Abstract
The frequency of low O2 (hypoxia) has increased in coastal marine areas but how fish avoid deleterious water masses is not yet clear. To assess whether the presence and oxygen pressure (PO2) level of an O2 refuge affects the hypoxia avoidance behaviour of fish, individual Atlantic cod (Gadus morhua L.) were exposed to a range of O2 choices in a 2-way choice chamber at 11.4°C over two different experiments. Cod in the first experiment were allowed access to a fixed O2 refuge (fully air-saturated seawater) whilst oxygen pressure (PO2) on the other side was reduced in steps to a critically low level, i.e. 4.3 kPa—a point where cod can no longer regulate O2 consumption. Under these conditions, cod did not avoid any level of hypoxia and fish swimming speed also remained unchanged. In contrast, strong avoidance reactions were exhibited in a second experiment when fish were again exposed to 4.3 kPa but the safety, i.e. PO2, of the refuge was reduced. Fish not only spent less time at 4.3 kPa as a result of fewer sampling visits but they also swam at considerably slower speeds. The presence of an avoidance response was thus strongly related to refuge PO2 and it is unlikely that cod, and possibly other fish species, would enter low O2 to feed in the wild if a sufficiently safe O2 refuge was not available. It is therefore hypothesized that the feeding range of fish may be heavily compressed if hypoxia expands and intensifies in future years.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anthropogenic activities affect the environment, and the frequency of low oxygen (i.e. hypoxia) has increased in coastal marine areas over the past century (see review by Diaz and Rosenberg 2008). Hypoxia is a serious concern because oxygen is essential for aerobic metabolic processes and its availability affects aquatic animals on many different levels (Wu 2002). The effects on fish are particularly well documented; hypoxia induces stress (Herbert and Steffensen 2005), impairs growth and reproduction (Thomas et al. 2007; Brandt et al. 2009), and there is a serious risk of death if low oxygen is not physically avoided (Schurmann and Steffensen 1992; Plante et al. 1998). Avoidance is clearly an important survival mechanism but accurate information on the degree of low O2 avoidance is perhaps limiting ecological simulation models at present (Rose et al. 2009).
Fish do avoid hypoxia and, as expected for a diverse group of ectotherms, their avoidance thresholds vary between species and temperature regimes according to their own prescribed tolerances and ecotype (Claireaux et al. 1995; Burleson et al. 2001; Eby and Crowder 2002; Skjæraasen et al. 2008; Stierhoff et al. 2009). Whilst interspecific variability and thermal dependence is not unexpected, there is also little evidence of any specific species showing consistent avoidance thresholds. For example, Atlantic cod (Gadus morhua) apparently avoid oxygen levels anywhere between 16 and 44% saturation (D’Amours 1993; Claireaux et al. 1995; Plante et al. 1998; Tomkiewicz et al. 1998; Neuenfeldt 2002), and this level of intraspecific variability is not explained by environmental temperatures. There are many ecological factors that could affect avoidance thresholds (e.g. access to food, predation) but a growing body of evidence now implicates time in hypoxia (vs. the absolute level of low O2) as a major factor modifying the behaviour and survival of fish in low O2 waters. For example, Jones (1952) and Höglund (1961) were possibly the first to observe diverse avoidance reactions when fish spend different amounts of time in hypoxia. Plante et al. (1998) also demonstrate that the O2 level required to kill 50% of their cod (i.e. LC50) varies as a function of hypoxic exposure time, with fish requiring higher O2 levels to survive the longer term. Interestingly, Claireaux et al. (1995) and Neuenfeldt et al. (2009) show that cod do avoid low O2 regions most of the time but they will also enter extreme hypoxia to feed over short-term periods. This “hypoxic diving” behaviour to feed has also been noted for the anchovy (Anchoa spp.) and the yellow perch (Perca flavescens) (Taylor et al. 2007; Roberts et al. 2009). Understanding how hypoxia avoidance behaviour is controlled and how fish divide their time under low O2 conditions may therefore provide ecological models with additional power to explore the low O2 response of fish.
