Abstract
External parental care is uncommon among actiniarians but common in Epiactis species. Here, several aspects of reproduction are analyzed for of one of them, Epiactis georgiana. Samples were collected in December, January, February, March, and April in the Antarctic Peninsula and the eastern Weddell Sea, during 1998, 2000, 2002, and 2003. Most sexually mature individuals of E. georgiana are male or female, but some are hermaphrodites. This is the first report of hermaphroditism in E. georgiana, which is the third species of the genus with this sexual pattern. The results suggest that oogenesis starts in December and that at least two generations of oocytes overlap; a third generation is often brooded externally. Putative fertilization is likely internal, and larvae and/or embryos are externally brooded on the distal part of the adult column until an advanced developmental stage. Apparently E. georgiana reproduces seasonally, probably releasing the embryos/larvae in the last months of the austral spring (December). Inter-individual variability was observed in gametogenesis. In addition, specimens from the Antarctic Peninsula were larger than those from the Weddell Sea. This study represents the first step in understanding the reproductive mode of E. georgiana.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The diversity and number of clades of marine benthic invertebrates employing parental care in the Southern Ocean is unusually high, leading to a wide array of hypotheses to explain the phenomenon (reviewed by Pearse et al. 2009). Although the reproduction of many Antarctic invertebrates has been studied in the last two decades (e.g., Arntz et al. 1992; Barthel and Gutt 1992; Gutt et al. 1992; Peck and Robinson 1994; Poulin and Féral 1996; Barnes and Clarke 1998; Chantiore et al. 2002; Strathmann et al. 2006; Kang et al. 2009 among others), anthozoans have received little attention. Only five studies have focused on the group (Brito et al. 1997; Orejas et al. 2001, 2002, 2007; Waller et al. 2008), despite their abundance (Arntz et al. 1994). In the deep sea, a habitat whose benthic invertebrate biology is often compared with that of Antarctica, some studies on anthozoan reproduction are available, most of them on octocorals (reviewed by Watling et al. 2011) and scleractinian corals (e.g., Waller 2005; Waller et al. 2008; Waller and Tyler 2011). The paucity of studies on reproductive biology of sea anemones (Actiniaria) is notable in these habitats: only one study reports details of the reproduction of a sub-Antarctic species (Riemann-Zürneck 1975) and a few studies address deep-sea species (e.g., Van Praët 1990; Bronsdon et al. 1993; Mercier and Hamel 2009).
The highly variable reproductive strategies documented for Cnidaria (Fautin 2002) are especially pronounced in Actiniaria, in which a combination of sexual and asexual mechanisms is favored by their anatomical and physiological simplicity (Stephenson 1928; Uchida and Yamada 1968; Fautin 1991). High inter- and intra-specific variability, and geographical and interannual variation (Edmands 1996) occur, and patterns can be influenced by factors such as light or food availability (Hand and Uhlinger 1992; Lin et al. 2001; Chen et al. 2008).
Both external and internal parental care (brooding) have been described for Actiniaria (e.g., Chia 1976); brooding is especially common in Actiniidae (e.g., Actinia, Epiactis, Aulactinia) and Actinostolidae (e.g., Stomphia, Actinostola) and has been reported for several Antarctic actiniarians (Carlgren 1927; Riemann-Zürneck 1975, 1978; Dunn 1983; Fautin 1984). The externally and internally brooded offspring in actiniarians were assumed to have a sexual origin until genetic studies revealed that at least some brooded juveniles were asexually produced (see Actinia equina: Carter and Funnell 1980; Orr et al. 1982; Actinia tenebrosa: Black and Johnson 1979; Sherman et al. 2007; Sherman and Ayre 2008). However, in Epiactis species, juveniles can be genetically different from the progenitor (Bucklin et al. 1984; Edmands 1995; Edmands and Potts 1997), indicating a sexual origin for brooded offspring. External brooding is an uncommon strategy among actiniarians, being mainly recorded in subtidal austral and boreal species (Stephenson 1928; Fautin et al. 1989). However, in the genus Epiactis, external brooding is the most common reproductive pattern (Edmands 1995, 1996; Edmands and Potts 1997).
Here, we study the reproductive biology of the Antarctic externally brooding sea anemone Epiactis georgiana. This is a medium-size actiniarian (to 56 mm in diameter and 76 mm height), whitish in color, usually with a distinct marginal collar in which offspring are brooded (Fig. 1a). Epiactis georgiana is circumpolar in the Antarctic and sub-Antarctic (Rodríguez et al. 2007) and inhabits soft and hard substrates in a wide bathymetric range (118–1,227 m depth), although it is especially abundant at 400–500 m depth (Dunn 1983). The number of specimens collected during several Antarctic cruises in different seasons over 4 years provided an exceptional opportunity to study the reproductive mode of an Antarctic sea anemone in an ecological context. We identify and describe the pattern of sexuality and the reproductive trends of E. georgiana through the months sampled and explore possible differences between the Antarctic Peninsula and Weddell Sea.
Epiactis georgiana; a living female specimen brooding juveniles (arrows); these have open mouths and developed tentacles, b longitudinal histological section of column margin of a female and attached juvenile (arrow), c detail of defined layer (arrow) between epidermis of parental column and pedal disk of attached juveniles; it probably corresponds to mucus with which larvae and embryos come out. Abbreviations: ep epidermis, ms mesoglea. Scale bars a 40 mm; b 3 mm; c 0.2 mm
Materials and methods
Study areas and sampling
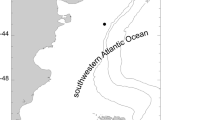
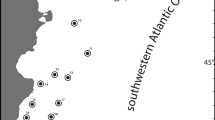
This study was conducted in the Antarctic Peninsula (AP) and the eastern Weddell Sea (WS) (Fig. 2). Sampling was carried out on board RV Polarstern during the EASIZ (Ecology of the Antarctic Sea Ice Zone) cruises II and III (in austral summer 1998 and 2000, respectively), ANDEEP-1 (Antarctic Benthic Deep-sea Biodiversity) cruise (in austral summer 2002), LAMPOS (Latin American “Polarstern” Study) cruise (in austral autumn 2002), and BENDEX (Benthic Disturbance Experiment) cruise (in late spring-summer 2003). For cruise details, see Arntz and Gutt (1999), Arntz and Brey (2001, 2003, 2005), and Fütterer et al. (2003). Specimens of Epiactis georgiana Carlgren, 1927 were sampled using Agassiz and bottom trawls. A total of 105 specimens collected from 36 stations have been analyzed (Table 1). Specimens were relaxed on board using menthol crystals, photographed alive, and subsequently preserved in 10 % sea-water-buffered formalin for histological analysis. The studied material has been deposited in the American Museum of Natural History (AMNH) in New York.
