Abstract
Hypoxic or oxygen-free zones are linked to large-scale mortalities of fauna in aquatic environments. Studies investigating the hypoxia tolerance of fish are limited and focused on marine species and short-term exposure. However, there has been minimal effort to understand the implications of long-term exposure on fish and their ability to acclimate. To test the effects of long-term exposure (months) of fish to hypoxia we devised a novel method to control the level of available oxygen. Juvenile golden perch (Macquaria ambigua ambigua), and silver perch (Bidyanus bidyanus), two key native species found within the Murray Darling Basin, Australia, were exposed to different temperatures (20, 24 and 28 °C) combined with normoxic (6–8 mgO2 L−1 or 12–14 kPa) and hypoxic (3–4 mgO2 L−1 or 7–9 kPa) conditions. After 10 months, fish were placed in individual respirometry chambers to measure standard and maximum metabolic rate (SMR and MMR), absolute aerobic scope (AAS) and hypoxia tolerance. Golden perch had a much higher tolerance to hypoxia exposure than silver perch, as most silver perch died after only 1 month exposure. Golden perch acclimated to hypoxia had reduced MMR at 20 and 28 °C, but there was no change to SMR. Long-term exposure to hypoxia improved the tolerance of golden perch to hypoxia, compared to individuals held under normoxic conditions suggesting that golden perch can acclimate to levels around 3 mgO2 L−1 (kPa ~ 7) and lower. The contrasting tolerance of two sympatric fish species to hypoxia highlights our lack of understanding of how hypoxia effects fish after long-term exposure.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hypoxia occurs when dissolved oxygen in the water goes below a level that can sustain the life of an organism and its natural capacity to function physiologically. Under such conditions organisms are unable to carry out vital processes such as feeding, reproduction, growth, migration and predator avoidance (Dean and Richardson 1999; Pollock et al. 2007). Hypoxia is not a new issue; in fact, it commonly occurs as a natural process, with anthropogenic activities contributing greatly to the severity and duration of hypoxic events in more recent times (King et al. 2012; Whitworth et al. 2012). Over 500 hypoxic areas or dead zones worldwide have been documented, predominantly associated with anthropogenic pressures and are expected to increase exponentially (by 5.54% per year Díaz and Rosenberg 2011; Vaquer-Sunyer and Duarte 2008).
The response of fish to hypoxia and temperature change is dependent on species and context, with conspecifics responding differently both physiologically and behaviourally (Collins et al. 2013; McNeil and Closs 2007; Nilsson et al. 2009). Fish exposed to hypoxia encounter a number of problems: short term (0.5–96 h) they face problems with maintaining oxygen uptake to meet basic metabolic maintenance requirements, reductions in aerobic scope, bactericidal activity, and antibody levels, the production of disease-fighting reactive oxygen species and a build-up of anaerobic by products (Díaz and Rosenberg 2011; Pörtner and Lannig 2009); long term (96 + h) they suffer reduced growth and fecundity, as well as altered behaviour and mortality (Breitburg et al. 2009; Pörtner and Lannig 2009). Indirectly, hypoxia can result in habitat loss (from forced migrations), increased predation pressure, and overall trophic changes, with some effects being irreversible or requiring extensive time for recovery (Collins et al. 2013; McCarthy et al. 2014; Vaquer-Sunyer and Duarte 2008). Fish can counter hypoxia by breathing air at the water’s surface (aquatic surface respiration, ASR), escaping hypoxic areas if possible and reducing oxygen demand by decreasing activity; as well as increasing the oxygen carrying capacity of haemoglobin (Hb), depressing their metabolism and changing cardiac function (Cook et al. 2013; Timmerman and Chapman 2004; Rogers et al. 2016). Cellular and tissue modifications can also improve performance under hypoxic conditions, evident through lowering of the critical oxygen tension (Pcrit), defined as the partial pressure of oxygen (Po 2 ) below which a stable rate of oxygen uptake can be maintained and is not reliant upon on ambient oxygen availability (Cook et al. 2013; Timmerman and Chapman 2004; Sollid et al. 2005). Furthermore, increased temperatures reduce the solubility of oxygen in water, thus reducing a fish’s capacity to supply oxygen to tissues, compounding the problem of a low-oxygen environment (Dean and Richardson 1999; Farrell 2016; McCarthy et al. 2014). Thus, the combined effects of temperature and hypoxia can be devastating and may affect ecological communities in complex ways and elicit highly species-specific responses.
The response of organisms to hypoxia can be difficult to measure, particularly in combination with temperature. Much of our understanding is based upon abundance presence–absence studies and short-term physiological and behavioural studies (King et al. 2012; Zhang et al. 2010). Presence–absence studies are limited by populations being present at time of sampling, typically small numbers and sizes of sample sites, and limited prior knowledge of fish assemblages of the sample area (King et al. 2012). While physiological and behavioural studies have focussed on short-term effects of different stressors (i.e. < 100 h exposure to hypoxic conditions) to metabolic performance and behavioural responses of individual fish species, few studies have considered the longer term (chronic) exposure to multiple stressors (Richardson et al. 2001).
