Abstract
Marine organisms are under threat globally from a suite of anthropogenic sources, but the current emphasis on global climate change has deflected the focus from local impacts. While the effect of increased sedimentation on the settlement of coral species is well studied, little is known about the impact on larval fish. Here, the effect of a laterite “red soil” sediment pollutant on settlement behaviour and post-settlement performance of reef fish was tested. In aquarium tests that isolated sensory cues, we found significant olfaction-based avoidance behaviour and disruption of visual cue use in settlement-stage larval fish at 50 mg L−1, a concentration regularly exceeded in situ during rain events. In situ light trap catches showed lower abundance and species richness in the presence of red soil, but were not significantly different due to high variance in the data. Prolonged exposure to red soil produced altered olfactory cue responses, whereby fish in red soil made a likely maladaptive choice for dead coral compared to controls where fish chose live coral. Other significant effects of prolonged exposure included decreased feeding rates and body condition. These effects on fish larvae reared over 5 days occurred in the presence of a minor drop in pH and may be due to the chemical influence of the sediment. Our results show that sediment pollution of coral reefs may have more complex effects on the ability of larval fish to successfully locate suitable habitat than previously thought, as well as impacting on their post-settlement performance and, ultimately, recruitment success.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coral-reef ecosystems, which comprise some of the world’s most species-rich environments, are facing threats to their survival on many fronts (Hughes et al. 2010). Apart from rising sea levels, surface temperatures, acidification and fishing pressure, coral reefs are also highly sensitive to the effects of anthropogenic land-based pollution (Bégin et al. 2014). Impacts of sediment pollution are already realised in some regions (Torres and Morelock 2002), but these effects are likely to worsen, as some 75 % of the world’s coral reefs are currently nearby human settlements and because human populations in nearly all countries with coral reefs are expected to double within the next 50–100 years (Mora et al. 2011). Sedimentation is one of the biggest localized sources of reef degradation because elevated amounts of sediment on coral reefs, generated by increased land development run-off and dredging projects, have resulted in reduced coral cover, diversity, health and productivity (Erftemeijer et al. 2012; Fabricius 2005). Sediment pollution also directly impacts on the fitness of coral reef inhabitants (Wong et al. 2013), though the indirect mechanisms leading to shifts in density and species richness of fish on sediment-impacted reefs are still being determined (Edinger and Risk 2013).
For coral reef fish populations to persist, their larvae first need to identify suitable settlement habitat and to then establish in this habitat as juveniles and adults. Coral-reef fish larvae have well-developed sensory systems capable of detecting habitat-relevant cues to allow orientation towards suitable settlement habitat, as well as the swimming capability to influence the direction of travel in ocean currents (Kingsford et al. 2002). Visual and olfactory senses play important roles in orienting the swimming direction of larval fish towards suitable habitat (Lecchini et al. 2014a, b). Therefore, sediment pollution likely influences coral reef fish recruitment by impacting the capacity of these fish to detect these cues (Siebeck et al. 2015). Suspended sediment can disrupt the ability of larval fish to select appropriate habitat, though our understanding of which cues are affected and the potential consequences for recruitment success is limited (Wenger et al. 2011).
Even if coral reef fish larvae can locate and settle on suitable habitat, sediment pollution may threaten the viability of reef fish populations through impacts on their post-settlement fitness. The growth and behavioural development of fish larvae during settlement not only determines their recruitment success (Shima and Findlay 2002), but also the ability of juvenile fish to compete for resources (i.e. food, shelter and living space) within coral reef environments (Thorrold and Milicich 1990). Larval growth may be disrupted by increased sediment levels (Wenger et al. 2014). Even in cases where larval development is not inhibited, the presence of suspended sediment may still increase predator-induced mortality of post-settlement individuals by disrupting sensory cues. Hence, sensory modalities are of primary importance for survival and therefore ecological fitness at both pre- and post-settlement stages (Wenger et al. 2013).
Here, we investigated the effects of red soil pollution on the settlement behaviour of coral reef fish larvae and the impacts of exposure to red soil pollution on post-settlement behaviour and performance. The term “red soil” refers to a laterite soil common to Okinawa, Japan, that inundates coastal areas with high levels of silt and turbidity during run-off events. These run-off events coincide with seasons of heavy rainfall, often three times that of other areas in Japan, from June to July and September to October (Higashi et al. 1985), corresponding with the seasonal recruitment of larval fish during May–September (Nanami and Nishihira 2002). Red soil pollution is regarded as having dramatically increased along with the land development of coastal sites in Okinawa. Although there are no quantitative data on pollution levels before and after development, surveys show that 97 % of respondees from local government and business believe it emerged after the intensive implementation of road and agriculture projects from the early 1970s (Okinawa Prefecture 1993). Although this run-off has been reduced since the enforcement of the Okinawa Prefecture Red Soil Erosion Ordinance in 1995, there is evidence that it is still a major contributor to the degradation of Okinawan coral reef communities, disrupting the settlement and growth of coral species, but the effect on larval fish remains largely unknown (Omori 2011).
Here we tested the hypothesis that red soil pollution affects the settlement dynamics of larval fish by applying red soil treatments in situ to light traps that caught larvae as they approached the reef to settle, specifically investigating the patterns of species abundance and richness. We then tested the effect of red soil on sensory cue use for habitat location and selection in the laboratory by observing the behavioural responses of newly caught settlement-stage larvae to visual and olfactory habitat cues in the presence and absence of red soil. To investigate the effects of prolonged exposure to red soil on post-settlement performance, we reared wild-caught fish larvae under different red soil concentrations and then applied similar choice experiments on sensory cue response, as well as monitoring feeding behaviour and body condition. This is the first study to combine in situ and ex situ techniques to investigate multifaceted effects of sediment pollution on early-stage fish from habitat selection to post-settlement fitness.
