Abstract
The annual reproductive cycle of the Antarctic soft-shelled clam, Laternula elliptica, in Marian Cove, King George Island was studied over a 2-year period from February 1998 to January 2000. Annual changes in the gametogenesis were investigated by measuring the percentage of area occupied by oocytes in a follicle [follicle index (FI)] and the oocyte size. In 1998, the monthly mean FI increased significantly from October to November, peaked in December, and decreased rapidly from December to January. In February and March 1999, degenerated eggs were observed in the spent follicles. Degeneration and resorption of residual eggs by phagocytosis occurred mostly in February and March in both 1998 and 1999, although the resorption process was observed year-round. The histology indicated that complete vitellogenic growth of L. ellpitica at Marian Cove takes at least a year and the clams spawn annually during the austral summer. The ripening and subsequent spawning of clams at Marian Cove in 1998 and 1999 coincided with the algal blooming (September–October 1998 and December and January 1999–2000) suggesting that in coastal Antarctica food supply is a crucial factor that governs gonad maturation and subsequent spawning along with the water temperature.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Antarctic soft-shelled clam, Laternula elliptica, is a simultaneous hermaphrodite and produces unusually large encapsulated eggs (Bosch and Pearse 1988). Like other benthic animals in Antarctica, the clam exhibits slow growth and slow gonad maturation (Pearse et al. 1991; Ansell and Harvey 1997). The slow physiological processes (Peck et al. 2000; Pearse and Lockhart 2004) and pelagic non-feeding larval development (Bosch and Pearse 1988; Pearse et al. 1991) of L. elliptica are considered as adaptation to the harsh Antarctic environment. As with other benthic Antarctic organisms, limited information is available on the growth and reproduction of L. elliptica because of the technical difficulties involved in direct observation and sampling. However, recent studies on reproduction of L. elliptica have reported that the clam spawns during the austral summer, although spawning may follow a latitudinal cline: mid-December to February at Marian Cove, King George Island (62°13′S, 58°45′W) (Ahn et al. 2000, 2003) and February to mid-May at McMurdo Sound (77°51′S, 166°40′W) (Bosch and Pearse 1988; Pearse et al. 1991). Urban and Mercuri (1998) histologically investigated annual gametogenesis over 1 year in L. elliptica collected from Potter Cove, King George Island, and reported spawning in February and March. Interestingly, they were unable to observe any early developing gametes in the gonad, whereas they found full-grown oocytes in the follicle year-round. Based on histological observations, they concluded that the oocyte development cycle took longer than 1 year (Urban and Mercuri 1998). Bigatti et al. (2001) measured the size of oocytes in the same clams sampled from Potter Cover by Urban and Mercuri (1998). Contrary to Urban and Mercuri (1998), they concluded that a complete oocyte growth cycle takes less than 7 months and that the clam might be ready for spawning at any time during the year, although intensive spawning is believed to occur in February. Interestingly, they were unable to observe any resorbed oocytes in the gonad, whereas they found full-grown oocytes in the follicles year-round.
Cyclic changes in gametogenesis are partly governed by changes in environmental parameters, such as water temperature, salinity, food availability, and photoperiod (Bayne and Newell 1983; Soniat and Ray 1985; Heffernan et al. 1989; Hofmann et al. 1992; Kang et al. 2000; Pearse and Bosch 2002). The amount of food in the water column available for filter feeders often shows strong seasonality in temperate regions, with water temperature playing a key role (Mann 1979; Clarke 1987; Heffernan et al. 1989; Hooker and Creese 1995; Saucedo et al. 2001). Numerous studies have reported that gonad development and subsequent spawning in marine bivalves are synchronized with seasonal changes in food availability in the water column (Hofmann et al. 1992; Grant and Creese 1995; Kennedy et al. 1996; Kang et al. 2000). Along with water temperature, the quantity of food in the water column determines the number of gametes produced (i.e., reproductive output) and the timing of spawning (Kang et al. 2000; Llodra 2002; Park and Choi 2004). Primary production in coastal Antarctic waters is extremely seasonal, and phytoplankton blooms occur during a short period in the austral summer (Chang et al. 1990; Rivkin 1990; Kang et al. 1997; Ahn et al. 2003). Therefore, food availability is important for the reproduction of various Antarctic marine invertebrates, including L. elliptica (Urban and Mercuri 1998; Peck et al. 2000; Ahn et al. 2003; Clarke et al. 2004).
