Abstract
We investigated whether individual rainbow trout (Oncorhynchus mykiss; 9–53 g) avoid a potentially lethal level of total dissolved gas (TDG) supersaturation using lateral movements during an acute exposure. As there is no mechanism by which fish can detect and avoid TDG supersaturation in shallow water, we hypothesize that rainbow trout do not directly detect TDG supersaturation. Most previous studies have tested TDG avoidance in groups of fish, which may confound responses because many fishes, such as juvenile rainbow trout, display territorial behavior. We placed rainbow trout individually into a flume and allowed them to swim freely between two channels for 6 min: one channel contained water at 145% TDG and the other contained air-equilibrated water. We then switched treatments between channels and tested fish in the same way for an additional 6 min. We quantified the duration spent by fish in each channel during the last 2 min of each 6 min exposure. Fish spent a mean duration of 11s longer in 145% TDG water compared to air-equilibrated water, and the inclusion of TDG treatment as a factor improved the model of the duration spent by fish in a channel. These results indicate that fish did not avoid a potentially lethal level of TDG. This suggests that some fishes in shallow water may be unable to avoid harmful TGD supersaturation generated by events such as spilling from hydroelectric dams.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Dams can generate total dissolved gas (TDG) supersaturation which can result in gas bubble trauma (GBT) in aquatic animals. The construction of dams is having a widespread effect on riverine landscapes. Approximately, 58,000 large dams (International Commission on Large Dams, 2019) and 82,000 small hydropower dams (Couto & Olden, 2018) fragment rivers worldwide, with over 3700 additional dams planned or under construction (Zarfl et al., 2015). Dams have multiple effects on aquatic ecosystems (reviewed in Baxter, 1977; Dynesius & Nilsson, 1994; Ligon et al., 1995), including loss of river connectivity, habitat alteration, altered flow regimes, and changes in sediment transport, but a lesser known effect is the generation of TDG supersaturation.
TDG supersaturation can be produced at dams during spilling or air injection into turbines (Ebel, 1969; Bouck et al., 1976). When air mixes with water passing through the spill gates or the turbines, bubbles can be entrained and pushed below the surface of the water in the plunge pool below the dam. Hydrostatic pressure causes these bubbles to dissolve at depth. When this water mixes into shallower depths, hydrostatic pressure is reduced, but air can remain dissolved, causing TDG supersaturation. The tissues of aquatic animals can become supersaturated with TDG in these conditions. In a process somewhat analogous to decompression sickness in divers, bubbles nucleate in the tissues of animals exposed to TDG supersaturation. These bubbles can cause damage, known as gas bubble trauma (GBT). Gas bubble trauma in fish is rare below 110% TDG (Pleizier et al., 2020a) and only occurs at 110% TDG after long exposures (e.g., Dawley et al., 1976; Gray et al., 1983; Shrimpton et al., 1990; Espmark et al., 2010). At 110% TDG, 10% mortality is predicted to occur in rainbow trout and steelhead trout (Oncorhynchus mykiss [Walbaum, 1792]) after 9.6 days in a surface treatment with a molar oxygen to nitrogen ratio of 0.52. The higher the TDG, the shorter the duration to mortality. For example, at 140% TDG, 50% mortality may occur in as little as 2 h (Nebeker et al., 1979; Pleizier et al., 2020a).
Gas bubble trauma can have multiple different effects on fish. Direct effects include gas bubbles in the lateral line, beneath the skin, in the mouth, in and on the gills, in the blood and the heart, behind the eyes, and in other tissues. Indirect effects include hemorrhaging, tissue necrosis (Stroud et al., 1975), infection (Schisler et al., 2000; Stroud et al., 1975), and impaired development (Cornacchia & Colt, 1984; Counihan et al., 1998; Geist et al., 2013). There have also been many reports on the effects of TDG on fish behavior (e.g., Bentley et al., 1976; Antcliffe et al., 2003; Chen et al., 2012), although much of the data are qualitative.
Knowledge of behavioral avoidance of deleterious environmental conditions can inform regulatory guidelines and management actions for protection of fish and wildlife (Buchholz, 2007; Cooke et al., 2014; Blumstein & Berger-Tal, 2015). Of the effects of TDG on fish behavior, it remains unclear whether fish can sense and choose to avoid TDG supersaturation. Whereas some studies provide evidence that fish increase depth during exposure to TDG supersaturation (e.g., Dawley et al., 1976; Chamberlain et al., 1980; Wang et al., 2015a; Table 1), others have not (Lund & Heggberget, 1985; Table 1). We note, however, that the TDG supersaturation levels tested differed by study (Table 1). Vertical movements to increase depth has the advantage of compressing gases in the swim bladder and reducing buoyancy in the event of swimbladder over-inflation, but this may be a response to changes in buoyancy rather than direct detection of TDG. Increasing depth also reduces other effects of GBT because hydrostatic pressure reduces the probability of bubble formation and compensates for the effects of TDG at a rate of 9.7% supersaturation per meter of freshwater (Pleizier et al., 2020b). Fish can also potentially reduce their exposure to unevenly distributed TDG supersaturation using lateral movements to swim away from areas with high TDG levels. However, this would require the ability to directly sense TDG supersaturation and respond. Lateral avoidance has been observed in some studies, but not in others (Table 1).