Materials and methods
Field experiments
Establishing the coral species important for settlement
To decide which coral species to use in settlement behaviour trials of C. viridis, in situ settlement preference was determined using underwater visual census. First, roaming surveys 20 min in length were conducted daily over a period of 5 days on reef habitat adjacent to the light trap positions at Sesoko Island, an area of Okinawa regarded as unimpacted by red soil pollution (Arakaki et al. 2005). The abundance of settlement stage individuals (~10 mm in length) encountered and the coral species they were associating with were recorded. Surveys were conducted within 2 h of first light, with surveys extending from the intertidal zone to a depth of 4 m, encompassing all subtidal habitats within the site. Surveys were conducted across a ~16,000 m2 patch of reef with water visibility >10 m and swell <0.5 m. Second, to determine whether C. viridis associated with particular species of corals by chance, the cover of reef by each coral species was quantified within the site. The proportion of reef covered by each coral species was quantified along ten haphazardly positioned 30-m transects laid perpendicular from shore ~20 m apart, recording coral species found directly under the transect tape at 50-cm intervals. Coral identification was conducted post hoc from photographs taken at each interval. The most common coral that C. viridis associated with was determined by comparing the frequencies of recruits on each coral species observed during surveys. To test if association of C. viridis recruits with a given coral species occurred by chance, a chi-squared test adapted for low values (P values evaluated by Monte Carlo simulation, n = 1,000) was used to compare the proportion of C. viridis that associated with a coral species to the proportion of reef it covered.
In situ choice experiment
To test the in situ settlement dynamics of larval fish in the presence of red soil, four light traps were deployed (for a more detailed description of these light traps, see Nakamura et al. 2009) adjacent to fringing coral reefs surrounding the Sesoko Station (Tropical Biosphere Research Center, University of the Ryukyus) on the south-east side of Sesoko Jima, Okinawa (see Fig. S1A in the Electronic supplementary material, ESM). Traps were set at one of four locations spaced at 50-m intervals and randomly assigned one of four treatments upon each deployment: (1) control, (2) red soil added, (3) live coral added and (4) live coral and red soil added. Red soil was added by the attachment of three slow-release 250-mL bottles filled with red soil suspended in sea water to the top, bottom and inner chamber of the light trap. The flow of red soil from the bottles was adjusted such that although water movement dispersed the red soil as it was released, there would be suspended red soil present around the trap from the time of deployment until collection (~12 h). Live coral was added with a Porites cylindrica coral head ~20 cm in diameter attached in a mesh bag suspended within the light trap (see Fig. S2A in the ESM). Slow-release bottles filled with clean sand and empty mesh bags were attached to treatments without red soil and/or live coral to account for potential visual bias on the trap. Total abundance and species richness from each trap catch was recorded for a period of 11 consecutive days (1–11 July 2013). A one-way ANOVA was used to test for differences in mean abundance and species richness between treatments. Chi-squared tests were used on rank–frequency data from the light trap catches to investigate bias in light-trap location, treatment type, and temporal effects. These data were then put into a general linear model (GLM) using the Poisson distribution (usually adapted for abundance data) to compare the effect of each treatment on larval catch patterns. Only fish caught in the control light traps were used in subsequent experiments to avoid prior conditioning to coral or red soil.
Settlement-stage larval sensory experiments
Effect of sediment extract on olfactory cue use
A two-channel Perspex choice flume of a similar design to that of Gerlach et al. (2007) was used to test preferences between olfactory cues in water sources with and without the presence of red soil. Each trial began with a larva being placed at the centre of the downstream end of the chamber to explore the chamber and acclimate to the water sources for a period of 2 min. Fish that did not swim actively or explore both sides of the chamber during these 2 min were discarded from the trials (<5 % of fish tested were discarded). After the acclimation period, the position of the fish was recorded every 5 s for another 2 min period as being on one side of the chamber or the other. Water sources entering the chamber from buckets gravity-feeding into the left and right side were then switched, with 1 min being allocated for the water sources to exchange and flush completely, in order to control for a side preference by individuals. After switching water sources, another 2 min acclimation period was given, followed by another 2 min observation period. In this way, it is possible to tease apart choice for chemical properties of the water sources and side preference of the apparatus. Each individual was tested only once, after which they were released back to the capture site and the chamber was rinsed thoroughly with freshwater. The flow rate was maintained at 200 mL min−1 and dye tests were conducted after each replicate to ensure a laminar flow on each side of the chamber without eddies or mixing of the two water sources.
Previous studies on olfactory preferences of C. viridis demonstrate, as with other coral reef species, a preference for chemical cues released from coral over blank seawater (Lecchini et al. 2014a). In this experiment, treatments for each fish consisted of an initial control testing for positional bias in the apparatus, where incoming water on both sides of the chamber was from the same source (i.e. coral-soaked water vs. coral-soaked water), followed by the experimental treatment testing preferences between water from two different sources, resulting in two comparable data sets for each fish. To test the effect of red soil on behavioural response to habitat cues, C. viridis larvae were given a choice between coral-soaked water and the same coral-soaked water with red soil added in two different concentrations. Twenty fish larvae were used per treatment. Coral-soaked water was produced in a 150-L tank containing approximately 6 kg of live P. cylindrica coral heads. After the flow-through system had been shut off for at least 2 h, water was transferred to two 60 L tanks. Depending on the treatment being tested, 50 or 200 mg L−1 of red soil were mixed into one of the tanks. The two water sources were then left to sit for a further 2–4 h to allow time for the water containing the suspended red soil to equilibrate and become “clear” in order to minimise the effect of turbidity, creating a red soil “extract”. Olfactory preference data was analysed using Wilcoxon’s signed rank test, a nonparametric test suited to the time proportion data and accounting for the repeated measures of the same individual.
