Abstract
Fatty acid analysis was used to study the trophic ecology of 10 demersal fish species in the South Georgia region. Principal component analysis grouped the species into three general clusters, revealing resource partitioning between species. Two groups were characterised by large proportions of either monounsaturated or polyunsaturated fatty acids, separating species according to their predominant feeding habitat. The third group showed fatty acid signatures overlapping with either or both of the previous two groups, suggesting a more opportunistic feeding behaviour for these species. Intraspecific comparisons furthermore revealed dietary variability with size, year and geographical location in several species. Mackerel icefish (Champsocephalus gunnari) in particular showed inter-annual differences in muscle lipid concentrations closely linked to prey availability with low lipid contents found in years of low krill (Euphausia superba) abundance. Despite the intraspecific differences the majority of species could be easily distinguished from each other, which indicates the utility of this method in the dietary analysis of higher predators.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The demersal fish community in the South Georgia archipelago is primarily comprised of species of the endemic demersal suborder Notothenioidei, which dominates the Antarctic ichthyofauna in shelf waters and includes nearly 70 % of all species and over 90 % of individuals (Kock 1992). Of the 4 prominent families (Nototheniidae, Channichthyidae, Harpagiferidae and Bathydraconidae) present in South Georgia waters the Nototheniidae and the Channichthyidae comprise the species most valuable to current international fisheries (Kock et al. 2007; Collins et al. 2010). This demersal fish community also supports large numbers of higher predators, particularly in years when krill is in low abundance (Everson et al. 1999; Barlow and Croxall 2002; Reid 2002). Fischer and Hureau (1985) reported that in the Southern Ocean, fish are the second most important prey for higher predators after krill, with predominantly demersal fish (e.g. Notothenioidei) being eaten in coastal waters.
Since commercial fishing in South Georgia waters started in the late 1960s the composition of the fish community has changed with a significant shift from a community dominated by a few large-bodied species to fewer, mostly small-bodied species with more even distributions (McKenna 1991). To effectively manage fish stocks and gauge the impact of the removal of a species on the ecosystem, it is crucial to investigate the trophic links between fish and other organisms in their environment. This has led to several studies investigating the distribution and trophic ecology of the demersal fish community in South Georgia waters (e.g. Permitin and Tarverdiyeva 1972; McKenna 1991; Everson et al. 1999).
The use of fatty acids as trophic biomarkers is based on the assumption that many fatty acids in the marine environment are characteristic of specific groups of phytoplankton or invertebrate species. These fatty acids can generally not be synthesised in higher trophic levels and are incorporated into the consumers’ tissues with minimal modification, thus retaining signatures of their dietary origin (Sargent et al. 1987). Once fatty acid patterns are established for prey, they can then be used to trace food webs and diets of higher predators. To investigate the trophic dynamics within the Scotia Sea ecosystem is particularly important as recent research has found evidence for rapid environmental change in the western Scotia Sea (Meredith and King 2005) and with it changes in predator population dynamics (e.g. Trathan et al. 2007).
Fatty acid analysis has previously been used to resolve trophic interactions in the South Georgia food web (e.g. Staniland and Pond 2005; Schmidt et al. 2006). Studies also exist on the fatty acid composition of notothenioid species (e.g. Lund and Sidell 1992; Sidell et al. 1995; Hagen et al. 2000) though not to our knowledge for the South Georgia region. However, most of these studies were based on small sample sizes and little is therefore known about intraspecific variation and the resulting consequences for higher predator studies.
In the present study, fatty acid analyses were used to investigate the trophic ecology of eight notothenioid, one muraenolepid and one morid species common to the South Georgia region. To establish the trophic position of individual species in the demersal fish community and their interactions, we compared fatty acid signatures between and within species. We examined evidence for intraspecific variation in regard to temporal, geographical and ontogenetic differences in the diet. These fatty acid data will also be useful in food web studies of higher predators and will help elucidate the importance of notothenioid species in their diet.
Materials and methods
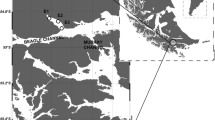
A total of 10 demersal fish species, comprising 230 specimens, were collected over the course of 3 years (2003, 2004, 2006, all January) from three cruises on the fisheries patrol and research vessel ‘FPV Dorada’ and one cruise (2004, March/April) on the ‘RRS James Clark Ross’ (NERC, British Antarctic Survey) in the northern Scotia Sea (Table 1; Fig. 1). On ‘RRS James Clark Ross’ samples were obtained by means of Rectangular Midwater Trawl (RMT) 25 (2.5 knots tow speed, codend mesh 5 mm; Baker et al. 1973), while on ‘FPV Dorada’ a FP120 bottom trawl net was used (4 knots tow speed, codend mesh 40 mm; Everson et al. 1999). Although caught with a pelagic net, the fish analysed from ‘RRS James Clark Ross (JCR)’ derived from 3 hauls carried out close to the seabed. Total length (TL) was measured in the majority of species. For Notothenia coriiceps (Nototheniidae) only standard length (SL) was available. Muscle tissue was collected dorso-laterally, immediately anterior to the dorsal fin of the fish. While samples collected on JCR were immediately stored at −80 °C samples collected on FPV Dorada were initially stored at −20 °C (max. 1 month) after which they were transferred to RRS James Clark Ross and also stored at −80 °C prior to fatty acid analysis.