Rainbow and steelhead trout are of interest in TDG avoidance studies because they commonly occur downstream of dams in North America. Stevens et al. (1980) found that a strain of rainbow trout avoided TDG supersaturation using lateral movements, but steelhead strains tested by Blahm et al. (1976) and Stevens et al. (1980) did not. Moreover, the rainbow trout that avoided TDG supersaturation in the study by Stevens et al. (1980) did not avoid it to the extent that it prevented high rates of mortality from GBT, despite regions of air-equilibrated water being available to these fish. In these studies of lateral avoidance, juvenile rainbow and steelhead trout were tested in groups, although juveniles of this species are known to be territorial and to form social hierarchies (reviewed by Sloman & Armstrong, 2002 and Gilmour et al., 2005). Thus, it is possible that interactions with conspecifics prevented fish in these experiments from avoiding TDG supersaturation.
To determine whether rainbow trout can detect and avoid TDG supersaturation in the first few minutes of exposure, we conducted a series of lateral choice behavior trials. As there is no known mechanism by which fish can detect TDG supersaturation in shallow water, we hypothesize that rainbow trout do not detect and avoid TDG supersaturation in shallow water. Results from preliminary experiments suggested that there may be a difference between strains in the ability of rainbow trout to detect and avoid TDG supersaturation; for this reason, we tested fish from the Blackwater and Fraser Valley strains. The Blackwater strain originates from the Blackwater River in the Cariboo region of British Columbia (BC), Canada. The Blackwater broodstock are reared in Dragon Lake near Quesnel, BC, Canada and are regularly crossed with wild source fish from the Blackwater River. The Fraser Valley strain is a domesticated strain developed in the 1940s with fish that may have originated from California, USA. We introduced rainbow trout individually into an arena where they were able to swim freely between two channels: one which contained control TDG and one which contained high TDG supersaturation (Fig. 1). For the latter, we chose a nominal TDG value of 145% as this level would result in mortality within a few h of exposure; for example, juvenile steelhead reach 50% mortality in 2 to 5 h of exposure to 140% TDG at depths less than 60 cm (Nebeker et al., 1979; Pleizier et al., 2020a). After 6 min of exposure, we switched treatments between the two channels to account for any effects of side preference, and we exposed the fish to these treatments for an additional 6 min. We analyzed video footage of their movements during the last 2 min of each trial to quantify the time that fish spent in each channel of the flume and to determine whether they make lateral movements to avoid TDG supersaturation. This is the first study to test the lateral avoidance of TDG supersaturation by individual rainbow trout. By testing individuals, we avoid the confounding effects of territorial behavior and dominance hierarchies among conspecifics.
Methods
Experimental subjects
We acquired Fraser Valley strain rainbow trout June 5th, 2019, and Blackwater strain rainbow trout March 6, 2020, from the Freshwater Fisheries Society of BC Fraser Valley Trout Hatchery in Abbotsford, BC, Canada. Fraser Valley strain were spawned at the Fraser Valley Trout Hatchery November 2018 and were 1 + years old at the time of the experiment (mean weight 28.48 g ± 1.88 SEM, mean fork length 132 mm ± 2.6 SEM). Blackwater strain were spawned in May 2019 in the Vancouver Island Trout Hatchery in Duncan, BC, Canada and were 1 + years old at the time of the experiment (mean weight 19.44 g ± 1.04, mean fork length 123.06 mm ± 2.04). We held fish at the University of British Columbia in a 15,000 l recirculating system supplied with dechlorinated Vancouver city water maintained at a mean temperature of 10.8°C for the Fraser Valley strain and 9.7°C for the Blackwater strain. The photoperiod was 12 h light, 12 h dark for all fish. We fed all fish a maintenance diet of 1.5% body weight three times a week with EWOS Pacific commercial fish feed pellets prior to the experiment. TDG in the holding tanks was generally 100–102% TDG.
Experimental system
We generated TDG supersaturation using a pressurized column (12″ pressurized packed column for supersaturated oxygen, model number X024656‐01, Pentair Aquatic Eco-systems). The system pumps water into the top of a 2.8 m tall, 0.3 m diameter pressurized stainless-steel column packed with bio balls. The water equilibrated with the air in the column as it trickled over the high surface area medium. A pressure transducer measured the pressure in the column and provided feedback to the variable frequency drive water pump which turned on when the internal pressure fell below 30 PSI. We used a level sensor attached to a sight glass to maintain the water depth in the column. When the water level rose above the sensor, the air input valve opened, and a compressor turned on and pumped air into the column at a rate of 11 l/min. When the water level fell below the sensor, the air input valve closed, and the compressor turned off. After the air input valve closed, there was a 5 s lag before it could be turned on again. The pressurized column supplied TDG supersaturated water (150% TDG) to a 38 l header tank. We supplied air-equilibrated water (100% TDG) to two additional 38 l header tanks. Water for both the TDG supersaturated header tank and the air-equilibrated header tanks originally came from the same 15,000 l recirculation system.
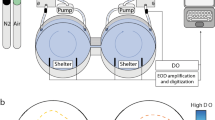
Two-channel flume
We used a two-channel flume (Fig. 1) which was constructed of sealed wood and painted white. The dimensions of the entire two-channel flume were 131 cm × 44 cm × 35 cm and was 11 cm deep, for a total water volume of approximately 63 l. Water of each respective TDG level flowed into one of the two channels created by a divider. We diffused the water inflow using a round plastic baffle attached to mesh and used a plastic collimator to straighten water flow (Fig. 1). The area available to the fish in each channel was 42 cm × 21 cm (× 11 cm deep). Water from the two channels flowed into the 44 cm × 44 cm mixed area before flowing through a mesh and over a ramp to the outflow. Water was supplied to the two-channel flume by header tanks containing either TDG supersaturated or air-equilibrated water.