Study area with detail of locations pooled into main areas of study. Dark gray line, subtropical front; light gray line, polar front. Abbreviations: AP Antarctic Peninsula, AUS Austasen, BFS Bransfield Strait, DI Drescher Inlet, DP Drake Passage, KN Kapp Norvegia, EI Elephant Island, S/VK South Vestkapp, WS Weddell Sea
Although in some cases, the distances between sampling stations within the AP and the WS were relatively great, due to the low number of specimens captured at some stations, we pooled specimens from sampling stations into two geographical zones for some of the data analyses: (1) “Antarctic Peninsula area” (AP) includes Drake Passage, Bransfield Strait, and Elephant Island; and (2) “Eastern Weddell Sea area” (WS) includes Kapp Norvergia, South Vestkapp, Drescher Inlet, and Austasen (Fig. 2).
Reproductive study
All specimens were examined to determine sex ratio and maturity classes. Sex ratio was calculated separately for each pooled area (65 specimens from AP, 40 from WS). Pedal disk diameter and column height were measured in preserved specimens; the number of pairs of mesenteries in a specimen was used to determine its maturity. The number of mesenteries is the most reliable way to determine maturity because although it is related to the size of the individual, it is not as variable as body size and might also relate to the sexual state (Dunn 1975a). In sea anemones, the number of pairs and cycles of mesenteries is characteristic at the genus or species level; the development of gametogenic tissue (sexual maturity) is usually associated with the development of a certain number of cycles (or pairs) of mesenteries (Stephenson 1928).
The development of gametogenic tissue was studied in histological sections of 51 specimens (29 from AP and 22 from WS, see Online Resource 1) sampled in December to April in different years. In subsequent discussion, we refer to the large, yolky structures inside females as “oocytes”, but it is possible that these are not meiotically produced eggs, being instead asexual buds, or parthenogenic eggs rather than participants in mictic reproduction. Furthermore, we call those products of the same size and structure as these oocytes produced by hermaphrodites oocytes, and those products similar in size and structure to spermatic cysts produced by hermaphrodites spermatic cysts. Fragments of specimens were dehydrated in butanol (Johansen 1940) and embedded in paraffin. Histological sections of 7–8 μm thick were stained with Ramón y Cajal’s Triple Stain (Gabe 1968). Individual sections were spaced at intervals of at least 200 μm (mean nucleus diameter from test sections), and intervening sections were discarded to avoid measuring the same cell repeatedly. Fifty gametes (when possible) were haphazardly selected from 30 female (16 from AP, 14 from WS) and 18 male (11 from AP, 7 from WS) specimens; however, in the case of oocytes, only those sectioned through the nucleus were measured; when the shape of the oocytes was irregular, the major axis was measured. Because oocytes and spermatic cysts should be equally affected by the decrease in size produced by the histological process (60–75 %, see Dunn 1975a), this shrinkage is factored out in comparisons. Oocytes inside the coelenteron were classified into three maturity classes: previtellogenic oocytes (<300 μm), early vitellogenic oocytes (300–600 μm), and late vitellogenic oocytes (600–1,000 μm). The development of the spermatic cysts was classified according to Wedi and Fautin Dunn (1983) as follows: stage 1 (E1), spermatic cysts only with spermatogonia; stage 2 (E2), spermatic cysts with spermatogonia, spermatocytes, and the first appreciable tailed sperm; stage 3 (E3), spermatic cysts completely mature with sperm dominance (see Online Resource 2). Putative gametes or zygotes free in the gastrovascular cavity of the female and those found in the brooding area were studied with scanning electron microscopy (SEM) to discriminate between large late vitellogenic oocytes and embryos/larvae and to infer the type of fertilization (external/internal). The number of brooded embryos/larvae or juveniles and the number of mesenteries in each of these were counted and studied in histological sections. Pedal disk diameter and column height of brooded juveniles were also measured.
Data analysis
A chi-square test (\( \chi_{2}^{2} \)) was used to test for significant differences in sex ratio between AP and WS. Measurements of pedal disk diameter, column height, and number of mesenteries were compared between sexes (pooled areas) and also between geographical zones (pooled sexes). Maximum relative frequencies of oocyte sizes and spermatic cysts stages (E1, E2, E3) were calculated for the sampled months, years, and geographical zones. Due to the lack of normality or variance homogeneity, the non-parametric tests U-Mann–Whitney (Mann and Whitney 1947) and Kruskal–Wallis (Kruskal and Wish 1978) with a Dunn post hoc test were applied in all cases. All results are presented as means (\( \bar{\rm X} \)) ± standard deviation (SD).
Results
Sexual pattern
The studied populations of Epiactis georgiana consisted of 55 % females and 41.7 % males in AP and 47.5 % females and 50 % males in WS. A small proportion of hermaphrodite individuals (3.3 % in AP, 2.5 % in WS) and some sterile individuals (5 specimens in AP) were also detected (Table 2, Fig. 3a). No significant differences in sex ratio were detected for AP (\( \chi_{2}^{2} \) = 1.103, P = 0.29) or WS (\( \chi_{2}^{2} \) = 0.256, P = 0.87), and thus between zones. The sex ratio did not deviate significantly from expected equal frequencies.
Epiactis georgiana; a detail of gametes in a mesentery of hermaphrodite specimen, b cross section at actinopharynx level showing cycles of mesenteries; numbers indicate pairs of mesenteries of different cycles. Abbreviations: ac actinopharynx, me mesenteries, oo oocyte, sp spermatic cyst. Scale bars a 0.3 mm; b 5 mm
Pedal disk diameter and column height ranged from 13.6 to 57.2 mm and 15.0–75.7 mm in females and males, respectively. We did not find a correlation between gamete and parent size (results not shown). The number of pairs of mesenteries ranged from 24 to 54. We found no significant differences in pedal disk diameter (H 2 = 0.862, P = 0.6498), column height (H 2 = 0.370, P = 0.8311), or number of pairs of mesenteries (H 2 = 0.736, P = 0.6921) between females and males. However, the comparison between AP and WS revealed a significantly higher pedal disk diameter (U = 184, N 1 = 32.4, N 2 = 20, P < 0.005), column height (U = 43.5, N 1 = 37.1, N 2 = 13.9, P < 0.0001), and number of mesenteries (U = 3.500, N 1 = 38.9, N 2 = 12.2, P < 0.0001) (Online Resource 3) in AP specimens.