Lipid content and fatty acid analysis
Muscle samples were freeze-dried for 24 h and subsequently homogenised in a 2:1 v/v chloroform/methanol solvent system, before being filtered through a pre-washed (chloroform/methanol) Whatman No.1 paper filter. Total Lipid was extracted as detailed by Folch et al. (1957) followed by trans-esterification in methanol, containing 1.5 % sulphuric acid, at 50 °C for 16 h to generate fatty acid methyl esters (FAME) (Christie 1982). FAME were purified by thin-layer chromatography (TLC) using a hexane/diethyl ether/acetic acid (90:10:1, v/v/v) solvent system. Purified FAME were extracted from the TLC in a hexane/diethyl ether solvent system (1:1, v/v). The solvent was evaporated under nitrogen and the samples dissolved in hexane to a concentration of 0.25 mg ml−1. Samples were analysed by on-column injection on a Thermo Finnigan Trace 2000 gas chromatograph, fitted with a ZBWAX column (30 m × 0.32 mm i.d.), with hydrogen as the carrier gas. Twenty-four fatty acids were identified by comparison to relative retention times of a known standard (Marinol) and comprise the fatty acid population chosen as representative for this study. Lipid concentrations are expressed as per cent lipid of dry mass of muscle tissue (% lipid DM) and fatty acids as percentages of the total array of fatty acids detected (normalised area percentages, % NA).
Data analysis
Principal component analysis (PCA) was performed to investigate the variation in fatty acid signatures between species, areas and years and identify the fatty acids most responsible for this variation. Data were normalised using an arcsine square root transformation. Cross-validation using discriminant analysis was applied to test the efficiency of fatty acid data in predicting group membership in the fish species analysed. For discriminant analysis the number of group variables has to exceed the number of predictor variables (Tabachnick and Fidell 2001). We therefore set the number of fatty acids to 15 to allow the four most abundant fish species Champsocephalus gunnari, Chaenocephalus aceratus, Patagonotothen guntheri and Dissostichus eleginoides to be included in the analysis. We used the 15 fatty acids that exhibited the greatest average variance across all species, accounting for approximately 96 % of total fatty acids identified. General linear models (GLM) using Tukey’s tests for pairwise comparisons were applied to examine inter-species as well as intraspecific variation in individual fatty acids. One-way ANOVA was applied to investigate inter-specific variation in n-3/n-6 fatty acid ratios. Intraspecific variation was tested for species where sufficient numbers and groupings were available (i.e. C. gunnari, C. aceratus, Notothenia rossii, Lepidonotothen squamifrons, P. guntheri and D. eleginoides). Size classes chosen for ontogenetic comparisons were selected according to the frequency distribution of fish lengths and resulting adequate sample size. For geographical comparisons of C. gunnari data a separation between South Georgia and Shag Rock waters was defined. There is a consistent northward flow through the trench between South Georgia and Shag Rocks, which separates the two shelf areas. This was demonstrated by the northward trajectory of eight Argo floats at 1,000 m and acoustic Doppler current profiler (ADCP) transects that also showed a north to north-west flow through the gap (Venables et al. 2012). Consequently all samples derived from hauls west of 40°W were designated as originating from Shag Rock waters and all samples derived from hauls east of 40°W as originating from South Georgia waters (Fig. 1).
Results
Between-species differences
Muscle tissue lipid concentrations were significantly different between species (ANOVA, df = 9, F = 33.7, p < 0.001). The lipid content of most species averaged between 3 and 6 % (DM) (Table 2). The lowest lipid content was found for Gobionotothen gibberifrons (2.5 %), and the highest average lipid content was found in D. eleginoides (21.7 %). N. coriiceps, N. rossii and P. guntheri all showed intermediate lipid levels of 11–13 %.
Principal component analysis separated species along the 1st and 2nd principal components (PC) (Fig. 2). Proportions of saturated fatty acids (SFAs) ranged from 24.4 to 36.3 %, with the highest proportions found for N. rossii and C. aceratus and the lowest levels found for D. eleginoides (Table 2). Palmitic acid (16:0) was the most common saturated fatty acid identified in all species followed by 14:0 and 18:0. A wide range of proportions were found for monounsaturated fatty acids with values ranging from 15.8 % in G. gibberifrons to 49.0 and 52.8 % in N. rossii and D. eleginoides, respectively. Of all monounsaturates (MUFAs) identified oleic acid 18:1(n-9) was the dominant MUFA for all species, followed by 16:1(n-7) and 18:1(n-7). The highest levels of long-chained MUFAs (i.e. 20:1(n-9), 20:1(n-7); 22:1(n-11) and 22:1(n-9)) were found for D. eleginoides, P. guntheri and N. rossii. The proportion of polyunsaturated fatty acids (PUFAs) was accordingly low for species with high MUFA and/or SFA content. Levels ranged from 14.8 % in N. rossii to 57.3 % in G. gibberifrons. The most prominent PUFAs identified were 20:5(n-3) and 22:6(n-3) for all species. Whereas the proportion of n-6 PUFAs showed relatively little variation between species (1.5–3.4 %) proportions of n-3 PUFAs were highly variable with values ranging from 9.6 % in N. rossii to 52.5 % in G. gibberifrons. Thus n-3/n-6 ratios were significantly different between species (ANOVA, p < 0.001). In accordance with high levels of n-6 PUFA and low levels of n-3 PUFAs Muraenolepis sp. and Antimora rostrata showed the highest n-3/n-6 ratios (23.2–27.0). In contrast the species with the lowest proportions of n-6 PUFAs (N. rossii and D. eleginoides) showed ratios of 6.5 and 11.4, respectively. Fatty acid signatures of all species are detailed in Table 2. A close relationship existed between the lipid content of muscle tissue of individual species and proportions of MUFAs and PUFAs (Fig. 3). The proportions of MUFAs (18:1(n-9) in particular) increased with increasing lipid content (r 2 = 0.756; p = 0.001), concurrent with a decrease in proportions of PUFAs (r 2 = 0.519; p = 0.019). No significant relationship existed between proportions of SFAs and lipid content (r 2 = 0.010; p = 0.785).