The Atlantic cod (Gadus morhua) is a commercially important finfish species that inhabits highly heterogeneous O2 environments. This is especially well documented in the Baltic Sea (Tomkiewicz et al. 1998; Neuenfeldt 2002; Neuenfeldt and Beyer 2003) and the Gulf of St. Lawrence (D’Amours 1993). The distribution of cod in the Gulf of St. Lawrence is influenced strongly by severe hypoxia and extreme low temperatures with both factors acting as barriers to movement (D’Amours 1993; Gilbert et al. 2005). The waters of the Baltic Sea, however, are stratified into two layers comprising a low salinity surface layer and a more deep saline layer, which also differs in temperature and dissolved oxygen (Tomkiewicz et al. 1998). The movements of Baltic cod are generally constrained to intermediate depths by hypoxia in bottom waters and reduced salinity in the shallows (Tomkiewicz et al. 1998). Whilst cod are shown to avoid hypoxia in the field, it is interesting to note that other environmental factors, such as low surface salinities in the Baltic and extreme low temperatures in the Gulf of St. Lawrence, can actually prevent cod from accessing a full O2 refuge (i.e. 100% O2 saturated seawater). Indeed, data show that cod can be constrained to refuge areas that are considerably less than 80% O2 saturated at certain times of the year (D’Amours 1993; Tomkiewicz et al. 1998; Neuenfeldt and Beyer 2003). Therefore, the available O2 refuge of cod may not always be truly “safe” and fish may be forced to spend increasing amounts of time in hypoxia in these regions. The role that O2 refuges play in the avoidance behaviour of fish is relatively unknown but Eby and Crowder (2002) did note a shift in the avoidance thresholds of several fish species as their available O2 refuge shrank. More insight into the regulatory role of O2 refuges and its relevance to hypoxia avoidance behaviour is thus warranted.
By providing and manipulating the O2 pressure (PO2) level of an O2 refuge and examining variability in the avoidance response of Atlantic cod to controlled O2 choices, we have set out to test whether the avoidance behaviour and hypoxic time management of cod varies as a function of refuge PO2. We specifically employed Atlantic cod as our model species because it is a commercially important finfish, it inhabits various hypoxic regions and the factors modulating its hypoxia avoidance behaviour are not fully understood (Claireaux et al. 1995; Schurmann and Steffensen 1997; Plante et al. 1998; Tomkiewicz et al. 1998; Neuenfeldt 2002; Herbert and Steffensen 2005; Skjæraasen et al. 2008). This study uses laboratory-based methods to explore a fundamental aspect of hypoxia avoidance behaviour but it does not necessarily reflect true in-field conditions as a result of the duration of low O2 exposures, size of tanks, etc. Experimental methods of this type are useful, however, to understand fish avoidance reactions and any advance in this area will ultimately help improve the projection of future change and management of valuable fish stocks in response to adverse environmental conditions.
Materials and methods
Experimental fish and initial acclimation
Seventeen juvenile Atlantic cod, G. morhua, (mean ± SD: 18.7 ± 7.96 g wet weight; 12.6 ± 1.61 cm standard length, SL) were obtained from coastal cod broodstock from around the Bergen area (60° 25′ N, 5° 20′ E) and acclimated in fully air-saturated seawater (8.0–10.0°C) for at least 8 weeks prior to experimentation at the University of Bergen, Norway. Fish were held under a simulated Bergen photoperiod and an approximate 8-h light: 16-h dark cycle was in effect at the time of experimentation. Two hypoxia experiments were run with fish of similar mass and length.
Experimental apparatus: the choice chamber and control of water oxygen levels
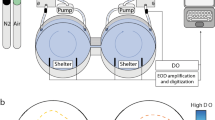
A diagrammatic representation of the experimental set-up and the behavioural choice chamber is given in Fig. 1. The behavioural apparatus was housed in a temperature-controlled room that maintained water temperature at 11.4 ± 0.3°C. Two cylindrical (75 l) mixing chambers and a flow meter were each connected to a separate side of the rectangular Plexiglas™ choice chamber (L × W × H = 120 × 40 × 11 cm) (Loligo, Denmark) and supplied each half of the test arena (L × W = 50 × 40 cm) with a laminar flow of seawater (1,000 l h−1). Strict laminar flow was maintained in the test arena by passing seawater through a screen diffuser and then a series of honeycomb baffles. Seawater from each side of the choice chamber drained under gravity into a 150-l reservoir tank but was almost immediately recycled back to each mixing chamber by a 1,000 l h−1 pump (Grundfoss, Denmark).
Diagrammatic overview of the behavioural apparatus. a An overhead view of the rectangular choice chamber with a single cod in the test arena (TA). The two cylindrical mixing towers (MT) equipped with a flow meter (FM) supplied the choice chamber with a laminar flow of seawater. Different PO2 was maintained on either side of the test arena by de-oxygenating seawater in 2 mixing chambers. Strict laminar flow was maintained by passing seawater through a screen diffuser (ScD) and then a series of honeycomb baffles (HCB). Another screen (Sc) demarked the downstream limit of the test arena. b A transverse view of a fish in the test arena (TA) showing only one of the two cylindrical mixing towers (MT), hypoxia control units and re-circulatory flow systems. The PO2 on each side of the choice chamber was monitored by an oximeter equipped with an O2 probe positioned between the honeycomb baffles. For controlled de-oxygenations, an O2 control unit (C) received the PO2 signal and, via a solenoid valve (S), controlled the flow of gas, hence the level of deoxygenation, from a compressed bottle of nitrogen (N2). Seawater was continuously passed back to the mixing tower by the pump (P) in the reservoir tank. The behaviour of the fish was monitored by a CCD video camera (V) and was analysed online using Lolitrack software on the PC
By de-oxygenating seawater in the two mixing towers (see below for methodology) and creating laminar flow, it was possible to maintain two different levels of oxygen partial pressure (PO2) on either side of the arena with a central separation zone of only 0.5 cm. PO2 on each side of the arena was monitored by a WTW 340i oximeter (Germany) with the oxygen probe positioned between the honeycomb baffles. For controlled de-oxygenations in the mixing chambers, a relay set-point control unit (Model 5514, PR Electronics, Denmark) received the PO2 signal from the oximeter and, via a solenoid valve, controlled the flow of gas from a compressed bottle of nitrogen and hence the desired level of PO2. The two PO2 streams had a width of 20.5 cm, and the 0.5 cm separation distance was stable over time and even in the presence of the most extreme PO2 choice (20.5–4.3 kPa). Cod were free to move between the two PO2 streams in the test arena, and the fish themselves did not disturb the stability of the PO2 difference. The stability of the 0.5 cm separation zone was confirmed visually during preliminary trials by creating a strong 20.5–4.3 kPa difference and adding kaolin to one side of the choice chamber. The stability of PO2 on either side of the 0.5 cm separation zone was also confirmed with an O2 probe during preliminary trials. These tests were carried out repeatedly with and without a fish in the choice chamber.