Respirometry, a physiological approach used to measure oxygen consumption rates (\( \dot{M}o_{2} \)) of aquatic organisms presents a unique opportunity to predict an organism’s response to long-term exposure to environmental stressors (Roche et al. 2013). The metabolic scope of an individual is of particular interest as it shows the total capacity for energy use by aerobic pathways and can be estimated indirectly through measurements of oxygen consumption (Norin and Clark 2016). Furthermore, it can be influenced by a number of intrinsic and extrinsic factors such as activity level, body mass, temperature, food consumption and environmental conditions like hypoxia (Norin and Clark 2016; Chabot et al. 2016). Metabolic scope is calculated using the standard (resting) metabolic rate (SMR or \( \dot{M}_{{O_{2} ,{ \hbox{min} }}} \) ) and the maximum metabolic rate (MMR or \( \dot{M}_{{O_{2} ,{ \hbox{max} }}} \)). The SMR represents the minimum rate of oxygen consumption (minimal cost of living) of a resting fish in a post-absorptive state at a given temperature (McBryan et al. 2013; Roche et al. 2013), while the MMR represents the maximum rate at which oxygen from the environment can be transported to the organism for consumption (McBryan et al. 2013; Roche et al. 2013). A fish’s total aerobic scope for activity, the range of metabolic energy available for aerobic activity, can then be determined from SMR and MMR; this is referred to as the absolute aerobic scope (AAS)(Roche et al. 2013). Fish exposed to hypoxic conditions will suffer a reduction to their total metabolic scope. As such, it is necessary to understand the severity of this reduction and if prolonged hypoxia exposure would allow organisms to initiate an acclimation response.
Understanding the thresholds of fish to hypoxic conditions is crucial for establishing management targets to avoid high mortalities (Vaquer-Sunyer and Duarte 2008). There have been few attempts to determine thresholds of hypoxia for fish species and identify a limit for management purposes (Dean and Richardson 1999). Of the possible limits proposed the majority of the literature refers to a value of ~ 2mgO2 L−1 for all aquatic environments (Breitburg et al. 2009; Helz and Adelson 2013; Vaquer-Sunyer and Duarte 2008). Species-specific responses to hypoxic conditions suggest that this value may be inadequate in supporting whole system survival, and suggests that ecosystems may require independent reviews before instituting a limit for management (Dean and Richardson 1999; Vaquer-Sunyer and Duarte 2008).
To date, there are no known studies that have considered freshwater species and their tolerance to the combined effects of elevated temperatures and hypoxia. We investigated the independent and interactive effects of long-term exposure to low dissolved oxygen and temperature on the metabolic scope and tolerance of two freshwater fish species. We used juvenile golden perch (Percichthyidae: Macquaria ambigua ambigua) and silver perch (Terapontidae: Bidyanus bidyanus) these are key species found throughout Australia’s largest inland freshwater river system, the Murray Darling Basin (MDB) and are classified as vulnerable, threatened or endangered dependent on region (for more species information see Supp. SSI 1, Lintermans 2007) were used. The MDB is a highly regulated system subject to extreme variations in environmental conditions. Prolonged periods of severe drought are punctuated by periods of high rainfall and flooding, conditions that can result in hypoxic events particularly during summer. Golden perch and silver perch were exposed to prolonged hypoxic conditions at different temperatures; however, due to mortality of silver perch physiological tests were only performed on golden perch. We expect long-term exposure to hypoxic conditions will allow fish to acclimate to hypoxia. Furthermore, higher temperature treatments are likely to limit the metabolic scope and acclimation ability of fish even after prolonged hypoxic exposure.
Methods
Experimental design
Silver perch (Bidyanus bidyanus, average length 45 mm, average body mass 2.7 ± 0.5 g) and golden perch (Macquaria ambigua ambigua, average length 35 mm, average body mass 2.1 ± 0.5 g) were sourced from aquaculture stock from the NSW Hatchery Quality Assurance Scheme (HQAS) accredited Silverwater Native Fish Hatchery, Grong Grong, NSW, in March 2014. Upon arrival at the University of Adelaide, fish were held in 250-L holding tanks at 20 °C. Aged (dechlorinated) tap water was used in tanks throughout the pre-experimental and experimental periods. Silver perch were fed hatchery pellet food until satiation, with any excess siphoned out an hour after feeding. Golden perch were fed live black worm (Lumbriculus variegatus), with waste siphoned out 24 h after feeding. Diets of both fish were matched to those at the hatchery: silver perch were fed pellets (sourced from Silverwater Native Fish Hatchery) and golden perch were fed live blackworm (sourced from Seaview Aquarium Centre, Plympton, SA). Fish were exposed to a 12:12-h light:dark cycle and room temperature was maintained at 20 °C. Water quality was monitored every second day for pH, ammonia and nitrite, with 25% water changes made daily for silver perch and every other day for golden perch. For both pre-experimental and experimental periods all tanks were aerated, and water in each tank was filtered using independent submersible aquarium filters for the duration of the experiment. Evaporation was minimised by covering tanks with clear plexiglass lids.
