Abstract
Marine invertebrates can display several reproductive strategies, from external reproduction to parental care. Internal brooding is particularly relevant in harsh conditions, like Antarctic/sub-Antarctic waters and deep-sea, since it maximizes the survival of the young. Actinostola crassicornis is an abundant and widely distributed sea anemone from the southwestern Atlantic Ocean. It can be found all along the Argentinean sea down to 1200 m depth, usually in large numbers. It is a unique species in the area, since it is a large white brooding sea anemone. We studied 75 specimens collected by the O/V Walther Herwig and the O/V Puerto Deseado all along its distribution, from about 60 m to 800 m depth, in different seasons of the year. All the specimens were sexed, and the presence of free oocytes and juveniles inside the coelenteron were assessed. Large oocytes (over 500 μm) and juveniles were found in samples from most of the sampled months. We found a larger number of female specimens, and most of the brooding specimens were female. No early developmental stages were found smaller than a sea anemone with about 12 tentacles. We conclude that A. crassicornis reproduces continuously throughout the year and that although most of the juveniles were found in females, male specimens can breed. Brooding has great benefits in terms of protecting the offspring, since predation upon the juveniles is prevented, but dispersal of the offspring is low, shown by the aggregated distribution of the species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Marine invertebrates can display several reproductive strategies, from external reproduction to parental care. Pelagic development of the larvae has been assumed to be plesiomorphic, so the particular reproductive strategy may be an adaptation to the particular environmental conditions or the reflection of a phyletic constraint (Pearse et al. 2009 and references herein). For example, despite the fact that parental care is quite common in the Southern Ocean, it seems not to be related with the conditions of the area (Pearse et al. 2009).
Sea anemones (Cnidaria: Actiniaria) are usually well represented in the benthic community. They are widely spread: from the intertidal zone to the oceanic depths and from the equator to the poles, but since their identification requires both morphological and histological examinations, they are usually under-represented in the scientific literature (Häussermann 2004a). Worldwide there are about 1200 known species, and about 50 were found in Argentinean waters (Fautin 2013; pers. obs.).
Brooding is not common in sea anemones (Chia 1976). At least 69 sea anemones species have been reported as brooders, both internal and external (Larson 2017). Internal seems to be more abundant than external brooding (50 spp. vs 19 spp.), and most of the cases have been reported within Actiniidae: 26 internal and 16 external (Larson 2017). Only eight species of Actinostolidae have been reported as brooders (all internal), including Actinostola crassicornis, a relative abundant species in the southwestern Atlantic Ocean (SAO) off Argentina.
The deep sea is the largest known habitat hosting, and it has turned to host plenty of species, many of them unknown (500,000 to 10,000,000 upon diverse estimation, Brökeland and George 2009). Information about diversity, ecology, feeding and reproduction is also scarce in this environment, and the SAO deep sea is no exception. Sea anemone abundance is usually low, and resampling in the same area is uncommon: deep-sea research is difficult and expensive, which leads to a scarce knowledge of deep-sea anemone biology.
Actinostola crassicornis (Hertwig, 1882) is one of the most abundant sea anemones from the Argentinean platform and upper slope (pers. obs.). It is a unique species in the area, since it is a large white sea anemone, with a smooth column (Fig. 2a), which sets it apart from all other known species in the area of study, and presents a wide geographic and batimetric distribution. It has been found in the SAO from 38º S to 54º S, and from 34 to 1200 m depth (Riemann-Zürneck 1978; Rodríguez and López-González 2013), and its distribution may also include Antarctic waters (Fautin 1984), but the identity of Antarctic specimens has been questioned (Häussermann 2004b; Rodríguez and López-González 2013). Previous studies have reported the presence of juveniles in the interior of the coelenteron of A. crassicornis (Riemann-Zürneck 1978; Fautin 1984; Rodríguez and López-González 2013); Zamponi (1984) studied the larval development of samples assigned to A. crassicornis based on planktonic samples (although it is not possible to confirm the identification of the larvae with the information provided in the paper). To date there is no detailed description of this phenomenon based on a large set of samples from the SAO collected throughout the year, including sex proportion and sex of the brooding specimens. Here we describe the brooding behavior of A. crassicornis based on samples from the SAO.