The temperature-controlled room was equipped with dimmed overhead (fluorescent) lighting but, to enhance video tracking under low light conditions (see below), fish were also backlit with a secondary source of light under the Plexiglass™ choice chamber. A high resolution CCD camera (WV BP550, Panasonic, USA), positioned 0.6 m above the choice chamber, was connected to a PC via an external framegrabber (Pinnacle PCTV USB2). Positional tracking software (Lolitrack, Loligo, Denmark) on the PC was used to determine the x, y position of the geometric centre of the fish which was recorded at a rate of 10 Hz. These x, y coordinates were used to calculate (1) the percentage of time spent on each side of the test arena (as an indicator of hypoxia avoidance), (2) the frequency of movements into the most hypoxic channel, (3) residence time in the most hypoxic channel and (4) the averaged rate of swimming speed in body lengths per second (BL s−1). We define hypoxia avoidance as fish spending less than 50% of their time on the most hypoxic side and their choice of side (space use) being significantly different from that observed under control conditions (i.e. no O2 difference).
Experimental design
Individual cod were transferred to the test arena without any air exposure and were allowed at least 16 h of overnight acclimation. Once fish were introduced to the choice chamber, there was no further disturbance until the end of the trial. Hypoxia avoidance behaviour and swimming speed were recorded in response to different PO2 choices during two separate experiments.
Experiment 1
Fully air-saturated seawater (i.e. 20.5 kPa) was held on both sides of the choice chamber at the start of experimentation, and the behaviour of nine individual cod was recorded under control (high PO2) conditions. Fish were then allowed to choose between fully air-saturated seawater (20.5 kPa) on one side of the choice chamber and a series of reduced oxygen pressures (17.1, 13.0, 10.8, 8.7, 6.5 and 4.3 kPa) on the other (Fig. 2a). Behavioural observations were made over 30-min periods when the desired PO2 difference was steady. The 30-min steady state periods were selected because cod do show a physiological (stress) response and can make rational behavioural decisions within this timeframe (Herbert and Steffensen 2005). A 15-min (unsteady PO2) period was required to reach the next steady PO2 state. Recordings always commenced with control observations at the same time of day (10:00am), and the side that received the reduction in PO2 was randomized between trials.
The hypoxia avoidance response of cod during experiment 1. a The different PO2 streams and choice levels across the two sides of the choice chamber. b The % time spent on the most hypoxic side of the choice chamber. c Frequency of movements into the side with lowest PO2. d The residence time of the fish in hypoxia per visit. e The total averaged rate of swimming speed. f The averaged rate of swimming speed on the two sides of the choice chamber experiencing different PO2. The closed circles represent the response of fish on the most hypoxic side. Data with an open square at the 20.5–20.5 kPa level (b–d only) describe the control reaction of the fish on the side destined to become most hypoxic. All data are mean values with 95% confidence intervals. No significant differences were observed
Experiment 2
Fully air-saturated seawater (i.e. 20.5 kPa) was held on both sides of the choice chamber at the start of experimentation and the behaviour of eight individual cod was recorded under control (high PO2) conditions for 30 min. Both sides of the choice chamber were then reduced to 4.3 kPa and this level was held for 30 min as part of a control (low PO2) condition. Thereafter, one side was held at 4.3 kPa whilst access to O2 refuge was provided in manipulated steps of increasing PO2 (i.e. 6.5, 8.6, 10.7, 12.8, 16.8 and 19.9 kPa) (Fig. 3a). Again behavioural observations were made over 30-min periods when the desired PO2 difference was steady. A 15-min (unsteady PO2) period was required to reach the next steady PO2 state (Fig. 3a). This experiment always commenced at 0830 h and the side which received the lowest level of PO2 was randomized between individual trials. In both experiments, 4.3 kPa was selected as the lowest PO2 level because it represents a dangerous and critically low level of hypoxia for Atlantic cod at 10°C (Schurmann and Steffensen 1997; Herbert and Steffensen 2005). And 4.3 kPa is also encountered by this species in their natural marine environment (Neuenfeldt 2002) and is therefore a relevant environmental condition.