Effect of suspended sediment on visual cue use
Larvae were tested in a three-compartment test chamber (60 × 12 × 10 cm). The side compartments were formed by placing two transparent plexiglass panels separated by 1 cm to create barriers 8 cm from each end, resulting in a central compartment 32 cm in length which was delimited into three equal parts (see Fig. S3A in the ESM). This experimental system isolated visual cues available to the individual placed in the central compartment from chemical cues present in the side compartments. Thus, only visual cues from coral colonies influenced larval movement in the central compartment.
Before each trial, one live and one dead coral colony were placed in the side compartments behind the plexiglass barriers. A single larva was introduced into the middle of the central compartment for a 1 min acclimation period during which an opaque screen was placed between the plexiglass barriers to block visual cues, after which the screens were removed and the trial commenced. The position of the larva in the central compartment was recorded every 5 s for a period of 1 min. This short test period also ensured that sediment added to the test chamber remained in suspension. The aquarium was emptied and washed with freshwater after each trial. To exclude a possible side bias of the fish, the order of adjacent compartments containing each coral type was randomised for each trial. Moreover, water samples were taken and the temperature, pH, salinity and turbidity were measured to ensure there was no biasing effect in these parameters.
Three treatments were applied as follows (n = 20 per treatment):
-
1.
Control—habitat compartments (at each end): live or dead coral, larval chamber (centre): no red soil added
-
2.
Low—50 mg L−1 red soil suspended in larval chamber
-
3.
High—200 mg L−1 red soil suspended in larval chamber
Each individual was only tested once. Visual preference data were analysed using Wilcoxon’s rank sum test, a nonparametric alternative to the two-sample t test.
Effect of prolonged exposure to sediment on sensory responses
Olfactory response
To test the olfactory responses of settlement-stage larvae after being acclimated to different pollution levels, C. viridis larvae caught in light traps were placed into aerated non-flow-through 10 L holding tanks and reared for 5 days, during which time they were fed twice daily ad libitum with newly hatched Artemia salina. Three different treatments were applied to the rearing tanks: (1) control (no red soil added), (2) 50 mg L−1 red soil mixed into solution and (3) 200 mg L−1 red soil mixed into solution. After the initial mixing, red soil was not resuspended during the rearing period and allowed to settle in the tank. The time during which the red soil stayed in suspension varied with concentration, but after 2 h the sediment had settled in all rearing tanks. Ten fish larvae from each treatment were then selected for olfactory choice testing using the same methods previously described, but a test between olfactory cues from water soaked with live coral and dead coral (coral rubble collected from the just below the intertidal zone of the reef area sampled in this study, rinsed thoroughly to remove any sediment or algae present) was implemented.
Visual response
Individuals from the same treatments as used for the olfactory experiments above were also tested in the visual cue test chamber with the same protocol as the newly caught larvae. Fifteen fish per rearing treatment were tested in clear water with live and dead coral colonies in the adjacent compartments at each end of the test chamber without red soil added. Instead of comparing red soil concentrations in the chamber, preferences of individuals swimming in clear water but from different rearing concentrations of red soil were analysed.
Red soil acclimation effects on post-settlement performance
Feeding rates and condition
To test the effect of red soil on the post-settlement performance of C. viridis recruits, aquaria trials were used to compare feeding rates and physiological condition. Following capture, ten individual larvae were randomly selected and allocated to one of sixteen 15-L plastic tanks—eight containing 50 mg L−1 of red soil and eight containing untreated seawater. Larvae were maintained for 7 days, during which time they were fed live A. salina ad libitum twice daily. Following this rearing period, fish were filmed using HD video from above tanks for 1 min following the addition of a standardised quantity of pellet food. Feeding rates (bites per minute) of all individuals within groups were counted whilst replaying this video footage. The wet weight (WW) and standard length (SL) of each fish was measured at the end of the conditioning period, allowing the calculation of Fulton’s condition (K) factor; K = 100(WW/S 3L ). Tanks were lit naturally, aerated and contained a small piece of coral for shelter. Water was changed daily, ensuring concentrations of red soil were consistent. Sediment was suspended by stirring tanks twice daily (controls were stirred to the same extent). Sediment settled out of suspension after approximately 2 h, with feeding trials conducted randomly across all treatments between 30 min and 1 h after the tanks were stirred. Feeding rates and body condition (Fulton’s) were compared between red soil and no red soil treatments (fixed) and groups (random; nested within red soil treatment) using a two-factor nested ANOVA (individual fish being the residual).
Post-experiment red soil pH analysis
Following the analysis of the data on the sensory responses of fish reared for a prolonged period under different sediment treatments, further measurements of the effect on pH levels were required. Measurements were done on site with a 713 pH meter (Metrohm Japan Ltd.) using six replicates in 1 L bottles for each of the three treatment concentrations of red soil employed for the rearing tanks (namely 0, 50 and 200 mg L−1). As with the rearing trials, sediment was initially mixed and not disturbed for the remainder of the experimental period. Measurements of pH were taken at 0 h (turbid water) and 2 h (clear water after sediment had settled to the bottom), and then at 3 and 7 days after the initial mixing.
Results
Field experiments
C. viridis coral association
Field surveys showed C. viridis associated with the branching coral P. cylindrica significantly more frequently than expected by chance; 119 of 184 individuals (64.7 %) observed were associated with P. cylindrica, which composed 4 % of the live hard corals (χ 2 = 1160, df = 10, P < 0.001). This preliminary survey justified the use of P. cylindrica as a habitat cue in settlement cue experiments.