Materials and methods
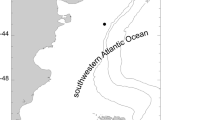
A total of 75 specimens of Actinostola crassicornis from the SAO deep sea off Argentina were analyzed, from 59 to 800 m depth (Fig. 1 and Table 1). Forty-four were collected by the authors onboard the O/V Puerto Deseado (CONICET) and 31 were collected by the O/V Walther Herwig expeditions (1966 and 1970/71) (Table 2). Photographs of selected specimens were taken using a Nikon D800 SRL digital camera with a Nikkor 60 mm F2.8 macro lens. Later, at least one cross section below the actinopharynx level of each specimen was made (Fig. 2b). The complete interior of each sea anemone was checked for oocytes, larvae, and/or juveniles (Fig. 2c, d) under a stereoscopic microscope. When any of those were found, they were counted, measured (length and width) and the number of tentacles was counted in selected specimens, as a proxy of developmental stage. Here we follow Larson (2017) definition for larva (a development stage from postembryonic stage to metamorphosis, deferring from the adult in morphology, nutrition or habitat), juvenile (prefertile individual with at least one cycle of tentacles and mesenteries, a mouth and a pedal disc), brooding (retention of an individual by the adult to at least a juvenile) and fostering (an individual brooding an individual from another adult).
Sampling stations along the Argentinean coast. Numbers next to the stations correspond to the code number in Table 1
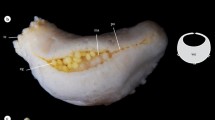
a Living Actinostola crassicornis in aquarium. b Cross section of adult with several juveniles free inside the coelenteron. c Size variation of juveniles from a single specimen of 55.5 mm of diameter, including the smaller and the larger juvenile inside the adult (the specimen had six broods in total). d Scanning electron microscopy image of a small juvenile (note the presence of 12 tentacles)
The sex of the 44 specimens stored at the Museo Argentino de Ciencias Naturales “Bernardino Rivadavia” (MACN) was determined using histology. Small fragments of the specimens were dehydrated and included in paraffin, sectioned at 5–10 μm and stained with Azocarmine triple stain or eosin-hematoxylin stain (Humason 1967; Suvarna et al. 2013). Digital images were taken using an AxioCam HRc camera on an Axio Imager Z1 Zeiss microscope. For the 31 specimens stored at the Zoological Museum of Hamburg (ZMH), the sex was inferred based on the examination of the mesenteries under a stereoscopic microscope. Males and females with well-developed gametes were easily identified, but for some specimens it was not possible to see any gametes (even after doing histological sections), so they were labeled as indeterminate. Differences in sex proportion were analyzed using a Chi-square test (χ2) (Zar 2010). Maximum oocyte diameter was measured in histological sections where nucleus and nucleolus were visible. To study the internal development of the juveniles (number of mesenterial cycles, muscle development, etc.), histological sections of selected whole specimens stored at the MACN were made. Scanning electron microscopy (SEM) was used to take images of small juveniles.
Results
Actinostola crassicornis is a gonochoric species. From 75 specimens, 45 were female (Fig. 3a, b), 20 were male (Fig. 3c, d) and in ten specimens no reproductive tissue could be found (labeled as indeterminate) (Table 2), so the female:male proportion is 2.25:1 (χ2 = 9.615, gl = 1, P < 0.01). Adults size ranged from 19.8 to 76.6 mm in column diameter (mean value of 40.4 mm ± 11.0 mm). Brooding specimens ranged from 19.8 to 76.6 mm in column diameter (mean value of 39.7 mm ± 12.2 mm). Juveniles were found in 28 specimens (37% of the studied specimens), most of them female, except for one male specimen, one indeterminate specimens (confirmed by histology) and one male specimen (in the ZMH) with gametes that we could not do histology to confirm its sex. Brooding specimens were found in all the seasons but spring (although samples from early September had larvae) (Fig. 4a–d). No correlation was found between the size (diameter) or the number of juveniles and the size (diameter) of the brooding adult (Fig. 4e, f). No blastula, gastrula, nor larvae were found in any of the examined specimens. Some oocytes were free inside the coelenteron, but it is possible that at least some of them were dislodged during dissection. Large oocytes (over 500 μm and up to 600 μm) were found in specimens from January, February, April, June, August, and September (Table 2, Fig. 3a, b). Most male sections (April, August and September) showed cysts with scarce mature sperm (Fig. 3c, d).