We directed water flow from the respective header tanks to the channel of choice using ball valves, which permitted switching of water types between channels as required. We monitored the flow rate from each header tank using flowmeters (Great Plains Industries TM050 Flowmeter/Totalizer) which we controlled manually using needle valves to achieve a flow rate of 4.5 l/min per channel, for a combined total flow rate of 9 l/min into the flume. We calibrated flowmeters at the beginning of the experiment and again partway through the experiment. Dye tests indicated mixing between the two channels; however, nominal TDG levels were relatively constant in the channels over the duration of the study. We conducted TDG measurements in both channels at the end of the trials for each fish. Dye tests indicated that it took approximately 4 min to flush out each channel after switching TDG treatments between channels.
Measuring TDG
We measured TDG using a Point Four Tracker Total Gas Pressure Meter (Pentair Aquatic Eco-Systems). The meter uses two sensors to simultaneously measure atmospheric pressure and the pressure of gas that has diffused into the silastic tubing of the submerged probe. We calibrated the sensors based on an adapted protocol from the US Geological Survey (Tanner & Johnston, 2001). We corrected the atmospheric pressure sensor to the prevailing atmospheric pressure reported by Environment and Climate Change Canada at the Vancouver International Airport (YVR). We calibrate the TDG pressure sensor in the silastic tube using a two-point calibration. We measured the first point with the dry probe at atmospheric pressure. We measured the second point by placing the probe in a chamber and pressurizing it up to 200 mmHg above atmospheric. We compared the reading of the probe to the reading of a separate pressure gauge attached to the chamber. The range of the two-point calibration is equivalent to 100–126% TDG. We increased the second point for the calibration to 300 mmHg (100–139% TDG) above atmospheric when calibrating prior to the trials using Blackwater strain rainbow trout. We measured TDG during the experiment by repeatedly knocking the submerged probe against the bottom of the flume to dislodge any bubbles on the silastic tubing. We assume the pressure in the silastic tubing was equilibrated with the TDG pressure in the water when the percent TDG reading was stable for 2 min.
Water quality
We measured the pH, ammonia, and nitrite levels in the two-channel flume at the beginning of the experiment for the Fraser Valley strain and both at the beginning of the experiment and mid-experiment for the Blackwater strain. We measured temperature, dissolved oxygen, and TDG in each channel of the flume at the end of the behavior trials for each fish (Table 2).
Behavioral protocol
To avoid including initial fright response behavior in the behavioral assays, we ran preliminary trials to determine the time necessary for fish to resume swimming behavior after being introduced to the flume. During preliminary trials, we placed single fish in the flume (Fraser Valley strain n = 5; Blackwater strain n = 5) and recorded their behavior using a video camera (Apexcam Action Camera M80) for 30 min. The fish displayed freezing behavior when first introduced into the flume and gradually resumed swimming. We deemed that fish had fully resumed swimming behavior when a fish swam continuously for 3 min without a pause greater than 3 s. The longest time to resume swimming behavior in the preliminary trials was 9 min; thus, we gave fish in the experimental trials 9 min to acclimate to the two-channel flume before beginning observations (Ou et al., 2015; Jutfelt et al., 2017).
We conducted behavioral trials between January 8 and 21, 2020, for the Fraser Valley strain rainbow trout (n = 37) and between June 19 and July 6, 2020, for the Blackwater strain rainbow trout (n = 41). We fasted fish for 48 h prior to the behavioral trials. We conducted trials between 10 AM and 5 PM. Behavior experiments consisted of three trials (Fig. 2); during the first, baseline trial both channels of the flume contained air-equilibrated water (100% TDG), during the second trial one channel of the flume contained air-equilibrated water and the other contained water at 145% TDG, and during the third trial we switched the air-equilibrated and the 145% TDG treatments between channels. At the beginning of each set of trials we flushed both channels of the flume with air-equilibrated water for at least 10 min. Next, we netted a single fish and quickly placed it in the flume at the downstream end in the “mixed area” (Fig. 1). We recorded fish behavior continuously with a video camera. During all trials, the experimenter was blocked from the view of the fish by an opaque screen. In most of the trials we monitored and adjusted flowrate continuously throughout the experiment to maintain consistent flowrates of 4.5 l/min in each channel. This was necessary because bubbles from TDG supersaturated water can form in the supply tubing and restrict flow. After 9 min of acclimation with both channels receiving air-equilibrated water, we began the baseline trial, during which we recorded the behavior of the fish for an additional 2 min (Ou et al., 2015) to determine whether the fish had a preference for one channel over the other. After this, we recorded the flowrate for both channels. The TDG supersaturated water from the pressurized column is approximately 1°C warmer than the control water; for this reason, after the first trial and before the beginning of the second trial we turned on heaters in one of the air-equilibrated header tanks so that the temperatures would be similar between the two treatments (~ 11°C, see Table 2). Immediately after turning on the heater, we switched the water to TDG supersaturated water in one of the channels. We assigned the channel that received TDG supersaturated water first using a random number generator. We determined that after 6 min the water in the air-equilibrated header tank reached the same temperature as the TDG supersaturated water and the TDG supersaturated water had also replaced the air-equilibrated water in one channel of the flume. At this time, we used a sign within view of the video camera, but not visible to the fish, to indicate the beginning of the second behavior trial.