Fish were randomly assigned to 20-L treatment tanks 10 days after arrival to give sufficient time to adjust to the new conditions, with about 11 fish per tank. The experimental design consisted of two oxygen treatments, normoxic (6–8 mgO2 L−1 or 12–14 kPa) and hypoxic (3–4 mgO2 L−1 or 7–9 kPa), combined with up to three temperature treatments (20, 24 and 28 °C). Treatments included all possible combinations of temperature and oxygen for golden perch, with duplicate tanks for each treatment (n = 12 tanks), while silver perch included all possible combinations of two temperatures (20 °C and 28 °C) and oxygen with duplicate tanks for each treatment (n = 8 tanks). The experimental design differed for silver perch as there were less individuals available. The temperatures chosen reflected a portion of the natural thermal range experienced by both species (from 4 to 34 °C across their entire natural range) and are most likely to be affected by hypoxic conditions (Lintermans 2007). All tanks with temperatures ≥ 24 °C were heated independently using submersible aquarium heaters; temperature was monitored regularly. The desired temperatures were reached by adjusting heaters by 2 °C per day. Oxygen levels were based on the globally accepted tolerance limit of 2 mgO2 L−1 which is expected to cause high levels of stress and mortality for most species (Vaquer-Sunyer and Duarte 2008). The experiment was designed to provide long-term exposure yet still subject species to low levels of oxygen; therefore, the hypoxic treatments were higher than this limit, enough to cause slight discomfort but not mortalities. At the completion of all experiments fish were measured and weighed to calculate a simple condition index, Fulton’s K, which assumes that the weight of a fish is proportional to the cube of its length:
where W is body wet weight (g) and L the total standard length (cm), 100 is used to bring the factor close to a value of one. Fulton’s K condition factor is widely used in fish biology studies to describe the condition of the individual (Nash et al. 2006).
Control of hypoxia
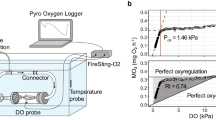
For the hypoxic treatments a simple and novel degassing system was developed, which used nitrogen gas to remove oxygen from the water (see Fig. 1). A combination of 9 L/min of nitrogen, split across 3 G-Class nitrogen cylinders (food grade, 3 L/min per cylinder) was mixed with 9 L/min of air in a loosely sealed 35-L mixing tank. Cylinders were changed on average every 3 days. The mixing tank contained two electric air pumps, with a combined flow rate of less than 18 L/min, which pumped the mixed gas to individual aquarium tanks, using air hosing (of equal distance) connected to a single air stone (of the same size) in each tank. Air was pumped into the mixing tank from an air compressor. Tanks were covered with plexiglass lids to minimise turbulence and limit diffusion of surrounding atmospheric air. This method allowed oxygen levels to be simultaneously controlled in all tanks over an extended period, and could be adjusted to suit both larger and smaller experiments.
Maximum exposure periods for silver perch and golden perch were 87 and 247 days, respectively. Exposure times varied as physiology experiments were carried out over 20 weeks, which included the initial experimental trials. Physiological trials were randomised such that there was little variation of exposure to experimental conditions among fish relative to the total exposure time.
Resting respirometry
Resting respirometry was only conducted on golden perch due to high mortalities of silver perch in the initial experiment. All fish were fasted for 24 h prior to experimental trials to evacuate the digestive tract so that only oxygen consumption rates (\( \dot{M}o_{2} \)) were recorded. Approximately 12 fish, per treatment, were randomly selected and subjected to respirometry experiments.
A four-chamber system was designed so that multiple fish could be tested simultaneously. Each resting chamber was custom made to fit the size of the fish based on a 1:10 ratio (1 kg of animal for every 10 L of water), and was 300 mL in volume. All four chambers were submerged in a larger water bath (139 × 52 × 20 cm), which was used to control temperature and oxygen levels. Individual chambers used a closed recirculation loop to pump low flowing water over the fish. A fibre optic oxygen probe (Pyroscience, OXROB3, Aachen, Germany) was fitted to the recirculation loop in each chamber; this recorded oxygen consumed during each \( \dot{M}o_{2} \) determination. Each chamber was connected to a flushing pump that circulated water intermittently after each \( \dot{M}o_{2} \) determination to completely replenish the chamber with oxygenated water from the water bath. A 5-min flushing period and a 20-min determination period was used for the 20 °C normoxic and hypoxic treatments; however, this was reduced to a 2-min flush and 20-min determination period for the remaining treatments as the 5-min flush caused stress for fish at higher temperatures. Stress was indicated by fish not maintaining a neutral or central position in the middle of the chamber during flushing. During the 20-min determination period oxygen was not reduced to less than 1 mgO2 L−1 and was above the background respiration rates. Dissolved oxygen concentration was recorded using four fibre optic oxygen probes (OXROB3) in a four-channel FireSting O2 Optical Oxygen Meter (Pyroscience, Aachen, Germany), with one probe fitted to the recirculation loop of each chamber.
To determine maximum metabolic rate (MMR), we used a method described by Roche et al. (2013), where fish were subjected to a 3-min exhaustive chase followed by a 1-min air exposure prior to being placed in respirometry chambers. This method forces fish to reach their MMR in a short time and can be determined using the highest \( \dot{M}o_{2} \) determination value. However, the 3- and 1-min combination was too stressful for the golden perch at higher temperatures, such that they were unable to recover from 3 min of chase and 1 min of air exposure; these times were, therefore, reduced to a 2-min exhaustive chase with 40-s air exposure for the 24 and 28 °C treatments. During the exhaustive chase, individual fish were placed in a 25-L bucket and were encouraged to continue swimming by gently touching the tip of the tail; fish were only encouraged if they slowed down or stopped swimming. Once the exhaustive chase was complete fish were suspended in a mesh aquarium net out of the water for 40–60 s and then immediately placed into a chamber. The first determination period was started 1 min after each fish was placed in a chamber, whereby maximum metabolic rate (MMR) was measured. Following this determination period, fish were left in the chamber for 8–12 h to allow fish to reach a resting state. Once the resting state was reached, the standard metabolic rate (SMR) was calculated using the equation below.