The examined specimens of Epiactis georgiana had up to five cycles of mesenteries (Table 2); only the first two cycles (and some pairs of the third cycle in several specimens) were perfect (reaching the actinopharynx, see Fig. 3b). All mesenteries (except those in the youngest cycles, present only proximally) were fertile (including the directives) in most individuals. Specimens (except juveniles) were fertile once the third cycle of mesenteries (24 pairs) developed (Fig. 3b). The regularity in the number of pairs of mesenteries and directives indicates that E. georgiana does not reproduce asexually by fission (irregularities in arrangement of mesenteries are often associated with species with asexual reproduction, see Stephenson 1928). In hermaphrodite specimens, gametes of both sexes occurred in the same mesentery without vertical separation (Fig. 3a).
Gametogenesis
Oogenesis
Oogonia (8.0–35 μm in diameter) with a relatively large nucleus (15–17.5 μm in diameter) grew and multiplied in the gastrodermis; afterward, they migrated into the mesoglea, increasing their size (while decreasing the nucleus size) and concentrating yolk grains (small oval bodies) around the germinal vesicle (Fig. 4). Three maturity classes were distinguished: previtellogenic oocytes (<300 μm), each with a large nucleus; early vitellogenic oocytes (300–600 μm), each with large yolk granules; late vitellogenic oocytes (600–1,000 μm), with a prominent nucleolus in the central nucleus (Fig. 4c).
Epiactis georgiana, histological sections of oogenesis; a first stage of development of female gametes: primary and secondary oogonia (8–10 μm and 30–35 μm in diameter, respectively) in gastrodermis with relatively large nucleus, b female cells (oocyte stage) migrated into mesoglea after reaching 30–45 μm diameter, c detail of two different sizes classes of oocyte (pre- and early-vitellogenic oocytes); note trophonema. Abbreviations: ga gastrodermis, ms mesoglea, nu nucleus, og oogonia, oo oocyte, tr trophonema, vi vitelo. Scale bars a, b 30 μm; c 0.25 mm
Spermatogenesis
Male gametes were recognizable when they formed spermatic cysts, each covered by mesoglea. The first developmental stage (E1) was a cyst with 24–28 spermatogonia. Visible spermatocytes (from 5 μm in diameter) in the center of the spermatogonia defined the next developmental stage (E2); at the end of this stage (spermatid), cells (to 2 μm in diameter) occupied the center of the spermatic cyst. In the final stage (E3), spermatozoids (to 1 μm in diameter) were visible in the mature follicles filling the center of the cyst with tails converging at the edge of the mesentery. The different stages of the process are summarized in Online Resource 2.
Although no oocytes or spermatic cysts were observed free in the gastrovascular cavity of Epiactis georgiana, we documented free embryos and/or larvae in the coelenteron of several specimens collected in December (see following section).
Reproductive trends
Based on measurements of 1,420 oocytes from 30 females, oocyte diameter ranged from 30 to 2,100 μm and did not differ significantly (pooling all specimens, sampling months and years, Online Resource 4) between AP and WS (U = 242,415, N 1 = 723.8, N 2 = 696.8, P > 0.05). However, results are presented separately to display the variability within and among months, years, and study areas (Fig. 5, Online Resources 4, 5, 6).
The frequency distributions of oocyte size differed among months (Fig. 5, Online Resources 4, 5). The diameter of oocytes from specimens collected in December (late spring) in WS showed the widest size range, followed by those from February and March in AP and WS. The diameter of oocytes from specimens collected in January (summer) in WS and April (autumn) in both AP and WS had the narrowest range. Samples for December and January were only available for WS. The most common stage in December (late spring) was the small previtellogenic oocyte (100–200 μm in diameter); we also found a small proportion of putative embryos/larvae (>1,000 μm in diameter) in this month (Fig. 5). The most prevalent oocyte size in the only specimen collected in January was ~600 μm, and there were no putative embryos/larvae. In WS, in February and March (summer), the most common oocytes were slightly larger than in December or January; this slight shift to a larger oocyte size continued in April (autumn) (Fig. 5). Although a similar trend was observed at AP in March and April, the distribution of oocyte sizes in February at AP mirrored that in December from WS. We observed high inter-individual variability within and among months and study areas (Online Resource 6); despite this variability, all studied females had previtellogenic and late vitellogenic oocytes, indicating at least two different oocyte cohorts (Figs. 4c, 5, Online Resource 5). However, caution in the interpretation of these results is advisable due to the low number of individuals analyzed for some months.
Based on measurements of 907 spermatic cysts from 18 individuals, spermatic cyst size was 35–380 μm and did not differ significantly in terms of the relative proportions of cyst stages (E1, E2, and E3) in a given month between AP and WS (H 2 = 3.964, P = 0.1378). However, results are presented separately to show the variability within and among months, years, and study areas (Fig. 6, Online Resources 4, 6). The frequency distribution of spermatic cysts in each month was related to the three defined maturity stages. Stage E1 was always the least common, followed by E2, and E3, except in March and April in WS, in which stages E2 and E3 were less common (Fig. 6).
SEM studies allowed the characterization of embryos and/or larvae in different developmental stages inside the coelenterons of two of the 52 females (both collected in December: Fig. 7, Online Resource 7). Furthermore, we found six additional females (not included in the study) externally brooding larvae (3–48 per polyp) in an early developmental stage in December (late spring) in WS; these females were collected in the same collection as seven recruits (free individuals completely developed but sexually immature).