Annual gametogensis of L. elliptica in Marian Cove, King George Island was monitored over a 2-year period during 1998 and 2000. The present study reports seasonal variation in oocyte growth and the gametogenic cycle. We also describe the process of resorption of relict eggs in specimens collected from Marian Cove during the sampling period.
Materials and methods
Sample collection
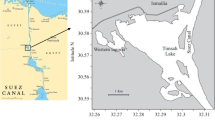
Clams were collected by SCUBA divers from depths of 20–30 m near King Sejong Station (62°13′S, 58°47′W) in Marian Cove, King George Island (Fig. 1). Clams were collected on a monthly basis between February 1998 and January 2000, except in April, July, and September 1998, when clams could not be collected because of inclement weather (Table 1). A total number of 637 clams were collected and fixed in 10% buffered formalin in situ for histology. Daily total chlorophyll concentration and water temperature data were obtained from a long-term monitoring program conducted at the station (KORDI 1999). The total chlorophyll (chlorophyll a + phaeopigments) concentration in the water column was determined in vivo using a fluorometer (Turner 10-AU-005). Water temperature and salinity was measured using a conductivity meter (YSI 610-D).
Histological preparation
Upon arrival at the laboratory, shell length and wet tissue weight were measured to 0.01 mm and 0.01 g, respectively. A longitudinal cross section of 1 cm thickness was made along the central part of the soft body. After dehydration, clearing, and embedding in paraffin, the block was sliced into 5-μm sections and stained with Harris hematoxylin and eosin Y. Degeneration of relict oocytes by hemocytes (i.e., phagocytosis) was examined using hemocyte-specific Giemsa staining of paraffin sections.
Analysis of annual gametogenesis
The reproductive condition of L. elliptica was examined under a light microscope equipped with a video camera connected to a personal computer. Gonad and oocyte development of the ovary was analyzed using a planimetric technique (Kang et al. 2003). From the digitized images of gonadal tissue, oocyte diameter and follicle area were measured using image analyzing software. Oocyte diameter was measured on a microscopic image of the ovary, and the mean oocyte diameter (MOD, micrometer) was calculated from 40 to 50 selected oocytes in each follicle. The frequency distribution of the oocytes was then analyzed to identify the monthly variation in oocyte size and growth. The normality of the frequency distribution of oocytes was tested using the Kolmogorov–Smirnov test (Sokal and Rohlf 1981). One-way ANOVA and Pearson’s correlation were used to compare the differences in the frequency distributions over the sampling period. Various sizes of oocytes were classified according to oogenic stage. The percentage area occupied by oocytes in a follicle, or follicle index (FI, %), was determined using three to six follicles selected from the histological slide of each clam. The percentage of total follicle area occupied by oocytes was then calculated. The average FI per month and standard deviation for each clam were calculated to analyze the temporal fecundity and spawning time of the clam. One-way ANOVA was used to analyze the annual and interannual differences in FI. Two-sample t tests were used for comparisons following one-way ANOVA.
Results
Seawater temperature and total chlorophyll
Water temperature and total chlorophyll concentrations in surface seawater varied monthly. Water temperature varied from −1.8°C (August 1998) to 4.5°C (January 1999, Fig. 2). Water temperature decreased from February to August, and gradually increased from October to January. Total chlorophyll concentrations recorded daily varied widely (0.1–12.2 μg/L), with an irregular seasonal pattern year-round. Unusually high levels of algal blooms (1.07–12.2 μg/L) occurred from late September to early October 1998 in the early austral spring; such a spring algal bloom was also considered unusual. In addition, another bloom occurred from December 1999 to January 2000 in the austral summer. Blooms were most intense in early spring.
Microscopic observation of gonads
There were seasonal changes in the female part of the gonadal tissues (Fig. 3). In the resting stage, shrunken or vacated follicles were common (Fig. 3a); primary oocytes (PO) and relic oocytes (RO) were infrequently observed along the follicle walls. In the developing stage, large numbers of early vitellogenic oocytes (EVO), previtellogenic oocytes (PVO), and vitellogenic oocytes (VO) were dominant along the germinal epithelium, resulting in expansion of the follicles (Fig. 3b). The developing stage was observed from March to September, and a few ripe eggs were often present in the follicles in this stage. The fully developed stage was characterized by follicles that were greatly expanded and completely filled with fully mature eggs (Fig. 3c). The proportion of mature females increased in December when the mature gonad enlarged into the connective tissues and occupied much of the gonadal space. The outer margins of ripe eggs were covered with a gelatinous membrane 12–20 μm in thickness. A few PO were often found along the germinal epithelium. Clams collected in January 1999 were in the spawning stage (Fig. 3d). Partly spawned ovaries were loosely packed with eggs in the follicles. Spent ovaries were observed in late February–March 1999 (Fig. 3e). Small numbers of RO and VO were observed in the follicles, and the follicles tended to decrease in size during this period. In the resorption stage, ROs were resorbed by phagocytic activity in the follicles (Fig. 3f). Some degenerating ova were surrounded by hemocytes, indicating phagocytosis of eggs. Resorption of eggs in the ovaries was observed throughout the year (2–28%).