The second behavior trial lasted for 6 min; the fish acclimated to the treatments during the first 4 min, after which we recorded behavior for an additional 2 min. After the 6 min trial, we recorded the flowrates in each channel of the flume and we switched the air-equilibrated and TDG supersaturated water between the channels of the flume. Once we adjusted the flows in each channel to the target flowrates, we used a sign in view of the video camera to indicate that the treatments had been switched between channels. As above, during the third trial the fish acclimated to the new conditions for the first 4 min and we recorded fish behavior for 2 min. After this behavior trial, we recorded the flowrates in each channel of the flume and removed the fish (Fig. 2). We euthanized the fish with MS-222 (200 mg/l MS-222 and 400 mg/l sodium bicarbonate) and we weighed the fish and measured their fork length. We measured TDG, temperature, and dissolved oxygen in the flume as described above after testing each fish.
Video analysis
We analyzed the videos using Ethovision XT video tracking software (version 13.0.1220, Noldus Information Technology, 2017), which tracked the mid-point of a fish and quantified the amount of time spent in each channel of the flume during the last 2 min of the first trial in air-equilibrated water only, as well as during the last 2 min of both trials with air-equilibrated water in one channel and TDG supersaturated water in the other. We excluded fish from the first day of testing from the dataset (Fraser Valley strain n = 3) because those fish displayed freezing behavior typical of a stress response throughout the experiment, possibly because they had been recently disturbed during preparations for the experiment. We also removed fish from all analyses if they expressed jumping behavior during the trials (Fraser Valley strain n = 2; Blackwater strain n = 5), because jumping can be caused by flow attraction as well as avoidance. We ran two models; for the first model we removed additional fish from the analysis if they did not enter both sides of the two-channel flume after TDG supersaturate water was introduced or did not enter either of the channels during both of the 2 min behavior trials with air-equilibrated and TDG supersaturated water (Fraser Valley strain n = 1; Blackwater strain n = 3). We did this because the fish would not have had the opportunity to experience the TDG level in each channel and to potentially avoid one treatment in favor of the other. The second model included fish whether they entered both channels during the experiment or not.
We concealed the treatments from a researcher who reanalyzed a subset of the behavior trials. The author NKP removed the treatment information from the video trials of 30 randomly selected fish. The author BRK used Ethovision to track the fish in the same way described above for each of the two trials with air-equilibrated and TDG supersaturated water per fish. There was no effect of concealing the treatments from one of the authors on the results from the video analysis of the time spent by fish in each treatment.
Statistical analysis
To determine whether there was a difference in the duration of time spent by fish in the TDG or air-equilibrated treatments, we modeled the time spent in each channel of the flume with fixed effects for treatment (TDG or air-equilibrated water), time (whether it was the second or third trial), the channel (channel A or B), fish strain, flowrate, the combined time spent in the channels during the baseline trial, and a random effect for fish ID. Because the data were zero-inflated we used the package ‘glmmTMB’ (Version 1.0.1, Brooks et al., 2017) from the R environment to make generalized linear mixed effect models with a Tweedie distribution and a log-link. We checked model assumptions using the ‘DHARMa’ package (version 0.2.7, Hartig, 2020, http://florianhartig.github.io/DHARMa/). We compared models with the drop1 method using AIC values with a threshold of 2 AIC units to determine if two models were different.
We compared the behavior data collected by two of the authors to determine whether concealing the treatments affected the results. We summed the time spent by the fish in each treatment between the second and third trial for each fish. We compared the time spent by the fish in each treatment using a robust between-subjects ANOVA on the 20% trimmed means (bwtrim(), package ‘WRS2’, version 1.0.0, Mair & Wilcox, 2019) with the observer, the treatment, and their interaction as fixed effects and a random effect for fish ID.
Results
Mean TDG levels at the end of behavioral trials were 106% TDG (± 0.5; mean pressure above ambient air pressure (ΔP) = 49 mmHg) for the air-equilibrated treatment and 144% TDG (± 0.5; ΔP = 334 mmHg) for the TDG supersaturated treatment. The TDG treatments differed somewhat between strains; the range of TDG values was greater for the Fraser Valley strain in both the air-equilibrated treatments (mean 107% TDG (± 1; ΔP = 55 mmHg); range 103–118% TDG) and the TDG supersaturated treatments (mean 143% TDG (± 1; ΔP = 326 mmHg); range 134–151% TDG) compared to the Blackwater strain air-equilibrated treatments (mean 106% TDG (± 0.3; ΔP = 44 mmHg); range 103–111% TDG) and the TDG supersaturated treatments (mean 145% TDG (± 0.3; ΔP = 341 mmHg); range 140–148% TDG). Air-equilibrated treatments above 104% TDG were the result of mixing in the flume. However, in all trials TDG treatments were at least 29% TDG greater than the air-equilibrated treatments. The mean water temperature for all subjects was 11.4°C (± 0.0) in the channels of the flume at the end of the trials. The mean temperature in the air-equilibrated treatment for all fish was slightly higher (0.2°C (± 0.0)) than the TDG supersaturated treatment at the end of the trials. Mean dissolved oxygen for all fish was 12.89 mg/l (± 0.05) in the air-equilibrated treatments and 15.53 mg/l (± 0.06) in the TDG supersaturated treatments. The mean flowrate into the channels of the flume for all fish and treatments during the trials was 4.50 l/min (± 0.0). For a comparison of water conditions between treatments and strains see Table 2. The pH in the flume ranged from 6.6 to 6.8 during the trials, and ammonia and nitrite were undetectable in the flume throughout the experiment.