Epiactis georgiana; a histological cross section of planula larva in gastrovascular cavity of female specimen, b embryos in gastrula phase (arrows) externally brooded in distal part of column (longitudinal section), c embryos in advanced developmental stage with cilia forming toward blastopore area (white arrow). Abbreviations: ba brooding area, bl blastopore, te tentacle; Scale bars a 0.15 mm; b 20 mm; c 0.2 mm
Thirteen females (of the total 52 studied) were brooding offspring externally in the distal part of the column (Fig. 1, Online Resource 7). Only mature females brooded. In the brooding females, the epidermis was slightly thickened and more compact in the brooding area than in the rest of the column. We found a total of 19 (13 plus 6 additional ones, see above) female specimens externally brooding. Two of the brooding females were incubating embryos in the column collar in February and March (summer months) (Fig. 7b). One specimen collected in January was brooding juveniles in an early stage of development. The rest of the specimens brooding juveniles in a more developed stage (from 2 to 44 juveniles) were collected in February (Online Resource 7). Attached juveniles had a pedal disk diameter 3.5–13.0 mm and 12–30 pairs of mesenteries (Fig. 1b, Online Resource 7). Although some juveniles had developed the third cycle of mesenteries, none were fertile. Brooded juveniles had cnidae, and glandular cells completely developed in tentacles by the time six pairs of mesenteries had developed.
The number of brooded juveniles differed greatly with time and zone; we found 200 well-developed juveniles in February and three in April (Online Resource 5); no juveniles were detected in December. Juveniles were significantly (U = 248, N 1 = 31, N 2 = 22.8, P = 0.05) more abundant at AP than WS. We found the highest number of brooded juveniles in females with 48 pairs of mesenteries. We did not find any correlation (results not shown) between maternal size and number of juveniles.
Discussion
This is the first documentation of hermaphroditism for Epiactis georgiana, and the third time, this pattern of sexuality has been reported for the genus. Our data suggest that E. georgiana reproduces seasonally; oogenesis probably begins in December (late spring); and presumably fertilization takes place in the gastrovascular cavity of the female. The relatively slow development of oocytes results in the overlap of at least two, and often three, different generations in female specimens (Fig. 8).
Schematic representation of hypothetic reproductive cycle of Epiactis georgiana. A brooding female is represented through seasons of two consecutive years; each pattern (depicted by asterisks, circles, dashes, etc.) represents a different generation. Broken timeline and question marks represent missing data from winter; late spring (December); summer (January, February, and March); autumn (April)
Epiactis georgiana has a mixed sexuality: populations consist of females, males, and a low proportion of hermaphroditic individuals. Hermaphroditic individuals were previously not encountered (Carlgren 1927; Dunn 1983), probably due to their rarity and the difficulty of distinguishing small oocytes and spermatic cysts except in histological sections. A similar pattern with females, males, and a very low proportion of hermaphroditic individuals (but only one hermaphrodite among hundreds of individuals) has been reported for only two actiniarians: Condylactis gigantea (Jennison 1981) and Paracalliactis stephensoni (Van Praët 1990). Like E. fernaldi, E. georgiana is a simultaneous hermaphrodite; however, E. fernaldi broods its offspring internally (Dunn 1975a; Fautin and Chia 1986). The other externally brooding species of the genus, E. prolifera, is gynodioecious (only females and hermaphroditic individuals) (Dunn 1975a, b). Thus, E. georgiana shows a combination of sexual states previously unknown for the genus. Expression of hermaphroditism was not related to individual size in E. georgiana in contrast to E. prolifera, where females become hermaphrodites after reaching a particular size (Dunn 1975a).
Hermaphroditism increases fertilization probability in extreme and variable environments (Ghiselin 1969, 1974; Clark 1978; Bucklin et al. 1984). The extreme and locally variable environmental conditions of AP and WS (e.g., topography, deep-sea currents, iceberg scouring impact, etc.) might explain the advantages of hermaphroditism for Epiactis georgiana. Furthermore, the patchy distribution of Antarctic actiniarians (Rodríguez, unpubl. data) can reduce the probability of cross-fertilization (Levitan 1991; Coma and Lasker 1997). Hermaphroditism is usually, but not always, associated with self-fertilization in sea anemones (Edmands 1995); however, it is always (only one exception, see Bronsdon et al. 1993) associated with vivipary or some kind of brooding in this group (Shick 1991). Self-fertilization allows reproduction in widely distributed individuals (e.g., Ghiselin 1969, 1974; Clark 1978) and favors faster colonization of new areas from a small number of specimens (e.g., zones affected by a recent iceberg scouring event). Although this could be the case in E. georgiana, molecular studies are necessary to confirm the occurrence of self-fertilization and to make inferences about the advantages this trait could provide to this species.
The observed relationship between hermaphroditism and relatively small size for marine invertebrates (Ghiselin 1969; Charnov 1982) has been pointed out for the genus Epiactis (see Shick 1991). However, E. georgiana does not follow this trend: its body size (pedal disk 57.2 mm in diameter) is more similar to the gonochoric species (e.g., in E. lisbethae and E. ritteri, pedal disk diameter is usually 50 mm or larger), rather than to the small bodied, hermaphroditic species (E. prolifera and E. fernaldi, pedal disk diameter usually 20 mm). Despite some studies that relate size and brooding strategy in marine invertebrates (e.g., Charnov 1982; Strathmann and Strathmann 1982; Strathmann et al. 1984), this relationship is still unclear (Poulin and Féral 1996). Nonetheless, the unusual and intermediate nature of external brooding could be linked with the allometry hypothesis, which seeks to explain the inverse relationship between brooding and small sizes in marine invertebrates (Strathmann and Strathmann 1982). We consider external brooding an intermediate reproductive strategy with respect to broadcast spawning and internal brooding: offspring develop in two different environments when externally brooded because part of the development occurs outside the parent, although with some parental protection. Furthermore, brooding offspring outside the adult alleviates space limitation for gamete production while still protecting the offspring; this might diminish the risk of the shift from the internal to external environments, probably the most critical time for offspring survival. The relatively large size of E. georgiana might represent an instance of the proposed trend toward “gigantism” in some marine invertebrates in the Antarctic (e.g., Chapelle and Peck 1999, 2004); however, this is only valid for some taxa (Woods et al. 2009) and requires detailed comparisons of sibling taxa. Although most Antarctic sea anemones tend to be relatively large (Rodríguez pers. obs.), a trend toward gigantism for actiniarians in Antarctica still has to be confirmed.
