density dependent effects on individual fitness and population demography. Ecology 76:91–106
Caley MJ, Carr MH, Hixon MA, Hughes TP, Jones GP, Menge BA (1996) Recruitment and the local dynamics of open marine populations. Annu Rev Ecol Syst 27:477–500
Dahlgren CP, Eggleston DB (2000) Ecological processes underlying ontogenetic habitat shifts in a coral reef fish. Ecology 81:2227–2240
Doherty PJ (2002) Variable replenishment and the dynamics of reef fish populations. In: Sale PF (ed) Coral reef fishes: dynamics in a complex ecosystem. Academic press, San Diego, pp 327–355
Doherty PJ, Dufour V, Galzin R, Hixon MA, Meekan MG, Planes S (2005) High mortality during settlement is a population bottleneck for a tropical surgeonfish. Ecology 85:2422–2428
Gillanders BM, Able KW, Brown JA, Eggleston DB, Sheridan PF (2003) Evidence of connectivity between juvenile and adult habitats for mobile marine fauna: an important component of nurseries. Mar Ecol Prog Ser 247:281–295
Jones GP (1987) Some interactions between residents and recruits in two coral reef fishes. J Exp Mar Biol Ecol 114:169–182
Lecchini D (2005) Spatial and behavioural patterns of reef habitat settlement by fish larvae. Mar Ecol Prog Ser 301:247–252
Lecchini D, Galzin R (2005) Spatial repartition and ontogenetic shifts in habitat use by coral reef fishes (Moorea, French Polynesia). Mar Biol 147:47–58
Lecchini D, Nakamura Y, Grignon J, Tsuchiya M (2006) Evidence of density-independent mortality in a settling coral reef damselfish. Ichthyol Res 53:298–300
Leis JM, McCormick MI (2002) The biology, behavior and ecology of the pelagic, larval stage of coral reef fishes. In: Sale PF (ed) Coral reef fishes: dynamics in a complex ecosystem. Academic press, San Diego, pp 171–199
McCormick MI, Makey LJ (1997) Post-settlement transition in coral reef fishes: overlooked complexity in niche shifts. Mar Ecol Prog Ser 153:247–257
Öhman MC, Munday PL, Jones GP, Caley MJ (1998) Settlement strategies and distribution patterns of coral-reef fishes. J Exp Mar Biol Ecol 225:219–238
Qian PY (1999) Larval settlement of polychaetes. Hydrobiologia 402:239–253
Sano M (2001) Short-term responses of fishes to macroalgal overgrowth on coral rubble on a degraded reef at Iriomote Island, Japan. Bull Mar Sci 68:543–556
ter Braak CJF (1986) Canonical correspondence analysis: a new eigenvector technique for multivariate direct gradient analysis. Ecology 67:1167–1179
Acknowledgments
This research was supported by the French Ministry of Foreign Affairs (Lavoisier Fellowship) and the Japanese Government (Centre Of Excellence Fellowship of University of the Ryukyus). The authors wish to thank J.P. Lecchini, F. Ravary and J. Le Breton for their assistance in the field.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Lecchini, D., Tsuchiya, M. Spatial structure of coral reef fish communities at Kudaka Island (Ryukyu Archipelago), Japan. Ichthyol Res 55, 321–327 (2008). https://doi.org/10.1007/s10228-008-0039-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10228-008-0039-0