The model of the time spent in each channel of the flume with air-equilibrated and TDG supersaturated treatments for the dataset in which all fish entered both channels was improved by the inclusion of TDG treatment, flowrate in each channel, and the baseline data as fixed effects, but not by the inclusion of channel, strain, or time (whether it was the second or third trial) (Table 3). The mean amount of time spent in the channel with TDG supersaturation was 11 s greater than the time spent in the channel with air-equilibrated water (Fig. 3), which indicates that fish did not avoid the TDG supersaturation treatment in favor of the air-equilibrated treatment. Fish preferred the mixed area of the flume near the outflow; on average, fish spent 72% (87 s, n = 64 fish, two trials per fish) of each 2 min trial in the mixed area of the flume during the trials with TDG supersaturated and air-equilibrated water.
The duration spent in air-equilibrated and TDG supersaturated channels by rainbow trout (Oncorhynchus mykiss) from two strains; a Fraser Valley, n = 31, b Blackwater n = 33, and c both strains combined, n = 64 in a two-channel flume. Each point is the sum of the durations for two trials for a single fish, where we switched air-equilibrated and TDG supersaturated treatments between channels for those two trials. There was no significant effect of channel or trial sequence on duration. Gray lines connect paired observations for individual fish. The combined duration of the two trials for each fish was 240 s; fish also had the choice to spend time in an arena with an intermediate TDG level that connected the two channels
The model of the time spent in each channel of the flume with air-equilibrated and TDG supersaturated treatments for the dataset in which all fish were included (n = 68), whether they entered both channels during the experiment or not, was improved by the inclusion of channel, flowrate in each channel, and the baseline data as fixed effects, but not by the inclusion of TDG treatment, strain, or time (whether it was the second or third trial) (Online Resource 1).
Discussion
Our results indicate that rainbow trout from two strains were unable to detect and avoid TDG supersaturation using lateral movements. Rainbow trout in our experiment spent slightly more time in the channel with TDG supersaturated water than the channel with air-equilibrated water during the trials. The results support our hypothesis that fish cannot detect and avoid harmful TDG supersaturation using lateral movements during an acute exposure.
Two similar studies also report a lack of lateral avoidance to TDG in steelhead. Blahm et al. (1976) found that steelhead exhibited 50% mortality from GBT after 43 h in a two-channel flume containing 130% TDG in one channel and air-equilibrated water in the other channel, indicating that fish were not able to detect and thus avoid lethal levels of TDG supersaturation. Stevens et al. (1980) reported that the number of steelhead observed in each compartment of a rosette-shaped avoidance test tank with 145%, 125%, 115%, and 100% TDG treatments did not differ significantly between treatments. In the same experiment, significantly fewer rainbow trout were observed in compartments with high levels of TDG relative to compartments with low levels of TDG. However, 80% of the rainbow trout in this experiment died from GBT, which suggests that the fish were unable to avoid lethal exposures to TDG despite some avoidance behavior. Both the experiments by Blahm et al. (1976) and Stevens et al. (1980) suggest that some strains of O. mykiss do not use lateral movements to avoid TDG supersaturation in shallow test arenas. However, the animals in these experiments were tested in groups and juvenile rainbow and steelhead trout are known to exhibit territorial behavior and social hierarchies, which has the potential to obscure avoidance behavior. For example, Stevens et al. (1980) observed territorial behavior in rainbow and steelhead trout during their lateral avoidance experiments. Ours is the first lateral TDG avoidance study to test rainbow trout individually, and our results reflect previous findings that most strains of rainbow and steelhead trout do not avoid TDG supersaturation.
Interestingly, other species have been reported to avoid TDG supersaturation as low as 125% in lateral choice tests, including black bullhead (Gray et al., 1983), coho salmon (Stevens et al., 1980), chinook salmon (Blahm et al., 1976; Stevens et al., 1980), rock carp (Huang et al., 2010; Wang et al., 2015a), and ya-fish (Wang et al., 2015b) (Table 1). We note that these experiments differ in the %TDG exposures, the number of fish tested in a group, and the duration of exposure. Our results indicate no avoidance of TDG supersaturation during a 2-min exposure after a 4-min acclimation period. However, studies that did detect lateral TDG supersaturation avoidance generally exposed fish to TDG for hours (between 2 and 48 h), with the exception of Huang et al. (2010) and Wang et al. (2015a, b), and reported avoidance only after multiple hours of exposure (Stevens et al., 1980; Gray et al., 1983). Thus, it is possible that fish have a mechanism for detecting TDG supersaturation over longer timescales, although no mechanism has been proposed by the authors of lateral avoidance studies. Fish may become positively buoyant during longer exposures to TDG supersaturation (e.g., Shrimpton et al., 1990), with unknown effects on lateral movements. The short duration of the TDG exposure in our study may preclude the potential behavioral effects of positive buoyancy. Whereas TDG supersaturation avoidance after several hours of exposure may be protective at lower TDG levels, O. mykiss reach 50% mortality in as few as 2 h of exposure to 140% TDG (Nebeker et al., 1979), which suggests that a lack of short-term TDG detection and avoidance at high TDG supersaturation exposures in shallow water can lead to sublethal and potentially lethal harm to fish.
Other studies have demonstrated vertical avoidance of TDG supersaturation in deep tanks (e.g., Dawley et al., 1976; Chamberlain et al., 1980; Wang et al., 2015a) including rainbow trout (Shrimpton et al., 1990). In this scenario, the TDG content of the water is uniform throughout the water column, but hydrostatic pressure reduces the effects of TDG on GBT as depth increases (Pleizier et al., 2020b). In vertical avoidance tests fish generally began avoiding TDG after > 1 h of exposure, which is much longer than our TDG exposure treatments. It is possible that fish tested for vertical avoidance become positively buoyant at the surface as a result of swimbladder over-inflation and then increase depth to maintain neutral buoyancy, rather than choosing to avoid TDG supersaturation per se. This mechanism would not apply to species without swimbladders; it is unknown whether species without swimbladders change their vertical position to avoid TDG supersaturation.