\( \dot{M}o_{2} \) (mgO2 kg−1 h−1) was calculated for each determination cycle using the equation:
where (t 0) is the oxygen content of the water measured at the end of a flushing cycle, and (t 1) is the oxygen content measured at the end of a determination period, just prior to the flush, both measured in mg O2/L. V is the volume of the chamber minus the volume of the experimental animal in L, t is t 0–t 1 and BW is body weight of the experimental animal in kg. The average of the lowest 10% of total measurements was used to calculate SMR. Three determinations were run before and after the testing period to record background values of bacterial respiration. Background rates were subtracted from \( \dot{M}o_{2} \) values. To reduce background respiration water was pumped through a heater/chiller unit fitted with a UV lamp that sterilized the water. The absolute aerobic scope (AAS) for activity of fish was calculated by subtracting SMR from MMR (MMR − SMR). The whole system was rinsed every third day to ensure background consumption of oxygen remained below 15% of the resting metabolic rate of fish.
Determining tolerances to hypoxia
To record tolerance limits among the different treatments, fish were left in chambers for an additional period of time with the intermittent flushing cycle turned off, which normally replenishes the system with oxygen. We defined the tolerance limit as the point at which an individual fish showed signs of stress at low oxygen levels. A sign of stress was indicated by a significant burst reaction in the chamber or loss of equilibrium. Fish were observed constantly during this period. At the first signs of stress, the oxygen level and time were recorded and the fish was immediately removed from the chamber. A tolerance limit was recorded for each fish.
Critical oxygen tension or Pcrit
The critical oxygen tension or Pcrit was measured using data from the closed respirometry phase of the experiment. To minimise the effects of CO2 accumulation, metabolic products and reductions in pH the chamber was flushed after acclimation. The Pcrit was defined as the point at which \( \dot{M}o_{2} \) was reduced below SMR and fish shifted to an oxy-conforming state. The Pcrit was determined for each fish by fitting a segmented regression using RStudio Version 1.0.143 (RStudio Team 2016). The critical tension was recorded as the point of intersection of the two lines as this indicated the breakpoint at which oxy-regulating changed to oxy-conforming. This measure differed from the hypoxia tolerance measure as it occurred prior to fish losing equilibrium.
Statistical analyses
Statistical analyses were conducted using PRIMER 6 and PERMANOVA + software (www.primer-e.com). Temperature, DO and oxygen saturation of the water, and Fulton’s K were analysed for both species at all possible temperature and treatment levels and for tank effects using a three-way permutational univariate analysis of variance (ANOVA) with unrestricted permutations. Temperature and hypoxia treatment levels (hypoxic or normoxic) were treated as fixed factors with replicate tanks treated as a random factor nested in temperature and hypoxia treatment. The same ANOVA design was used to analyse data regarding MMR, SMR, AAS, Pcrit and tolerance limits. Where tank effects were not detected, data were pooled and re-analysed using a two-way permutational univariate ANOVA with unrestricted permutations. Post hoc pairwise tests were conducted where significant differences were found. All analyses included Monte Carlo tests to ensure that there were sufficient permutations to detect significant differences.
Results
Rearing conditions
Treatment conditions remained consistent throughout the experimental period for both species and fish length and weight were similar among treatments for each species (< 0.5 g/cm, Table S1). Significant tank effects were detected; however, these were generally less than the variation among treatments (Table S2). All significant effects of temperature and hypoxia on water conditions were in line with the experimental treatment designs and changes had minimal variation (temperature < 0.7 °C, dissolved oxygen < 1 mgO2 L−1 and saturation < 8%) over the course of the experiment (Table S1).
Fish condition
Body condition, Fulton’s K, did not vary for golden perch despite long-term exposure to varied environmental conditions (Table S3, P ≤ 0.05). Silver perch in hypoxic conditions had a significantly lower Fulton’s K than those in normoxic conditions (Table S3, Fig. 2). A between-species comparison also shows that golden perch overall had a poorer body condition than silver perch; this may be because of the length of time species were exposed to treatment conditions (Fig. 2).
Survivorship
Mortality recorded during the rearing period for both species showed variation between the two species (golden perch and silver perch) (Fig. 3). Silver perch suffered significant high levels of mortality during the rearing period with no fish surviving beyond week 14 (P < 0.0001, Kaplan–Meier, IBM SPSS Statistics 24.0.0.1). Silver perch treated under hypoxic conditions suffered more than 50% mortality by week 2 at 28 °C, and by week 3 at 20 °C. In contrast, silver perch in normoxic conditions suffered more than 50% mortalities in week 5 at 28 °C and week 12 at 20 °C. Comparatively, golden perch treated under the same conditions (Table S2) suffered few mortalities over the full 40-week period.
Weekly mean survival (± SE) for replicate tanks of each treatment for a silver perch (n = 93) and b golden perch (n = 127). Survival is calculated as a percentage of fish surviving in each tank and then the mean of the two tanks is shown. Silver perch were only exposed to two of the three temperature treatments
Metabolic scope
As silver perch suffered high mortalities during the rearing period, metabolic variables were only measured on golden perch. Long-term exposure to hypoxia at 20 and 28 °C resulted in lower SMR and MMRs compared to 24 °C for golden perch (Fig. 4, Table S4). Maximum metabolic rate and SMR showed no significant effects of hypoxia although there was a significant interaction between temperature and hypoxia for MMR (Table S4). Significant differences were detected among temperature treatments for both SMR and MMR (Table S4, Fig. 4). Lower AAS measures occurred at 20 and 28 °C in fish exposed to hypoxia long term compared to those treated under normoxia, while fish exposed to hypoxia at 24 °C had higher AAS than those treated under normoxia (Fig. 5).