Histological sections of Actinostola crassicornis showing gametogenic tissue. a Cross section of a fertile mesentery full of oocytes in different developmental stages. b Detail of a large vitelogenic oocyte with nucleus and nucleolus. c Cross section of a fertile mesentery full of spermatic cysts. d Detail of a spermatic cyst. Pvo previtelogenic oocyte, S spermatozoids, Sc spermatic cyst, Vo vitelogenic oocyte
a Number of specimens per month (black, all the adults; grey, brooders). b Number of specimens per season (black, all the adults; grey, brooders). c Mean number of juveniles per month. d Mean number of juveniles per season. e Correlation between the number of juveniles per brooding adult and the adult’s size (diameter). f Correlation between the mean size of the juveniles per adult and the adult’s size (diameter)
A total of 646 juveniles were found (but only 639 were measured due to the contraction of some of them specimens). They were found free inside the coelenteron or between the mesenteries, and no special orientation was detected. The largest number of juveniles in an adult was 210, and the lowest was 1 (average 23 per brooding adult). Juveniles size and developmental stage (i.e., number of mesenteries and tentacles) were very variable. Juveniles ranged from 0.48 mm (with 10?/12 tentacles visible) to 17.35 in column diameter (3.2 mm ± 2.1).
Juveniles were presented during most of the studied months (February, April, June, August, and September), but were more abundant during August (winter) (Table 3, Fig. 4b, c). No juveniles were found in samples from January, July, November, or December. Histological sections of the juveniles revealed a typical polyp organization (i.e., no larvae organization).
Discussion
Actinostola crassicornis is a gonochoric brooding deep-sea anemone, as reported by previous authors (Riemann-Zürneck 1978; Rodríguez and López-González 2013). A bias toward females was detected in the samples. Larger number of females in sea anemone populations is not rare. A larger number of females has been reported for populations of Epiactis prolifera (Dunn 1975a, b), Actinia equina (Chia and Rostron 1970) and A. tenebrosa (Ottaway 1979), among others. Dunn (1983) found a sex ratio of 5:1 (females: males) in a subtidal population of Anthopleura handi. Nevertheless, since there are many indeterminate specimens in the samples it is possible that many of those specimens were actually males (or females) with no gametes, and that the female:male ratio is closer to 1:1 or higher.
We found oocytes up to 600 μm inside the mesenteries and free in the coelenteron, which is in line with previous reports. Riemann-Zürneck (1978) reported the presence of five free “eggs” inside a female specimen, one of which turned to be a blastula (i.e., she found four oocytes and one blastula), so it is likely that the fertilization is internal. We also found free oocytes inside the coelenteron of some females, but we failed to find any blastula, nor any other developmental stage until juvenile of about 1 mm with 10–12 tentacles (some juveniles were completely or partially contracted, so it was not possible to be sure of the number of tentacles). It is possible that the first stages of development of A. crassicornis occurs in a short period of time, which explains why we only found juveniles. It is also possible that free living juveniles are released (or escape) from the female and may eventually be swallowed by adult anemones (both males and females in our case) like in Aulactinia stella (Bocharova 2015), where the development continues. If this is the case, then it would be a case of fostering, and brooding and fostering can co-occur. Brooding (or fostering) occurs mostly inside females, but males may also have juveniles in their coelenteron. This type of development has been reported for Actinia equina, where the fertilization is internal but juveniles are found inside males, females and undifferentiated (indeterminate in this work) specimens (Chia and Rostron 1970). Moreover, Rostron and Rostron (1978) reported that juveniles in A. equina do not correspond with the “brooding” adult, based on the difference in coloration between juveniles and brooder. Brooding male specimens have also been found in Phymanthus crucifer (Jennison 1981). Using molecular techniques Bocharova and Mugue (2012) showed that about 1/3 of the broods found in adult specimens of A. stella do not correspond to the “brooding” adult. Further research in A. crassicornis will be focused on the relationship between the brooders and the juveniles, in order to determine if each female broods its own offspring (and if the broods inside the male specimens are rare cases) or if adults brood juveniles from other specimens as a rule (fostering) or a mix of both.