Fish spent 72% more time in the mixed area compared to the channels of the flume, but the motivation to spend more time in this area is unknown. TDG was not measured in the mixed area during experiments but based on measurements made during preliminary experiments and on mixing during dye tests, we assume that TDG in this area was intermediate between the two channels. Intermediate values of TDG in the mixed area were likely above the threshold for GBT (76 mmHg or 110% TDG at an atmospheric pressure of 760 mmHg, Fidler, 1988), and thus the mixed area was unlikely to be a refuge from harmful levels of TDG supersaturation. We note, however, that in some experiments fish avoided high levels of TDG but not lower levels that could nevertheless be harmful over time (Huang et al., 2010; Wang et al., 2015a, b; see Table 1). If fish did display avoidance of TDG, this response might have been weaker toward the lower TDG level in the mixed area compared to the high TDG level in the treatment channel. Our results, however, do not indicate avoidance of high levels of TDG in the TDG supersaturation treatment channel compared to the air-equilibrated channel, which suggests that they were unable to detect and avoid harmful levels of TDG supersaturation in this scenario.
Dissolved oxygen in the air-equilibrated treatment channel had a mean saturation of 119% (although mean TDG was 106%), with potential effects on fish behavior. Atlantic salmon exposed to low levels of hyperoxia can display increased activity and small differences in spatial distribution relative to fish in control treatments (Espmark et al., 2009, 2010), although exposures in these experiments were 1 week or longer and dissolved oxygen levels tested differed from our own. Thus, we cannot discount the possibility that the behavior of fish in the air-equilibrated treatment in our experiment differs somewhat from fish exposed to 100% TDG treated water at normoxia; however, this would require further investigation.
Whereas fish spent a mean duration of 11 s longer in the TDG supersaturated treatment channel over a 240 s period compared to the air-equilibrated treatment channel, there were individual differences in the duration spent in each treatment. If we sum the time spent in each treatment over the two trials, we observe that 31 individuals of the 64 tested spent more time in air-equilibrated treatment than the TDG supersaturated treatment. We also observe an outlier; one fish spent 197 s in the TDG supersaturated treatment, which is almost twice as long as any other individual. If we remove this individual from the dataset, fish spent a mean duration of only 8 s longer in the TDG supersaturated treatment compared to the air-equilibrated treatment (n = 64). Individual differences in time spent in each treatment were not explained by strain and may be the outcome of either chance or some unknown variable.
Our results indicate that some fish species, such as rainbow trout, may be unable to detect and avoid acute TDG supersaturation in shallow water during events such as spilling from hydroelectric dams. This could be problematic for certain species and life stages that are restricted to shallow environments where lateral movements would be necessary to avoid TDG supersaturation. Fish in our experiment did not avoid TDG supersaturation (144% TDG) during an acute exposure to what would otherwise be a lethal level during longer exposures; Nebeker et al. (1979) report that juvenile steelhead reach 50% mortality in 2 to 5 h in 140% TDG at depths less than 60 cm. Fish in deep water have the potential to avoid GBT by increasing depth as a response to positive buoyancy, although experimental evidence suggests that not all individuals will depth compensate when exposed to TDG supersaturation (e.g., Shrimpton et al., 1990). Based on our results, we cannot assume that fish will seek deeper water due to detection of TDG supersaturation exposures in the field. Whereas some fish will maintain their depth in TDG supersaturated conditions (e.g., Johnson et al., 2005, 2010), others have the potential to become positively buoyant as a result of GBT (Shrimpton et al., 1990), which may cause them to occupy shallower depths during TDG exposure. For this reason, we recommend that managers consider the effects of exposure at the surface when estimating the effects of TDG supersaturation on wild fish to make conservative estimates of potential impacts of TDG on fish.
We recommend additional studies to confirm whether other species can avoid TDG supersaturation using lateral and vertical movements when tested as individuals. If certain species can avoid TDG supersaturation using lateral movements, as indicated by some previous studies, it would be of interest to determine the mechanism by which they detect TDG supersaturation. Understanding TDG detection mechanisms would allow us to predict which additional species may be able to avoid TDG supersaturation. In our study, both strains of rainbow trout have no known history of exposure to TDG supersaturation. Lateral and vertical avoidance tests comparing species from different populations in which one has a long history of exposure to TDG supersaturation would be of interest to determine whether populations can have behavioral adaptations to TDG supersaturation.
Data availability
The datasets generated during this study are available in the UBC Research Data Collection, Scholars Portal Dataverse [https://doi.org/10.5683/SP2/HGE71B].
References
Antcliffe, B. L., L. E. Fidler & I. K. Birtwell, 2003. Lethal and sublethal responses of rainbow trout (Oncorhynchus mykiss) and coho (Oncorhynchus kisutch) fry to elevated dissolved gas supersaturation and temperature. Vancouver, British Columbia: Fisheries and Oceans Canada, Habitat and Enhancement Branch.
Baxter, R. M., 1977. Environmental effects of dams and impoundments. Annual Review of Ecology, Evolution, and Systematics 8: 255–283.
Bentley, W. W., E. M. Dawley & T. W. Newcomb, 1976. Some effects of excess dissolved gas on squawfish, Ptychocheilus oregonensis (Richardson). In Fickeisen, D. H. & M. J. Schneider (eds). Gas Bubble Disease. Richland, Washington: Technical Information Center, Office of Public Affairs Energy Research and Development Administration. pp. 41–46.