In situ larval abundance and species richness trends
Forty-seven species of larval fish were caught in the light traps during the field experiment. No significant experimental bias of light trap position (χ 2 = 16.8, df = 3, P > 0.05) or time of deployment (global χ 2 = 0.6894) was detected. Species abundance and richness between light trap treatments were not significantly different (F = 0.36, df = 3,36, P = 0.78 and F = 1.57, df = 3,36 P = 0.21, respectively) due to high levels of variance in the data (Welch’s test for homogeneity of variance in abundance, P = 0.49) and species richness (P = 0.24). Though light trap catches were highly variable, a similar trend for both species richness and abundance was apparent between treatments (see Fig. S4A in the ESM). Total abundance was highest in treatments without red soil, with the live coral treatment attracting the most larvae (32 %), followed by control (empty light trap) (26 %), red soil (24 %) and live coral with red soil (18 %). Light traps containing live coral only also attracted the highest species richness (30 %), followed by control (28 %), red soil and live coral with red soil (21 % each).
One of the four light trap positions showed an effect of treatment type on the number of larvae caught that approached significance (χ 2 = 18.6, df = NA, P = 0.07) and a significant difference in species richness (χ 2 = 37.9, df = NA, P = 0.01). Comparing catches at this location highlighted a significant difference in larval abundance among traps that contained live coral and those that did not (χ 2 = 10, df = NA, P = 0.01). General linear modelling indicated that this difference was driven by an increasing trend in catch by light trap treatment, predicting increasing catch abundance with treatments as follows: control + red soil < live coral + red soil < control < live coral, a similar trend seen to that for the nonsignificant mean abundance and species richness among treatments (see Table S1A in the ESM). Comparing the rank frequencies of larval abundance at each of the four light trap positions for each day with chi-squared tests showed no difference in three of the four light traps (χ 2 = 6.2, df = NA, P = 0.69), but the southerly light trap position showed a significantly higher catch over time irrespective of which treatment was being applied (χ 2 = 10.8, df = NA, P = 0.02). It should be noted that this light trap was excluded from the GLM due to its potential bias.
Settlement-stage larvae sensory experiments
Larvae avoid habitat chemical cues with sediment extract present
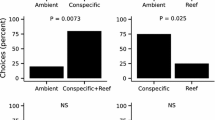
No bias with respect to the chamber or experimental conditions was detected in C. viridis larvae, and no side preference was shown when the same habitat cue was presented on both sides of the chamber. Larvae spent approximately the same time on each side (mean ± SE percentage of time spent in the left flow: 52 ± 0.63 %; in the right flow: 48 ± 0.63 %), justifying this comparison as a control treatment to test for experimental bias. Larvae responded to the red soil infused treatment water by spending significantly more time in the live coral soaked water (>70 %), strongly avoiding the water infused with 50 mg L−1 of red soil (Z = −3.643, P < 0.001). This effect increased when the concentration of red soil was increased to 200 mg L−1, with >74 % of the time (Fig. 1) spent in the live coral water (Z = −3.642, P < 0.001). Turbidity measurements did show a slight increase in turbidity with red soil concentration from live coral water to 50 and 200 mg L−1 (0.12, 0.15 and 0.19 NTU/FTU, respectively), but this difference was regarded as nominal.
Visual cue use disrupted by suspended sediment
Larvae presented with visual cues of both live coral and dead coral spent significantly more time swimming near the live coral than the dead coral (Wilcoxon’s rank sum test, Z = 3.0, P = 0.002), spending twice as much time exploring the compartment closest to the live coral chamber than the compartment near the dead coral (56 % vs. 26 %, respectively). When 50 mg L−1 of red soil solution were added to the central compartment with the larvae, this ability to visually discriminate between the live and dead coral chambers disappeared (see Fig. 2), with no significant difference seen in the time spent at each end of the choice chamber (Wilcoxon’s test, Z = 0.05, P = 0.96). Time spent exploring the chamber dropped dramatically when 200 mg L−1 of red soil solution were added to the chamber: larvae were in the central compartment 75 % of the time, as opposed to only 28 % under the 50 mg L−1 conditions. Again, approximately the same time was spent exploring compartments close to both live and dead coral (Wilcoxon’s test, Z = 0.05, P = 0.957).
Effect of prolonged exposure to sediment on sensory responses
Choice behaviour in response to reversed or eliminated olfactory cues
After 5 days of being reared in test aquaria, fish under red soil conditions displayed significantly different behaviour from those raised in “clean” control water (see Fig. 3). Individuals reared under control conditions strongly preferred live coral cues over dead coral cues (Wilcoxon’s rank sum; Z = 2.81, P = 0.01), spending over 70 % of the time in the flow containing live coral cues. By comparison, the fish reared in water containing 50 mg L−1 shifted their preference, displaying significant choice towards dead coral cues over live coral cues (Wilcoxon’s rank sum; Z = 2.20, P = 0.03), with 63 % of their time spent in the flow containing dead coral cues. After being reared in 200 mg L−1 conditions, there was no significant choice between the two cues (Wilcoxon’s rank sum; Z = −0.05, P = 0.96).
Undetectable choice behaviour in response to visual cues
Larvae reared under control conditions spent the same amount of time near the live coral compartment as near the dead coral compartment (31 % each). The responses of larvae reared with 50 mg L−1 of sediment were similar, with 32 and 29 % of the time spent near the live and dead coral compartments, respectively (Fig. 4). Under 200 mg L−1 rearing conditions, this dynamic changed somewhat, with the largest proportion of the time spent near the dead coral compartment (40 %). This change in choice behaviour is similar to what occurred in the olfaction trials, though the difference was not statistically significant (Wilcoxon’s test, Z = 0.86, P = 0.39). Furthermore, position distributions were not significantly different between the rearing treatments for time spent close to both live coral (K–S test, P = 0.99) and dead coral (K–S test, P = 0.93).