The hypoxia avoidance behaviour of fish during experiment 2. a The different PO2 streams and choice levels across the two sides of the choice chamber. b The % time spent on the most hypoxic side of the choice chamber. c Frequency of movements into the side with lowest PO2. d The residence time of the fish in hypoxia per visit. e The total averaged rate of swimming speed. f The averaged rate of swimming speed on the two sides of the choice chamber experiencing different PO2. The closed circles represent the response of fish on the most hypoxic side. Data with an open square at the 20.5–20.5 and 4.3–4.3 kPa level (b–d only) describe the control reactions of fish on the side destined to become most hypoxic. All data are mean values with 95% confidence intervals. An asterisk represents either a significant difference from the high PO2 control group (b–e) or a difference in swimming speed across the two sides of the choice chamber (f)
Data analyses
For each fish, we analysed (1) the percentage of time spent in the most hypoxic side of the choice chamber, (2) the frequency of visits to the most hypoxic side of the choice chamber and (3) the average duration of each visit, i.e. average residence time and (4) the total averaged rate of swimming speed (standardized to BL s−1). At each PO2 level, we also examined the swimming speed of fish in the least and most hypoxic sides of the choice chamber and based our statistical analysis on the calculated difference (S diff).
We commenced our statistical analyses by applying a linear mixed effect variance (LMEV) model to both sets of experimental data and incorporated the initial 20.5–20.5 kPa level as our high PO2 control value. No further analyses were carried out if no significant difference was found between the control value and any other PO2 level. If a significant difference was discovered at only one level of PO2, a Bonferroni correction was carried out before any significance level was assigned. If our initial model revealed a range of significant differences at several PO2 levels, we attempted to fit more advanced quantitative models to the data to disentangle general patterns in the response of the individual fish. Final model selection was based on the Akaike and Bayesian information criterion (Pinheiro and Bates 2000). Significance was accepted at p < 0.05. A detailed description of these models is provided in the supplementary material (Tables S1, S2). For residence time, we did not only compare the average residence time across oxygen levels, but also tested if any pattern could be detected within each oxygen level, i.e. did the duration of residence time change with visit number. This was tested by a linear mixed effect (LME) regression model where residence time was treated as the response variable, visit number as the explanatory variable and individual fish as a random effect.
Results
Experiment 1
Oxygen pressures as low as 4.3 kPa were not avoided whilst cod were also allowed access to a fixed and fully saturated O2 refuge (i.e. 20.5 kPa, fully air-saturated seawater). This is clearly demonstrated in Figs. 2a–c, e where swimming speed and even time spent in any level of reduced O2 pressure were similar to the response of fish during the control (high PO2) condition (p > 0.05). There was also no difference in swimming speed between the hypoxic and normoxic area and average residence time did not change between the different experimental levels (p > 0.05) (Figs. 2d, f). Only one event appeared to be significantly different during experiment 1, and this was the number of visits to the most hypoxic side during the 20.5–6.5 kPa level (p = 0.017, Fig. 2c). However, after performing a Bonferroni correction (0.05/6 = 0.0083), this value was not significant. As a result of fish moving regularly between the two sides of the chamber (Fig. 2c), the average amount of time spent in the area of lowest PO2 ranged from 7 to 8 s (Fig. 2d). No conclusive pattern was found when considering residence time within each oxygen level as a function of visit number. For example, at the control 20.5–20.5 and the 20.5–13.0 kPa level, residence time decreased with the number of visits (p < 0.05), whereas residence time increased with visit number at the 20.5–6.5 kPa level. No significant pattern was found for the other experimental oxygen levels.
Experiment 2: percentage of time spent in lowest PO2 (Fig. 3b)
Our LMEV model indicated that the % of time spent on the side with lowest PO2 was significantly less than the control (high PO2) level at every choice level. This was also apparent after Bonferroni corrections were applied (p < 0.01) and a more complex model was therefore applied to the data. The time spent on the side destined to become most hypoxic did not change during the first two control levels (as expected when PO2 is equal on both sides). However, the time spent on the most hypoxic side dropped to its lowest value during the 4.3–6.5 kPa level (i.e. 2.5% time) but then increased linearly until the end of the experiment as refuge PO2 was increased (p < 0.01). The latter pattern was recognized in all parameters studied, our quantitative models therefore contained two parts; the first part consisted of the two initial control levels (high and low PO2) either pooled or kept separate and a second, linear part containing the remaining oxygen levels when refuge PO2 was increased. Further details of the modelling process (i.e. methodology and results) are available as supplementary material (Tables S1, S2).