Principal component analysis of fatty acid composition for ten demersal fish species from the Scotia Sea. Analysis is based on the fatty acid signatures of 230 fish, using the 24 fatty acids presented in Table 2. a Score plot indicating relationships between individual fish and b loading plot indicating fatty acids contributing to the distribution of sample data points in the score plot
percentage composition of fatty acid classes SFA (saturated fatty acids), MUFA (monounsaturated fatty acids), PUFA (polyunsaturated fatty acids) and MUFA 18:1(n-9) in relation to lipid content of muscle tissue (% Lipid dry mass (DM)) of demersal fish species of the Scotia Sea. In increasing order of % lipid: Gg = Gobionotothen gibberifrons, Ar = Antimora rostrata, Msp = Muraenolepis sp., Ca = Chaenocephalus aceratus, Cg = Champsocephalus gunnari, Ls = Lepidonotothen squamifrons, Nc = Notothenia coriiceps, Pg = Patagonotothen guntheri, Nr = Notothenia rossii, De = Dissostichus eleginoides. Legend: Asterisks indicate statistically significant relationships
Discriminant analysis separated species with 98.6 % (204 of 207) of individuals correctly classified. Misclassifications were found in 2 of the 4 analysed species. These misclassifications occurred where strong overlap in fatty acid signatures existed between species (see also Fig. 2). One specimen of C. aceratus (1 out of 23) was identified as C. gunnari and two specimens of D. eleginoides (2 out of 36) as P. guntheri.
Within-species differences
High within-species variability was found in the lipid contents of muscle tissue of N. rossii, P. guntheri, L. squamifrons and D. eleginoides. However, no relationship was found with sex, body size, water depth, area or year for any of these species. Intraspecific separation in fatty acid signatures was found along the 1st PC for L. squamifrons. This variation in fatty acid signatures was correlated with lipid content of the muscle tissue but independent of sex, body size, geographical location or year of catch (all p > 0.05). While SFA 16:0 and PUFAs (20:4(n-6), 20:5(n-3), 22:6(n-3)) decreased with increasing lipid content SFA 14:0 and MUFAs 14:1(n-5), 16:1(n-7) and 18:1(n-9) were found in higher proportions in lipid-rich fish (Table 2; Fig. 3).
The two specimens of D. eleginoides with the lowest lipid levels had associated with it lower levels of 16:0 and MUFAs (14:1(n-5), 16:1(n-7) and 18:1(n-9)) and highly elevated levels of 14:0 and PUFAs 20:4(n-6), 20:5(n-3) and in particular 22:6(n-3), thus grouping them closer to C. gunnari than to their conspecifics. Across the whole size range MUFA 18:1(n-9) (df = 4, F = 32.82, p < 0.001) increased with increasing size and both 20:5(n-3) (df = 4, F = 14.57, p < 0.001) and 22:6(n-3) (df = 4, F = 9.45, p < 0.001) decreased. No differences in fatty acid signatures were found with respect to year, water depth or area of capture.
The separation along the 2nd PC for samples of N. rossii was due to elevated levels of SFA 16:0 and MUFA 18:1(n-7) and low levels of C18, C20 and C22 PUFAs in the two samples closest to C. aceratus. Specimens of C. aceratus were separated through the 1st and 2nd PC, based on higher proportions of PUFAs for specimens grouped closely to C. gunnari and higher proportions of MUFAs and C16:0 for samples separated along the 2nd PC. No relationship in fatty acid variation was found with lipid content, sex, size of the fish, maturity or geographical location for either N. rossii or C. aceratus (all p > 0.05).
Champsocephalus gunnari exhibited significant inter-annual variation in the lipid content of muscle tissue (df = 2, F = 5.42, p < 0.01) with specimens caught in 2003 (5.9 ± 2.4 % DM) showing higher lipid concentrations than fish caught in the two subsequently sampled years (2004: 5.0 ± 2.1 % DM; 2006: 4.6 ± 0.9 % DM). Fatty acid signatures were significantly different between C. gunnari from Shag Rock and South Georgia waters for fish collected in 2004 and 2006, respectively (Fig. 4). In 2004 the major differences between the two regions were elevated levels of 18:1(n-7) (df = 1, F = 7.23, p < 0.05) and 20:5(n-3) (df = 1, F = 6.71, p < 0.05) and lower proportions of 16:1(n-7) (df = 1, F = 4.54, p < 0.05) in fish caught in South Georgia as opposed to fish caught at Shag Rocks. In contrast in 2006 fish caught in South Georgia waters had significantly higher proportions of 22:6(n-3) (df = 1, F = 85.81, p < 0.001) in their tissues in conjunction with lower proportions of 16:1(n-7) (df = 1, F = 103.19, p < 0.001) and 20:1(n-9)(df = 1, F = 8.42, p < 0.01). There was no significant difference in body size between the two regions in either year (T tests, South Georgia: df = 54, p > 0.05; Shag Rocks: df = 16, p > 0.05). C. gunnari furthermore showed strong inter-annual (df = 2; F = 77.17; p < 0.01) and ontogenetic (df = 4; F = 5.05; p < 0.01) variation in n-3/n-6 ratios with mean ratios of fish collected in 2003 (7.4) and 2004 (8.1) being significantly lower than those of fish collected in 2006 (10.6) and larger fish exhibiting lower n-3/n-6 ratios. However, body size was strongly correlated with year of catch with the majority of small fish collected in 2006.
Variation in selected fatty acids with geographical location for Champsocephalus gunnari in 2004 and 2006. Analysis was based on the following groupings: South Georgia 2004 (n = 9); Shag Rocks 2004 (n = 10); South Georgia 2006 (n = 65); Shag Rocks 2006 (n = 34). Asterisks indicate statistically significant differences
Discussion
This study demonstrated that the majority of demersal fish species investigated could be readily distinguished by their fatty acid signatures. Furthermore inter- and intraspecific comparisons revealed resource partitioning between species and dietary variability within species. Species were separated into two main groups, with tissue profiles being characterised either by large proportions of MUFAs or large proportions of long-chained PUFAs separating species according to their predominant feeding habitat. The remainder of species showed fatty acid profiles that overlapped with either group, indicating a more opportunistic feeding behaviour within species.