Red soil acclimation effects on post-settlement performance
Decreased feeding rates and body condition
Feeding rates and condition were significantly impacted by the presence of red soil (see Fig. 5). Feeding rates were significantly slower for red soil conditioned fish than control fish (F 1,144 = 7.12; P = 0.04), whereas, within the sediment-treated tanks, there were significant differences among groups (F 1,144 = 3.65; P = 0.02). Condition was also significantly poorer in red soil conditioned fish than control fish (F 1,99 = 5.52; P = 0.04), though it did not significantly differ amongst groups within the sediment-treated tanks (F 1,99 = 0.15; P = 0.12). There was no difference in mortality between the control and red soil treated fish (25 and 20 individuals from 80, respectively) in the sediment-treated tanks (t 14 = 0.90; P = 0.38) (see Fig. 5).
Post-experimental pH analysis
Average pH level declined with increasing sediment concentration. Immediately after mixing, the mean pH values of the control samples and the 50 and 200 mg L−1 treated samples were 8.1, 8.09 and 8.08, respectively. Measurements were similar at 2 h after the sediment had settled out of suspension. However, while the mean pH of the control water remained stable over time, the water treated with red soil became more acidic over time, reaching 8.08 and 8.04 after 7 days for the 50 and 200 mg L−1 treatments, respectively (see Table S2A in the ESM).
Discussion
Larval fish behaviour is likely to influence their dispersal and orientation to a suitable settlement habitat (Cowen et al. 2006), yet the impacts of sediment pollution on this process are largely unknown. We showed that in the presence of sediment pollution, such as that caused by red soil, tropical reef fish larvae could be inhibited from locating suitable coral habitats during settlement. Specifically, we found that in the presence of red soil, C. viridis larvae failed to distinguish suitable coral habitat visual cues and avoided coral habitat olfactory cues. This may lead to decreased chances for recruitment in areas prone to recurrent sediment pollution, such as Okinawa, Japan, compared to more pristine areas. Previous studies have reported avoidance behaviour in settlement-stage fish of either degraded (by algal phase shift: Lecchini et al. 2013) or dying (Feary et al. 2007) coral reef habitat, and our results suggest that sensory cue use may be an important factor. Evidence for chemically mediated avoidance behaviour in situ has been shown using cues from degraded seaweed-dominated reef habitats, suggesting that olfactory cues from sediment-degraded reefs could affect recruitment densities and reef resilience (Dixson et al. 2014).
Evidence of negative impacts of sediment pollution on the settlement behaviour of settlement-stage reef fish larvae has already been found (Wenger and McCormick 2013), yet the field relevance of these results was previously untested. Here, we provide the initial evidence that the presence of red soil near a coral habitat, even in small amounts, may reduce settlement success in the field. Untreated light traps containing live coral with red soil absent caught a higher abundance of fish with a higher species richness than light traps with coral in the presence of red soil. The statistical significance of this result may have been affected by a positional bias of the light traps, with the southerly light trap position (the direction from which the prevailing current flows) catching more larvae than the other three positions. The high levels of variation in catch abundance seen in our results is typical of reliable light trap sampling (Leis et al. 2002). The nonsignificant trend of a negative response of settling fish to red soil treatments was congruent with results obtained in the laboratory experiments.
Results showing either an avoidance of olfactory cues from a habitat with red soil present or a disruption of the ability or inclination to utilise visual habitat cues in settlement-stage coral reef fish larvae indicate that such pollution of coral reef environments may impact larval recruitment and survival. A reluctance or inability to approach potential habitats can extend the period spent by fish larvae in close proximity to the reef running the “predation gauntlet”, increasing the risk of predation (Almany and Webster 2006). Even prolonged exposure to nonpredatory fish can be detrimental, with harassment of recruiting fish larvae by territorial residents observed to result in elevated predation risk or avoidance of the habitat altogether (Leis and Yerman 2012). In addition to this, previous studies have shown an effect of sediment pollution on larval development whereby the PLD is increased (Wenger et al. 2014). This effect could alter the timing and location of larval settlement, changing the natural distribution and recruitment of fish larvae through the modifications of their movement towards habitat, which could affect the spatial and community demography of fish populations.
Even if fish larvae manage to settle on a coral reef habitat degraded by sediment pollution, our results show that prolonged exposure to the presence of sediment could impact post-settlement behaviour and survival. Five days of exposure to water containing red soil significantly altered the response to olfactory cues from different habitat types, reversing the strong preference for live coral cues under control conditions to a preference for those from dead corals. At a higher rearing concentration of red soil, a response to olfactory cues was completely absent. As the habitat cues presented to individuals here after the rearing period contained no influence of sediment, it is assumed that the change in behaviour was due to physiological effects of exposure on the sense organ, rather than impairment of transmission of the cues themselves. The response to visual cues in individuals exposed for 5 days to sediment differed from the response of newly caught larvae, as they no longer showed significant choice behaviour towards live coral over dead coral. This reduced exploration of the visual cue chamber may be an artefact of rearing in captivity or an ontogenetic change in behaviour, perhaps due to a reduced inclination of juveniles to use visual habitat cues to orient movement as compared to settling larvae (Lecchini et al. 2007).
A reversal of normal behavioural responses to olfactory cues has been shown in response to increased water acidification, as a normal avoidance of predator cues became a strong attraction in fish larvae reared in water with higher acidity (Dixson et al. 2010). Furthermore, when the acidity of the rearing conditions was increased further, the fish larvae no longer responded to olfactory cues (Munday et al. 2009), similar to the trend observed in our results. The mechanism suggested for this is an alteration caused by the increased acidity of anion gradients across neuronal membranes, which reverses normal receptor function (Nilsson et al. 2012). The changes in behaviour seen in the reared post-settlement fish may be a similar response due to the acidity of red soil. Behavioural avoidance of acid sulfate soils has been shown in juveniles of various fishes when pH levels were well within the range exhibited in natural systems (Kroon 2005). Red soil in Okinawa is acidic, with a pH of ~5 (Mkadam et al. 2006), and analysis of the rearing conditions used here showed that the pH decreased with both increasing concentration of red soil and time. Reductions in pH of a similar magnitude (0.07) were shown to elicit similar behavioural changes in juvenile coral trout (Plectropomus leopardus) (Munday et al. 2013). This acidic soil could influence the pH of the water surrounding the reef on which it deposits, as has been shown with organic-rich soils (Weber et al. 2012).