Boxplots (95% quantile) indicating maximum metabolic rate (MMR) and standard metabolic rate (SMR) recorded for golden perch at all temperatures and hypoxia treatments; replicate tanks are pooled. A) MMR where light grey bars represent hypoxic conditions (4 mg L−1), and dark grey bars represent normoxia (8 mg L−1, n = 69). b SMR where data were pooled for each temperature because significant differences were not found for hypoxia at each temperature (n = 69). Circles (○) represent outliers that fell between 1.5 and 3 interquartile ranges from the nearest edge of the box and stars (*) represent outliers beyond 3 interquartile ranges from the nearest edge of the box, and lines in the centre of box represent the median point
Boxplot (95% quantile) showing absolute aerobic scope (AAS) for golden perch at all temperature and hypoxia treatments; replicate tanks are pooled (n = 69). Light grey bars represent hypoxic conditions (4 mg L−1), and dark grey bars represent normoxia (8 mg L−1). Circles (○) represent outliers, and lines in the centre of box represent the median point
Hypoxia tolerance limits and Pcrit
Hypoxia tolerance limits and critical oxygen tension (Pcrit) of fish were significantly higher at normoxic conditions but did not vary among temperatures (Table S5, Figs. 6 and 7).
Boxplot (95% quantile) showing the hypoxia tolerance limits for individual golden perch at normoxic (dark grey, 8 mg L−1) and hypoxic (light grey, 4 mg L−1) conditions; replicate tanks and temperatures are pooled, as there were no significant temperature or tank effects detected (n = 69). Circles (○) represent outliers, and lines in the centre of box represent the median point
Discussion
Our study showed that sympatric species have different responses to thermal and hypoxic stress. Despite exposure to the same conditions as golden perch in our initial experimental rearing period, silver perch were unable to cope and suffered high levels of mortality (50% mortality by week 3 in hypoxic treatments). Golden perch, however, suffered few mortalities over the whole experimental period. Additionally, golden perch were able to tolerate hypoxic conditions after prolonged exposure. The behaviour and tolerance of fish exposed to hypoxia often differs among and within species and also with size (Eliason and Farrell 2016; McCarthy et al. 2014; Metcalfe et al. 2016). Multiple studies have demonstrated highly species-specific responses to thermal and hypoxic stress, in some cases influenced by fish making physiological trade-offs associated with specific natal conditions (Eliason and Farrell 2016; McNeil and Closs 2007). We observed that thermal stress accelerated the decline of silver perch, particularly those treated under hypoxic conditions. Due to mortality during the initial experiment, physiological performance of silver perch was not tested; however, the effect of hypoxia on body condition of this species may indicate that exposure to hypoxic conditions quickly degenerates their overall health. In comparison, there was minimal impact on the body condition of golden perch held under the same conditions, but their overall condition was lower than silver perch. As all measures on body condition were conducted after the 40-week exposure period differences in condition between species may be attributed to golden perch withstanding the full 40 weeks of exposure. Differences in the diet between the two species may have also driven differences in body condition. Silver perch were fed a pellet food specifically designed to enhance fitness in this species (Rowland 2008, 2009; Rowland and Tully 2004), while golden perch were fed unenriched live food. Golden perch and silver perch inhabit similar zones in the MDB, including lowland, turbid and slow-flowing rivers with snags and rocky outcrops (Lintermans 2007). Historically, the distribution of silver and golden perch was similar; however, changes to river regulation, reproductive behaviour, migration patterns through the addition of weirs and dams, threat of alien species, thermal pollution, hypoxic episodes and flow regime have resulted in a severe decline in silver perch distribution, while golden perch are still widespread, albeit at reduced numbers (for more species information see Supp. SSI 1, Koehn and Nicol 2016; Lintermans 2007). Without knowing the complete genetic history of silver perch used in our study it is possible they do not fully reflect wild populations, although the hatchery fish were sourced from are one of many involved in restocking wild populations throughout the MDB. However, given the dramatic decline seen in this species over the last 50-year exposure to hypoxia and high temperatures in our laboratory experiment may explain why their natural range has diminished (Lintermans 2007; Rowland 2008, 2009; Rowland and Tully 2004).