Photomicrographs of ovaries. a Resting stage: gonadal tissues are sparse or absent along the thin follicle wall. b Developing stage: a growing oocytesry with primary oocytes (PO), previtellogenic oocytes (PVO), and vitellogenic oocytes (VO) along the follicle wall (FW). c Fully developed stage: mature ovaries packed with oocytes (O) and a thin layer along the follicle wall. Very small PO are present in the germinal epithelium. d Spawning stage: partly spawned ovaries with loosely packed oocytes and vacated spaces in the lumen (LM). e Spent stage: ovaries shrinking and largely devoid of oocytes, with VO and relict oocytes (RO). f Resorbing stage: relict oocytes are degenerated and resorbed (RSO); follicle epithelium is thin. Scale bar 100 μm
There were also seasonal changes in the male parts of the gonadal tissues (Fig. 4). In the resting stage, most of the follicle walls were thin and the lumens were empty (Fig. 4a). In the developing stage, spermatocytes (SC) began to proliferate in the center of the lumen, and maturation of spermatozoa (SZ) progressed rapidly in the center of the follicle. Thick differentiated bands between SC and SZ were observed in the follicles (Fig. 4b). In mature spermaries, the mass of mature SZ increased in volume (Fig. 4c). Most of the SZ were observed in the center of the lumen. In spawning spermaries, follicles had numerous gaps with spawning SZ; relict spermatogenic cells still occupied the lumen (Fig. 4d). In spent testes, the follicle walls were constricted and the lumens were empty, except for relict SZ (Fig. 4e). In the resorption stage, shrunken follicles were observed with relict or degenerating spermatogenic cells (Fig. 4f).
Photomicrographs of testes. a Resting stage: resting testes have a vacated LM and thin FW. b Developing stage: columns of spermatocytes (SC) present; spermatozoa (SZ) move to the central part of growing testes. c Fully developed stage: mature testes filled with SZ and largely devoid of nutritive tissue. d Spawning stage: partly spawned testes with spaces vacated by spawned SZ. e Spent stage: testes mostly devoid of content, although relict spermatozoa (RSZ) may be present. f Resorbing stage: shrinking testes with thin follicle wall. Scale bar 100 μm
Annual variation in oocyte size and growth
The proportion of oocytes in each size class varied monthly (Fig. 5). The class cohorts showed a clear growth pattern of small oocytes. Small oocytes (<60 μm in diameter) were dominant in March in both years. Oocytes in the 61- to 100-μm diameter size class moved up to the 81- to 120-μm size class from May to October 1998. A high frequency of oocytes 81–150 μm in diameter was observed in October and November 1998 (P < 0.05). The frequency of oocytes 121–150 μm in diameter peaked in December 1998 (48%). The size of oocytes decreased in January 1999, whereas the 101- to 120-μm size class increased by about 20% in February 1999. A thick gelatinous membrane was associated with the 81- to 100-μm size class (Fig. 6), indicating that the membrane-covered oocytes were close to maximum growth and ready to spawn. In March 1999, the female gonad resumed oogenesis, and small oocytes 21–60 μm in diameter were most frequent (65%). The 61- to 100-μm size class began to increase from April to July 1999. The 81- to 120-μm size class moved up to the 121- to 150-μm size class from November 1999 to January 2000. The 120- to 150-μm size class reached a peak (35%) in January 2000. Asymmetric distributions of oocyte size were observed from February to June 1998 and from March to May 1999; small oocytes developed mainly in these months. Symmetric or less asymmetric distributions occurred in December 1998, January–February 1999, and June 1999–January 2000. The number of large oocytes (>121 μm) increased from October to December 1998, whereas the number of large oocytes decreased suddenly from December 1998 to January 1999. In 1999, the number of large oocytes (101–120 μm) decreased rapidly from February to March, indicating that the clams spawned during this period.