The differences in size between Epiactis georgiana from AP and WS (larger in the former) do not have a clear explanation based on previous comparative studies between the two zones. No differences in biomass and abundance for macro- and mega-benthos have been found between these two regions; depth, food supply, and seabed features seem to be more influential factors than latitude (Dayton 1990; Piepenburg et al. 2002). Linse et al. (2006) did not find any clear relationship between body size and latitude in gastropods, echinoderms, or bryozoans; nevertheless, they found a correlation with food availability and fecundity in one of the studied gastropods. As Brey and Haine (1992) found for Antarctic bivalves, statistically significant differences in size between specimens of E. georgiana from the two zones could be related to depth: more specimens from AP (particularly in the Drake Passage) were from greater depths (18 individuals came from 1,047 to 1,227 m and 30 from 454 m) than those from WS (maximum sampling depth 920 m). However, studies of gastropods in the North Atlantic showed an inverse relationship between size and depth (Olabarria and Thurston 2003). Observed differences could also just be due to samples coming from different years in each zone.
Epiactis georgiana starts developing oogonia and spermatic cysts once the third cycle of mesenteries (24 pairs of mesenteries) is completely developed, a pattern common for actiniarians (Carlgren 1949). However, maturity does not seem to be related to the number of developed cycles of mesenteries as immature (non-reproductive) brooded juveniles as well as mature (reproductive) adult individuals both showed 24–30 pairs of mesenteries; this suggests a relationship between maturity and size of the organisms. Epiactis prolifera also develops oocytes only once the fourth cycle of mesenteries is completed, with no relationship to season (Dunn 1975a). According to Dunn (1975a), the presence of fertile individuals of E. prolifera year round is due to the continuous presence of different size classes of individuals in the population (there is always some individual completing the development of the fourth cycle of mesenteries and starting oocyte production). Similarly, fertile individuals of E. georgiana were found in all collection months. However, in the case of E. georgiana, this seems due to the overlap of different generations of sexual products—as observed in other Antarctic cnidarians (e.g., gorgonians, Orejas et al. 2002, 2007)—rather than continuous reproduction.
The gradual increase in frequencies of larger oocytes from December (late spring) to April (autumn) suggests that oogenesis starts in the early austral spring (or end of the winter) and that reproduction in Epiactis georgiana occurs seasonally. Several studies of deep-sea actiniarians support this pattern: six of seven studied species reproduce seasonally (Van Praët 1990; Van Praët et al. 1990; Bronsdon et al. 1993; Fautin 1997; Mercier and Hamel 2009). This suggests that a seasonal reproductive cycle may be correlated with maximum phytodetritus abundance, as has already been suggested for deep-sea organisms in the North East Atlantic (Gage and Tyler 1991) and hypothesized for Antarctic ecosystems (food bank theory; Smith et al. 2006; Galley et al. 2008). However, for logistical reasons, samples in this study came from different years and sampling localities (sometimes far apart) with likely local environmental differences among them; in addition, sample size in some months and areas (e.g., January, April, December in AP, etc.) was small. These methodological limitations might affect the interpretation of the data, and reproductive asynchrony among years may occur. Indeed, these limitations could explain the observed difference between the oocyte size frequency distribution in February between AP and WS: the slight delay in the increase in larger oocytes in AP (mirroring data from December in WS) might be due to samples from February coming from different years in each zone.
Our data suggest that Epiactis georgiana probably releases embryos from the previous year in the last months of the austral winter or beginning of spring (December). The frequency distributions of oocyte diameter in the different months suggest that at least two generations of developing sexual products overlap in an adult female because two size classes can be distinguished from December to April despite the high observed inter-individual variability. Thus, up to three different generations (two phases of gametogenesis and brooded embryos on the exterior of the column) may exist in one female; each generation takes about 2 years to develop and be released from the parent (Fig. 8). The two-year reproductive cycle suggested here for E. georgiana is shared with several deep-sea and other actiniarian species with parental care (Dunn 1975a; Van Praët 1990; Van Praët et al. 1990), as well as with other polar invertebrates such as isopods (Luxmoore 1982), caridid decapods (Gorny et al. 1993), and gorgonians (Orejas et al. 2002, 2007).
The slight decrease in the relative frequency of spermatic cysts in stage E3 in March and April in WS suggests their liberation at the end of the austral summer. However, males from AP did not follow this trend. These differences between AP and WS could be due to environmental conditions but also to methodological limitations: only two male samples were available for those months and they came from different years in each zone. The early embryos/larvae inside the coelenterons suggest internal fertilization; however, molecular analyses are necessary to test whether they are of sexual or asexual origin (e.g., parthenogenesis, self-fertilization).
The developmental stage of externally brooded offspring in Epiactis georgiana suggests that embryos and/or larvae are released synchronously in all individuals around December (late austral spring) or a little earlier. This means that offspring are usually in an advanced developmental stage by summer. The approximately simultaneous development of the brooded offspring in the two study areas suggests similar size classes among the offspring. The release of embryos and/or larvae in austral spring is supported by (1) the presence of free embryos and larvae inside the female coelenterons in December, and (2) the intermediate level of development of the externally brooded offspring collected in January.