Between-species differences
The 10 species investigated in this study spanned a wide range of lipid contents. Closely linked to lipid content were the relative contributions of MUFAs and PUFAs. Species with high lipid content in their muscle tissues showed high concentrations of MUFAs with correspondingly low proportions of PUFAs. The amount of lipid stored and the preferential retention of certain fatty acids in the muscle tissue have previously been linked to energy requirements and habitat use in Antarctic fish (Lund and Sidell 1992; Friedrich and Hagen 1994, Nichols et al. 1994). Notothenioids for example are known to deposit their lipid reserves as triacylglycerols, mainly in the form of SFAs and MUFAs, with 16:0 and 18:1(n-9) contributing the bulk of fatty acids (e.g. Clarke et al. 1984; Hagen et al. 2000). Although MUFA deposits may be used for energy metabolism in Antarctic fish (e.g. Sidell et al. 1995), their accumulation likely plays a more important role in improving buoyancy (Hazel and Sidell 2004), a characteristic of particular importance to notothenioid species due to their lack of a swim bladder. It is therefore not surprising that the species with the highest lipid contents and proportions of MUFAs inhabited predominantly pelagic trophic niches(i.e. D. eleginoides, P. guntheri, N. rossii and N. coriiceps), the supportive evidence of which will be described for each species individually below.
High proportions of long-chained MUFAs in the tissues of marine predators are generally derived from marine zooplankton in particular calanoid copepods such as Calanoides acutus and Calanus propinquus in Antarctic waters (Pond and Tarling 2011; Pond et al. 2012). D. eleginoides, the species with the highest levels of long-chained MUFAs, has previously been classified as an opportunistic predator mainly feeding on fish, squid and, to a smaller degree, on Euphausia superba (Antarctic krill) (e.g. Pilling et al. 2001; Collins et al. 2007). The majority of toothfish investigated here were juveniles caught on the Shag Rocks shelf, which is where the juveniles are particularly abundant (Collins et al. 2007). These juvenile toothfish are mostly piscivorous and their primary prey on the Shag Rocks shelf is Patagonotothen guntheri (Collins et al. 2007). P. guntheri feeds on copepods and other macrozooplankton (Collins et al. 2008) and is therefore the likely source of long-chained MUFAs in D. eleginoides.
In contrast, N. rossii has been described as an omnivorous planktonic feeder with krill, hyperiid amphipods, mysids and gelatinous zooplankton identified from stomach contents (e.g. Tarverdiyeva 1972; McKenna 1991). Although Antarctic krill plays an important role in the diet of N. rossii, in seasons and areas of low krill abundance the species was found to feed mainly on a diet of amphipods and gelatinous zooplankton (e.g. Tarverdiyeva 1972). Neither hyperiid amphipods nor Antarctic krill store large amounts of long-chained MUFAs in their tissues (e.g. Hagen et al. 2001; Nelson et al. 2001). However, considerable proportions of long-chained MUFAs are found in gelatinous zooplankton, which in turn derive these fatty acids through feeding on calanoid copepod species (Nelson et al. 2000). Since krill abundance was low in the season our samples were collected (summer 2004, see Main et al. 2009) the fatty acid signatures of N. rossii suggest that gelatinous zooplankton could have contributed a major part to the diet of these fish. The overlap of some specimens of N. rossii with species C. aceratus was mainly based on elevated levels of 16:0 and 18:1(n-7), trophic markers particularly high in herbivorous prey (Kattner and Hagen 1995).
Of the three species that exhibited the highest levels of long-chained MUFAs (D. eleginoides, P. guntheri and N. rossii) only P. guntheri is known to feed directly on copepod prey (e.g. Collins et al. 2008). This species is considered a benthopelagic feeder with bigger fish feeding mostly on krill and amphipods (Laptikhovsky 2004) and smaller fish (<180 mm) feeding predominantly on copepods (see Collins et al. 2008). Fish analysed in this study were small (mean total length: 160 mm) and fatty acid signatures showed elevated levels of typical phytoplankton (16:1(n-7), 18:4(n-3)) and copepod markers (20:1(n-9), 22:1(n-11)), suggesting that herbivorous copepods comprised an important part of their diet. Several specimens of P. guntheri also showed higher proportions of short-chained MUFAs (C16, C18) together with lower proportions of long-chained MUFAs (C20, C22), a combination of biomarkers which is generally found in Antarctic krill or the hyperiid amphipod Themisto gaudichaudii (Hagen et al. 2001; Nelson et al. 2001). This suggests that these prey species also contributed to the diet of P. guntheri, which would in turn account for the overlap in fatty acid signatures with C. gunnari and N. coriiceps in the present study.
N. coriiceps is rarely caught in South Georgia groundfish surveys (M. Collins pers. comm.), and to our knowledge, no dietary data exists for this species from South Georgia waters. In other regions of the Southern Ocean N. coriiceps actively grazes on macroalgae throughout the year and feeds on benthic and pelagic invertebrates in varying proportions, depending on the season (Casaux et al. 1990; Iken et al. 1997). The fatty acid signatures found for the two juvenile specimens caught in autumn 2004 showed a combination of trophic markers indicative of a diet of Antarctic krill and T. gaudichaudii (Phleger et al. 1998) rather than trophic markers typical for Antarctic macroalgae or benthic feeding (Graeve et al. 1997, 2002), which is not surprising as the two specimens were caught offshore in Shag Rock waters. The presence of relatively large proportions of C20 MUFAs furthermore indicated a copepod component to the food chain, which would account for the overlap in fatty acid signatures with both copepod and krill and amphipod feeders (see Fig. 2a).