There was seasonal variation in the monthly FI from February 1998 to January 2000 (Fig. 7). The highest FI was recorded in December 1998 (70%), and was similar to that in December 1999 (68%). The minimum FI was estimated in March of both years (19%), when the clams were in the spent and resorption stages. The FI did not differ from May to August 1998 or from May to September 1999. The FI increased significantly from October (38%) 1998 (P < 0.01). The maximum FI was observed in December 1998 (70%), when the clams were sexually mature and ready to spawn. The FI decreased significantly to 35% in January 1999, and also decreased by about 20% between February and March 1999 (P < 0.01). The main spawning period commenced from December 1998 to January 1999 and continued through February and March 1999; 90% of the ripe eggs were released for spawning in two spawning peaks. In addition, the variation in FI was strongly correlated with the variation in total chlorophyll concentration in the water column (P < 0.01).
Resorption of relict oocytes
The resorption stage was observed year-round, whereas degeneration and resorption of residual eggs was observed mainly in February–March 1999. Typical degenerating ovaries of L. elliptica collected in February 1999 are shown (Fig. 8). Invading hemocytes began phagocytic activity toward the relict oocytes (RO) (Fig. 8a). The resorption process began with the degeneration of the gelatinous membranes. The membranes and germinal vesicle of degenerative oocytes were phagocytosed by hemocytes, although some RO remained in the follicular lumen. Hemocytes invaded the follicular lumen and began phagocytosis of the remaining yolk granules (Fig. 8b). Hemocytes were round in shape, with a biased basophilic oval nucleus (Fig. 8c). After phagocytosis, resorption was completed in the inner membrane. Hemocytes were also observed around the follicular epithelium (Fig. 8d). At the end of the process, relics of the phagocytosed materials were observed at the follicular epithelium as rounded, opaque, eosinophilic corps (Fig. 8e, f).
Photomicrographs showing the resorption process in degenerative ovaries of Laternula elliptica. a–b Infiltration and encapsulation of oocytes. Hemocytes (H), relict oocytes (RO), nucleus (N), yolk granules (YG), gelatinous layer (GL). c Eosinophil granules (EG) transported by H and a wounded GL. d RO and phagocytosed oocytes (PHO) remaining in the follicle; phagocytosed materials (PM) transported to the follicular epithelium (FE). e Magnified image of PM transported by H. f Magnified image of PM deposited in follicle. Scale bar 20 μm
Discussion
Large lecithotrophic eggs of L. elliptica
Laternula elliptica eggs, excluding the gelatinous layer, varied from 120 to 150 μm in size, and were much larger than eggs of the Antarctic scallop, Adamussium colbeki, which exhibits planktotrophic larval development (approximately 55 μm in diameter; Berkman et al. 1991). The relatively large egg size of L. elliptica suggests that these clams produce lecithotrophic eggs (Bosch and Pearse 1988; Berkman et al. 1991; Arntz et al. 1994; Ansell and Harvey 1997). According to Pearse et al. (1991), the pelagic lecithotrophy might be an adaptation during evolutionary times to poor food availability in Antarctic waters. Poulin et al. (2002) also suggested that the lecithotrophy commonly observed in the Antarctic water could be a result of the selective extinction during the glacial maxima. Ansell and Harvey (1997) reported that the encapsulated trochophore embryos of the clam were observed 5 days after fertilization, and that by 18 days after fertilization, they had developed into shelled “larvae” within the egg capsules about 200 μm in size. Juvenile settlement of L. elliptica has been observed mainly in late April and May (Bosch and Pearse 1988). The size of shelled embryos of L. elliptica reported by Bosch and Pearse (1988) is somewhat larger than the size of mature eggs (120–150 μm in diameter) observed in this study, but as Ansell and Harvey (1997) pointed out, that is probably because the capsule was included in the measurement.
Food availability and clam reproduction
Microscopic examination of the gonads revealed that the clams became sexually mature in 1998, just before the summer phytoplankton bloom. Gonad maturation rapidly increased from October in both years, possibly as a consequence of optimal nutritional conditions in the same months. The phytoplankton blooms lead to high somatic growth rates, and then a peak in gonad maturation occurs in the austral summer (Ahn et al. 2003). In the present study, most of the ripe eggs occur in December and January. However, oocyte growth and gonad maturation were intensive during October and December in both years studied (P < 0.01). Ahn et al. (2003) reported that clam gonad mass increased significantly in October 1998 relative to the same time in 1999, indicating that food availability is a key factor regulating gonad growth. Thompson (1972) reported that the energetic transfer of ingested food from the digestive gland to the gonad in mussel occurred within approximately 7 days when adequate food was presented. This rapid conversion could support the maintenance of gametogenesis. Gonad maturation in L. elliptica occurred rapidly, within 3 months from October to December 1998, when food levels in the water column became sufficient; the peak of gamete production coincided with maximum food availability.