References
Arntz WE, Brey T (2001) The expedition ANTARKTIS XVII/3 (EASIZ III) of RV “Polarstern” to the eastern Weddell Sea in 2000 (eds). Ber Polar Meeresforsch 402:1–181
Arntz WE, Brey T (2003) The expedition ANTARKTIS XIX/5 (LAMPOS) of the R/V “Polarstern” in 2002 (eds). Ber Polar Meeresforsch 462:1–120
Arntz WE, Brey T (2005) The expedition ANTARKTIS XXI/2 (BENDEX) on RV ‘‘Polarstern’’ in 2003/2004 (eds). Ber Polar Meeresforsch 503:1–149
Arntz WE, Gutt J (1999) The expedition ANTARKTIS XV/3 (EASIZ II) of RV “Polarstern” to the eastern Weddell Sea in 1998 (eds). Ber Polarforsch 301:1–229
Arntz WE, Brey T, Gerdes D, Gorny M, Gutt J, Hain S, Klages M (1992) Patterns of life history and population dynamics of benthic invertebrates under the high Antarctic conditions of the Weddell Sea. In: Colombo G, Ferrari I, Ceccherelli VU, Rossi R (eds) Marine eutrophication and population dynamics. Proceedings of 25th EMB symposium, Ferrara, Italy, vol 370, pp 221–230
Arntz WE, Brey T, Gallardo V (1994) Antarctic zoobenthos. Oceanogr Mar Biol Ann Rev 32:241–304
Barnes DKA, Clarke A (1998) The ecology of an assemblage dominant: the encrusting bryozoan Fenestrulina rugula. Invertebr Biol 117:331–340
Barthel D, Gutt J (1992) Sponge association in the eastern Weddell Sea. Ant Sci 4:137–150
Black R, Johnson MS (1979) Asexual viviparity and population genetics of Actinia tenebrosa. Mar Biol 53:27–31
Brey T, Haine S (1992) Growth, reproduction and production of Lissarca notorcadensis (Bivalvia: Philobryidae) in the Weddell Sea, Antarctica. Mar Ecol Prog Ser 82:219–226
Brito TAS, Tyler PA, Clarke A (1997) Reproductive biology of the Antarctic octocoral Thouarella variabilis Wright and Studer, 1889. In: Proceedings of 6th International Conference on Coelent, pp 63–69
Bronsdon SK, Tyler PA, Gage JD, Rice AL (1993) Reproductive biology of two epizoic anemones from the deep north-eastern Atlantic ocean. J Mar Biol Ass UK 73:531–542
Bucklin A, Hedgecock D, Hand C (1984) Genetic evidence of self-fertilization in the sea anemone Epiactis prolifera. Mar Biol 84:175–182
Carlgren O (1927) Actiniaria and Zoantharia. In: Odhner T (ed). Further Zool Res Swed Ant Exp 1901–1903 2(3):1–102
Carlgren O (1949) A survey of the Ptychodactiaria, Corallimorpharia and Actiniaria. K Svenska Vetenskaps-Akad Handl Ser 4(1):1–121
Carter MA, Funnell F (1980) Reproduction and brooding in Actinia. In: Tardent P, Tardent R (eds) Development and cellular biology of coelenterates. Elsevier North Holland Inc, New York, pp 17–23
Chantiore M, Cattaneo-Vietti R, Elia L, Guidetti M, Antonini M (2002) Reproduction and condition of the scallop Adamussium colbecki (Smith 1902), the sea-urchin Sterechius neumayeri (Meissner 1900) and the sea-star Odontaster validus (Koehler 1911). Polar Biol 25:251–255
Chapelle G, Peck LS (1999) Polar gigantism dictated by oxygen availability. Nature 399:114–115
Chapelle G, Peck LS (2004) Amphipod crustacean size spectra: new insights in the relationship between size and oxygen. Oikos 106:167–175. doi:10.1111/j.0030-1299.2004.12934.x
Charnov EL (1982) The theory of sex allocation. Princeton University Press, Princeton, NJ
Chen C, Soong K, Chen CA (2008) The smallest oocytes among broadcast-spawning actiniarians and a unique lunar reproductive cycle in a unisexual population of the sea anemone, Aiptasia pulchella (Anthozoa: Actiniaria). Zool Stud 47:37–45
Chia FS (1976) Sea anemone reproduction: patterns and adaptative radiations. In: Mackie GO (ed) Coelenterate ecology and behaviour. Plenum Press, New York, pp 261–270
Clark AB (1978) Sex ratio and local resource competition in a prosimian primate. Science 201:163–165
Coma R, Lasker HR (1997) Effects of spatial distribution and reproductive biology on in situ fertilisation rates of a broadcast-spawning invertebrate. Biol Bull 193:20–29
Dayton PK (1990) Polar benthos. In: Smith WO (ed) Polar oceanography. Part B. Chemistry, biology and geology. Academic Press, San Diego, pp 631–685
Dunn DF (1975a) Reproduction of the externally brooding sea anemone Epiactis prolifera Verrill, 1869. Biol Bull 148:199–218
Dunn DF (1975b) Gynodioecy in an animal. Nature Lond 253:528–529
Dunn DG (1983) Some Antarctic and sub-Antarctic sea anemones (Coelenterata: Ptychodactiaria and Actiniaria). Ant Res Ser 39:1–67
Edmands S (1995) Mating systems in the sea anemone genus Epiactis. Mar Biol 123:723–733
Edmands S (1996) The evolution of mating systems in a group of brooding sea anemones (Epiactis). Invertebr Reprod Dev 30:227–237
Edmands S, Potts DC (1997) Population genetic structure in brooding sea anemones (Epiactis spp.) with contrasting reproductive modes. Mar Biol 127:485–498
Fautin DG (1984) More Antarctic and Subantarctic sea anemones (Coelenterata: Corallimorpharia and Actiniaria). Ant Res Ser 41:1–42
Fautin DG (1991) Developmental pathways of anthozoans. Hydrobiologia 216(217):143–149
Fautin DG (1997) Cnidarian reproduction: assumptions and their implications. In: Hartog den JC (ed) Coelenterate biology. Proceedings of 6th International Conference on Coelent, Leiden, pp 151–162
Fautin DG (2002) Reproduction of Cnidaria. Can J Zool 80:1735–1754
Fautin DG, Chia FS (1986) Revision of sea anemone genus Epiactis (Coelenterata: Actiniaria) on the Pacific coast of North America, with descriptions of two new brooding species. Can J Zool 64:1665–1674
Fautin DG, Spaulding JG, Chia FS (1989) Cnidaria (chapter 2). In: Adiyodi KG, Adiyodi RG (eds) Reproductive biology of invertebrates, 4A: fertilization, development, and parental care. Oxford and IBH Publishing Company, New Delhi, pp 43–62
Fütterer DK, Brandt A, Poore GCB (2003) The expeditions Antarktis-XIX/3 and XIX/4 of the Research Vessel POLARSTERN in 2002 (ANDEEP I and II: Antarctic Benthic deep-sea biodiversity: colonisation history and recent community patterns) (eds). Ber Polar Meeresforsch 470:1–174