Experiment 2: movements into the area with lowest PO2 (Fig. 3c)
With exception to the 4.3–16.8 and 4.3–19.9 kPa levels, the LMEV model showed that the frequency of visits to the most hypoxic side of the choice chamber was significantly less than control (high PO2) level throughout all choice presentations (p < 0.01). There was also a fairly significant linear increase in the frequency of visits from the initial 4.3–6.5 kPa level until the end of the experiment as refuge PO2 increased (p < 0.01).
Experiment 2: residence time (Fig. 3d)
With the exception of the 4.3–4.3 kPa level average residence time was generally shorter than our control (high PO2) level and this was significant for the 4.3–8.6, the 4.3–10.7 and the 4.3–12.8 kPa levels. The quantitative model again indicated that residence times increased from its lowest values at the 4.3–8.6 level in a linear way until the end of the experiment (p < 0.01). Within each oxygen level, no effect on residence time with visit number was detected at any experimental oxygen level.
Experiment 2: fish swimming speed (Fig. 3e)
The sudden onset of hypoxia from the high to low PO2 control level was associated with a marked reduction in swimming speed (p < 0.01). However, swimming increased significantly in a linear fashion when refuge PO2 was raised back to the near-fully air-saturated level (p < 0.01). The slight drop in swimming speed between the control (low PO2) condition and the initial 4.3–6.5 kPa level was not significant (p > 0.05).
Experiment 2: swimming speed as a result of different PO2 on either side of the choice chamber (Fig. 3f)
The initial LMEV model indicated that there was no difference in swimming speed on either side of the choice chamber at either 20.5–20.5 kPa or 4.3–4.3 kPa (i.e. S diff was not significantly different from zero) (p > 0.05). Therefore, without an established PO2 difference, cod had the same swimming speed on both sides of the tank. However, once a 4.3–6.5 kPa PO2 difference was formed and a slight O2 refuge was offered, a clear difference in speed was observed across the two sides of the choice chamber and this was the case for all other PO2 choice offerings except the 4.3–19.9 kPa level. Where a significant difference in speed was observed, fish always swam faster on the most hypoxic side of the choice chamber. The largest difference in speed occurred at the choice level of 4.3–6.5 kPa (p < 0.01) but this difference decreased gradually in a fairly linear manner and eventually disappeared as refuge PO2 was increased to a normoxic level of 19.9 kPa. Whilst swimming speed was seen to be higher on the most hypoxic side of the chamber (as above), it is important to note that hypoxic swimming speed was still generally lower than control speed, except at the 4.3–12.8 and 4.3–16.8 kPa level (p < 0.05), which showed comparable speed to the control level.
Discussion
Low O2 episodes have increased over the last two decades (Wu 2002; Hagy et al. 2004; Turner et al. 2005) but Atlantic cod is endowed with the capacity to avoid hypoxia, which presumably helps to offset the deleterious effects of aerobic stress (D’Amours 1993; Claireaux et al. 1995; Tomkiewicz et al. 1998; Neuenfeldt 2002; Skjæraasen et al. 2008). But what level of low O2 would be considered sufficiently dangerous to initiate a strong avoidance reaction in this species? The growth of cod is reduced from relatively high oxygen saturations (<65% saturation, Chabot and Dutil 1999), but this is not likely life-threatening during short-term exposures. On the other hand, the reduced rate of basal metabolism at pressures below critical oxygen tension (P crit) undoubtedly threatens life and should be avoided because metabolic scope is non-existent and basal demands are no longer sustained aerobically (Schurmann and Steffensen 1997; Claireaux et al. 2000; Chabot and Claireaux 2008). If we therefore assume that the 4.8 kPa P crit of cod at 10°C represents a very dangerous O2 level which should be avoided (Schurmann and Steffensen 1997; Claireaux et al. 2000; Chabot and Claireaux 2008), it is highly surprising that cod showed no behavioural reaction to 4.3 kPa in the first experiment (Fig. 2). It would therefore appear that cod do not necessarily avoid dangerous O2 levels if they also have access to a sufficiently safe O2 refuge. Having access to an O2 refuge and only entering hypoxia for short periods would effectively minimize time spent in hypoxia.
The O2 environment of cod is heterogeneous and dynamic and, although cod can avoid hypoxia by choosing more well-oxygenated areas, other environmental barriers such as low temperatures or salinity often restrict access to fully air-saturated seawater, the safest possible O2 refuge (D’Amours 1993; Tomkiewicz et al. 1998; Neuenfeldt 2002). Recognising that the O2 environments of cod in the wild are complex, we deliberately aimed to resolve how refuge PO2 affects fish hypoxia avoidance behaviour under controlled laboratory conditions. Compared to the lack of response in the first experiment, cod in the second experiment did avoid extreme low hypoxia (4.3 kPa) but the strength of the avoidance reaction was inversely related to refuge PO2. The fact that cod avoided 4.3 kPa more strongly when refuge PO2 was low demonstrates a high degree of context-dependency, which mirrors the in-field data of Eby and Crowder (2002). These researchers noted that several fish species avoided low O2 more readily when their useable habitat shrank following a decline in the spatial extent of the O2 refuge. In effect, when refuge PO2 becomes limiting and time in hypoxia is extended, initiating avoidance movements from the lowest to highest O2 level would effectively help conserve metabolic scope and delay the onset of stress. Indeed, without access to fully air-saturated seawater, increased time in hypoxia would be unavoidable and severe metabolic stress could be incurred (Herbert and Steffensen 2005). Future models that attempt to predict the low O2 response of fish should therefore focus on the context of low O2 exposures, paying particular attention to hypoxic residence times regulated by the provision and PO2 level of an O2 refuge.