Due to its commercial value and its importance to higher predators the general biology and trophic position of C. gunnari have been studied in detail over the last four decades (e.g. Permitin and Tarverdiyeva 1972; Kock et al. 1994; Main et al. 2009). In the northern part of the species distribution range (e.g. South Georgia) Antarctic krill was found to be the main prey species (Kock et al. 1994; Main et al. 2009). Smaller euphausid species, T. gaudichaudii, mysids and pelagic fish comprised the remainder of the diet (e.g. Kock 2005 and references therein; Main et al. 2009). Fatty acid profiles of C. gunnari supported findings from stomach content analysis and indicated a dominance of Antarctic krill and possibly T. gaudichaudii in the diet.
The fatty acid signature of the single specimen of Muraenolepis sp. was almost indistinguishable from those found for C. gunnari. All previous dietary studies of Muraenolepis sp. identified predominantly benthic prey from the stomachs with small numbers of pelagic amphipods and krill also found (e.g. Kompowski 1993). The lack of large proportions of typical benthic biomarkers (e.g. 18:2(n-6), 20:4(n-6), Graeve et al. 1997) in the specimen of Muraenolepis analysed in the current study suggests that benthic invertebrates were not the main prey for this individual. This is further supported by Kompowski (1993) who found that in larger specimens of the genus, such as analysed in this study (see Table 1; max. reported length 35 cm Cohen et al. 1990), the importance of fish and krill in the diet increases and the importance of benthic invertebrates decreases.
The notothenid G. gibberifrons showed the largest proportions of benthic biomarkers (i.e. 18:2(n-6), 20:4(n-6)) and long-chained PUFAs of all species analysed. The presence of benthic biomarkers in the tissues of G. gibberifrons concurs with findings from stomach content studies, where benthic infauna and sedentary polychaetes comprised the major part of the diet (e.g. Moreno and Osorio 1977; McKenna 1991).
The remainder of species had fatty acid compositions that overlapped with both copepod and krill and amphipod feeders. While two-thirds of the specimens of C. aceratus showed strong overlap in fatty acid signatures with C. gunnari the remainder were well separated through their high levels of short-chained SFAs and total MUFAs in the tissue. C. aceratus is the largest icefish species in South Georgia waters and considered an opportunistic epibenthic predator (McKenna 1991). It feeds predominantly on fish, mysids and Antarctic krill. Juvenile C. aceratus feed mainly on krill with mysids and fish adding a minor component to the diet and adults take primarily fish (notothenids and some myctophids) and krill and mysids become less important (Reid et al. 2007). These ontogenetic differences in feeding were, however, not reflected in the fatty acid signatures as intraspecific separation was independent of body size or maturity. Results rather indicate that over an extended period of time C. aceratus grouped with C. gunnari fed on a diet of Antarctic krill and/or their predators while the outlying specimens fed on a more planktivorous diet. The high levels of short-chained SFAs and total MUFAs indicate the contribution of zooplankton rich in wax esters in the diet of these specimens (e.g. Graeve et al. 1994; Hagen and Kattner 1998).
Lepidonotothen squamifrons and A. rostrata showed high intraspecific variation with individual specimens high in either pelagic or benthic trophic markers. A dietary study conducted at South Georgia identified L. squamifrons as a benthopelagic invertebrate feeder with stomach contents comprising mainly salps (46 % frequency of occurrence), amphipods, benthic invertebrates (mainly polychaetes) and a very small fraction of Antarctic krill (McKenna 1991). A similar diet was found for specimens collected at the same time as the fish analysed in the current study with krill, gelatinous zooplankton and amphipods comprising the bulk of the diet (British Antarctic Survey, unpublished data). Specimens caught at the Prince Edward Islands also contained a significant proportion of myctophid remains (56 % FO) in their stomachs and were classified as a planktivorous species that occasionally supplemented its diet with benthos and mesopelagic fish (Pakhomov et al. 2006). Copepod predators such as myctophids and amphipods likely contributed an important part to the diet of specimens that show high levels of long-chained MUFAs and thus group closely with D. eleginoides and P. guntheri.
To our knowledge no published dietary data exists on A. rostrata from South Atlantic waters. In the North Atlantic A. rostrata is considered a benthopelagic feeder with fish and squid constituting the main prey (Mauchline and Gordon 1986). A fatty acid study on the trophic position of deep sea fish in North Atlantic waters also identified A. rostrata as a benthopelagic feeder with a stronger link to the pelagic based on the proportions of benthic and pelagic biomarkers found in their tissue (Stowasser et al. 2009). Specimens investigated in the present study overall showed higher proportions of long-chained MUFAs and lower proportions of benthic markers (e.g. 18:2(n-6), 20:4(n-6)) than their North Atlantic conspecifics, suggesting that copepods and their predators played a bigger part in the food chain of A. rostrata in South Georgia waters than found in the North Atlantic.
Within-species differences
For a number of species fatty acid signatures varied in accordance with ontogenetic changes in feeding and geographical and temporal variations of the environment. Intraspecific variation in the fatty acids of L. squamifrons and D. eleginoides was linked to lipid content of the muscle tissue. Higher lipid contents correlated with higher proportions of MUFAs while fish with low lipid contents had muscle tissue with higher proportions of PUFAs. Sidell et al. (1995) have shown that monounsaturated fatty acids are preferentially used for energy metabolism in Antarctic fish. Particularly in areas of highly fluctuating food availability it seems advantageous for fish to retain these MUFAs and store them as lipid reserves in their tissues. Lipid reserves in fatty tissues generally increase with increasing size of the fish. However, in L. squamifrons lipid concentrations and fatty acid composition seemed to be independent of body size. A fluctuation in food availability and resultant mobilisation of stored fatty acids for metabolic energy could account for the lack of correlation between muscle lipid content and size in this species.