Previous studies on the effect of red soil on water chemistry showed that as the red soil concentration in a solution increased, the availability of H+ ions (increasing acidity) and heavy metals, particularly Al3+, also increased (Kombo et al. 2005). Exposure to elevated concentrations of heavy metals can also lead to changes in the behavioural responses of fish to olfactory cues (Scott and Sloman 2004). Increased Al3+ concentrations can exacerbate the damage done to the olfactory epithelium by increased acidity alone, as they work synergistically to disrupt olfactory abilities in salmonid fish (Klaprat et al. 1988). Our results suggest that the chemical properties of sediment pollution may have a greater impact on coral reef environments than turbidity alone. Of course, it is not only the use of habitat cues that can affect the ecology of early-stage fish. Chemical pollution of the aquatic environment may also disrupt communication, social recognition and shoaling in fish, which can have drastic effects on population fitness (Fisher et al. 2006; Ward et al. 2008).
Exposure to sediment pollution negatively impacted the condition and performance of C. viridis. Both feeding rate and condition reduced with increasing concentration of red soil. The sensory disruption detected in other individuals of the same cohort during this experiment as well as other physiological effects from prolonged exposure to sediment pollution may have played a role in the reduced foraging success. As evidence suggests that growth is a critical variable in the recruitment success and survival of post-settlement fish, this is another aspect in which sediment pollution can impact affected fish populations (Bergenius et al. 2002). Our results concur with data on juvenile reef fish reared under similar concentrations of suspended sediment (0–180 mg L−1), where reduced foraging behaviour led to reduced growth, condition and survival (Wenger et al. 2012). This effect has also been shown to reduce foraging success in situ when prey detection may be limited by absolute light levels rather then contrast (Fiksen et al. 2002).
In conclusion, this experiment used a variety of techniques and life history stages to investigate the effect of sediment pollution on coral reef fish larvae around the crucial settlement period. At the settlement stage, we found evidence of disruption of the natural use of sensory cues employed by fish larvae to locate suitable habitat in the presence of increased sediment levels. Following settlement (after 5 days of captive rearing), metamorphosing post-settlement fish exhibited reduced feeding behaviour and condition under the sediment treatments. Response to habitat-relevant sensory cues became confused, reversing the preference from live coral to dead coral in some cases in individuals which had experienced prolonged exposure to sediment concentrations. These results are similar to those reported for elevated levels of CO2, which can also simultaneously affect a range of fish behaviours (Jutfelt et al. 2013). These include avoidance behaviour, reversal of behavioural responses to ecologically relevant sensory cues, reduced growth and reduced feeding rates (Baumann et al. 2012; Briffa et al. 2012; Cripps et al. 2011; Dixson et al. 2010; Munday et al. 2009). This suggests that the interaction of red soil with the surrounding water can cause chemically altered environments that are detrimental to fish survival.
In any case, these behavioural changes can be biomarkers for significant biological effects of sediment pollution in marine environments (Galloway et al. 2004). If the impacts on sensory cue use in recruiting larval fish influence the recruitment success of coral reef fish populations, this can have implications for both fisheries and marine park management, as import and export of larvae between populations and habitats may be constrained by degraded habitat (Dixson et al. 2014). This is the first study assessing the effects of red soil pollution on the settlement behaviour of larval fish, and further work is needed to assess how these impacts are influencing recruitment on sediment-polluted reefs.
The concentrations of sediment used in this experiment are regularly encountered in coral reef ecosystems across the world; for example, on the Great Barrier Reef (GBR), Australia, sediment concentrations are regularly recorded as being near to the lower level used here (50 mg L−1), and can exceed the higher level (200 mg L−1) during the wet season or as a result of dredging (Bak 1978; Wenger and McCormick 2013). This indicates that negative impacts on the settlement behaviour and success of reef fish larvae may be exacerbated if the sediment load influx to coral reef environments increases due to anthropogenic activities. The close proximity of acidic sediment similar to Okinawa’s red soil to coral reef environments (e.g. over 600,000 ha of acid sulfate soil resides within the GBR (Queensland) catchment alone: Powell and Martens 2005), means that chemical effects of sediment are likely to have substantial impacts on coral reefs and their inhabitants. Future work is required to better understand these effects and how they interplay with effects of sediment-induced turbidity.
Author contribution statement
YN, DL, DB, JO, HB and GC conceived and designed the experiments. DL, JO, HB and GW performed the experiments. GL, JO, DL, HB and GC analyzed the data. JO wrote the manuscript; other authors provided editorial advice.