Long-term exposure to hypoxia directly affected the tolerance thresholds of golden perch. Fish exposed to hypoxia were able to tolerate lower levels of dissolved oxygen, while a lack of exposure (long-term normoxia) resulted in fish reaching their tolerance thresholds at higher levels of dissolved oxygen. Critical oxygen tensions (Pcrit) of golden perch also followed the same pattern, suggesting that long-term exposure to hypoxia induced an acclimation response (for other examples see: Cook et al. 2013, Timmerman and Chapman 2004; Rogers et al. 2016). Additionally, higher temperatures had no effect on the hypoxia tolerance of golden perch, which may be due to a thermal acclimation response from prolonged exposure (McMaster and Bond 2008). Although golden perch may persist in low levels of dissolved oxygen and partial pressure (Po 2 ) it is necessary to consider at what cost this occurs and if they use other options in the wild such as aquatic surface respiration (ASR), increasing gill ventilation or simply move away. For example, a study on the tolerance of zebrafish (Danio rerio) found that individuals could persist to levels of 1 mgO2 L−1 and lower; however, it was at the cost of poor-performing antioxidant enzymes, which resulted in oxidative damage (Feng et al. 2016). Environmental factors such as temperature, food intake and diet composition may also act to change this value in wild fish; therefore, oxygen thresholds of fish should always be used carefully for management. For example, our results suggest golden perch would be protected under the current universal threshold limit (2 mgO2 L−1), but silver perch would be unlikely to survive (Vaquer-Sunyer and Duarte 2008). The threshold limit for silver perch may be higher than 4 mgO2 L−1 given the high mortalities during the experimental rearing period even under normoxia; however, they are known to continue feeding at dissolved oxygen concentrations below 3mgO2 L−1 (Rowland 2009). Even though the global limit provides a standard for managers to work from it is difficult to predict what this value means for wild populations. Adapting this information to existing models for the MDB would allow managers to predict larger scale impacts of hypoxic events and appropriate dissolved oxygen levels and Po 2 for highly oxygenated water releases to combat hypoxic events spreading. Models could further incorporate physiological details such as thermal thresholds, oxygen carrying capacity, aerobic scope, and the growth, digestion and reproductive requirements of fish to provide a comprehensive way of predicting impacts on local populations. There are some examples of models being used effectively in this way for predicting how future environmental conditions will impact Pacific salmonids (Eliason and Farrell 2016; Hague et al. 2011; Rand et al. 2006). However, there may be some limits to this method due to the clear species-specific reactions to hypoxia, thus models considering the most sensitive species among local populations would be most appropriate. Other researchers have also observed fish under hypoxia reaching tolerance points much faster than those treated under normoxia (Dean and Richardson 1999; Fu et al. 2011).
Temperature is widely considered the principal controlling factor for aerobic and metabolic capacity, while hypoxia is considered the primary limiting factor (Claireaux and Chabot 2016; Pörtner and Lannig 2009). Long-term exposure to hypoxia typically limited the aerobic and metabolic capacity (AAS and MMR) of golden perch at 20 and 28 °C, while overall fish had a lower basal oxygen demand (standard metabolic rate, SMR) at the same temperatures. Therefore, aerobic and metabolic capacity may be limited beyond these points, such that it deleteriously impacts normal functioning (Farrell 2016; Neuheimer et al. 2011). However, at 24 °C prolonged hypoxia exposure typically improved the aerobic and metabolic capacity of golden perch, compared to those fish treated under normoxia. However, possible thermal acclimation of golden perch could confound some of our results, particularly the higher MMRs observed under normoxia at 28 °C (Farrell 2016; McBryan et al. 2013; McMaster and Bond 2008). Thermal acclimation can be achieved by reducing general metabolism via reduced feeding and movement, the downregulation of protein synthesis or the decrease and/or modification of certain regulatory enzymes in aerobic and anaerobic pathways (McMaster and Bond 2008; Wu 2002). For example, Chilko, Oncorhynchus nerka, have an enhanced cardiac capacity due to a higher density of adrenaline-binding ventricular β-adrenoreceptors, giving them a broader thermal range compared to co-migrators Nechako, O. nerka (Eliason et al. 2011). When fish are also exposed to hypoxia the capacity for oxygen transfer through haemoglobin and the circulatory system is reduced, this would seem to be the case for golden perch AAS and MMR, suggesting their thermal range may be limited by prolonged hypoxia exposure. The lower SMRs observed in golden perch overall at 28 °C could also be a result of thermal acclimation as fish at higher temperatures would be expected to grow and metabolise faster than those at lower temperatures. In a marine system, elevated levels of ambient CO2 would have a similar limiting effect as hypoxia, highlighting the necessity for future physiological studies to consider the synergistic effects of environmental factors (Pörtner and Lannig 2009).
Fish respond to hypoxia in many different ways and while the lethal endpoint has been used in the past to assess safe levels of dissolved oxygen for fish, the sub-lethal tolerance limits are likely to be the most useful (Feng et al. 2016). Fish tolerance to a sub-lethal point will indicate the sensitivity of other vital functions such as growth and reproduction (Feng et al. 2016). Organisms recovery from hypoxic events can be partially attributed to the availability of nearby refuges and species capacity to adapt, exploit oxygen-rich zones and recolonize an area successfully after an event (Conley et al. 2009; McMaster and Bond 2008). However, if those organisms are unable to relocate or survive a hypoxic event there is very little chance of system recovery. Conley et al. (2009) suggested that systems which have been previously exposed to hypoxia are more prone to experience it in the future and suffer a slower recovery with each incidence. For example, hypoxic events resulting in large-scale losses of benthic communities lead to a change in overall trophic structure, with smaller, fast-growing species recolonizing an area first, impacting not only community structure but complete system functioning with deleterious effects to the storage capacity of sediments (Conley et al. 2009; Diaz and Rosenberg 2008). Only ~ 4% of the 500-plus systems affected by hypoxia worldwide have shown signs of recovery (Diaz and Rosenberg 2008).