Significant inter-annual differences in both lipid concentrations and fatty acid composition were found for C. gunnari. Muscle lipid levels were significantly higher in 2003 than in the two following years even though fish were larger in 2004. A trophic study conducted in South Georgia waters found that in the summer of 2003/2004 C. gunnari had low numbers of krill in their stomachs and fish were in overall poor condition (Main et al. 2009). In years of low krill abundance C. gunnari has been found to feed on higher numbers of small euphausiids such as Thysanoessa sp. and the amphipod T. gaudichaudii (Permitin and Tarverdiyeva 1972; Kock et al. 1994; Main et al. 2009). Although similar in lipid content and thus energetic value to E. superba (Reinhardt and Van Vleet 1986), both Thysanoessa sp. and T. gaudichaudii are only a fraction of the size of a medium-sized krill and therefore yield less energy for the time spent on foraging. Thus the low lipid contents in fish caught in 2004 are likely due to higher energy expenditure during foraging. Although by 2006 both krill and C. gunnari fish stocks had recovered (Main et al. 2009) the fish caught were on average smaller than in the previous two years and thus, as expected, had lower lipid levels.
Differences in n-3/n-6 ratios between years are likely based on body size and thus ontogenetic differences in feeding. The fatty acids mostly contributing to these differences were higher amounts of the benthic biomarkers 18:2(n-6) and 20:4(n-6) (Graeve et al. 1997) in larger fish, thus lowering n-3/n-6 ratios in C. gunnari from 2003 to 2004. In large C. gunnari these benthic biomarkers could be derived from fish and mysid prey as larger specimens are reported to feed primarily on krill, small demersal and pelagic fish and mysids while juvenile C. gunnari take primarily hyperiid amphipods and small euphausiid species (Kock 2005).
Geographical variation in fatty acid composition was found for C. gunnari caught in 2004 and 2006, with differences apparent between Shag Rocks and South Georgia caught fish. Dietary differences between the two regions have previously been found with a higher frequency of Thysanoessa sp. and T. gaudichaudii found in the stomach contents of fish caught at Shag Rocks. This geographical difference, however, seemed to be dependent on the year of catch and general prey availability (Kock et al. 1994; Main et al. 2009). Since no ontogenetic difference was found for fish between South Georgia and Shag Rocks in either year, differences in fatty acids are likely due to variations in prey availability in the two regions. The analysis of historic catch data has shown that Antarctic krill is generally more abundant to the north of South Georgia than at Shag Rocks (Atkinson et al. 2008). In both years the fish at Shag Rocks showed higher proportions of 16:1(n-7) in their tissues and in 2006 also higher proportions of 20:1(n-9). In planktonic systems 16:1(n-7) is generally of diatom origin and together with 20:1(n-9) can be found in high proportions in calanoid copepod species frequently found in the stomach contents of T. gaudichaudii and Thysanoessa sp. (Reinhardt and Van Vleet 1986; Pakhomov and Perissinotto 1996). C. gunnari in South Georgia waters showed higher levels of 18:1(n-7) and long-chained PUFAs in their muscle tissue, which indicates the contribution of prey feeding on a phytoplankton diet (Graeve et al. 1994). At the same time the proportions of long-chained MUFAs were lower, it is therefore likely that prey other than copepods contributed to the diet in this area.
In summary fatty acid composition not only supported the results previously gained from stomach content analysis, but also found dietary specialisations and species overlap not previously detected. The demersal fish community in South Georgia waters seems to feed to a large extent in the pelagic realm with either Antarctic krill or copepods and their predators constituting the major components of the diet. Recent research into the trophic ecology of Antarctic krill, however, has shown that migration to the seabed and benthic feeding are an important part of the life of adult krill (Schmidt et al. 2011) and rather than leaving the seabed the fish species showing predominantly pelagic biomarkers might just take advantage of these benthopelagic aggregations (e.g. Main and Collins 2011). The lack of correlation of fatty acid signatures with either morphological or environmental factors has furthermore stressed the presence of highly opportunistic feeding behaviour in some of the species analysed. Although spatial, temporal and ontogenetic differences in diet were found in some species inter-specific variability was greater than intraspecific variability which has important implications for the investigation of food web interactions of higher predators. While our analysis has shown that the majority of species could be readily distinguished by their fatty acid signatures, the results also indicate that some species (e.g. Muraenolepis sp. versus C. gunnari) will be more difficult to distinguish from each other. However, alternative investigative approaches such as stomach content or stable isotope analysis have proven to be powerful complementary methods and could be useful additional tools when investigating the feeding ecology of higher predators.