Gabe M (1968) Technique histologique. Massou et Cie, Paris
Gage JD, Tyler PA (1991) Deep-sea biology: a natural history of organisms at the deep-sea floor. Cambridge University Press, London
Galley EA, Tyler PA, Smith CR, Clarke A (2008) Reproductive biology of two species of holothurian from the deep-sea order Elasipoda, on the Antarctic continental shelf. Deep-Sea Res II 55:2515–2526
Ghiselin M (1969) The evolution of hermaphroditism among animals. Q Rev Biol 44:189–208
Ghiselin M (1974) The economy of nature and the evolution of sex. University of California Press, Berkeley
Gorny M, Brey T, Arntz WE, Bruns T (1993) Growth, development and productivity of Chorismus antarcticus (Pfeffer) (Crustacea: Decapoda: Natantia) in the eastern Weddell Sea, Antarctica. J Exp Mar Biol Ecol 174:261–275
Gutt J, Gerdes D, Klages M (1992) Seasonality and spatial variability in the reproduction of two Antarctic holothurians (Echinodermata). Polar Biol 11:533–544
Hand C, Uhlinger K (1992) Culture, sexual and asexual reproduction, and growth of the sea anemone Nematostella vectensis. Biol Bull 182:169–176
Jennison BL (1981) Reproduction in three species of sea anemones from Key West, Florida. Can J Zool 59:1708–1719
Johansen DA (1940) Plant microtechniques. McGraw-Hill, New York
Kang DH, Ahn IY, Choi KS (2009) The annual reproductive pattern of the Antarctic clam Laternula elliptica from Marian Cove, King George Island. Polar Biol 32:517–528. doi:10.1007/s00300-008-0544-7
Kruskal JB, Wish M (1978) Multidimensional scaling. Sage Publications, Beverly Hills
Levitan DR (1991) Influence of body size and population density on fertilization success and reproductive output in a freespawning invertebrate. Biol Bull 181:261–268
Lin MD, Chen CA, Fang LS (2001) Distribution and sexual reproduction of a seagrass-bed-inhabiting Actiniarian, Phymanthus strandesi (Cnidaria: Anthozoa: Actiniaria: Phymantidae), at Hsiao-Liuchiu Island, Taiwan. Zool Stud 40:254–261
Linse K, Barnes KA, Enderlein P (2006) Body size and growth of benthic invertebrates along an Antarctic latitudinal gradient. Deep-Sea Res II 53:921–931
Luxmoore RA (1982) The reproductive biology of some serolid isopods from the Antarctic. Polar Biol 1:3–11
Mann HB, Whitney DR (1947) On a test of whether one of two random variables is stochastically larger than the other. Ann Math Statist 18:50–60
Mercier A, Hamel JF (2009) Reproductive periodicity and host-specific settlement and growth of a deep-water symbiotic sea anemone. Can J Zool 87:967–980
Olabarria C, Thurston MH (2003) Latitudinal and bathymetric trends in body size of the deep-sea gastropod Troschelia berniciensis (King). Mar Biol 143:723–730. doi:10.1007/s00227-003-1116-6
Orejas C, Gili JM, López-González PJ, Arntz WE (2001) Feeding strategies and diet composition of four species of Antarctic benthic cnidarians. Polar Biol 24:473–485
Orejas C, López-González PJ, Gili JM, Teixidó N, Gutt J, Arntz WE (2002) Distribution and reproductive ecology of the Antarctic octocoral Ainigmaptilon antarcticum in the Weddell Sea. Mar Ecol Progr Ser 250:105–116
Orejas C, Gili JM, López-González PJ, Hasemann C, Arntz WE (2007) Reproduction patterns of four Antarctic octocorals in the Weddell Sea: an inter-specific, shape, and latitudinal comparison. Mar Biol 150:551–563. doi:10.1007/s00227-006-0370-9
Orr J, Thorpe JP, Carter MA (1982) Biochemical genetic confirmation of the asexual reproduction of brooded offspring in the sea anemone Actinia equina. Mar Ecol Prog Ser 7:227–229
Pearse JS, Richard Mooi R, Lockhart SJ, Brandt A (2009) Brooding and species diversity in the Southern Ocean: selection for brooders or speciation within brooding clades? In: Krupnik I, Lang MA, Miller SE (eds) Smithsonian at the Poles: Contributions to International Polar Year Science. Proceedings of Smithsonian at the Poles Symposium, Smithsonian Institution, Washington, DC, 3–4 May 2007. Smithsonian Institution Scholarly Press, Washington, DC, pp 181–196
Peck LS, Robinson K (1994) Pelagic larval development in the brooding brachiopod Liothyrella uva. Mar Biol 120:279–286
Piepenburg D, Schmid MK, Gerdes D (2002) The benthos off King George Island (South Shetland Islands, Antarctica): further evidence for a lack of a latitudinal biomass cline in the Southern Ocean. Polar Biol 25:146–158
Poulin E, Féral JP (1996) Why are there so many species of brooding Antarctic echinoids? Evolution 50:820–830
Riemann-Zürneck K (1975) Actiniaria des Südwestatlantik II. Sagartiidae und Metridiidae. Helgol Wiss Meeres 27:70–95
Riemann-Zürneck K (1978) Actiniaria des Südwestatlantik. IV. Actinostola crassicornis (Hertwig, 1882) mit einer Diskussion verwandter Arten. Veröff Inst Meeresforsch Brem 17:65–85
Rodríguez E, López-González PJ, Gili JM (2007) Biogeography of Antarctic sea anemones (Anthozoa, Actinairia): what do they tell us about the origin of the Antarctic benthic fauna? Deep-Sea Res II 54(16–17):1876–1904
Sherman CDH, Ayre DJ (2008) Fine-scale adaptation in a clonal sea anemone. Evolution 62:1373–1380
Sherman CDH, Peucker AJ, Ayre DJ (2007) Do reproductive tactics vary with habitat heterogeneity in the intertidal sea anemone Actinia tenebrosa? J Exp Mar Biol Ecol 340:259–267
Shick JM (1991) A functional biology of sea anemones. In: Calow P (ed) Functional biology series. Chapman & Hall, London
Smith CR, Mincks SL, DeMaster DJ (2006) A synthesis of benthopelagic coupling on the Antarctic Shelf: food banks, ecosystem inertia and global climate change. Deep-Sea Res II 53:875894
Stephenson TA (1928) The British sea anemones. I. The Ray Society, London, p 148
Strathmann RR, Strathmann MF (1982) The relationship between adult size and brooding in marine invertebrates. Am Nat 119:91–101