Blahm, T. H., B. McConnell & G. R. Snyder, 1976. Gas Supersaturation Research, National Marine Fisheries Service Prescott Facility-1971 to 1974. In Fickeisen, D. H. & M. J. Schneider (eds). Gas Bubble Disease. Richland, Washington: Technical Information Center, Office of Public Affairs Energy Research and Development Administration. pp. 11–19.
Blumstein, D. T. & O. Berger-Tal, 2015. Understanding sensory mechanisms to develop effective conservation and management tools. Current Opinion in Behavioral Sciences 6: 13–18.
Bouck, G. R., G. A. Chapman, P. W. Schneider Jr. & D. G. Stevens, 1976. Observations on gas bubble disease among wild adult Columbia River fishes. Transactions of the American Fisheries Society 105: 114–115.
Brooks, M. E., K. Kristensen, K. J. van Benthem, A. Magnusson, C. W. Berg, A. Nielsen, H. J. Skaug, M. Maechler & B. M. Bolker, 2017. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R Journal, 9: 378–400.
Buchholz, R., 2007. Behavioural biology: an effective and relevant conservation tool. Trends in Ecology and Evolution 22: 401–407.
Chamberlain, G. W., W. H. Neill, P. A. Romanowsky & K. Strawn, 1980. Vertical responses of Atlantic croaker to gas supersaturation and temperature change. Transactions of the American Fisheries Society 109: 737–750.
Chen, S., X. Liu, W. Jiang, K. Li, J. Du, D. Shen & Q. Gong, 2012. Effects of total dissolved gas supersaturated water on lethality and catalase activity of Chinese sucker (Myxocyprinus asiaticus Bleeker). Journal of Zhejiang University Science B 13: 791–796.
Cooke, S. J., Blumstein, D. T., Buchholz, R., Caro, T., Fernandez-Juricic, E., Franklin, C. E., Metcalfe, J., O’Connor, C. M., St. Clair, C. C., Sutherland, W. J. & M. Wikelski, 2014. Physiology, behavior, and conservation. Physiological and Biochemical Zoology 87: 1–14.
Cornacchia, J. W. & J. E. Colt, 1984. The effects of dissolved gas supersaturation on larval striped bass, Morone saxatilis (Walbaum). Journal of Fish Diseases 7: 15–27.
Counihan, T. D., Miller, A. I., Mesa, M. G., & M. J. Parsley, 1998. The effects of dissolved gas supersaturation on white sturgeon larvae. Transactions of the American Fisheries Society 127: 316–322.
Couto, T. B. & J. D. Olden, 2018. Global proliferation of small hydropower plants–science and policy. Frontiers in Ecology and the Environment 16: 91–100.
Dawley, E. M., M. Schiewe & B. Monk, 1976. Effects of long-term exposure to supersaturation of dissolved atmospheric gases on juvenile chinook salmon and steelhead trout in deep and shallow test tanks. In Fickeisen, D. H. & M. J. Schneider (eds). Gas Bubble Disease. Richland, Washington: Technical Information Center, Office of Public Affairs Energy Research and Development Administration. pp. 1–20.
Dixson, D. L., P. L. Munday & G. P. Jones, 2010. Ocean acidification disrupts the innate ability of fish to detect predator olfactory cues. Ecology Letters 13: 68–75.
Dynesius, M. & C. Nilsson, 1994. Fragmentation and flow regulation of river systems in the northern third of the world. Science 266: 753–762.
Ebel, W. J., 1969. Supersaturation of nitrogen in the Columbia River and its effect on salmon and steelhead trout. Fishery Bulletin 68: 1–11.
Espmark, Å. M. & G. Baeverfjord, 2009. Effects of hyperoxia on behavioural and physiological variables in farmed Atlantic salmon (Salmo salar) parr. Aquaculture International 17: 341–353.
Espmark, Å. M., K. Hjelde, & G. Baeverfjord, 2010. Development of gas bubble disease in juvenile Atlantic salmon exposed to water supersaturated with oxygen. Aquaculture 306: 198–204.
Geist, D. R., T. J. Linley, V. Cullinan & Z. Q. Deng, 2013. Effects of total dissolved gas on chum salmon fry survival, growth, gas bubble disease, and seawater tolerance. North American Journal of Fisheries Management 33: 200–215.
Gilmour, K. M., J. DiBattista & J. Thomas, 2005. Physiological causes and consequences of social status in salmonid fish. Integrative and Comparative Biology 45: 263–273.
Gray, R. H., T. L. Page & M. G. Saroglia, 1983. Behavioral response of carp, Cyprinus carpio, and black bullhead, Ictalurus melas, from Italy to gas supersaturated water. Environmental Biology of Fishes 8: 163–167.
Hartig, F., 2020. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level / Mixed) Regression Models. R package version 0.2.7. https://CRAN.R-project.org/package=DHARMa.
Huang, X., K. Li, J. Du & R. Li, 2010. Effects of gas supersaturation on lethality and avoidance responses in juvenile rock carp (Procypris rabaudi Tchang). Journal of Zhejiang University Science B 11: 806–811.
International Commission On Large Dams, 2019. General synthesis. Retrieved April 9, 2020, from https://www.icold-cigb.org/GB/world_register/general_synthesis.asp.
Johnson, E. L., T. S. Clabough, D. H. Bennett, T. C. Jornn, C. A. Peery, C. C. Caudil & L. C. Stuehrenberg, 2005. Migration depths of adult spring and summer chinook salmon in the lower Columbia and Snake Rivers in relation to dissolved gas supersaturation. Transactions of the American Fisheries Society 134: 1213–1227.