Individual fish species that spend different amounts of time in hypoxia show diverse avoidance reactions (Jones 1952; Höglund 1961), and it is speculated that some form of internal systemic signal might be involved in the activation of this behaviour. Fish in the first experiment did not avoid any level of low O2 but may not have experienced any physiological change if they were allowed free and open access to the 20.5 kPa refuge. Indeed, as a result of the fish moving regularly between the two sides of the chamber (Figs. 2b, c), the average amount of time spent in hypoxia was only 7–8 s per visit (Fig. 2d), which is probably not sufficiently long to affect blood PO2 markedly. By contrast, fish denied access to an O2 refuge in the second experiment would have been under low O2 conditions for an extended period and may have had their hypoxia avoidance behaviour activated as a result of internal (systemic) stress (Herbert and Steffensen 2005). The likely manifestation and persistence of stress over the course of experiment 2 may also explain why fish still avoided 4.3 kPa when they had access to a normoxic refuge (19.9 kPa). Future experiments that test the additional role of systemic signals in the induction and control of hypoxia avoidance behaviour may therefore provide a valuable insight into the motivational basis of fish movements during hypoxic challenge.
Physiological studies provide clear evidence that fish possess a well-developed oxygen sensing capacity (see Burleson 1995; Perry and Gilmour 2002 for a review). Branchial oxygen receptors are present in almost all fish species. These are orientated both externally and internally on the gills and have an important role in modulating cardiorespiratory reflexes during hypoxia. Burleson et al. (2001) first hypothesized that external oxygen receptors are involved in hypoxia avoidance. Our experiments, however, do not support the idea that avoidance behaviour is triggered by external O2 signals alone since fish entered the critical 4.3 kPa level in the first experiment without any form of behavioural response. The variable avoidance response seen in the second experiment might therefore suggest that hypoxia avoidance is under the control of a more complex integrated signal. Shingles et al. (2005) feasibly add support to this argument by showing that low O2 avoidance (via aquatic surface respiration) was modulated by external O2 receptors and the threat of predation in flathead grey mullet (Mugil cephalus). However, whilst more detailed studies are lacking, we should probably also bear in mind that external chemoreceptors might function differently as the spatial extent of hypoxia varies across studies.
The fact that cod enter dangerous levels of low O2 without any apparent sign of avoidance (Fig. 2) might initially seem counter-intuitive but not having their movements constrained by the absolute level of low O2 would feasibly allow access to rich feeding areas during short-term forays. For example, cod in the Baltic Sea would not have access to benthic crabs and molluscs if they responded only to a low external PO2 signal and never entered hypoxia in the deeper layers. This is clearly not the case since Claireaux et al. (1995) and Neuenfeldt et al. (2009) have both observed cod penetrating deep hypoxic waters during short feeding excursions. Anchovy (Anchoa spp.), sprat (Sprattus sprattus) and the yellow perch (P. flavescens) also exhibit similar behaviour (Taylor et al. 2007; Roberts et al. 2009; Kaartvedt et al. 2009). Our experiments provide evidence that cod do not base their avoidance solely on an external PO2 signal and that “hypoxic diving” to feed would probably only occur if fish are allowed access to an O 2 refuge. This would ultimately allow fish to regulate their total time in hypoxia within safe physiological limits.
Experiment 2 implies that cod possess an excellent hypoxia survival strategy because rapid hypoxia avoidance is likely achieved with a simultaneous drop in energetic expenditure. This arises from the fact that cod generally reduced their swimming speed from control levels, which is known to reduce metabolic expenditure and offset the manifestation of major metabolic stress (Schurmann and Steffensen 1994; Herbert and Steffensen 2005). Whilst metabolic expenditure was generally reduced cod did actually swim faster on the more hypoxic side of the chamber choice, which may have endowed fish with a fast and efficient form of escape. There appears to be adaptive value in the avoidance response of cod but the generally reduced swimming speed of cod is not in line with other studies suggesting that effective low O2 avoidance occurs only with heightened swimming speed (i.e. above routine control levels) (Domenici et al. 2000; Herbert and Steffensen 2005; Johansen et al. 2006; Brady et al. 2009). It should be stated, however, that all previous studies never presented fish with a choice or opportunity to escape under progressive hypoxia. Therefore, until more studies have examined avoidance and swimming speed in a wider range of species, major speed increases should cautiously be considered part of a functional avoidance response. When considering the relative size of the choice chambers to the body size of our fish, we also treat the absolute swimming speed values of our fish with caution and make no attempt to compare these speeds against other fish or studies.