Big size oocytes (> 500 μm) were present in females throughout the year. Some oocytes were free inside the coelenteron, but it is possible that at least some of them were a consequence of the dissection of the specimen rather than real free oocytes. Juveniles were present during most of the studied months, and were more abundant during August. No juveniles were found in samples from January, July, November, or December, but this may be due to the low number of samples from those months (5, 2, 4, and 1 respectively) (Table 3). Riemann-Zürneck (1978) found up to 50 juveniles per adult and the largest one was about 3 cm, meanwhile we found up to 210 juveniles per adult, and the largest was 16.8 mm, so there is a large variability in number of juveniles and sizes, and there is no correlation between the size of the adult and the number or size of the juveniles. All this evidence may indicate the lack of an annual brooding (and gametogenic) cycle, and that the juveniles are produced throughout the year, which is in line with the results/conclusions obtained by Riemann-Zürneck (1978). It is also possible that the apparent lack of a reproductive cycle in A. crassicornis is the result of the large temporal and geographic range of the samples analyzed by Riemann-Zürneck (1978) and us. This type of data is not unusual for deep-sea fauna (e.g., Martinez et al. 2018), but it is not possible to discard that environmental differences along the species distribution’ (from about 38º to 54º) added to particular conditions in the different years when the samples were taken (1966, 1970, 1971, 2009, 2012 and 2014) may be hiding the presence of a non-continuous reproductive cycle. Nevertheless, monthly samples for at least one year from the same location are needed to confirm this hypothesis.
The number of brooding adults (and the number of juveniles per brooding adult) may have been underestimated, given the possibility that juveniles may have been released or escaped from the adult during collection. Although it would seem unlikely that no larvae or juveniles at early stages remained inside brooding adults, their absence could also be justified by their release/escape during sampling.
Zamponi (1984) studied the planktonic development of samples assigned to A. crassicornis based on samples taken by the O/V Walther Herwig (1978), from “egg” (huevo in the original text, probably a zygote) to halcampoid larvae. As Riemann-Zürneck pointed out (1986, p. 134), it is not possible to be sure of the identity of the larvae studied by Zamponi. Moreover, it is possible that Zamponi’s works were made with larvae of several species, but it is not possible to be sure. Nevertheless “egg” sizes measured by Zamponi are much smaller than the oocytes found in the mesenteries by Riemann-Zürneck and by us (30 to 180 μm in Zamponi’s works vs 500 μm in Riemann-Zürneck’s and up to 600 μm in this work), so we can be sure that at least that part of the development reported does not correspond to A. crassicornis.
Based on the information that we have so far, fertilization in A. crassicornis seems to be internal (i.e., inside the coelenteron of the female). Then, there are two possibilities (1) the larvae develop inside females, but some larvae or juveniles are released/escape into the water column, and re-enter the adult's coelenteron, which will explain the broods inside male and indeterminate specimens. In this case, this will be a case of fostering; or (2) the larvae develop in the water and re-enter the adults when they are at a stage with about 12 tentacles (also fostering), in which case we would expect for a larger number of non-females specimens brooding. Finally, the juveniles leave the adults as small sea anemones with at least three cycles of tentacles and settle. Studies including specimens in aquaria could confirm one of these two hypotheses. Parental care has great benefits in terms of protecting the offspring, since predation upon the juveniles is prevented and lack of food do not affect them as much, but it presents a low dispersal of the broods, which explains the aggregated distribution of the species.