References
Atkinson A, Siegel V, Pakhomov EA, Rothery P, Loeb V, Ross RM, Quetin LB, Schmidt K, Fretwell P, Murphy EJ, Tarling GA, Fleming AH (2008) Oceanic circumpolar habitats of Antarctic krill. Mar Ecol Prog Ser 362:1–23
Baker ADC, Clarke MR, Harris MJ (1973) The N. I. 0. combination net (RMT 1 + 8) and further developments of rectangular midwater trawls. J Mar Biol Assess UK 53:167–184
Barlow KE, Croxall JP (2002) Seasonal and interannual variation in foraging range and habitat of macaroni penguins Eudyptes chrysolophus at South Georgia. Mar Ecol Prog Ser 232:291–304
Casaux RJ, Mazzotta AS, Barreraoro ER (1990) Seasonal aspects of the biology and diet of nearshore nototheniid fish at Potter Cove, South Shetland Islands, Antarctica. Polar Biol 11:63–72
Christie WW (1982) Lipid analysis. Pergamon Press, Oxford
Clarke A, Doherty N, Devries AL, Eastman JT (1984) Lipid-content and composition of 3 species of Antarctic fish in relation to buoyancy. Polar Biol 3:77–83
Cohen DM, Inada T, Iwamoto T, Scialabba N (1990) FAO Species Catalogue Vol 10 Gadiform fishes of the world (Order Gadiformes). An annotated and illustrated catalogue of cods, hakes, grenadiers and other gadiform fishes known to date. FAO Fish Synop 10(125):442
Collins MA, Ross KA, Belchier M, Reid K (2007) Distribution and diet of juvenile patagonian toothfish on the South Georgia and Shag Rocks shelves (Southern Ocean). Mar Biol 152:135–147
Collins MA, Shreeve RS, Fielding S, Thurston MH (2008) Distribution, growth, diet and foraging behaviour of the yellow-fin notothen Patagonotothen guntheri (Norman) on the Shag Rocks shelf (Southern Ocean). J Fish Biol 72:271–286
Collins MA, Brickle P, Brown J, Belchier M (2010) The Patagonian Toothfish: Biology, ecology and fishery. Adv Mar Biol 58:227–300
Everson I, Parkes G, Kock KH, Boyd IL (1999) Variation in standing stock of the mackerel icefish Champsocephalus gunnari at South Georgia. J Appl Ecol 36:591–603
Fischer W, Hureau J-C (eds) (1985) FAO species identification sheets for fishery purposes, Southern Ocean, CCAMLR Convention Area, Fishing Areas 48, 58, and 88. Food and Agriculture Organization, Rome
Folch J, Lees M, Stanley GHS (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–509
Friedrich C, Hagen W (1994) Lipid contents of 5 species of notothenioid fish from high-Antarctic waters and ecological implications. Polar Biol 14:359–369
Gon O, Heemstra PC (1990) Fishes of the Southern Ocean. Institute of Ichthyology, Grahamstown
Graeve M, Hagen W, Kattner G (1994) Herbivorous or omnivorous? On the significance of lipid compositions as trophic markers in Antarctic copepods. Deep Sea Res I 41:915–924
Graeve M, Kattner G, Piepenburg D (1997) Lipids in Arctic benthos: does the fatty acid and alcohol composition reflect feeding and trophic interactions? Polar Biol 18:53–61
Graeve M, Kattner G, Wiencke C, Karsten U (2002) Fatty acid composition of Arctic and Antarctic macroalgae: indicator of phylogenetic and trophic relationships. Mar Ecol Prog Ser 231:67–74
Hagen W, Kattner G (1998) Lipid metabolism of the Antarctic euphausiid Thysanoessa macrura and its ecological implications. Limnol Oceanogr 43:1894–1901
Hagen W, Kattner G, Friedrich C (2000) The lipid compositions of high-Antarctic notothenioid fish species with different life strategies. Polar Biol 23:785–791
Hagen W, Kattner G, Terbruggen A, Van Vleet ES (2001) Lipid metabolism of the Antarctic krill Euphausia superba and its ecological implications. Mar Biol 139:95–104
Hazel JR, Sidell BD (2004) The substrate specificity of hormone-sensitive lipase from adipose tissue of the Antarctic fish Trematomus newnesi. J Exp Biol 207:897–903
Iken K, Barrera-Oro ER, Quartino ML, Casaux RJ, Brey T (1997) Grazing by the Antarctic fish Notothenia coriiceps: evidence for selective feeding on macroalgae. Antarc Sci 9:386–391
Kattner G, Hagen W (1995) Polar herbivorous copepods—different pathways in lipid biosynthesis. ICES J Mar Sci 52:329–335
Kock KH (1992) Antarctic fish and fisheries. Cambridge University Press, Cambridge
Kock KH (2005) Antarctic icefishes (Channichthyidae): a unique family of fishes. A review, Part I. Polar Biol 28:862–895
Kock KH, Wilhelms S, Everson I, Groger J (1994) Variations in the diet composition and feeding intensity of mackerel icefish Champsocephalus gunnari at South Georgia (Antarctic). Mar Ecol Prog Ser 108:43–57
Kock KH, Reid K, Croxall JP, Nicol S (2007) Fisheries in the Southern Ocean: an ecosystem approach. Phil Trans R Soc B 363:2333–2349
Kompowski A (1993) Food and feeding behaviour of eel-cod Muraenolepis sp., (Pisces, Gadiformes, Muraenolepididae) from the region of South Georgia. Acta Ichthyol Piscat 23:59–68
Laptikhovsky VV (2004) A comparative study of diet in three sympatric populations of Patagonotothen species (Pisces: Nototheniidae). Polar Biol 27:202–205
Lund ED, Sidell BD (1992) Neutral lipid compositions of Antarctic fish tissues may reflect use of fatty acyl substrates by catabolic systems. Mar Biol 112:377–382
Main CE, Collins MA (2011) Diet of the starry skate Amblyraja georgiana (Rajidae) at South Georgia. Pol Biol 34:389–396
Main CE, Collins MA, Mitchell R, Belchier M (2009) Identifying patterns in the diet of mackerel icefish (Champsocephalus gunnari) at South Georgia using bootstrapped confidence intervals of a dietary index. Polar Biol 32:569–581