References
Almany GR, Webster MS (2006) The predation gauntlet: early post-settlement mortality in reef fishes. Coral Reefs 25:19–22. doi:10.1007/s00338-005-0044-y
Arakaki T et al (2005) Simultaneous measurement of hydrogen peroxide and Fe species (Fe(II) and Fe(tot)) in Okinawa Island Seawater: impacts of red soil pollution. J Oceanogr 61:561–568
Bak RP (1978) Lethal and sublethal effects of dredging on reef corals. Mar Pollut Bull 9:14–16. doi:10.1016/0025-326X(78)90275-8
Baumann H, Talmage SC, Gobler CJ (2012) Reduced early life growth and survival in a fish in direct response to increased carbon dioxide. Nat Clim Change 2:38–41. doi:10.1038/nclimate1291
Bégin C, Brooks G, Larson RA, Dragićević S, Ramos Scharrón CE, Côté IM (2014) Increased sediment loads over coral reefs in Saint Lucia in relation to land use change in contributing watersheds. Ocean Coast Manag 95:35–45. doi:10.1016/j.ocecoaman.2014.03.018
Bergenius MA, Meekan MG, Robertson RD, McCormick MI (2002) Larval growth predicts the recruitment success of a coral reef fish. Oecologia 131:521–525. doi:10.1007/s00442-002-0918-4
Briffa M, de la Haye K, Munday PL (2012) High CO2 and marine animal behaviour: potential mechanisms and ecological consequences. Mar Pollut Bull 64:1519–1528. doi:10.1016/j.marpolbul.2012.05.032
Cowen R, Paris C, Srinivasan A (2006) Scaling of connectivity in marine populations. Science 311:522–527
Cripps IL, Munday PL, McCormick MI (2011) Ocean acidification affects prey detection by a predatory reef fish. PLoS ONE 6:e22736. doi:10.1371/journal.pone.0022736
Dixson DL, Munday PL, Jones GP (2010) Ocean acidification disrupts the innate ability of fish to detect predator olfactory cues. Ecol Lett 13:68–75. doi:10.1111/j.1461-0248.2009.01400.x
Dixson DL, Abrego D, Hay ME (2014) Chemically mediated behavior of recruiting corals and fishes: a tipping point that may limit reef recovery. Science 345:892–897. doi:10.1126/science.1255057
Edinger EN, Risk MJ (2013) Effect of land-based pollution on central Java coral reefs. Coast Dev 3:593–613
Erftemeijer PL, Riegl B, Hoeksema BW, Todd PA (2012) Environmental impacts of dredging and other sediment disturbances on corals: a review. Mar Pollut Bull 64:1737–1765. doi:10.1016/j.marpolbul.2012.05.008
Fabricius KE (2005) Effects of terrestrial runoff on the ecology of corals and coral reefs: review and synthesis. Mar Pollut Bull 50:125–146
Feary DA, Almany GR, McCormick MI, Jones GP (2007) Habitat choice, recruitment and the response of coral reef fishes to coral degradation. Oecologia 153:727–737. doi:10.1007/s00442-007-0773-4
Fiksen Ø, Aksnes DL, Flyum MH, Giske J (2002) The influence of turbidity on growth and survival of fish larvae: a numerical analysis. Sustainable increase of marine harvesting: fundamental mechanisms and new concepts. Springer, Berlin, pp 49–59
Fisher HS, Wong BB, Rosenthal GG (2006) Alteration of the chemical environment disrupts communication in a freshwater fish. Proc R Soc B 273:1187–1193. doi:10.1098/rspb.2005.3406
Galloway TS et al (2004) Ecosystem management bioindicators: the ECOMAN project—a multi-biomarker approach to ecosystem management. Mar Environ Res 58:233–237. doi:10.1016/j.marenvres.2004.03.064
Gerlach G, Atema J, Kingsford MJ, Black KP, Miller-Sims V (2007) Smelling home can prevent dispersal of reef fish larvae. Proc Natl Acad Sci USA 104:858–863. doi:10.1073/pnas.0606777104
Higashi T, Katayama TC, Shinagawa A (1985) Land development works and soil erosion in Okinawa Prefecture. Mem Kagoshima Univ Res Center S Pac 6:26–36
Hughes TP, Graham NA, Jackson JB, Mumby PJ, Steneck RS (2010) Rising to the challenge of sustaining coral reef resilience. Trends Ecol Evol 25:633–642. doi:10.1016/j.tree.2010.07.011
Jutfelt F, de Souza KB, Vuylsteke A, Sturve J (2013) Behavioural disturbances in a temperate fish exposed to sustained high-CO2 levels. PLoS ONE 8:e65825. doi:10.1371/journal.pone.0065825
Kingsford MJ, Leis JM, Shanks A, Lindeman KC, Morgan SG, Pineda J (2002) Sensory environments, larval abilities and local self-recruitment. Bull Mar Sci 70:309–340
Klaprat DA, Brown SB, Hara TJ (1988) The effect of low pH and aluminum on the olfactory organ or rainbow trout Salmo gairdneri. Environ Biol Fishes 22:69–78. doi:10.1007/BF00000544
Kombo MM, Vuai SA, Ishiki M, Tokuyama A (2005) Influence of salinity on pH and aluminum concentration on the interaction of acidic red soil with seawater. J Oceanogr 61:591–601. doi:10.1007/s10872-005-0067-6
Kroon FJ (2005) Behavioural avoidance of acidified water by juveniles of four commercial fish and prawn species with migratory life stages. Mar Ecol Prog Ser 285:193–204
Lecchini D, Osenberg CW, Shima JS, St Mary CM, Galzin G (2007) Ontogenetic changes in habitat selection during settlement in a coral reef fish: ecological determinants and sensory mechanisms. Coral Reefs 26:423–432. doi:10.1007/s00338-007-0212-3
Lecchini D, Waqalevu VP, Parmentier E, Radford CA, Banaigs B (2013) Fish larvae prefer coral over algal water cues: implications of coral reef degradation. Mar Ecol Prog Ser 475:303–307
Lecchini D, Miura T, Lecellier G, Banaigs B, Nakamura Y (2014a) Transmission distance of chemical cues from coral habitats: implications for marine larval settlement in context of reef degradation. Mar Biol 161:1677–1686
Lecchini D, Peyrusse K, Lanyon RG, Lecellier G (2014b) Importance of visual cues of conspecifics and predators during the habitat selection of coral reef fish larvae. C R Biol 337:345–351. doi:10.1016/j.crvi.2014.03.007
Leis JM, Yerman MN (2012) Behavior of larval butterflyfishes (Teleostei, Chaetodontidae) at settlement on coral reefs. Copeia 2012:212–222. doi:10.1643/CE-10-185