Several studies have estimated the spawning time of L. elliptica by investigating the reproductive condition of individuals collected from different Antarctic nearshore areas using direct and histological techniques (Table 2). These studies reported that the major spawning of L. elliptica occurred during the summer months. In Potter Cove, a small bay only a few kilometers from Marian Cove, the major spawning of L. elliptica occurred in January and February; up to 75% of the clams collected in these months exhibited spent gonads (Urban and Mercuri 1998). At Marian Cove, spawning of the clam has been observed in December, January, and February (Ahn et al. 2003). However, south of Marian Cove in McMurdo Sound, the spawning activity of L. elliptica was found to be highest in late March to early April, and it continued to mid-May (Bosch and Pearse 1988), indicating some latitudinal differences in spawning times.
We found that intensive spawning of L. elliptica occurs from December to February after phytoplankton blooms, and about 90% of the mature eggs in follicles were discharged at this time (Fig. 7). In addition, gonad mass also decreases by about 50% at this time (Ahn et al. 2003). Soniat and Ray (1985) reported that oyster fecundity was closely correlated with food availability during the annual gametogenic cycle. Park and Choi (2004) also suggested that the fecundity and spawning frequency of Ruditapes philippinarum tracks the frequency of plankton blooms. We observed two spawning peaks: during December and January 1999, and January and February 1999 (Figs. 5, 7). Ahn et al. (2003) also reported that the major spawning season of L. elliptica occurred when proteins and lipids in the storage tissues of clams were decreasing.
Kang et al. (2003) reported that FI measurement using a planimetric technique provided reliable quantitative information on L. elliptica reproduction. Seasonal variation in FI indicates variation in the reproduction of L. elliptica. Monthly changes in FI varied seasonally (Fig. 7), indicating that gametogenesis has a seasonality within an annual gonad growth pattern. Urban and Mercuri (1998) suggested that cyclic oogenesis took more than 1 year. In contrast, Bigatti et al. (2001) reported that complete growth of small oocytes was possible in about 7 months. We found, however, that the FI increased significantly from October in 1998 and 1999, and peaked in December in 1998 and 1999 (P < 0.01). The maximum FI was observed in December 1998, 3 months after the total chlorophyll maximum in the water column, indicating that the chlorophyll maximum caused rapid gonad growth in the clams. Our data suggest that the time required for eggs to complete vitellogenesis and be ready for spawning at Marian Cove is 3 months under phytoplankton bloom conditions. Ahn et al. (2003) reported that oocyte development and the increase in gonad mass proceeded at a much slower rate from March to August 1999. After October, however, gonad mass began to increase rapidly, and the mass peaked in December 1999. Laternula elliptica spawning occurred from December 1998 to March 1999 and coincided with the sudden decrease in FI (90%) in this season.
Resorption of residual eggs
The annual gametogenic cycle in marine bivalves is often categorized into several stages, according to the microscopic appearance of the ovary or testis. In brief, the cycle can be summarized in four phases: a resting phase, when gonadal tissues are nearly absent; the development and growth of ovary or testis; spawning to discharge eggs or sperm; and a resorption phase, when residual or relic gametes are degenerated and resorbed (Loosanoff 1942; Seed and Brown 1977; Sastry 1979). After major spawning periods, residual eggs are often degenerated by hemocytes and resorbed to recycle the nutrient-rich cells. Such resorption activity is considered a reproductive strategy of bivalves (Loosanoff 1942; de Jong Brink et al. 1983; Dorange et al. 1989; Ituarte 1997). We observed resorption in the gonad of L. elliptica. Relict eggs rapidly shrunk and were degenerated by hemocytes (Fig. 8); degenerating eggs were observed throughout the year. Dorange et al. (1989) suggested that the reuse of phagocytosed material from degenerated eggs was important in regulating valuable nutrients in the scallop, Pecten maximus, in which ripe eggs can be observed in the gonads year-round (Poulet et al. 1988; Mackie and Ansell 1993; Strohmeier et al. 2000) and the eggs are resorbed at any time in the reproductive cycle (Dorange et al. 1989). According to Thorson (1950) and Strathmann (1990, 1993), these resorbed materials would allocate alternative energetic resources for gametogenesis.