There is also the possibility that asexual reproduction is occurring in A. crassicornis. Somatic embryogenesis has been proposed to explain the presence of juveniles in non-female specimens of Actinia equina (Orr et al. 1982). This will explain the presence of broods inside non-female specimens. Although we cannot test this hypothesis at the moment, if the juveniles were the result of asexual reproduction, we would expect a larger number of non-female specimens brooding with a similar number of juveniles in each one, and we only found a few broods in the male specimens. In consequence, we believe that the juveniles found in our specimens are not the result of asexual reproduction, and that their presence in the non-female specimens is the result of larvae/juveniles from the water being shallowed by other specimens. The use of molecular techniques (e.g., Bocharova and Mugue 2012; Bocharova 2015) is needed to properly evaluate this hypothesis and to clearly determine if A. crassicornis is a brooding or fostering species, as defined by Larson (2017).
Brooding overall seems to be present in about 5% of the species, with more than twice as many internal than external brooders, and species from the same genus may present different brooding strategies (Larson 2017). Internal brooding seems to be a simpler way, since the juveniles are retained inside the coelenteron, without the development of any particular structure. In contrast, external brooding may involve the development of special structures like chambers, pits or grooves (reviewed in Larson 2017). In recent years, several researches have been focused in this phenomenon in sea anemones (e.g., Bocharova and Mugue 2012; Rodríguez et al. 2012; Bocharova 2015; Larson et al. 2012; Larson and Daly 2016), and a review of the knowledge has been recently published (Larson 2017). Nevertheless, many of the brooding reports came from taxonomical works, and no detailed study of it has been done in several species (e.g., Antholoba achates in Fautin (1984). Despite the lack of monthly samples (which are hard to get for a deep-sea species) we describe the internal brooding (and fostering) behavior of A. crassicornis from the SAO deep-sea and identify open question for this species.
In the past 3 years, over ten cases of parental care/lecitotrophic development have been reported from several phyla in the SAO, including the sub-Antarctic waters. These reports include species of cnidarians (one black coral), mollusks (six snails) and echinoderms (six species within sea cucumbers, sea stars, brittle stars, sea urchin, and feather stars) (Berecoechea et al. 2017; Lauretta and Penchaszadeh 2017; Martinez and Penchaszadeh 2017; Penchaszadeh et al. 2017, 2019; Rivadeneira et al. 2017; Averbuj et al. 2018; Martinez et al. 2018; Sánchez et al. 2018; Flores et al. 2019; Pertossi et al. 2019; Teso and Penchaszadeh 2019). All these reports may indicate a high proportion of non-pelagic development in the SAO deep-sea, although more data are necessary to support this idea and, if proven, to determinate its causes.
Data availability
The datasets used during the current study are available from the corresponding author on reasonable request.
References
Averbuj A, Penchaszadeh P, Pastorino G (2018) Egg masses and development of Falsilunatia eltanini (Mollusca: Gastropoda): A deep-sea naticid from southwestern Atlantic Canyon. Mar Biol. https://doi.org/10.1007/s00227-018-3337-8
Berecoechea JJ, Brogger M, Penchaszadeh P (2017) New evidence of brooding in the deep-sea brittle star Astrotoma agassizii Lyman, 1876 from a South Western Atlantic Canyon. Deep Sea Res Part I 127:105–110
Bocharova ES (2015) Reproductive biology and genetic diversity of the sea anemone Aulactinia stella (Verrill, 1864). Hydrobiologia 759:27–38
Bocharova ES, Mugue NS (2012) Sea anemones Aulactinia stella (Verrill, 1864) (Hexacorallia, Actiniidae) can brood offspring from other individual of the same species. Dokl Biol Sci 444:173–175