By observing the speed and distribution of cod presented with controlled O2 choices, we have gained an insight into the response of fish to hypoxia. It is important that we understand these responses because severe hypoxia is becoming increasingly frequent in coastal regions and hypoxia is also likely to persist in the future with other environmental stressors (e.g. global warming and pollution) (Diaz and Rosenberg 2008; Kidwell et al. 2009). This presents a worrying situation because cod might require an O2 refuge if they are to thrive and exploit feeding opportunities across a range of different O2 levels, especially hypoxic deep-water habitats with an abundance of fish and invertebrate prey (Claireaux et al. 1995). Further experiments should therefore test whether wild fish populations are negatively affected by impoverished refuge areas.
Abbreviations
- PO2:
-
Partial pressure of oxygen
References
Brady DC, Targett TE, Tuzzolino DM (2009) Behavioral responses of juvenile weakfish (Cynoscion regalis) to diel-cycling hypoxia: swimming speed, angular correlation, expected displacement, and effects of hypoxia acclimation. Can J Fish Aquat Sci 66:415–424
Brandt SB, Gerken M, Hartman KJ, Demers E (2009) Effects of hypoxia on food consumption and growth of juvenile striped bass (Morone saxatilis). J Exp Mar Biol Ecol 381:S143–S149
Burleson M (1995) Oxygen availability: sensory systems. In: Hochachka PW, Mommsen TP (eds) Environmental and ecological biochemistry. Elsevier, New York, pp 1–18
Burleson ML, Wilhelm DR, Smatresk NJ (2001) The influence of fish size on the avoidance of hypoxia and oxygen selection by largemouth bass. J Fish Biol 59:1336–1349
Chabot D, Claireaux G (2008) Environmental hypoxia as a metabolic constraint on fish: the case of Atlantic cod, Gadus morhua. Mar Pollut Bull 57:287–294
Chabot D, Dutil JD (1999) Reduced growth of Atlantic cod in non-lethal hypoxic conditions. J Fish Biol 55:472–491
Claireaux G, Webber DM, Kerr SR, Boutilier RG (1995) Physiology and behavior of free swimming Atlantic cod (Gadus morhua) facing fluctuating salinity and oxygenation conditions. J Exp Biol 198:61–69
Claireaux G, Webber DM, Lagardere JP, Kerr SR (2000) Influence of water temperature and oxygenation on the aerobic metabolic scope of Atlantic cod (Gadus morhua). J Sea Res 44:257–265
D’Amours D (1993) The distribution of cod (Gadus morhua) in relation to temperature and oxygen in the Gulf of St. Lawrence. Fish Oceanogr 2:24–29
Diaz RJ, Rosenberg R (2008) Spreading dead zones and consequences for marine ecosystems. Science 321:926–929
Domenici P, Steffensen JF, Batty RS (2000) The effect of progressive hypoxia on swimming activity and schooling in Atlantic herring. J Fish Biol 57:1526–1538
Eby LA, Crowder LB (2002) Hypoxia-based habitat compression in the Neuse River Estuary: context-dependent shifts in behavioral avoidance thresholds. Can J Fish Aquat Sci 59:952–965
Gilbert D, Sunby B, Gobeil C, Mucci A, Tremblay G-H (2005) A seventy-two-year record of diminishing deep-water oxygen in the St. Lawrence estuary: the Northwest Atlantic connection. Limnol Oceanogr 50:1654–1666
Hagy JD, Boynton WR, Keefe CW, Wood KV (2004) Hypoxia in Chesapeake Bay, 1950–2001: long-term change in relation to nutrient loading and river flow. Estuaries 27:634–658
Herbert NA, Steffensen JF (2005) The response of Atlantic cod, Gadus morhua, to progressive hypoxia: fish swimming speed and physiological stress. Mar Biol 147:1403–1412
Höglund LB (1961) The reactions of fish in concentration gradients. Fish Board Swed Rep 43:1–147
Johansen JL, Herbert NA, Steffensen JF (2006) The behavioural and physiological response of Atlantic cod (Gadus morhua L.) to short-term acute hypoxia. J Fish Biol 68:1918–1924
Jones JRE (1952) The reactions of fish to water of low oxygen concentration. J Exp Biol 29:403–415
Kaartvedt S, Røstad A, Klevjer TA (2009) Sprat Sprattus sprattus can exploit low oxygen waters for overwintering. Mar Ecol Prog Ser 390:237–249
Kidwell DM, Lewitus AJ, Jewett EB, Brandt S, Mason DM (2009) Ecological impacts of hypoxia on living resources. J Exp Mar Biol Ecol 381:S1–S3