Urban and Mercuri (1998) observed that 60–80% of ovaries were ripe throughout the year in clams collected from Potter Cove, King George Island. Bigatti et al. (2001) re-examined Urban and Mercuri (1998) histological data and concluded that L. elliptica at Potter Cove were possibly prepared to spawn at any time in suitable conditions because mature eggs were observed all year round. Contrary to the observations of Urban and Mercuri (1998) and Bigatti et al. (2001), the spawning activity of clams collected from Marian Cove (Fig. 1) was rather confined to spring and summer (from October 1998 to February 1999 and from December 1999 to January 2000). We also found a number of clams in the resorption stage that exhibited degenerating ovaries. The resorption stage could be identified year-round, although intensive degeneration and resorption of relic or residual oocytes mostly occurred in February and March. The degeneration and resorption process was detected on serial sections of soft tissues after observing some trace of phagocytic activity in the first tissue section. Phagocytic activity was further confirmed using the hemocyte-specific Giemsa staining technique, and the degeneration of residual eggs was visualized (Fig. 8).
It is unclear why the resorption process was not observed in previous studies at Potter Cove, located only a few kilometers from Marian Cove. Because Potter Cove and Marian Cove are in close proximity, it is unlikely that different environmental conditions between the two locations, such as water temperature and food availability, resulted in the reported differences. It is also unlikely that the resorption stage was absent in previous studies because of year to year variation in gametogenesis in the clam, because the gametogenic pattern in the reproductive strategy of marine bivalves is thought to be a consequence of long-term evolutionary adaptation (Clarke 1987; Pearse et al. 1991; Poulin et al. 2002). Alternatively, the discrepancy could be the result of different processes used in the preparation of the histological slides. Often, only a small part of the gonadal tissue is taken from the body of marine bivalves for preparation of histological slides; thus, some cells or tissues may be overlooked.
Because the eggs of L. elliptica are large and lecithotrophic, egg production may require abundant nutritional energy to support vitelline synthesis. Thus, energy may be a limited resource for adult L. elliptica. And it depends on the time period of gametogenesis among other things. Our data demonstrate that the phagocytic activity by hemocytes in L. elliptica affects only relict eggs, and phagocytosed materials derived from relict eggs should be deposited in follicular cells as was observed in other species (Dorange et al. 1989; Ituarte 1997). This process could benefit the clam by increasing energy availability in follicular cells after spawning, for use in subsequent vitelline synthesis at a low energetic cost.
References
Ahn I-Y, Cho K-W, Choi K-S, Seo Y-W, Shin J-H (2000) Lipid content and composition of the Antarctic lamellibranch, Laternula elliptica (King & Broderip) (Anomalodesmata: Laternulidae), in King George Island during an austral summer. Polar Biol 23:24–33
Ahn I-Y, Surh J, Park Y-G, Kwon H, Choi K-S, Kang S-H, Choi HJ, Kim K-W, Chung H (2003) Growth and seasonal energetics of the Antarctic bivalve Laternula elliptica (King & Broderip) from King George Island, Antarctica. Mar Ecol Progr Ser 257:99–110
Ansell AD, Harvey R (1997) Protected larval development in the Antarctic bivalve Laternula elliptica (King and Broderip) (Anomalodesmata: Laternulidae). J Moll Stud 63:285–286
Arntz WE, Brey T, Gallardo VA (1994) Antarctic zoobenthos. Oceanogr Mar Biol Ann Rev 32:241–304
Bayne BL (1976) Marine mussels: their ecology and physiology. Cambridge University Press, Cambridge
Bayne BL, Newell RC (1983) Physiological energetics of marine molluscs. In: Saleuddin ASM, Wilbur KM (eds) The mollusca. Academic Press, London, pp 407–515
Berkman PA, Waller TR, Alexander SP (1991) Unprotected larval development in the Antarctic scallop Adamussium colbecki (Mollusca: Bivalvia: Pectinidae). Antarct Sci 3:151–157