Brökeland W, George K (2009) Deep-sea taxonomy—a contribution to our knowledge of biodiversity. Zootaxa 2096:6–8
Chia F (1976) Sea anemone reproduction: patterns and adaptive radiations. In: Mackie GO (ed) Coelenterate ecology and behavior. Plenum Press, New York, pp 261–270
Chia F, Rostron M (1970) Some Aspect of the reproductive biology of Actinia equina (Cnidaria: Anthozoa). J Mar Biol Assoc U K 50:253–264
Dunn D (1975a) Gynodioecy in an animal. Nature 253:528–529
Dunn D (1975b) Reproduction of the externally brooding sea anemone Epiactis prolifera Verrill, 1869. Biol Bull 148:199–218
Dunn D (1983) Sexual reproduction of two intertidal sea anemones (Coelenterata: Actiniaria) in Malaysia. Biotrópico 14:262–271
Fautin D (1984) More Antarctic and Subantarctic sea anemones (Coelenterata: Corallimorpharia and Actiniaria). Antarct Res Ser 41:1–42
Fautin D (2013) Hexacorallians of the World. https://geoportal.kgs.ku.edu/hexacoral/anemone2/index.cfm. Accessed October 2018
Flores J, Brogger M, Penchaszadeh PE (2019) Reproduction and development of the brooding sea urchin Austrocidaris canaliculata from deep-sea off Argentina. Deep Sea Res Part I 143:35–42
Häussermann V (2004a) Identification and taxonomy of soft-bodied hexacorals exemplified by Chilean sea anemones; including guidelines for sampling, preservation and examination. J Mar Biol Assoc U K 84:931–936
Häussermann V (2004b) The sea anemone genus Actinostola (Verrill 1883): variability and utility of traditional taxonomic features, and a re-description of Actinostola chilensis (McMurrich 1904). Polar Biol 28:26–38
Hertwig R (1882) Die Actinien der Challenger Expedition. Gustav Fischer, Jena
Humason G (1967) Animal tissue techniques. Freeman and Company, San Francisco
Jennison B (1981) Reproduction in three species of sea anemones from Key West, Florida. Can J Zool 59:1708–1719
Larson P (2017) Brooding sea anemones (Cnidaria: Anthozoa: Actiniaria): paragons of diversity in mode, morphology, and maternity. Invertebr Biol 136(1):92–112
Larson PG, Daly M (2016) Phylogenetic analysis reveals an evolutionary transition from internal to external brooding in Epiactis Verrill (Cnidaria: Anthozoa: Actiniaria) and rejects the validity of the genus Cnidopus Carlgren. Mol Phylo Evol 94:548–558
Larson PG, Hamel J-F, Mercier A (2012) Redescription and notes on the reproductive biology of the sea anemone Urticina fecunda (Verrill, 1899), comb. nov. (Cnidaria: Actiniaria: Actiniidae). Zootaxa 3523(1):69–79
Lauretta D, Penchaszadeh PE (2017) Gigantic oocytes in the deep sea black coral Dendrobathypathes grandis (Antipatharia) from the Mar del Plata submarine canyon area (southwestern Atlantic). Deep Sea Res Part I 128:109–114
Martinez M, Penchaszadeh PE (2017) A new species of brooding Psolidae (Echinodermata: Holothuroidea) from deep-sea off Argentina, Southwestern Atlantic Ocean. Deep Sea Res Part II 146:13–17
Martinez M, Alba-Posse E, Lauretta D, Penchaszadeh PE (2018) Developmental stages in the brooding sea cucumber Cladodactyla crocea (Lesson, 1830) in the Southwestern Atlantic Ocean. Polar Biol 41:1237–1244. https://doi.org/10.1007/s00300-018-2280-y
Orr J, Thorpe JP, Carter MA (1982) Biochemical genetic confirmation of the asexual reproduction of brooded offspring in the sea anemone Actinia equine. Mar Ecol Prog Ser 7:227–229
Ottaway J (1979) Population ecology of the intertidal anemone Actinia tenebrosa II. Geographical distribution, synonymy, reproductive cycle and fecundity. Aust J Zool 27:273–290
Pearse J, Mooi R, Lockhart S, Brandt A (2009) Brooding and species diversity in the Southern Ocean: selection for brooders or speciation within brooding clades? In: Krupnik I, Lang M, Miller S (eds) Smithsonian at the poles: contributions to international polar year science. Smithsonian Institution Scholarly Press, Washington, pp 181–196