Supply of oxygen to aquatic organisms worldwide is going to be affected by climate change, with models predicting substantial warming and deoxygenation throughout much of the world’s oceans and terrestrial water bodies (Deutsch et al. 2015). Due to the disparity observed among our two case study species, it will be necessary to consider management targets carefully to ensure the survival of all species within any one system (Vaquer-Sunyer and Duarte 2008). We show some species may be able to develop natural resistance to poor oxygen conditions over time; however, this may be limited to only those with a naturally higher tolerance to hypoxia. Furthermore, future research should be targeted towards understanding the individual tolerance of known sensitive species within a system. We recommend that it is valuable to consider each system individually in terms of species and the effects of large-scale water allocation on dissolved oxygen content. Poor management of re-allocated waters will influence local fauna where managers do not consider the complete spectrum of organism tolerance to hypoxia. Sympatric populations of fish under hypoxic stress in our system exhibited distinctly different responses to prolonged hypoxia exposure, and while it appears acclimation is achievable it remains species specific.
References
Breitburg DL, Hondorp DW, Davias LA, Diaz RJ (2009) Hypoxia, nitrogen, and fisheries: integrating effects across local and global landscapes. Ann Rev Mar Sci 1:329–349
Chabot D, Steffensen JF, Farrell AP (2016) The determination of standard metabolic rate in fishes. J Fish Biol 88(1):81–121
Claireaux G, Chabot D (2016) Responses by fishes to environmental hypoxia: integration through Fry’s concept of aerobic metabolic scope. J Fish Biol 88(1):232–251
Collins GM, Clark TD, Rummer JL, Carton AG (2013) Hypoxia tolerance is conserved across genetically distinct sub-populations of an iconic, tropical Australian teleost (Lates calcarifer). Conserv Physiol 1(1):1–9
Conley D, Carstensen J, Vaquer-Sunyer R, Duarte C (2009) Ecosystem thresholds with hypoxia. In: Andersen JH, Conley DJ (eds) Eutrophication in coastal ecosystems, vol 207. Springer, Netherlands, pp 21–29
Cook DG, Iftikar FI, Baker DW, Hickey AJ, Herbert NA (2013) Low-O2 acclimation shifts the hypoxia avoidance behaviour of snapper (Pagrus auratus) with only subtle changes in aerobic and anaerobic function. J Exp Biol 216(3):369–378
Dean TL, Richardson J (1999) Responses of seven species of native freshwater fish and a shrimp to low levels of dissolved oxygen. N Z J Mar Freshw Res 33(1):99–106
Deutsch C, Ferrel A, Seibel B, Pörtner H-O, Huey RB (2015) Climate change tightens a metabolic constraint on marine habitats. Science 348(6239):1132–1135
Diaz RJ, Rosenberg R (2008) Spreading dead zones and consequences for marine ecosystems. Science 321(5891):926–929
Díaz RJ, Rosenberg R (2011) Introduction to environmental and economic consequences of hypoxia. Int J Water Resour Dev 27(1):71–82
Eliason EJ, Farrell AP (2016) Oxygen uptake in Pacific salmon Oncorhynchus spp.: when ecology and physiology meet. J Fish Biol 88(1):359–388
Eliason EJ, Clark TD, Hague MJ, Hanson LM, Gallagher ZS, Jeffries KM, Gale MK, Patterson DA, Hinch SG, Farrell AP (2011) Differences in thermal tolerance among sockeye salmon populations. Science 332(6025):109–112
Farrell AP (2016) Pragmatic perspective on aerobic scope: peaking, plummeting, pejus and apportioning. J Fish Biol 88(1):322–343
Feng J, Guo Y, Gao Y, Zhu L (2016) Effects of hypoxia on the physiology of zebrafish (Danio rerio): initial responses, acclimation and recovery. Bull Environ Contam Toxicol 96(1):43–48
Fu S-J, Brauner CJ, Cao Z-D, Richards JG, Peng J-L, Dhillon R, Wang Y-X (2011) The effect of acclimation to hypoxia and sustained exercise on subsequent hypoxia tolerance and swimming performance in goldfish (Carassius auratus). J Exp Biol 214(12):2080–2088
Hague MJ, Ferrari MR, Miller JR, Patterson DA, Russell GL, Farrell AP, Hinch SG (2011) Modelling the future hydroclimatology of the lower Fraser River and its impacts on the spawning migration survival of sockeye salmon. Glob Change Biol 17(1):87–98
Helz GR, Adelson JM (2013) Trace element profiles in sediments as proxies of dead zone history; rhenium compared to molybdenum. Environ Sci Technol 47(3):1257–1264
King AJ, Tonkin Z, Lieshcke J (2012) Short-term effects of a prolonged blackwater event on aquatic fauna in the Murray River, Australia: considerations for future events. Mar Freshw Res 63(7):576–586
Koehn JD, Nicol SJ (2016) Comparative movements of four large fish species in a lowland river. J Fish Biol 88(4):1350–1368
Lintermans M (2007) Fishes of the Murray-Darling basin: an introductory guide. Murray Darling Basin Comission Publication, Canberra, ACT, pp 1–131
McBryan TL, Anttila K, Healy TM, Schulte PM (2013) Responses to temperature and hypoxia as interacting stressors in fish: implications for adaptation to environmental change. Integr Comp Biol 53(4):648–659
McCarthy B, Zukowski S, Whiterod N, Vilizzi L, Beesley L, King A (2014) Hypoxic blackwater event severely impacts Murray crayfish (Euastacus armatus) populations in the Murray River, Australia. Austral Ecol 39(5):491–500