Mauchline J, Gordon JDM (1986) Foraging strategies of deep-sea fish. Mar Ecol Prog Ser 27:227–238
McKenna JE (1991) Trophic relationships within the Antarctic demersal fish community of South Georgia Island. Fish B-NOAA 89:643–654
Meredith MP, King JC (2005) Rapid climate change in the ocean west of the Antarctic Peninsula during the second half of the 20th century. Geophys Res Lett 32. doi:10.1029/2005GL024042
Moreno CA, Osorio HH (1977) Bathymetric food habit changes in the Antarctic fish, Notothenia gibberifrons Lönnberg (Pisces:Nototheniidae). Hydrobiologia 55:139–144
Nelson MM, Phleger CF, Mooney BD, Nichols PD (2000) Lipids of gelatinous Antarctic zooplankton: Cnidaria and Ctenophora. Lipids 35:551–559
Nelson MM, Mooney BD, Nichols PD, Phleger CF (2001) Lipids of Antarctic Ocean amphipods: food chain interactions and the occurrence of novel biomarkers. Mar Chem 73:53–64
Nichols DS, Williams D, Dunstan GA, Nichols PD, Volkman JK (1994) Fatty acid composition of Antarctic and temperate fish of commercial interest. Comp Biochem Physiol B 107:357–363
Pakhomov EA, Perissinotto R (1996) Trophodynamics of the hyperiid amphipod Themisto gaudichaudii in the South Georgia region during late austral summer. Mar Ecol Prog Ser 134:91–100
Pakhomov EA, Bushula T, Kaehler S, Watkins BP, Leslie RW (2006) Structure and distribution of the slope fish community in the vicinity of the sub-Antarctic Prince Edward Archipelago. J Fish Biol 68:1834–1866
Permitin YY, Tarverdiyeva MI (1972) The food of some Antarctic fish in the South Georgia area. J Ichthyol 12:104–114
Phleger CF, Nichols PD, Virtue P (1998) Lipids and trophodynamics of Antarctic zooplankton. Comp Biochem Physiol B 120:311–323
Pilling GM, Purves MG, Daw TM, Agnew DA, Xavier JC (2001) The stomach contents of Patagonian toothfish around South Georgia (South Atlantic). J Fish Biol 59:1370–1384
Pond DW, Tarling GA (2011) Phase transitions of wax esters adjust buoyancy in diapausing Calanoides acutus. Limnol Oceanogr 56:1310–1318
Pond DW, Tarling GA, Ward P, Mayor D (2012) Wax ester composition influences the diapause patterns in the copepod, Calanoides acutus. Deep-Sea Res II 59–60:93–104
Reid K (2002) Growth rates of Antarctic fur seals as indices of environmental conditions. Mar Mammal Sci 18:469–482
Reid WDK, Clarke S, Collins MA, Belchier M (2007) Distribution and ecology of Chaenocephalus aceratus (Channichthyidae) around South Georgia and Shag Rocks (Southern Ocean). Polar Biol 30:1523–1533
Reinhardt SB, Van Vleet ES (1986) Lipid composition of twenty-two species of Antarctic midwater zooplankton and fish. Mar Biol 91:149–159
Sargent JR, Parkes JR, Mueller-Harvey I, Henderson RJ (1987) Lipid biomarkers in marine ecology. In: Sleigh MA (ed) Microbes in the sea. Ellis Horwood Ltd., Chichester, pp 119–138
Schmidt K, Atkinson A, Petzke KJ, Voss M, Pond DW (2006) Protozoans as a food source for Antarctic krill, Euphausia superba: Complementary insights from stomach content, fatty acids, and stable isotopes. Limnol Oceanogr 51:2409–2427
Schmidt K, Atkinson A, Steigenberger S, Fielding S, Lindsay MCM, Pond DW, Tarling GA, Klevjer TA, Allen CS, Nicol S, Achterberg EP (2011) Seabed foraging by Antarctic krill: Implications for stock assessment, bentho-pelagic coupling, and the vertical transfer of iron. Limnol Oceanogr 56:1411–1428
Sidell BD, Crockett EL, Driedzic WR (1995) Antarctic fish tissues preferentially catabolize monoenic fatty acids. J Exp Zool 271:73–81
Staniland IJ, Pond DW (2005) Investigating the use of milk fatty acids to detect dietary changes: a comparison with faecal analysis in Antarctic fur seals. Mar Ecol Prog Ser 294:283–294
Stowasser G, McAllen R, Pierce GJ, Collins MA, Moffat CF, Priede IG, Pond DW (2009) Trophic position of deep-sea fish-Assessment through fatty acid and stable isotope analyses. Deep Sea Res I 56:812–826
Tabachnick BG, Fidell LS (2001) Using multivariate statistics. Allyn and Bacon, Boston
Tarverdiyeva MI (1972) Daily food consumption and feeding pattern of the South Georgian cod (Notothenia rossii marmorata Fischer) and the Patagonian toothfish (Dissostichus eleginoides Smitt) (family Nototheniidae) in the South Georgia area. J Ichthyol 12:684–692
Trathan PN, Forcada J, Murphy EJ (2007) Environmental forcing and Southern Ocean marine predator populations: effects of climate change and variability. Phil T Roy Soci B 362:2351–2365
Venables HJ, Meredith MP, Atkinson A, Ward P (2012) Fronts and habitat zones in the Scotia Sea. Deep-Sea Res II(59–60):14–24
Acknowledgments
The authors thank the captain, crew and scientists on board RRS James Clark Ross during cruise JR 100 and on FPV Dorada during the South Georgia groundfish surveys in 2003, 2004 and 2006 (funded by the Government of South Georgia and the South Sandwich Islands). Thanks also to Peter Rothery for advice on statistical analysis and Peter Fretwell for creating the map in Fig. 1. This is a contribution to the Ecosystems Science programme at the British Antarctic Survey.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. A. Peck.
Rights and permissions
About this article
Cite this article
Stowasser, G., Pond, D.W. & Collins, M.A. Fatty acid trophic markers elucidate resource partitioning within the demersal fish community of South Georgia and Shag Rocks (Southern Ocean). Mar Biol 159, 2299–2310 (2012). https://doi.org/10.1007/s00227-012-2015-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-012-2015-5