Penchaszadeh PE, Teso V, Pastorino G (2017) Spawn in two deep-sea volute gastropods (Neogastropoda: Volutidae) from southwestern Atlantic waters. Deep Sea Res Part I 130:55–62
Penchaszadeh PE, Pastorino G, Martinez M, Miloslavich P (2019) Spawn and development of the gastropod Americominella longisetosa (Castellanos and Fernández, 1972) (Mollusca: Buccinidae) from the Southwestern Atlantic deep sea. Deep Sea Res Part I 143:43–49
Pertossi R, Brogger M, Penchaszadeh PE, Martinez M (2019) Reproduction and developmental stages in the crinoid Isometra vivipara Mortensen, 1917 from the southwestern Atlantic. Polar Biol 42:807–816
Riemann-Zürneck K (1978) Actiniaria des Südwestatlantik IV. Actinostola crassicornis (Hertwig, 1882) mit einer Diskussion verwandter Arten. Veröff Inst Meeresforsch Bremer 17:65–85
Riemann-Zürneck K (1986) Zur Biogeographie des Südwestatlantik mit besonderer Berücksichtigung dera Seeanemonen (Coelenterata: Actiniaria). Helgol Meeresunter 40:91–149
Rivadeneira P, Brogger M, Penchaszadeh PE (2017) Aboral brooding in the deep water sea star Ctenodiscus australis Lütken, 1871 (Asteroidea) from the Southwestern Atlantic. Deep Sea Res Part I 123:105–109
Rodríguez E, Orejas C, López-González PJ, Gili JM (2012) Reproduction in the externally brooding sea anemone Epiactis georgiana in the Antarctic Peninsula and the Weddell Sea. Mar Biol 160:1–14
Rodríguez E, López-González P (2013) New records of Antarctic and Sub-Antarctic sea anemones (Cnidaria, Anthozoa, Actiniaria and Corallimorpharia) from the Weddell Sea, Antarctic Peninsula, and Scotia Arc. Zootaxa 3624:1–100
Rostron MA, Rostron J (1978) Fecundity and reproductive ecology of a natural population of Actinia equina (Cnidaria: Anthozoa). J Exp Mar Biol Ecol 33:251–259
Sánchez N, Pastorino G, Penchaszadeh PE (2018) Giant eggs in the gastropod Aforia obesa (Conoidea: Cochlespiridae) in Southwestern Atlantic deep-waters. Zool Anz 276:94–99
Suvarna SK, Layton C, Bancroft JD (2013) Bancroft's theory and practice of histological techniques. Elsevier, Churchill Livingstore
Teso V, Penchaszadeh PE (2019) Development of the gastropod Trochita pileus (Calyptraeidae) in the sub-Antarctic Southwestern Atlantic. Polar Biol 42:171–178
Zar J (2010) Biostatistical analysis, 5th edn. Pearson Prentice Hall, New Jersey
Zamponi M (1984) Los primeros estadios del desarrollo en Actinostola crassicornis (Hertwig, 1882) (Actiniaria: Actinostolidae). Neotrópica 30:111–120
Acknowledgements
Many thanks to the crew of the O/V Puerto Deseado for their assistance during the expeditions and to the curator of the ZMH (Andreas Schmidt-Rhaesa) for the access to the collection and specimens. Thanks to Alejandra Lauretta for improving the English of the manuscript. This manuscript was greatly improved by the comments and suggestion of Ekaterina Bocharova, Vreni Häussermann and a third anonymous review.
Funding
This work was partially founded by PICT 2013-2504 from the Agencia Nacional de Promoción Científica y Tecnológica to Pablo Penchaszadeh, a PIP 2017-0643 from the National Scientific and Technical Research Council to DL and MM, a DAAD (German Academic Exchange Service) to DL and a PADI foundation grant to DL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Research involving human and animal rights
All the specimens of Actinostola crassicornis used in this study were approved by the corresponding authorities both in the Museo Argentino de Ciencias Naturales “Bernardino Rivadavia” and in the Zoological Museum of Hamburg.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lauretta, D., Vidos, C., Martinez, M.I. et al. Brooding in the deep-sea sea anemone Actinostola crassicornis (Hertwig, 1882) (Cnidaria: Anthozoa: Actiniaria) from the southwestern Atlantic Ocean. Polar Biol 43, 1353–1361 (2020). https://doi.org/10.1007/s00300-020-02713-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-020-02713-3