Bigatti G, Penchaszadeh PE, Mercuri G (2001) Aspects of the gonadal cycle in the Antarctic bivalve Laternula elliptica. J Shellfish Res 20:283–287
Bosch I, Pearse JS (1988) Seasonal pelagic development and juvenile recruitment of the bivalve Laternula elliptica in McMurdo Sound, Antarctica. Am Zool 28:89A
Chang KI, Jun HK, Park GT, So YS (1990) Oceanographic conditions of Maxwell Bay, King George Island, Antarctica (Austral Summer 1989). Korean J Polar Res 1:27–46
Cheng TC (1981) Bivalves. In: Ratcliffe NA, Rowley AF (eds) Invertebrate blood cells. Academic Press, London, pp 233–300
Clarke A (1987) Temperature, latitude and reproductive effort. Mar Ecol Prog Ser 38:89–99
Clarke A, Prothero-Thomas E, Beaumont JC, Chapman AL, Brey T (2004) Growth in the limpet Nacella concinna from contrasting sites in Antarctica. Polar Biol 28:62–71
de Jong Brink MH, Boer HH, Joosse J (1983) Mollusca. In: Adiyodi KG, Adiyodi RG (eds) Reproductive biology of invertebrates, 1: oogenesis, oviposition and oosorption. Wiley, New York, pp 297–355
Dorange G, Paulet YM, Le Pennec M (1989) Etude cytologique de la partie femelle de la gonade de Pecten maximus recolte en baie de Saint-Brieuc. 2. Ovogenese et lyse ovocytaire. Haliotis 19:299–314
Grant CM, Creese RG (1995) The reproductive cycle of the tuatua-Paphies subtriangulata (Wood, 1828), in New Zealand. J Shellfish Res 14:287–292
Heffernan PB, Walker RL, Carr JL (1989) Gametogenic cycle of three marine bivalves in Wassaw Sound, Georgia: I Mercenaria mercenaria. J Shellfish Res 8:51–60
Hofmann EE, Powell EN, Klinck JM, Wilson EA (1992) Modeling oyster populations III. Critical feeding periods, growth and reproduction. J Shellfish Res 11:399–416
Hooker SH, Creese RG (1995) The reproductive biology of pipi, Paphies australis (Gmelin, 1790) (Bivalvia: Mesodesmatidae). I. Temporal patterns of the reproductive cycle. J Shellfish Res 14:7–15
Howard D, Smith C (1983) Histological techniques for marine bivalve mollusks. NOAA technical memorandum NMFS-F/NEC-25
Humason GL (1972) Staining hematologic elements and related tissues. In: Kennedy D, Park R (eds) Animal tissue techniques, 3rd edn edn. WH Freeman and Company, San Francisco, pp 255–259
Ituarte CF (1997) The role of follicular epithelium in the oosorption process in Eupera platensis Doello Jurado, 1921 (Bivalvia: Sphaeriidae): a light microscopic approach. Veliger 40:47–54
Kang S-H, Kang J-S, Chung K-H, Lee M-Y, Lee B-Y, Chung H, Kim Y, Kim D-Y (1997) Seasonal variation of nearshore Antarctic microalgae and environmental factors in Marian Cove, King George Island, 1996. Korean J Polar Res 8:9–27
Kang C-K, Park M-S, Lee P-Y, Choi W-J, Choi W-C (2000) Seasonal variations in condition, reproductive activity, and biochemical composition of the Pacific oyster, Crassostrea gigas (Thunberg), in suspended culture in two coastal bays of Korea. J Shellfish Res 19:771–778
Kang D-H, Ahn I-Y, Choi K-S (2003) Quantitative assessment of reproductive condition of the Antarctic clam, Laternula elliptica (King & Broderip), using image analysis. Invertebr Reprod Dev 44:71–78
Kennedy VS, Newell RIE, Eble AF (1996) The eastern oyster Crassostrea virginica. Maryland Sea Grant College Publication UM-SG-TS-TS-96-01, pp 734
KORDI (1999) The studies on natural environment and conservation of polar region. Korea Ocean Research & Development Institute (KORDI), ECPP 99001-03
Llodra ER (2002) Fecundity and life-history strategies in marine invertebrates. Adv Mar Biol 43:87–170
Loosanoff VL (1942) Seasonal gonadal changes in the adult oysters, Ostrea virginica, of Long Island Sound. Biol Bull 82:195–206
McCormick-Ray MG (1987) Hemocytes of Mytilus edulis affected by Prudhoe Bay crude oil emulsion. Mar Environ Res 22:107–122
Mackie LA, Ansell AD (1993) Differences in reproductive ecology in natural and transplanted populations of Pecten maximus: evidence for the existence of separate stocks. J Exp Mar Biol Ecol 169:57–75
Mann R (1979) Some biochemical and physiological aspects of growth and gametogenesis in Crassostrea gigas and Ostrea edulis grown at sustained elevated temperatures. J Mar Biol Assoc UK 59:95–110
Park K-I, Choi K-S (2004) Application of enzyme-linked immunosorbent assay for studying of reproduction in the Manila clam Ruditapes philippinarum (Mollusca: Bivalvia): I. Quantifying eggs. Aquaculture 241:667–687