McMaster D, Bond N (2008) A field and experimental study on the tolerances of fish to Eucalyptus camaldulensis leachate and low dissolved oxygen concentrations. Mar Freshw Res 59(2):177–185
McNeil DG, Closs GP (2007) Behavioural responses of a south-east Australian floodplain fish community to gradual hypoxia. Freshw Biol 52(3):412–420
Metcalfe NB, Van Leeuwen TE, Killen SS (2016) Does individual variation in metabolic phenotype predict fish behaviour and performance? J Fish Biol 88(1):298–321
Nash RD, Valencia AH, Geffen AJ (2006) The origin of Fulton’s condition factor—setting the record straight. Fisheries 31(5):236–238
Neuheimer AB, Thresher RE, Lyle JM, Semmens JM (2011) Tolerance limit for fish growth exceeded by warming waters. Nat Clim Change 1(2):110–113
Nilsson GE, Crawley N, Lunde IG, Munday PL (2009) Elevated temperature reduces the respiratory scope of coral reef fishes. Glob Change Biol 15(6):1405–1412
Norin T, Clark TD (2016) Measurement and relevance of maximum metabolic rate in fishes. J Fish Biol 88(1):122–151
Pollock MS, Clarke LMJ, Dubé MG (2007) The effects of hypoxia on fishes: from ecological relevance to physiological effects. Environ Rev 15:1–14
Pörtner HO, Lannig G (2009) Oxygen and capacity limited thermal tolerance. In: Jeffrey APF, Richards G, Colin JB (eds) Fish physiology, vol 27. Academic Press, Cambridge, pp 143–191
Rand PS, Hinch SG, Morrison J, Foreman MGG, MacNutt MJ, Macdonald JS, Healey MC, Farrell AP, Higgs DA (2006) Effects of river discharge, temperature, and future climates on energetics and mortality of adult migrating Fraser River sockeye salmon. Trans Am Fish Soc 135(3):655–667
Richardson J, Williams EK, Hickey CW (2001) Avoidance behaviour of freshwater fish and shrimp exposed to ammonia and low dissolved oxygen separately and in combination. N Z J Mar Freshw Res 35(3):625–633
Roche DG, Binning SA, Bosiger Y, Johansen JL, Rummer JL (2013) Finding the best estimates of metabolic rates in a coral reef fish. J Exp Biol 216(11):2103–2110
Rogers NJ, Urbina MA, Reardon EE, McKenzie DJ, Wilson RW (2016) A new analysis of hypoxia tolerance in fishes using a database of critical oxygen level (Pcrit). Conserv Physiol 4(1):1–19
Rowland SJ (2008) Domestication of silver perch, Bidyanus bidyanus, broodfish. J Appl Aquac 16(1–2):75–83
Rowland SJ (2009) Review of aquaculture research and development of the Australian freshwater fish silver perch, Bidyanus bidyanus. J World Aquac Soc 40(3):291–324
Rowland SJ, Tully P (2004) Hatchery quality assurance program for Murray cod (Maccullochella peelii peelii), golden perch (Macquaria ambigua), and silver perch (Bidyanus bidyanus). NSW Department of Primary Industries, Sydney
R Studio Team (2016) RStudio: integrated development for R. RStudio, Inc., Boston, MA. http://www.rstudio.com/
Sollid J, Weber RE, Nilsson GE (2005) Temperature alters the respiratory surface area of crucian carp Carassius carassius and goldfish Carassius auratus. J Exp Biol 208(6):1109–1116
Timmerman CM, Chapman LJ (2004) Behavioural and physiological compensation for chronic hypoxia in the Sailfin molly (Poecilia latipinna). Physiol Biochem Zool 77(4):601–610
Vaquer-Sunyer R, Duarte CM (2008) Thresholds of hypoxia for marine biodiversity. Proc Natl Acad Sci 105(40):15452–15457
Whitworth KL, Baldwin DS, Kerr JL (2012) Drought, floods and water quality: drivers of a severe hypoxic blackwater event in a major river system (the southern Murray-Darling Basin, Australia). J Hydrol 450:190–198
Wu RSS (2002) Hypoxia: from molecular responses to ecosystem responses. Mar Poll Bull 45(1):35–45
Zhang W, Cao Z-D, Peng J-L, Chen B-J, Fu S-J (2010) The effects of dissolved oxygen level on the metabolic interaction between digestion and locomotion in juvenile southern catfish (Silurus meridionalis Chen). Comp Biochem Physiol Mol Integr Physiol 157(3):212–219
Acknowledgements
We would like to thank the Goyder Institute for Water research for funding which contributed to the success of this research. Funding from an ARC Future Fellowship (FT100100767) is also acknowledged. We would also like to thank Owen Burnell for his assistance in developing the novel approach to degassing multiple tanks to create hypoxic conditions long term in aquaria, and Lincoln Gilmore for building the respirometry chambers. Fish were kept according to the Australian Code of Practice for the care and use of animals for scientific purposes (8th Edition), and approved by the University of Adelaide’s animal care and ethics committee (AEC project approval S-2013-183).
Author information
Authors and Affiliations
Contributions
KLG, BMG and ZAD conceived the experiment, KLG was responsible for the design of the experiment, performing the experiment and analysing data. KLG wrote the manuscript; BMG and ZAD were crucial in reviewing work and provided editorial advice.
Corresponding author
Additional information
Communicated by Donovan P. German.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gilmore, K.L., Doubleday, Z.A. & Gillanders, B.M. Testing hypoxia: physiological effects of long-term exposure in two freshwater fishes. Oecologia 186, 37–47 (2018). https://doi.org/10.1007/s00442-017-3992-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-017-3992-3