Leis JM, Carson-Ewart BM, Cato DH (2002) Sound detection in situ by the larvae of a coral-reef damselfish (Pomacentridae). Mar Ecol Prog Ser 232:259–268
Mkadam KM, Yonaha T, Ali VS, Tokuyama A (2006) Dissolved aluminum and silica release on the interaction of Okinawan subtropical red soil and seawater at different salinities: experimental and field observations. Geochem J 40:333–343. doi:10.2343/geochemj.40.33
Mora C et al (2011) Global human footprint on the linkage between biodiversity and ecosystem functioning in reef fishes. PLoS Biol 9:e1000606. doi:10.1371/journal.pbio.1000606
Munday PL et al (2009) Ocean acidification impairs olfactory discrimination and homing ability of a marine fish. Proc Natl Acad Sci USA 106:1848–1852. doi:10.1073/pnas.0809996106
Munday PL et al (2013) Elevated CO2 affects the behavior of an ecologically and economically important coral reef fish. Mar Biol 160:2137–2144. doi:10.1007/s00227-012-2111-6
Nakamura Y, Shibuno T, Lecchini D, Kawamura T, Watanabe Y (2009) Spatial variability in habitat associations of pre- and post-settlement stages of coral reef fishes at Ishigaki Island, Japan. Mar Biol 156:2413–2419. doi:10.1007/s00227-009-1257-3
Nanami A, Nishihira M (2002) The structures and dynamics of fish communities in an Okinawan coral reef: effects of coral-based habitat structures at sites with rocky and sandy sea bottoms. Environ Biol Fishes 63:353–372
Nilsson GE et al (2012) Near-future carbon dioxide levels alter fish behaviour by interfering with neurotransmitter function. Nat Clim Change 2:201–204. doi:10.1038/nclimate1352
Okinawa Prefecture (1993) Survey report on the state of red soil pollution and its damage. Department of Health and Environment Okinawa, Naha, p 204
Omori M (2011) Degradation and restoration of coral reefs: experience in Okinawa, Japan. Mar Biol Res 7:3–12. doi:10.1080/17451001003642317
Powell B, Martens M (2005) A review of acid sulfate soil impacts, actions and policies that impact on water quality in Great Barrier Reef catchments, including a case study on remediation at East Trinity. Mar Pollut Bull 51:149–164. doi:10.1016/j.marpolbul.2004.10.047
Scott GR, Sloman KA (2004) The effects of environmental pollutants on complex fish behaviour: integrating behavioural and physiological indicators of toxicity. Aquat Toxicol 68:369–392. doi:10.1016/j.aquatox.2004.03.016
Shima JS, Findlay AM (2002) Pelagic larval growth rate impacts benthic settlement and survival of a temperate reef fish. Mar Ecol Prog Ser 235:303–309
Siebeck UE, O’Connor J, Braun C, Leis JM (2015) Do human activities influence survival and orientation abilities of larval fishes in the ocean? Integr Zool 10:65–82. doi:10.1111/1749-4877.12096
Thorrold SR, Milicich MJ (1990) Comparison of larval duration and pre- and post-settlement growth in two species of damselfish, Chromis atripectoralis and Pomacentrus coelestis (Pisces: Pomacentridae) from the Great Barrier Reef. Mar Biol 105:375–384. doi:10.1007/BF01316308
Torres JL, Morelock J (2002) Effect of terrigenous sediment influx on coral cover and linear extension rates of three Caribbean massive coral species. Caribb J Sci 38:222–229
Ward AJ, Duff AJ, Horsfall JS, Currie S (2008) Scents and scents-ability: pollution disrupts chemical social recognition and shoaling in fish. Proc R Soc B 275:101–105. doi:10.1098/rspb.2007.1283
Weber M et al (2012) Mechanisms of damage to corals exposed to sedimentation. Proc Natl Acad Sci USA 109:E1558–E1567. doi:10.1073/pnas.1100715109
Wenger AS, McCormick MI (2013) Determining trigger values of suspended sediment for behavioral changes in a coral reef fish. Mar Pollut Bull 70:73–80. doi:10.1016/j.marpolbul.2013.02.014
Wenger AS, Johansen J, Jones G (2011) Suspended sediment impairs habitat choice and chemosensory discrimination in two coral reef fishes. Coral Reefs 30:879–887. doi:10.1007/s00338-011-0773-z
Wenger AS, Johansen JL, Jones GP (2012) Increasing suspended sediment reduces foraging, growth and condition of a planktivorous damselfish. J Exp Mar Biol Ecol 428:43–48. doi:10.1016/j.jembe.2012.06.004
Wenger A, McCormick M, McLeod I, Jones G (2013) Suspended sediment alters predator–prey interactions between two coral reef fishes. Coral Reefs 32:369–374. doi:10.1007/s00338-012-0991-z
Wenger A, McCormick M, Endo G, McLeod I, Kroon F, Jones G (2014) Suspended sediment prolongs larval development in a coral reef fish. J Exp Biol 217:1122–1128. doi:10.1242/jeb.094409
Wong CK, Pak IAP, Jiang Liu X (2013) Gill damage to juvenile orange-spotted grouper Epinephelus coioides (Hamilton, 1822) following exposure to suspended sediments. Aquac Res 44:1685–1695. doi:10.1111/j.1365-2109.2012.03173.x
Acknowledgments
The authors would like to thank the staff at the Tropical Biosphere Research Centre (University of the Ryukyus) for their logistical support, and the Australian Museum, the Sydney Institute of Marine Science and the University of Technology Sydney for their financial support. We also thank Ryuta Suzuki and Yasuaki Tanaka for assistance with the field and laboratory experiments. This research was carried out in accordance with Japanese law and was supported by the Japan Society for the Promotion of Science (Grant No. 24780188). All applicable institutional and/or national guidelines for the care and use of animals were followed.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Deron E. Burkepile.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
O’Connor, J.J., Lecchini, D., Beck, H.J. et al. Sediment pollution impacts sensory ability and performance of settling coral-reef fish. Oecologia 180, 11–21 (2016). https://doi.org/10.1007/s00442-015-3367-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-015-3367-6