Johnson, E. L., T. S. Clabough, C. C. Caudill, M. L. Keefer, C. A. Peery & M. C. Richmond, 2010. Migration depths of adult steelhead Oncorhynchus mykiss in relation to dissolved gas supersaturation in a regulated river system. Journal of Fish Biology 76: 1520–1528.
Jutfelt, F., J. Sundin, G. D. Raby, A. S. Krång & T. D. Clark, 2017. Two‐current choice flumes for testing avoidance and preference in aquatic animals. Methods in Ecology and Evolution 8: 379–390.
Ligon, F. K., W. E. Dietrich & W. J. Trush, 1995. Downstream ecological effects of dams. BioScience 45: 183–192.
Lund, M. & T. G. Heggberget, 1985. Avoidance response of two-year-old rainbow trout, Salmo gairdneri R., to air-supersaturated water: hydrostatic compensation. Journal of Fish Biology 26: 193–200.
Mair, P. & R. Wilcox, 2019. Robust statistical methods in R using the WRS2 package. Behaviour Research Methods 52: 464–488.
Nebeker, A. V., K. A. Hauck & F. D. Baker, 1979. Temperature and oxygen-nitrogen gas ratios affect fish survival in air-supersaturated water. Water Research 13: 299–303.
Ou, M., T. J. Hamilton, J. Eom, E. M. Lyall, J. Gallup, A. Jiang, J. Lee, D. A. Close, S. Yun & C. J. Brauner, 2015. Responses of pink salmon to CO2-induced aquatic acidification. Nature Climate Change 5: 950–955.
Pleizier, N. K., D. Algera, S. J. Cooke & C. J. Brauner, 2020a. A meta-analysis of gas bubble trauma in fish. Fish & Fisheries 21: 1175–1194.
Pleizier, N. K., C. Nelson, S. J. Cooke & C. J. Brauner, 2020b. Understanding gas bubble trauma in an era of hydropower expansion: how do fish compensate at depth? Canadian Journal of Fisheries and Aquatic Sciences 77: 556–563.
Schisler, G. J., E. P. Bergersen & P. G. Walker, 2000. Effects of multiple stressors on morbidity and mortality of fingerling rainbow trout infected with Myxobolus cerebralis. Transactions of the American Fisheries Society 129: 859–865.
Shrimpton, J. M., D. J. Randall & L. E. Fidler, 1990. Assessing the effects of positive buoyancy on rainbow trout (Oncorhynchus mykiss) held in gas supersaturated water. Canadian Journal of Zoology 68: 969–973.
Sloman, K. A. & J. D. Armstrong. 2002. Physiological effects of dominance hierarchies: laboratory artefacts or natural phenomena. Journal of Fish Biology 61: 1–23.
Stevens, D. G., A. V. Nebeker & R. J. Baker, 1980. Avoidance responses of salmon and trout to air‐supersaturated water. Transactions of the American Fisheries Society 109: 751–754.
Stroud, R. K., G. R. Bouck & A. V. Nebeker, 1975. Pathology of acute and chronic exposure of salmonid fishes to supersaturated water. In Chemistry and Physics of Aqueous Gas Solutions. Princeton, New Jersey: Electrothermics and Metallurgy and Industrial Electrolytic Divisions, Electrochemical Society. pp. 435–449.
Tanner, D. Q. & M. W. Johnston, 2001. Data-collection methods, quality assurance data, and site considerations for total dissolved gas monitoring, lower Columbia River, Oregon and Washington, 2000. Oregon Water Resources Department, Portland. Investigation Report 01-4005, p. 1–19.
Wang, Y., R. Liang, Y. Tuo, K. Li, & B. Hodges, 2015a. Tolerance and avoidance behavior towards gas supersaturation in rock carp Procypris rabaudi with a history of previous exposure. North American Journal of Aquaculture 77: 478–484.
Wang, Y., K. Li, J. Li, R. Li & Y. Deng, 2015b. Tolerance and avoidance characteristics of Prenant’s schizothoracin Schizothorax prenanti to total dissolved gas supersaturated water. North American Journal of Fisheries Management 35: 827–834.
Zarfl, C., A. E. Lumsdon, J. Berlekamp, L. Tydecks, & K. Tockner, 2015. A global boom in hydropower dam construction. Aquatic Science 77: 161–170.
Acknowledgments
The authors acknowledge the technical assistance of Bruce Gillespie, Vincent Grant, and Pak Chan. We also thank the Freshwater Fisheries Society of BC for supplying the study animals.
Funding
This work was supported by a Natural Sciences and Engineering Research Council (NSERC) Collaborative Research and Development (CRD) Grant (CRDPJ 474297-14) to CJB and SJC in partnership with BC Hydro. NP was supported by an NSERC Post-Graduate Scholarship (Grant No. PGS 6564) and the University of British Columbia Zoology Graduate Fellowship and Tuition Award (Grant Nos. 4YF 6569, FYF 6456).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
To the best of our knowledge, none of the authors have any conflict of interest that might influence their objectivity on the topic of this manuscript.
Ethical approval
All experiments were conducted in accordance with the guidelines of the Canadian Council on Animal Care as administered by the University of British Columbia (A19-0284).
Additional information
Handling editor: Fernando M. Pelicice
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pleizier, N.K., Rost-Komiya, B., Cooke, S.J. et al. The lack of avoidance of total dissolved gas supersaturation in juvenile rainbow trout. Hydrobiologia 848, 4837–4850 (2021). https://doi.org/10.1007/s10750-021-04676-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-021-04676-w