Neuenfeldt S (2002) The influence of oxygen saturation on the distributional overlap of predator (cod, Gadus morhua) and prey (herring, Clupea harengus) in the Bornholm Basin of the Baltic Sea. Fish Oceanogr 11:11–17
Neuenfeldt S, Beyer JE (2003) Oxygen and salinity characteristics of predator-prey distributional overlaps shown by predatory Baltic cod during spawning. J Fish Biol 62:168–183
Neuenfeldt S, Andersen KH, Hinrichsen H-H (2009) Some Atlantic cod Gadus morhua in the Baltic Sea visit hypoxic water briefly but often. J Fish Biol 75:290–294
Perry SF, Gilmour KM (2002) Sensing and transfer of respiratory gases at the fish gill. J Exp Zool 293:249–263
Pinheiro JC, Bates DM (2000) Mixed effects models in S and S-Plus. Springer, London
Plante S, Chabot D, Dutil JD (1998) Hypoxia tolerance in Atlantic cod. J Fish Biol 53:1342–1356
Roberts JJ, Höök TO, Ludsin SA, Pothoven SA, Vanderploeg HA, Brandt SB (2009) Effects of hypolimnetic hypoxia on foraging and distributions of Lake Erie yellow perch. J Exp Mar Biol Ecol 381:S132–S142
Rose KA, Adamack AT, Murphy CA, Sable SE, Kolesar SE, Craig JK, Breitburg DL, Thomas P, Brouwer MH, Cerco CF, Diamond S (2009) Does hypoxia have population-level effects on coastal fish? Musings from the virtual world. J Exp Mar Biol Ecol 381:S188–S203
Schurmann H, Steffensen JF (1992) Lethal oxygen levels at different temperatures and the preferred temperature during hypoxia of the Atlantic cod, Gadus morhua. J Fish Biol 41:927–934
Schurmann H, Steffensen JF (1994) Spontaneous swimming activity of Atlantic cod Gadus morhua exposed to graded hypoxia at 3 temperatures. J Exp Biol 197:129–142
Schurmann H, Steffensen JF (1997) Effects of temperature, hypoxia and activity on the metabolism of juvenile Atlantic cod. J Fish Biol 50:1166–1180
Shingles A, McKenzie DJ, Claireaux G, Domenici P (2005) Reflex cardioventilatory responses to hypoxia in the flathead gray mullet (Mugil cephalus) and their behavioral modulation by perceived threat of predation and water turbidity. Physiol Biochem Zool 78:744–755
Skjæraasen JE, Nilsen T, Meager JJ, Herbert NA, Moberg O, Tronci V, Johansen T, Salvanes AGV (2008) Hypoxic avoidance behaviour in cod (Gadus morhua L.): the effect of temperature and haemoglobin genotype. J Exp Mar Biol Ecol 358:70–77
Stierhoff KL, Tyler RM, Targett TE (2009) Hypoxia tolerance of juvenile weakfish (Cynoscion regalis): laboratory assessment of growth and behavioral avoidance responses. J Exp Mar Biol Ecol 381:S173–S179
Taylor JC, Rand PS, Jenkins J (2007) Swimming behavior of juvenile anchovies (Anchoa spp.) in an episodically hypoxic estuary: implications for individual energetics and trophic dynamics. Mar Biol 152:939–957
Thomas P, Rahman MS, Khan IA, Kummer JA (2007) Widespread endocrine disruption and reproductive impairment in an estuarine fish population exposed to seasonal hypoxia. Proc R Soc B Biol Sci 274:2693–2702
Tomkiewicz J, Lehmann KM, St John MA (1998) Oceanographic influences on the distribution of Baltic cod, Gadus morhua, during spawning in the Bornholm Basin of the Baltic Sea. Fish Oceanogr 7:48–62
Turner RE, Rabalais NN, Swenson EM, Kasprzak M, Romaire T (2005) Summer hypoxia in the northern Gulf of Mexico and its prediction from 1978 to 1995. Mar Environ Res 59:65–77
Wu RSS (2002) Hypoxia: from molecular responses to ecosystem responses. Mar Pollut Bull 45:35–45
Acknowledgments
We gratefully acknowledge funding from the European Union Fisheries Directorate (via contract QLRS-2002-00799, Project ETHOFISH), as well as the Norweigan Research Council (152862/120). Ethical approval was granted by the Experimental Animal Committee (Ministry of Justice, Denmark), through license numbers 1999/561 -232 and 2004/561 -894, and the Norwegian Veterinary Authorities for Experimental Research (Site license number 18).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. A. Peck.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Herbert, N.A., Skjæraasen, J.E., Nilsen, T. et al. The hypoxia avoidance behaviour of juvenile Atlantic cod (Gadus morhua L.) depends on the provision and pressure level of an O2 refuge. Mar Biol 158, 737–746 (2011). https://doi.org/10.1007/s00227-010-1601-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-010-1601-7