Pearse JS, Bosch I (2002) Photoperiodic regulation of gametogenesis in the Antarctic sea star Odontaster validus Koehler: evidence for a circannual rhythm modulated by light. Invert Repro Dev 41:73–81
Pearse JS, Lockhart SJ (2004) Reproduction in cold water: paradigm changes in the 20th century and a role for cidaroid sea urchins. Deep Sea Res II 51:1533–1549
Pearse JS, McClintock JB, Bosch I (1991) Reproduction of Antarctic benthic marine invertebrates: tempos, modes, and timing. Am Zool 31:65–80
Peck LS, Colman JGA, Murray WA (2000) Growth and tissue mass cycles in the infaunal bivalve Yoldia eightsi at Signy Island, Antarctica. Polar Biol 23:420–428
Poulet YM, Lucas A, Gerard A (1988) Reproduction and larval development in two Pecten maximus (L.) populations from Brittany. J Exp Mar Biol Ecol 119:145–156
Poulin E, Palma AT, Féral J-P (2002) Evolutionary versus ecological success in Antarctic benthic invertebrates. Trends Ecol Evol 17:218–222
Powell DK, Tyler PA, Peck LS (2001) Effect of sperm concentratation and sperm ageing on fertilization success in the Antarctic soft-shelled clam Laternula elliptica and the Antarctic limpet Nacella concinna. Mar Ecol Prog Ser 215:191–200
Rivkin RB (1990) Seasonal patterns of planktonic production in McMurdo Sound, Antarctica. Am Zool 30:5–16
Sastry AN (1979) Pelecypoda (excluding Ostreidae). In: Giese AC, Pearse JS (eds) Reproduction of marine invertebrates. Academic Press, New York, 5:113–265
Saucedo PC, Rodríguez-Jaramillo C, Aldana-Avilés P, Monsalvo-Spencer THV, Monteforte M (2001) Gonadic conditioning of the calafia mother-of-pearl oyster, Pinctada mazatlanica (Hanley, 1856), under two temperature regimes. Aquaculture 195:103–119
Seed R, Brown RA (1977) A comparison of the reproductive cycles of Modiolus modiolus (L.), Cerastoderma (= Cardium) edule (L.), and Mytilus edulis L. in Strangford Lough, Northern Ireland. Oecologia 30:173–188
Sokal RR, Rohlf FJ (1981) Biometry. The principles and practice of statistics in biological research, 2nd edn. W. H. Freeman and Co., San Francisco
Soniat TM, Ray SM (1985) Relationships between possible available food and the composition, condition and reproductive state of oysters from Galveston Bay, Texas. Contrib Mar Sci 28:109–121
Strathmann RR (1990) Why life histories evolve differently in the sea. Am Zool 30:197–207
Strathmann RR (1993) Hypotheses on the origins of marine larvae. A Rev Ecol Syst 24:89–117
Strohmeier T, Duinker A, Lie Q (2000) Seasonal variations in chemical composition of the female gonad and storage organs in Pecten maximus (L.) suggesting that somatic and reproductive growth are separated in time. J Shellfish Res 19:741–747
Thompson RJ (1972) Feeding and metabolism in the mussel Mytilus edulis. L. PhD Thesis, University of Leicester
Thorson G (1950) Reproductive and larval ecology of marine bottom invertebrates. Biol Rev 25:1–45
Urban H-J, Mercuri G (1998) Population dynamics of the bivalve Laternula elliptica from Potter Cove, King George Island, South Shetland Islands. Antartic Sci 10:153–160
Yonge CM (1937) Evolution and adaptation in the digestive system of the Metazoa. Biol Rev 12:87–115
Yonge CM (1946) Digestion of animals by Lamellibranchs. Nature 157:729
Acknowledgments
This research was part of the project Status and changes of polar indicator species and coastal/terrestrial ecosystems (PE08040) and was supported by the Korea Polar Research Institute (KOPRI) of the Korea Ocean Research & Development Institute (KORDI). This study was also supported in part by the Korea Research Foundation (KRF) through The Second Stage of BK21 and KRF-2005-213-F00005 to Kang DH. We are grateful to the divers, Mr. Hyunsoo Kim and Mr. Sungsoo Han, for collecting the clams, and to Dr. Joseph G. Loesch for reviewing an early draft of this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kang, DH., Ahn, IY. & Choi, KS. The annual reproductive pattern of the Antarctic clam, Laternula elliptica from Marian Cove, King George Island. Polar Biol 32, 517–528 (2009). https://doi.org/10.1007/s00300-008-0544-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-008-0544-7