Strathmann RR, Strathmann MF, Emson RH (1984) Does limited brood capacity link adult size, brooding and simultaneous hermaphroditism? A test with the starfish Asterina phylactica. Am Nat 123:796–818
Strathmann RR, Lindsay RK, Marsh AG (2006) Embryonic and larval development of a cold adapted Antarctic ascidian. Polar Biol 29:495–501
Uchida T, Yamada M (1968) Cnidaria. In: Kume M, Dan K (eds) Invertebrate embryology. NOLIT Publishing House, Belgrade, pp 86–116
Van Praët M (1990) Gametogenesis and the reproductive cycle in the deep-sea anemone Paracalliactis stephensoni (Cnidaria: Actiniara). J Mar Biol Ass UK 70:163–172
Van Praët M, Rice AL, Thurston MH (1990) Reproduction in two deep-sea anemones (Actiniaria); Phelliactis hertwigi and P. robusta. Prog Oceanogr 24:207–222
Waller RG (2005) Deep water scleractinians: current knowledge of reproductive processes. In: Freiwald A, Roberts JM (eds) Cold-water corals and ecosystems. Springer, Heidelberg, pp 691–700
Waller RG, Tyler PA (2011) Reproductive patterns in two deep-water solitary corals from the North-east Atlantic—Flabellum alabastrum and F. angulare (Cnidaria: Anthozoa: Scleractinia). J Mar Biol Assoc UK 91:669–675
Waller RG, Tyler PA, Smith CR (2008) Fecundity and embryo development of three Antarctic deep-water scleractinians: Flabellum thouarsii, F. curvatum and F. impensum. Deep-Sea Res II 55:2527–2534
Watling L, France SC, Pante E, Simpson A (2011) Biology of deep-water octocorals. Adv Mar Biol 60:41–122
Wedi SE, Fautin Dunn D (1983) Gametogenesis and reproductive periodicity of the subtidal sea anemone Urticina lofotensis (Coelenterata: Actiniaria) in California. Biol Bull 165:458–472
Woods HA, Moran AL, Arango CP, Mullen L, Shields C (2009) Oxygen hypothesis of polar gigantism not supported by performance of Antarctic pycnogonids in hypoxia. Proc R Soc B 276:1069–1075. doi:10.1098/rspb.2008.1489
Acknowledgments
Special thanks are addressed to Prof. Dr. Wolf Arntz (Alfred-Wegener-Institute, Bremerhaven, Germany) who made possible our participation in several Antarctic projects and cruises. We extend our acknowledgements to the officers and crew of the R/V Polarstern and many colleagues on board during the EASIZ, ANDEEP, and BENDEX cruises for their valuable assistance. Thanks to M. Conradi (Universidad de Sevilla) who collected a considerable amount of the material analyzed in this manuscript. Comments from M. Daly, D. Fautin, and an anonymous reviewer substantially improved this manuscript. Support was provided by a MCT-CSIC grant (I3P-BPD2001-1) to E. Rodríguez and Spanish CICYT projects: ANT97-1533-E, ANT98-1739-E, ANT99-1608-E, REN2001-4269-E/ANT, REN2003-04236, and CGL2004-20141-E. This is a contribution to the SCAR program, Ecology of the Antarctic Sea Ice Zone (EASIZ) and ANDEEP contribution 159.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. P. Grassle.
E. Rodríguez and C. Orejas have equally contributed to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
227_2012_2063_MOESM1_ESM.pdf
EMS 1 Data of specimens used in histological study. N is number of individuals studied. AP, Antarctic Peninsula; F, females; HP, Hermaphrodites; M, males; WS, Weddell Sea (PDF 84 kb)
227_2012_2063_MOESM2_ESM.tif
EMS 2 Epiactis georgiana, histological sections of spermatogenesis; a male gametes recognizable once grouped in cysts with 24–28 spermatogonia (E1), each covered by mesoglea, b spermatocytes visible after reaching a diameter of 5 µm in center of spermatogonia (E2), c spermatid (to 2 µm of diameter) in center of spermatic cyst, d at the end of spermatogenesis, spermatozoids (1 µm in diameter) visible in mature follicles (E3) filling center of cysts with tails converging to mesentery edge. Abbreviations: ga, gastrodermis; ms, mesoglea; sc, spermatocytes; sg, spermatogonia; sp, spermatic cyst; st, spermatid; sz, spermatozoids; t: spermatozoid tail. Scale bars: A, B, 50 µm; C, D, 0.1 mm (TIFF 58479 kb)
227_2012_2063_MOESM3_ESM.tif
EMS 3 Average measurements (± SD) of pedal disk size (mm), column height (mm) (right axis), and number of mesenteries (left axis) of female (F) and male (M) specimens in each pooled area (AP, Antarctic Peninsula; WS, Weddell Sea). N, number of individuals measured (TIFF 5983 kb)
227_2012_2063_MOESM4_ESM.pdf
EMS 4 Minimum and maximum values of range of diameter (µm) for oocytes and spermatic cysts for each month in each pooled area. Average diameter is expressed as mean (\( \bar{\rm X} \)) ± standard deviation (SD) for each oocyte category (previtellogenic, early vitellogenic, late vitellogenic, and early embryos/larvae) and spermatic cyst state defined (E1, E2, E3) in each studied month. N = number of female individuals; AP, Antarctic Peninsula; WS, Weddell Sea (PDF 96 kb)
227_2012_2063_MOESM5_ESM.tiff
EMS 5 Proportion (%) of different oocyte maturity stages, embryos/larvae, and juveniles in studied months and pooled areas. AP, Antarctic Peninsula; WS, Weddell Sea (TIFF 2022 kb)
227_2012_2063_MOESM6_ESM.tif
EMS 6 Example of observed variability in oocyte and spermatic cyst size frequency distribution among individuals in December and February between areas of study. AP, Antarctic Peninsula; WS, Weddell Sea (TIFF 15338 kb)
227_2012_2063_MOESM7_ESM.tif
EMS 7 Epiactis georgiana brooding specimens and juveniles; a, oral view of preserved female with embryos and/or larvae free in gastrovascular cavity (arrows), b preserved brooding female, c, detail of brooded juveniles. Scale bars: A, 10 mm; B, 50 mm; C, 20 mm (TIFF 28804 kb)
Rights and permissions
About this article
Cite this article
Rodríguez, E., Orejas, C., López-González, P.J. et al. Reproduction in the externally brooding sea anemone Epiactis georgiana in the Antarctic Peninsula and the Weddell Sea. Mar Biol 160, 67–80 (2013). https://doi.org/10.1007/s00227-012-2063-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-012-2063-x